Abstract
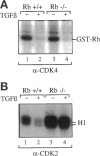
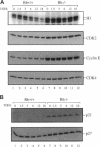
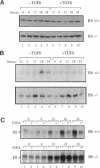
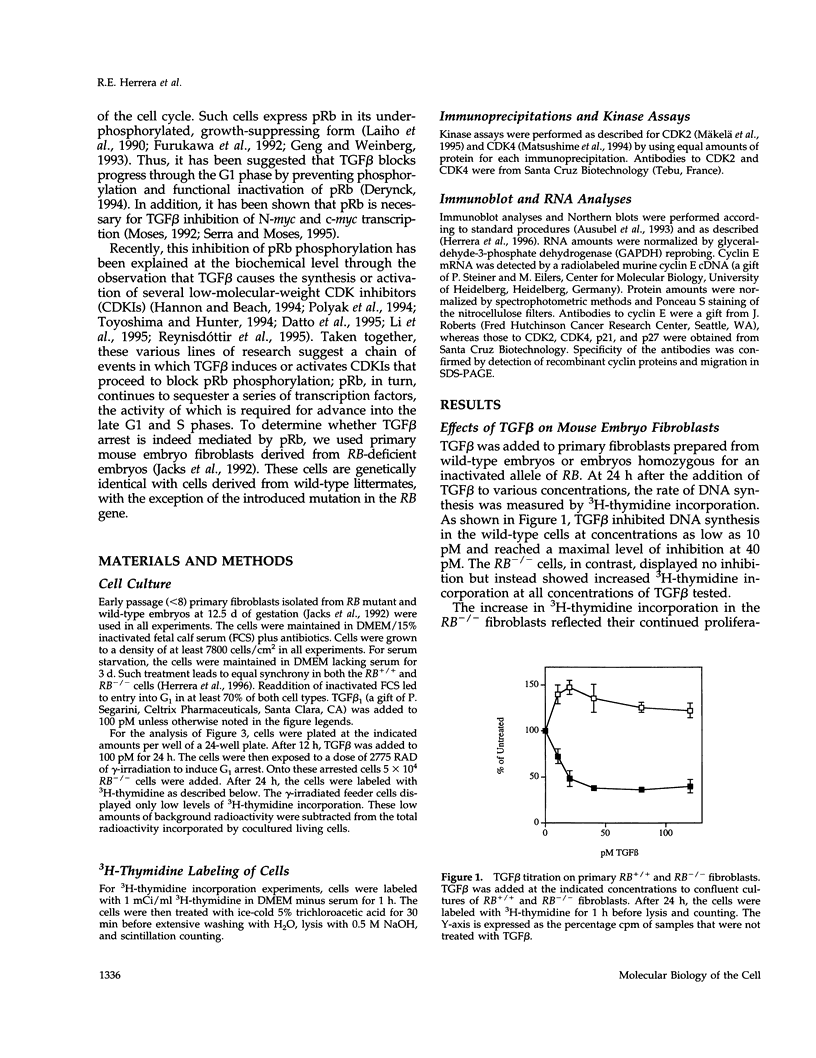
Transforming growth factor beta (TGF beta) inhibits cell proliferation by inducing a G1 cell-cycle arrest. Cyclin/CDK complexes have been implicated in this arrest, because TGF beta treatment leads to inhibition of cyclin/CDK activity. We have investigated the role of the retinoblastoma protein (pRb) in TGF beta-induced growth arrest by using RB+/+ and RB-/- primary mouse embryo fibroblasts. In both of these cell types, TGF beta inhibits CDK4-associated kinase activity. However, whereas CDK2-associated kinase activity was completely inhibited by TGF beta in the wild-type cells, it was reduced only slightly in the RB mutant cells. In addition, at high-cell density the growth-inhibitory effects of TGF beta are no longer observed in the RB-/- cells; on the contrary, TGF beta treatment promotes the growth of these mutant fibroblasts. Thus, under certain cellular growth conditions, elimination of pRb transforms the growth-inhibitory effects of TGF beta into growth-stimulatory effects. These observations could help to explain why TGF beta is often found to enhance tumorigenicity in vivo and why inactivation of the RB gene leads to tumorigenesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrick B. A., Lopez A. R., Elfman F., Ebner R., Damsky C. H., Derynck R. Altered metabolic and adhesive properties and increased tumorigenesis associated with increased expression of transforming growth factor beta 1. J Cell Biol. 1992 Aug;118(3):715–726. doi: 10.1083/jcb.118.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteaga C. L., Carty-Dugger T., Moses H. L., Hurd S. D., Pietenpol J. A. Transforming growth factor beta 1 can induce estrogen-independent tumorigenicity of human breast cancer cells in athymic mice. Cell Growth Differ. 1993 Mar;4(3):193–201. [PubMed] [Google Scholar]
- Arteaga C. L., Hurd S. D., Winnier A. R., Johnson M. D., Fendly B. M., Forbes J. T. Anti-transforming growth factor (TGF)-beta antibodies inhibit breast cancer cell tumorigenicity and increase mouse spleen natural killer cell activity. Implications for a possible role of tumor cell/host TGF-beta interactions in human breast cancer progression. J Clin Invest. 1993 Dec;92(6):2569–2576. doi: 10.1172/JCI116871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchkovich K., Duffy L. A., Harlow E. The retinoblastoma protein is phosphorylated during specific phases of the cell cycle. Cell. 1989 Sep 22;58(6):1097–1105. doi: 10.1016/0092-8674(89)90508-4. [DOI] [PubMed] [Google Scholar]
- Chang H. L., Gillett N., Figari I., Lopez A. R., Palladino M. A., Derynck R. Increased transforming growth factor beta expression inhibits cell proliferation in vitro, yet increases tumorigenicity and tumor growth of Meth A sarcoma cells. Cancer Res. 1993 Sep 15;53(18):4391–4398. [PubMed] [Google Scholar]
- Chen P. L., Scully P., Shew J. Y., Wang J. Y., Lee W. H. Phosphorylation of the retinoblastoma gene product is modulated during the cell cycle and cellular differentiation. Cell. 1989 Sep 22;58(6):1193–1198. doi: 10.1016/0092-8674(89)90517-5. [DOI] [PubMed] [Google Scholar]
- Datto M. B., Li Y., Panus J. F., Howe D. J., Xiong Y., Wang X. F. Transforming growth factor beta induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5545–5549. doi: 10.1073/pnas.92.12.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Lynch D., Furukawa Y., Griffin J., Piwnica-Worms H., Huang C. M., Livingston D. M. The product of the retinoblastoma susceptibility gene has properties of a cell cycle regulatory element. Cell. 1989 Sep 22;58(6):1085–1095. doi: 10.1016/0092-8674(89)90507-2. [DOI] [PubMed] [Google Scholar]
- Derynck R. TGF-beta-receptor-mediated signaling. Trends Biochem Sci. 1994 Dec;19(12):548–553. doi: 10.1016/0968-0004(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Ewen M. E., Sluss H. K., Sherr C. J., Matsushime H., Kato J., Livingston D. M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993 May 7;73(3):487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- Ewen M. E., Sluss H. K., Whitehouse L. L., Livingston D. M. TGF beta inhibition of Cdk4 synthesis is linked to cell cycle arrest. Cell. 1993 Sep 24;74(6):1009–1020. doi: 10.1016/0092-8674(93)90723-4. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick D. R., Bielefeldt-Ohmann H., Himbeck R. P., Jarnicki A. G., Marzo A. L., Robinson B. W. Transforming growth factor-beta: antisense RNA-mediated inhibition affects anchorage-independent growth, tumorigenicity and tumor-infiltrating T-cells in malignant mesothelioma. Growth Factors. 1994;11(1):29–44. doi: 10.3109/08977199409015049. [DOI] [PubMed] [Google Scholar]
- Furukawa Y., Uenoyama S., Ohta M., Tsunoda A., Griffin J. D., Saito M. Transforming growth factor-beta inhibits phosphorylation of the retinoblastoma susceptibility gene product in human monocytic leukemia cell line JOSK-I. J Biol Chem. 1992 Aug 25;267(24):17121–17127. [PubMed] [Google Scholar]
- Fynan T. M., Reiss M. Resistance to inhibition of cell growth by transforming growth factor-beta and its role in oncogenesis. Crit Rev Oncog. 1993;4(5):493–540. [PubMed] [Google Scholar]
- Geng Y., Eaton E. N., Picón M., Roberts J. M., Lundberg A. S., Gifford A., Sardet C., Weinberg R. A. Regulation of cyclin E transcription by E2Fs and retinoblastoma protein. Oncogene. 1996 Mar 21;12(6):1173–1180. [PubMed] [Google Scholar]
- Geng Y., Weinberg R. A. Transforming growth factor beta effects on expression of G1 cyclins and cyclin-dependent protein kinases. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10315–10319. doi: 10.1073/pnas.90.21.10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon G. J., Beach D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature. 1994 Sep 15;371(6494):257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- Herrera R. E., Sah V. P., Williams B. O., Mäkelä T. P., Weinberg R. A., Jacks T. Altered cell cycle kinetics, gene expression, and G1 restriction point regulation in Rb-deficient fibroblasts. Mol Cell Biol. 1996 May;16(5):2402–2407. doi: 10.1128/mcb.16.5.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds P. W., Mittnacht S., Dulic V., Arnold A., Reed S. I., Weinberg R. A. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992 Sep 18;70(6):993–1006. doi: 10.1016/0092-8674(92)90249-c. [DOI] [PubMed] [Google Scholar]
- Huggett A. C., Ellis P. A., Ford C. P., Hampton L. L., Rimoldi D., Thorgeirsson S. S. Development of resistance to the growth inhibitory effects of transforming growth factor beta 1 during the spontaneous transformation of rat liver epithelial cells. Cancer Res. 1991 Nov 1;51(21):5929–5936. [PubMed] [Google Scholar]
- Jacks T., Fazeli A., Schmitt E. M., Bronson R. T., Goodell M. A., Weinberg R. A. Effects of an Rb mutation in the mouse. Nature. 1992 Sep 24;359(6393):295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- Kato J., Matsushime H., Hiebert S. W., Ewen M. E., Sherr C. J. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993 Mar;7(3):331–342. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- Koff A., Ohtsuki M., Polyak K., Roberts J. M., Massagué J. Negative regulation of G1 in mammalian cells: inhibition of cyclin E-dependent kinase by TGF-beta. Science. 1993 Apr 23;260(5107):536–539. doi: 10.1126/science.8475385. [DOI] [PubMed] [Google Scholar]
- Laiho M., DeCaprio J. A., Ludlow J. W., Livingston D. M., Massagué J. Growth inhibition by TGF-beta linked to suppression of retinoblastoma protein phosphorylation. Cell. 1990 Jul 13;62(1):175–185. doi: 10.1016/0092-8674(90)90251-9. [DOI] [PubMed] [Google Scholar]
- Li C. Y., Suardet L., Little J. B. Potential role of WAF1/Cip1/p21 as a mediator of TGF-beta cytoinhibitory effect. J Biol Chem. 1995 Mar 10;270(10):4971–4974. doi: 10.1074/jbc.270.10.4971. [DOI] [PubMed] [Google Scholar]
- Lukas J., Bartkova J., Rohde M., Strauss M., Bartek J. Cyclin D1 is dispensable for G1 control in retinoblastoma gene-deficient cells independently of cdk4 activity. Mol Cell Biol. 1995 May;15(5):2600–2611. doi: 10.1128/mcb.15.5.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J., Cheifetz S., Laiho M., Ralph D. A., Weis F. M., Zentella A. Transforming growth factor-beta. Cancer Surv. 1992;12:81–103. [PubMed] [Google Scholar]
- Matsushime H., Quelle D. E., Shurtleff S. A., Shibuya M., Sherr C. J., Kato J. Y. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994 Mar;14(3):2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton D. M., Barrack E. R. Modulation of transforming growth factor beta 1 effects on prostate cancer cell proliferation by growth factors and extracellular matrix. Cancer Res. 1995 Jun 15;55(12):2596–2602. [PubMed] [Google Scholar]
- Moses H. L. TGF-beta regulation of epithelial cell proliferation. Mol Reprod Dev. 1992 Jun;32(2):179–184. doi: 10.1002/mrd.1080320215. [DOI] [PubMed] [Google Scholar]
- Mäkelä T. P., Parvin J. D., Kim J., Huber L. J., Sharp P. A., Weinberg R. A. A kinase-deficient transcription factor TFIIH is functional in basal and activated transcription. Proc Natl Acad Sci U S A. 1995 May 23;92(11):5174–5178. doi: 10.1073/pnas.92.11.5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M. J. Transforming growth factor beta and the cell surface in tumor progression. Cancer Metastasis Rev. 1993 Sep;12(3-4):239–254. doi: 10.1007/BF00665956. [DOI] [PubMed] [Google Scholar]
- Park K., Kim S. J., Bang Y. J., Park J. G., Kim N. K., Roberts A. B., Sporn M. B. Genetic changes in the transforming growth factor beta (TGF-beta) type II receptor gene in human gastric cancer cells: correlation with sensitivity to growth inhibition by TGF-beta. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8772–8776. doi: 10.1073/pnas.91.19.8772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K., Kato J. Y., Solomon M. J., Sherr C. J., Massague J., Roberts J. M., Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994 Jan;8(1):9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- Resnitzky D., Reed S. I. Different roles for cyclins D1 and E in regulation of the G1-to-S transition. Mol Cell Biol. 1995 Jul;15(7):3463–3469. doi: 10.1128/mcb.15.7.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynisdóttir I., Polyak K., Iavarone A., Massagué J. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-beta. Genes Dev. 1995 Aug 1;9(15):1831–1845. doi: 10.1101/gad.9.15.1831. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., McCune B. K., Sporn M. B. TGF-beta: regulation of extracellular matrix. Kidney Int. 1992 Mar;41(3):557–559. doi: 10.1038/ki.1992.81. [DOI] [PubMed] [Google Scholar]
- Rodeck U., Bossler A., Graeven U., Fox F. E., Nowell P. C., Knabbe C., Kari C. Transforming growth factor beta production and responsiveness in normal human melanocytes and melanoma cells. Cancer Res. 1994 Jan 15;54(2):575–581. [PubMed] [Google Scholar]
- Serra R., Moses H. L. pRb is necessary for inhibition of N-myc expression by TGF-beta 1 in embryonic lung organ cultures. Development. 1995 Sep;121(9):3057–3066. doi: 10.1242/dev.121.9.3057. [DOI] [PubMed] [Google Scholar]
- Smith R. D. The anti-proliferative action of transforming growth factor-beta 1 on a rat intestinal epithelial cell line (RIE-1) is dependent on cell population density and culture passage number. Int J Biochem. 1994 Jul;26(7):899–903. doi: 10.1016/0020-711x(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Steiner M. S., Barrack E. R. Transforming growth factor-beta 1 overproduction in prostate cancer: effects on growth in vivo and in vitro. Mol Endocrinol. 1992 Jan;6(1):15–25. doi: 10.1210/mend.6.1.1738367. [DOI] [PubMed] [Google Scholar]
- Suardet L., Gaide A. C., Calmès J. M., Sordat B., Givel J. C., Eliason J. F., Odartchenko N. Responsiveness of three newly established human colorectal cancer cell lines to transforming growth factors beta 1 and beta 2. Cancer Res. 1992 Jul 1;52(13):3705–3712. [PubMed] [Google Scholar]
- Toyoshima H., Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994 Jul 15;78(1):67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- Tsai L. H., Lees E., Faha B., Harlow E., Riabowol K. The cdk2 kinase is required for the G1-to-S transition in mammalian cells. Oncogene. 1993 Jun;8(6):1593–1602. [PubMed] [Google Scholar]
- Ueki N., Nakazato M., Ohkawa T., Ikeda T., Amuro Y., Hada T., Higashino K. Excessive production of transforming growth-factor beta 1 can play an important role in the development of tumorigenesis by its action for angiogenesis: validity of neutralizing antibodies to block tumor growth. Biochim Biophys Acta. 1992 Oct 27;1137(2):189–196. doi: 10.1016/0167-4889(92)90201-l. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. The retinoblastoma protein and cell cycle control. Cell. 1995 May 5;81(3):323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]