Abstract
Background
Several studies have examined serum creatinine as a marker for prostate cancer stage, recurrence, and prognosis. We evaluated whether serum creatinine concentration was associated with risk of developing prostate cancer in a prospective cohort of male smokers.
Methods
A nested case-control study within the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study of 50–69 year old Finnish men was conducted. Two controls (n=464) were matched to each case (n=232) on study center, intervention group, date of baseline blood draw (±45 days), and age (±5 years). Conditional logistic regression was used to calculate odds ratios (OR) and 95% confidence intervals (CI). All p-values were two-sided.
Results
Cases had significantly higher pre-diagnostic serum creatinine concentrations compared with controls (medians of 1.13 vs 1.10 mg/dL, respectively; p=0.004). Serum creatinine was associated with a significantly greater risk of prostate cancer (multivariate OR=2.23, 95% CI 1.33–3.75 for highest vs. lowest quartile), with a significant trend (p-trend = 0.0008). Exclusion of subjects with a reported history of diabetes, benign prostatic hyperplasia, or hypertension, or whose cancer was diagnosed within the first five years of follow-up, did not alter the association. Risk did not differ by disease stage or time from blood draw to diagnosis.
Conclusion
Prospectively measured serum creatinine, within normal ranges, is positively related to prostate cancer risk. Future research should reexamine the association in other populations, including any inter-relationship with serum prostate-specific antigen.
Keywords: prostate cancer, creatinine
Introduction
Prostate cancer is the most commonly diagnosed cancer in American men and the second leading cause of cancer mortality, following lung cancer (1). The only established risk factors for this malignancy are age, family history, race, and some common genetic variants. Studies among prostate cancer patients have shown that serum creatinine is associated with more advanced disease (2,3) and with decreased survival (3,4), although this relationship is not supported by some studies (5–8). Serum creatinine could be a marker for homocysteine status and one carbon metabolism, as synthesis of the fomer results in removal of a methyl group from S-adenosylmethionine, creation of homocysteine, and potentially reducing availability of one-carbon groups for DNA methylation, synthesis, and repair (9,10). We investigated whether baseline serum creatinine concentrations were associated with prostate cancer risk in a prospective cohort study.
Subjects and Methods
The Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study included 29,133 male Finnish smokers, ages 50–69 years, recruited between 1985 and 1988. Study exclusion factors included prior cancer other than non-melanoma skin cancer, severe angina on exertion, chronic renal insufficiency, liver cirrhosis, chronic alcoholism, anticoagulant therapy, other medical problems such as physical disabilities or psychiatric disorders which might limit participation, and current vitamin use greater than pre-defined levels (11). Study participants were provided α-tocopherol and/or β-carotene supplements or placebo for 5–8 years, with post-intervention follow-up continuing through the Finnish Cancer Registry (12). Participants completed detailed dietary questionnaires at baseline (13) and provided fasting serum samples (stored at −70 °C). Height, weight, and blood pressure were measured at baseline and annually during the trial (except height). The study was approved by the institutional review boards of the National Cancer Institute and the National Public Health Institute of Finland. Written informed consent was obtained from all participants.
The cases (n=232) were defined as incident prostate cancers diagnosed through December 1994, with available serum. Medical records were reviewed centrally by two study oncologists for diagnostic confirmation and staging, and cases with histopathologic and cytologic specimens available were reviewed and confirmed by pathologists. Finland has not adopted population-based prostate-specific antigen (PSA) screening programs, and only 1 of the 246 cases that occurred during the trial period was detected by PSA (14).
Two controls (n=464) were matched to each case based on clinic, intervention group, date of baseline blood draw (±45 days), age (±5 years), and serum availability. Cases and controls were assayed in succession within batches along with blinded quality control samples (n=28). Serum creatinine assays were performed using a standard Jaffé reaction method (15). The coefficient of variation, calculated from masked quality control samples, was 3.1% (interbatch) and 1.5% (intrabatch). Serum folate, homocysteine, β-carotene, α-tocopherol, retinol, and cholesterol were previously measured (11,16). Serum data from eight cases and ten controls were unavailable due to cracked serum vials, and was missing for one additional control. In addition, we excluded one control with a reported history of acute renal failure, leaving a total of 224 cases and 452 controls.
Statistical Analysis
Chi-squared tests (for categorical variables) and Wilcoxon rank sum tests (for continuous variables) were used to compare various characteristics of cases and controls. Correlations were determined using Spearman’s rank order coefficient among the control subjects. Conditional logistic regression was used to calculate odds ratios (OR) and 95% confidence intervals (CI), with serum creatinine quartiles based on the distribution among the controls. The unit of assessment for the continuous model was expressed as ½ the interquartile range, based on the control distribution. Hypertension was defined as diastolic blood pressure >= 90 mm Hg and/or systolic blood pressure >= 140 mm Hg. All variables listed in Table 1 were tested for confounding. Potential confounders were defined as variables that were correlated with serum creatinine or those resulting in a >10% change in the creatinine coefficients upon addition to the crude model. These included serum α-tocopherol, serum homocysteine, serum retinol, serum folate, urban residence, and history of diabetes. In addition, we included age at randomization, body mass index (BMI), height, history of benign prostatic hyperplasia (BPH), hypertension at baseline, history of hypertension, and family history of prostate cancer (categorical with a separate category for those missing data) as potential confounders. Backward step-wise assessment indicated that serum homocysteine and family history of prostate cancer had the greatest influence as confounders and are the only factors, in addition to age, included in multivariate-adjusted model I, which is the model used for all additional analyses. The multivariate-adjusted model II includes all potential confounders listed above. Effect modification was evaluated by including the cross-product term of the creatinine quartiles and the effect modifier (split at the median or yes/no) and by stratified analysis, conducted using an unconditional logistic model additionally adjusted for the matching factors. All analyses were performed using SAS Software version 9.1.3 (SAS Institute, Inc., Cary, North Carolina) and all p-values were two-sided.
Table 1.
Selected baseline characteristics (medians and interquartile ranges, or percents) for cases and controls
| Median (25%-75%) or percents |
|||
|---|---|---|---|
| Characteristic | Cases, n=232 | Controls, n=464 | p value* |
| Age (y) | 62 (57–65) | 61 (57–64) | 0.60 |
| Height (cm) | 174 (170–178) | 174 (169–178) | 0.65 |
| Weight (kg) | 79 (71–86) | 78 (70–86) | 0.20 |
| Body mass index (kg/m2) | 26.0 (23.9–28.6) | 25.7 (23.3–28.2) | 0.15 |
| No. of cigarettes smoked per day | 20 (13–25) | 20 (12–25) | 0.95 |
| Years of smoking | 40 (35–45) | 40 (35–45) | 0.67 |
| Benign prostatic hyperplasia (%) | 8.2 | 5.4 | 0.15 |
| Family history of prostate cancer (%)† | 5.4 | 4.3 | 0.62 |
| Physical activity (% active) | 9.9 | 11.6 | 0.49 |
| Urban residence (%) | 65.5 | 64.0 | 0.70 |
| Married (%) | 78.5 | 82.3 | 0.22 |
| Education (% >elementary school) | 19.8 | 23.7 | 0.25 |
| History of chronic renal failure (%) | 0 | 0.43 | 0.32 |
| History of diabetes mellitus (%) | 5.2 | 4.5 | 0.71 |
| History of hypertension (%) | 22.0 | 19.3 | 0.40 |
| Hypertension at baseline (%) | 67.2 | 63.4 | 0.32 |
| Blood pressure – systolic (mm Hg) | 142 (130–158) | 142 (130–156) | 0.77 |
| Blood pressure – diastolic (mm Hg) | 88 (80–96) | 88 (80–94) | 0.32 |
| Daily dietary intake‡ | |||
| Energy (kcal) | 2537 (2069–3065) | 2486 (2091–2994) | 0.66 |
| Protein(g) | 91 (84–99) | 91 (83–99) | 0.95 |
| Fat (g) | 119 (111–130) | 119 (108–128) | 0.22 |
| Carbohydrate (g) | 259 (238–281) | 261 (240–284) | 0.44 |
| Folate (ug) | 328 (291–358) | 330 (292–365) | 0.24 |
| Methionine (mg) | 1923 (1762–2210) | 1957 (1769–2194) | 0.78 |
| Alcohol (g) | 7.9 (1.7–20.5) | 9.3 (1.7–22.9) | 0.37 |
| Meat (g) | 181 | 174 | 0.23 |
| Serum biomarker concentrations§ | |||
| Creatinine (mg/dL) | 1.13 (1.05–1.23) | 1.10 (1.02–1.19) | 0.004 |
| α-Tocopherol (mg/L) | 11.4 (9.7–13.4) | 11.8 (10.0–13.8) | 0.19 |
| β-Carotene (ug/L) | 173 (119–274) | 178 (111–260) | 0.71 |
| Retinol (ug/L) | 575 (508–648) | 572 (493–650) | 0.49 |
| Cholesterol (mmol/L) | 6.25 (5.38–7.04) | 6.14 (5.42–6.90) | 0.66 |
| Folate (ng/mL) | 3.85 (3.04–4.82) | 3.84 (3.03–4.76) | 0.75 |
| Homocysteine (nmol/mL) | 12.2 (10.3–14.3) | 11.8 (10.1–14.2) | 0.74 |
p-value based on Chi-squared tests (for categorical variables) and Wilcoxon rank sum tests (for continuous variables)
Family history data available on only 65% of these subjects
Dietary variables (except alcohol) are energy adjusted (n=222 cases and 443 controls)
Eight cases and 10 controls were lost due to cracked vials
Results
Cases had significantly higher pre-diagnostic serum creatinine concentrations compared with controls (p=0.004; Table 1), but did not differ from controls with respect to other characteristics, including dietary and serum factors. There was substantial overlap in the case and control distributions for baseline serum creatinine, although as Figure 1 shows, the case distribution displayed a right shift compared with controls. Among controls, serum creatinine was weakly, though significantly, correlated with serum homocysteine (r = 0.24, p=<0.0001), serum α-tocopherol (r = 0.10, p = 0.04), and serum retinol (r = 0.20, p = <0.0001). In addition, serum creatinine was significantly higher among controls living in an urban compared with rural area (p=0.02), among those with no reported history of diabetes (p=0.04), and among those randomized to receive the trial α-tocopherol supplement (p=0.005). None of the other factors listed in Table 1 were correlated or associated with creatinine.
Figure 1.
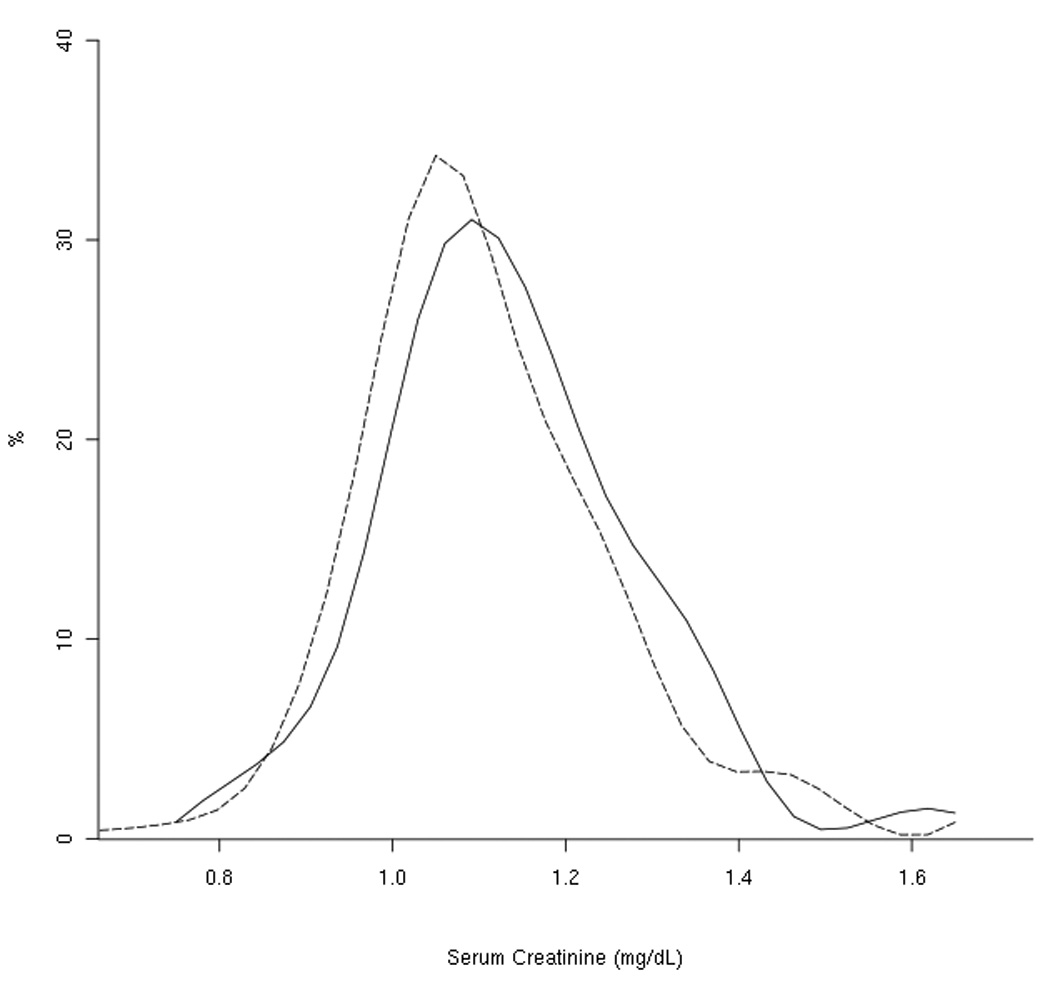
Distribution of baseline serum creatinine values by case/control status
--------- controls
——— cases
Serum creatinine was associated with a significantly greater risk of prostate cancer (age-adjusted OR=1.82, 95% CI 1.12–2.95 for highest vs. lowest quartile, p-trend = 0.006). Adjustment for confounders strengthened the association (OR =2.23, 95% CI 1.33–3.75 for highest vs. lowest quartile, p-trend=0.0008). Further adjustment for all potential confounders (Table 2), or for hypertension or BPH during follow-up (data not shown) did not materially alter the risks. When examined on a continuous scale, the risks remained significant (OR = 1.19, 95% CI 1.07–1.33 based on one-half the interquartile range). These odds ratios were also not materially affected by hypothesis-based exclusion of specific study subjects, including 21 cases/10 controls with a reported history of diabetes (multivariate-adjusted I OR for highest vs. lowest quartile = 2.08, 95% CI 1.22–3.56), 19 cases/24 controls with a reported history of BPH at baseline (OR 2.23, 95% CI 1.33–3.75), 71 cases/28 controls with BPH during follow-up (OR 2.08, 95% CI 1.06–4.08), or 47 cases/86 controls with a reported history of hypertension (OR 2.21, 95% CI 1.18–4.15). Because of the close relationship between serum creatinine and renal function and disease, we identified and excluded all subjects diagnosed with kidney cancer at any time during follow-up, with no resulting change in the odds ratio for high serum creatinine (multivariate model I OR for the highest quartile = 2.22, 95% CI 1.31–3.75).
Table 2.
Odds ratios and 95% confidence intervals of prostate cancer according to baseline serum creatinine
| OR (95% CI) by quartile of serum creatinine | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | P-trend | Continuous OR* | |
| Serum creatinine (mg/dL) | < =1.02 | >1.02 & < =1.10 | >1.10 & < =1.19 | >1.19 | ||
| #cases/#controls | 44/117 | 53/129 | 55/98 | 72/108 | ||
| Age-adjusted † | 1.00 | 1.09 (0.68–1.76) | 1.54 (0.94–2.50) | 1.82 (1.12–2.95) | 0.006 | 1.14 (1.03–1.26) |
| Multivariable model I ‡ | 1.00 | 1.23 (0.75–2.02) | 1.80 (1.09–2.98) | 2.23 (1.33–3.75) | 0.0008 | 1.19 (1.07–1.33) |
| Multivariable model II § | 1.00 | 1.24 (0.75–2.08) | 1.93 (1.15–3.25) | 2.34 (1.36–4.03) | 0.0007 | 1.20 (1.07–1.35) |
Conditioned on age, clinic, intervention group, and date of baseline blood draw.
½ the interquartile range
Adjusted for age at randomization
Adjusted for age at randomization, serum homocysteine, and family history of prostate cancer
Adjusted for age at randomization, BMI, height, serum α-tocopherol, serum homocysteine, serum retinol, serum folate, urban residence, hypertension at baseline, history of hypertension, history of BPH, history of diabetes, and family history of prostate cancer
To test whether the creatinine-prostate cancer relationship was due to reverse-causality, we first considered whether symptoms related to elevated creatinine could have prompted a physician visit and a resulting diagnosis of prostate cancer which may not have been otherwise detected. While serum creatinine concentration was nominally higher among the 79% of men whose prostate cancer was found due to symptoms (median = 1.15 mg/dL) compared with men whose prostate cancer was found by chance (1.12 mg/dL), these were not significantly different (p=0.49). Serum creatinine concentrations also did not differ by stage at diagnosis (median = 1.12 mg/dL vs. 1.15 mg/dL for non-advanced and advanced, respectively, p=0.81) or by time to diagnosis (age-adjusted mean = 1.13 mg/dL vs. 1.15 mg/dL, respectively, for cases diagnosed less than five years and five or more years after blood draw, respectively, p=0.71).
We examined whether the prostate cancer-serum creatinine relationship differed across subgroups defined by age, smoking, protein and alcohol intake, BMI, height, physical activity, blood pressure, serum α-tocopherol, serum homocysteine, intervention assignment, history of diabetes, hypertension at baseline, history of hypertension, hypertension during follow-up (both incident and prevalent), family history of prostate cancer, history of BPH, stage of disease, and follow-up time. While none of the interaction terms were statistically significant (all p for interaction > 0.05), some interesting qualitative differences were revealed (Table 3). For example, the relationship between serum creatinine and prostate cancer appeared stronger among those who were older, had greater height or BMI, no history of benign prostatic hyperplasia, higher diastolic blood pressure, lower baseline serum α-tocopherol, and greater protein or alcohol intakes, Risk was elevated in the subgroup of men with no family history of prostate cancer (OR=3.18, 95% CI 1.65–6.14 for highest vs. lowest quartile), however, there were too few men with a family history to run the model in that stratum. Most interestingly and striking, the association was very strong among those men not randomized to receive α-tocopherol supplementation during the trial phase of the study (highest quartile OR = 3.36, p-trend = 0.0003) compared with those randomized to the α-tocopherol supplementation group (OR = 1.02, p-trend = 0.66), with a p-value for interaction of 0.06. Consistent with this interaction and as noted earlier, serum creatinine was significantly lower among controls not randomized to receive the trial α-tocopherol supplement compared with those who were supplemented. By contrast, the creatinine association did not materially differ by β-carotene supplementation status, nor by disease stage or follow-up time.
Table 3.
Odds ratios and 95% confidence intervals of prostate cancer according to baseline serum creatinine, stratified by selected characteristics
| OR (95% CI) by quartile of serum creatinine |
|||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | p-trend | |
| Age (y) | |||||
| < 61 | 19/55* | 24/56 | 32/43 | 22/46 | |
| 1.00† | 1.25 (0.59–2.64) | 2.65 (1.25–5.61) | 1.54 (0.70–3.38) | 0.13 | |
| > = 61 | 25/62 | 29/73 | 23/55 | 50/62 | |
| 1.00 | 1.07 (0.56–2.04) | 1.08 (0.54–2.17) | 2.38 (1.26–4.51) | 0.005 | |
| Cigarettes/d | |||||
| < 20 | 20/50 | 22/52 | 24/42 | 38/57 | |
| 1.00 | 1.13 (0.52–2.43) | 1.51 (0.70–3.27) | 2.00 (0.97–4.14) | 0.04 | |
| > = 20 | 24/67 | 31/77 | 31/56 | 34/51 | |
| 1.00 | 1.07 (0.56–2.04) | 1.56 (0.79–3.06) | 1.96 (0.97–3.92) | 0.03 | |
| Height (cm) | |||||
| < 174 | 23/68 | 32/54 | 20/48 | 28/52 | |
| 1.00 | 1.79 (0.90–3.58) | 1.17 (0.56–2.47) | 1.67 (0.83–3.38) | 0.10 | |
| > = 174 | 20/49 | 21/75 | 35/50 | 44/56 | |
| 1.00 | 0.74 (0.35–1.54) | 1.99 (0.97–4.08) | 2.20 (1.08–4.49) | 0.003 | |
| Body mass index (kg/m2) | |||||
| < 26 | 25/61 | 20/61 | 24/50 | 30/54 | |
| 1.00 | 0.80 (0.38–1.67) | 1.29 (0.63–2.68) | 1.70 (0.83–3.48) | 0.08 | |
| > = 26 | 18/56 | 33/68 | 31/48 | 42/54 | |
| 1.00 | 1.45 (0.72–2.91) | 1.98 (0.96–4.09) | 2.41 (1.19–4.88) | 0.01 | |
| Disease stage | |||||
| Non-aggressive | 23/71 | 30/57 | 24/46 | 37/53 | |
| 1.00 | 1.76 (0.91–3.40) | 1.91 (0.95–3.86) | 2.71 (1.34–5.48) | 0.006 | |
| Aggressive | 20/46 | 23/71 | 30/50 | 35/54 | |
| 1.00 | 0.84 (0.39–1.81) | 1.66 (0.78–3.51) | 1.84 (0.83–4.11) | 0.04 | |
| Follow-up time | |||||
| < 5 years | |||||
| 1.00 | 0.61 (0.25–1.51) | 1.12 (0.48–2.62) | 2.00 (0.78–5.11) | 0.09 | |
| > = 5 years | |||||
| 1.00 | 1.70 (0.91–3.17) | 2.09 (1.08– 4.05) | 2.44 (1.28– 4.65) | 0.007 | |
| History of benign prostatic hyperplasia (BPH) | |||||
| no | 41/108 | 49/127 | 50/96 | 65/97 | |
| 1.00 | 1.08 (0.66–1.78) | 1.49 (0.89–2.49) | 2.00 (1.21–3.30) | 0.003 | |
| yes | 3/9 | 4/2 | 5/2 | 7/11 | |
| 1.00 | 2.93 (0.16–52.82) | 9.81 (0.33–288.22) | 1.11 (0.12–10.21) | 0.80 | |
| Diastolic blood pressure (mm Hg) | |||||
| < 90 | 29/67 | 26/65 | 27/63 | 37/59 | |
| 1.00 | 0.97 (0.50–1.88) | 1.01 (0.52–1.96) | 1.71 (0.89–3.28) | 0.11 | |
| > = 90 | 15/50 | 27/64 | 28/35 | 35/49 | |
| 1.00 | 1.56 (0.73–3.37) | 3.35 (1.49–7.56) | 3.09 (1.41–6.80) | 0.002 | |
| Systolic blood pressure (mm Hg) | |||||
| < 140 | 19/52 | 21/49 | 24/43 | 30/48 | |
| 1.00 | 1.32 (0.60–2.90) | 1.77 (0.81–3.85) | 1.97 (0.91–4.24) | 0.06 | |
| > = 140 | 25/65 | 32/80 | 31/55 | 42/60 | |
| 1.00 | 1.09 (0.58–2.07) | 1.62 (0.84–3.15) | 2.20 (1.15–4.20) | 0.01 | |
| History of hypertension | |||||
| no | 38/95 | 40/105 | 45/83 | 54/83 | |
| 1.00 | 1.00 (0.58–1.71) | 1.43 (0.83–2.47) | 1.85 (1.08–3.16) | 0.01 | |
| yes | 6/22 | 13/24 | 10/15 | 18/25 | |
| 1.00 | 5.42 (1.35–21.81) | 4.26 (1.00–18.12) | 3.88 (1.02–14.68) | 0.11 | |
| Alpha-tocopherol supplementation | |||||
| no | 24/75 | 37/82 | 28/60 | 45/52 | |
| 1.00 | 1.48 (0.79–2.75) | 1.53 (0.78–2.99) | 3.36 (1.74–6.46) | 0.0003 | |
| yes | 20/42 | 16/47 | 27/38 | 27/56 | |
| 1.00 | 0.71 (0.31–1.61) | 1.47 (0.67–3.23) | 1.02 (0.47–2.21) | 0.66 | |
| Beta-carotene supplementation | |||||
| no | 19/49 | 22/56 | 22/46 | 36/48 | |
| 1.00 | 1.01 (0.48–2.14) | 1.35 (0.63–2.89) | 2.46 (1.17–5.14) | 0.01 | |
| yes | 25/68 | 31/73 | 33/52 | 36/60 | |
| 1.00 | 1.21 (0.64–2.31) | 1.90 (0.98–3.69) | 1.82 (0.95–3.48) | 0.04 | |
| Serum alpha-tocopherol (mg/L) | |||||
| < 11.75 | 23/65 | 31/71 | 33/45 | 36/44 | |
| 1.00 | 1.29 (0.66–2.49) | 2.28 (1.14–4.56) | 2.55 (1.28–5.10) | 0.003 | |
| > = 11.75 | 21/52 | 22/58 | 22/53 | 36/64 | |
| 1.00 | 0.99 (0.47–2.09) | 1.29 (0.61–2.73) | 1.82 (0.90–3.67) | 0.06 | |
| Serum homocysteine (nmol/ml) | |||||
| < 11.8 | 31/71 | 21/72 | 26/50 | 26/33 | |
| 1.00 | 0.67 (0.34–1.33) | 1.22 (0.62–2.40) | 1.96 (0.96–3.98) | 0.04 | |
| > = 11.8 | 13/46 | 32/57 | 29/48 | 46/75 | |
| 1.00 | 1.95 (0.89–4.25) | 2.18 (0.98–4.87) | 2.43 (1.13–5.20) | 0.03 | |
| Proteine intake (g/d) | |||||
| < 91 | 23/57 | 23/66 | 26/38 | 40/56 | |
| 1.00 | 0.85 (0.41–1.77) | 2.06 (0.98–4.35) | 1.86 (0.94–3.69) | 0.02 | |
| > = 91 | 16/56 | 29/59 | 27/57 | 30/43 | |
| 1.00 | 1.77 (0.84–3.76) | 1.77 (0.82–3.86) | 3.38 (1.49–7.67) | 0.005 | |
| Alcohol intake (g ethanol/d) | |||||
| < 9.3 | 23/52 | 29/56 | 29/46 | 38/59 | |
| 1.00 | 1.24 (0.62–2.49) | 1.39 (0.68–2.86) | 1.55 (0.79–3.07) | 0.20 | |
| > = 9.3 | 16/61 | 23/69 | 24/49 | 32/40 | |
| 1.00 | 1.29 (0.59–2.79) | 1.98 (0.89–4.39) | 3.03 (1.34–6.82) | 0.004 | |
number of cases and controls
unconditioned logistic regression models, adjusting for age at randomization, clinic, intervention group, date of baseline blood draw, serum homocysteine, and family history of prostate cancer
Discussion
Higher baseline serum creatinine concentrations were strongly related to higher risk of prostate cancer in this prospective study. We observed a two-fold increase in risk among subjects with serum creatinine concentrations greater than 1.19 mg/dL compared with those with serum creatinine less than or equal to 1.02 mg/dL. This association appeared to be dose-dependent and was also significant when examined on a continuous scale.
Serum creatinine is a measure of renal function, but it is also influenced by other factors and therefore not a specific or sensitive indicator of renal disease within normal ranges (17–19). In addition to the glomerular filtration rate, for example, creatinine concentrations are influenced by age, sex, muscle mass, and intake and absorption of dietary creatine and creatinine, which are consumed in meat (17,18). Other reported correlates of serum creatinine include ethnicity, weight, BMI, lean mass, and upper arm circumference; serum triglycerides and total cholesterol; physical activity; blood pressure; diseases such as diabetes, hypertension, and heart disease; and use of antihypertensive medication, statins, cimetidine, or diuretics (17–23). Interestingly, serum creatinine was lower among heavy smokers in one cohort study (23). It is also an important risk factor for cardiovascular disease and related mortality (23–26).
The median serum creatinine concentrations in the U.S. National Health and Nutrition Examination Survey III were 1.11 mg/dL for men aged 40–59 years and 1.18 mg/dL for men aged 60 years and older (27), which was similar to that reported for men (mean age = 53 years) in the Framingham Heart Study (mean 1.2 mg/dL)(20). In the Hordaland Health Study in Norway, median serum creatinine was 1.13 mg/dL in men aged 71–74 years (23). Several values have been used to define creatininemia, depending partially on the laboratory assay methods, but thresholds are typically in the 1.5–2.0 mg/dL range (17,19,27). In the current study, only 1% of all subjects had creatinine values greater than 1.5 mg/dL with the rest being within the normal range.
Several clinical investigations have examined serum creatinine as a potential prostate cancer staging and prognostic marker (2–8). For example, creatinine concentrations predicted advanced prostate carcinoma and decreased survival in one study (3) and were elevated in patients presenting with high PSA and locally advanced or metastatic disease, compared with those with low initial PSA (2). Elevated serum creatinine was also associated with reduced survival in a group of men with hormone-resistant prostate cancer (4). In other studies, significant relationships between elevated serum creatinine and disease stage, recurrence, progression, or mortality were attenuated when adjusted for other factors (e.g., age, stage, race, or PSA)(5,7), or were only marginally significant (6) or not associated (8). While impaired renal function may explain the association between creatinine levels and poor prognosis among prostate cancer patients, it is unlikely that underlying renal disease explains the prospective associations we observed here.
In a study of Korean men, serum creatinine was positively, though weakly (r=0.05, p=0.02), correlated with serum PSA (28), but, in a similar study, was no longer correlated once adjusted for age (29). We did not have serum PSA data for all men in the current study and therefore could not rule out confounding of the serum creatinine association by PSA. Baseline serum PSA had been measured for 118 cases and 7 controls in our study, however, and it was not correlated with serum creatinine (r=−0.0007, p=0.99). It is unclear if, or how, the association between serum creatinine and prostate cancer risk might differ in a population routinely screened through PSA serology.
In the present investigation, as in two previous studies (30,31), serum creatinine was correlated with serum homocysteine. The synthesis of creatine, which is converted to creatinine in muscle cells, requires a methyl group from S-adenosylmethionine, which in turn is converted via adenosylhomocysteine to homocysteine (9). It is therefore conceivable that creatinine could serve as a marker for homocysteine status. Homocysteine (and other one-carbon biomarkers) were not associated with prostate cancer in this sample (16), however, and adjustment for homocysteine did not impact the present findings. Creatinine could also be considered a proxy for meat consumption, where lower levels are associated with reduced intake (17), but neither meat nor protein intake were associated with serum creatinine or prostate cancer in our study, and did not confound the risk association.
To assess whether uncontrolled confounding explained our findings, we identified and tested factors known to be associated with serum creatinine for which we had data. Inclusion of homocysteine in the regression model strengthened the association (mulitvariable model I), while inclusion of numerous other potential confounding factors (e.g., hypertension, diabetes) had no additional effect on the risk estimates (multivariable model II), suggesting that uncontrolled confounding is unlikely to explain the association. Elevated creatinine could be due to diabetes, and we only had insulin and glucose measurements for 23 subjects so we can not rule out this possibility. However, the exclusion of men with reported history of diabetes, as well as acute renal failure, hypertension, or BPH, or any renal cancer diagnoses during follow-up did not alter our findings. In addition, diabetes has been associated with reduced risk of prostate cancer (32), so any undiagnosed diabetes at baseline would likely attenuate the creatinine-prostate association. To further address the possibility that undiagnosed disease led to elevated baseline serum creatinine, we stratified subjects based on follow-up time and observed no material difference in the association when either the earlier or the later cases were excluded. Moreover, the relationship did not differ among those diagnosed with aggressive compared with non-aggressive disease. Due to the long period over which prostate cancer develops, however, it remains conceivable that chronic urinary obstruction due to BPH or undiagnosed prostate cancer led to the elevated baseline serum creatinine.
We identified a number of potential effect modifiers of the serum creatinine - prostate cancer association which may be chance findings, but which should be evaluated in other studies. For example, the association appeared limited to men who were not randomized to receive α-tocopherol supplementation during the trial phase of the ATBC Study. Similarly, there was a slightly greater risk with high creatinine among men with lower baseline serum α-tocopherol. Since prostate cancer incidence was reduced by the α-tocopherol supplement in the ATBC trial (14), it could be that the beneficial effect of supplementation overshadowed any risk related to creatinine, such as through α-tocopherol inhibition of protein kinase C activity, with resulting prostate smooth muscle cell relaxation, and reduced symptoms of urinary obstruction (14,33). Related to this, baseline BPH prevalence in the non-α-tocopherol supplemented group was greater among cases (9%) than among controls (4%, p=0.03), compared with 7% prevalence among both cases and controls in the α-tocopherol supplement group; however, removal of subjects with reported BPH (or adjustment for BPH) did not alter the interaction.
Strengths of our study include the prospective design, which minimizes the potential impact of cancer on baseline creatinine concentrations, and our ability to test numerous potential confounding factors. All participants were cigarette smokers, potentially limiting the generalizability of the findings; however, smoking dose and duration were not correlated with creatinine concentrations, and did not confound or modify the association. We could not fully examine confounding by, or interaction with, serum PSA, and as our study population was not routinely screened with PSA testing, whether a similar creatinine association exists in screened populations is unknown.
We report herein a strong prospective relationship between higher serum creatinine, within normal ranges, and an increased prostate cancer risk. Additional studies are needed to retest the hypothesis, determine whether the association is evident in nonsmokers and in other racial groups, and explore potential mechanisms, including any interaction with serum PSA.
Acknowledgments
This work was supported in part by the Intramural Research Program of the National Institutes of Health and the National Cancer Institute. Additionally, this research was supported by Public Health Service contracts N01-CN-45165, N01-RC-45035, and N01-RC-37004 from the National Cancer Institute, Department of Health and Human Services.
Abbreviations
- ATBC
Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study
- BPH
benign prostatic hyperplasia
- BMI
body mass index
- CI
confidence intervals
- OR
odds ratios
- PSA
prostate-specific antigen
Reference List
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Chiong E, Wong AF, Chan YH, Chin CM. Review of clinical manifestations of biochemically-advanced prostate cancer cases. Asian J Surg. 2005;28:202–206. doi: 10.1016/S1015-9584(09)60344-4. [DOI] [PubMed] [Google Scholar]
- 3.Vesalainen S, Lipponen P, Talja M, Syrjanen K. Biochemical parameters as prognostic factors in prostatic adenocarcinoma. Acta Oncol. 1995;34:53–59. doi: 10.3109/02841869509093639. [DOI] [PubMed] [Google Scholar]
- 4.Fossa SD, Dearnaley DP, Law M, Gad J, Newling DW, Tveter K. Prognostic factors in hormone-resistant progressing cancer of the prostate. Ann Oncol. 1992;3:361–366. doi: 10.1093/oxfordjournals.annonc.a058207. [DOI] [PubMed] [Google Scholar]
- 5.Merseburger AS, Connelly RR, Sun L, Richter E, Moul JW. Use of serum creatinine to predict pathologic stage and recurrence among radical prostatectomy patients. Urology. 2001;58:729–734. doi: 10.1016/s0090-4295(01)01370-x. [DOI] [PubMed] [Google Scholar]
- 6.Sandhu DP, Mayor PE, Sambrook PA, George NJ. Outcome and prognostic factors in patients with advanced prostate cancer and obstructive uropathy. Br J Urol. 1992;70:412–416. doi: 10.1111/j.1464-410x.1992.tb15799.x. [DOI] [PubMed] [Google Scholar]
- 7.Johansson JE, Andersson SO, Holmberg L, Bergstrom R. Prognostic factors in progression-free survival and corrected survival in patients with advanced prostatic cancer: results from a randomized study comprising 150 patients treated with orchiectomy or estrogens. J Urol. 1991;146:1327–1332. doi: 10.1016/s0022-5347(17)38081-3. [DOI] [PubMed] [Google Scholar]
- 8.Ribeiro M, Ruff P, Falkson G. Low serum testosterone anda younger age predict for a poor outcome in metastatic prostate cancer. Am J Clin Oncol. 1997;20:605–608. doi: 10.1097/00000421-199712000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Stead LM, Brosnan JT, Brosnan ME, Vance DE, Jacobs RL. Isit time to reevaluate methyl balance in humans? Am J Clin Nutr. 2006;83:5–10. doi: 10.1093/ajcn/83.1.5. [DOI] [PubMed] [Google Scholar]
- 10.Eto I, Krumdieck CL. Role of vitamin B12 and folate deficienciesin carcinogenesis. Adv Exp Med Biol. 1986;206:313–330. doi: 10.1007/978-1-4613-1835-4_23. [DOI] [PubMed] [Google Scholar]
- 11.The ATBC Cancer Prevention Study Group. The alpha-tocopherol beta-carotene lung cancer prevention study: design, methods, participant characteristics, and compliance. Ann Epidemiol. 1994;4:1–10. doi: 10.1016/1047-2797(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 12.Korhonen P, Malila N, Pukkala E, Teppo L, Albanes D, Virtamo J. The Finnish Cancer Registry as follow-up source of a large trialcohort--accuracy and delay. Acta Oncol. 2002;41:381–388. doi: 10.1080/028418602760169442. [DOI] [PubMed] [Google Scholar]
- 13.Pietinen P, Hartman AM, Haapa E, et al. Reproducibility and validity of dietary assessment instruments.I A self-administered food use questionnaire with a portion size picture booklet. Am J Epidemiol. 1988;128:655–666. doi: 10.1093/oxfordjournals.aje.a115013. [DOI] [PubMed] [Google Scholar]
- 14.Heinonen OP, Albanes D, Virtamo J, et al. Prostate cancer and supplementation withalpha-tocopherol beta-carotene: incidence mortality in a controlled trial. J Natl Cancer Inst. 1998;90:440–446. doi: 10.1093/jnci/90.6.440. [DOI] [PubMed] [Google Scholar]
- 15.Larsen K. Creatinine assay by a reaction-kinetic principle. Clin Chim Acta. 1972;41:209–217. doi: 10.1016/0009-8981(72)90513-x. [DOI] [PubMed] [Google Scholar]
- 16.Weinstein SJ, Hartman TJ, Stolzenberg-Solomon R, et al. Null association between prostate cancer and serum folate, vitamin B(6), vitamin B(12), and homocysteine. Cancer Epidemiol Biomarkers Prev. 2003;12:1271–1272. [PubMed] [Google Scholar]
- 17.Levey AS, Perrone RD, Madias NE. Serum creatinine and renal function. Annu Rev Med. 1988;39:465–490. doi: 10.1146/annurev.me.39.020188.002341. [DOI] [PubMed] [Google Scholar]
- 18.Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38:1933–1953. [PubMed] [Google Scholar]
- 19.Coresh J, Wei GL, McQuillan G, et al. Prevalence of high bloodpressure elevated serum creatinine level in the United States: findings from the third NationalHealth Nutrition Examination Survey (1988–1994) Arch Intern Med. 2001;161:1207–1216. doi: 10.1001/archinte.161.9.1207. [DOI] [PubMed] [Google Scholar]
- 20.Culleton BF, Larson MG, Evans JC, et al. Prevalence correlates of elevated serum creatinine levels: the Framingham Heart Study. Arch Intern Med. 1999;159:1785–1790. doi: 10.1001/archinte.159.15.1785. [DOI] [PubMed] [Google Scholar]
- 21.Baxmann AC, Ahmed MS, Marques NC, et al. Influence of muscle mass physical activity on serum urinary creatinine serum cystatin. C Clin J Am Soc Nephrol. 2008;3:348–354. doi: 10.2215/CJN.02870707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salive ME, Jones CA, Guralnik JM, Agodoa LY, Pahor M, Wallace RB. Serum creatinine levels in older adults: relationship with healthstatus medications. Age Ageing. 1995;24:142–150. doi: 10.1093/ageing/24.2.142. [DOI] [PubMed] [Google Scholar]
- 23.Vikse BE, Vollset SE, Tell GS, Refsum H, Iversen BM. Distribution and determinants of serum creatinine in the general population: the Hordaland Health Study. Scand J Clin Lab Invest. 2004;64:709–722. doi: 10.1080/00365510410003057. [DOI] [PubMed] [Google Scholar]
- 24.Schillaci G, Reboldi G, Verdecchia P. High-normal serum creatinine concentration is a predictor of cardiovascular risk in essential hypertension. Arch Intern Med. 2001;161:886–891. doi: 10.1001/archinte.161.6.886. [DOI] [PubMed] [Google Scholar]
- 25.Walsh CR, O'Donnell CJ, Camargo CA, Jr, Giugliano RP, Lloyd-Jones DM. Elevated serum creatinine is associated with 1-year mortality after acute myocardial infarction. Am Heart J. 2002;144:1003–1011. doi: 10.1067/mhj.2002.125504. [DOI] [PubMed] [Google Scholar]
- 26.Praught ML, Shlipak MG. Are small changes in serum creatinine an important risk factor? Curr Opin Nephrol Hypertens. 2005;14:265–270. doi: 10.1097/01.mnh.0000165894.90748.72. [DOI] [PubMed] [Google Scholar]
- 27.Jones CA, McQuillan GM, Kusek JW. Serum creatinine levels in the US population: third National Health Nutrition Examination Survey. Am J Kidney Dis. 1998;32:992–999. doi: 10.1016/s0272-6386(98)70074-5. [DOI] [PubMed] [Google Scholar]
- 28.Ahn JO, Ku JH. Relationship between serum prostate-specific antigen levels and body mass index in healthy younger men. Urology. 2006;68:570–574. doi: 10.1016/j.urology.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 29.Ku JH, Kim ME, Lee NK, Park YH, Ahn JO. Influence of age, anthropometry, and hepatic and renal function on serum prostate-specific antigen levels in healthy middle-age men. Urology. 2003;61:132–136. doi: 10.1016/s0090-4295(02)02001-0. [DOI] [PubMed] [Google Scholar]
- 30.Elshorbagy AK, Oulhaj A, Konstantinova S, et al. Plasma creatinine as a determinant of plasma total homocysteine concentrations in the Hordaland Homocysteine Study: use of statistical modeling to determine reference limits. Clin Biochem. 2007;40:1209–1218. doi: 10.1016/j.clinbiochem.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 31.Jacques PF, Bostom AG, Wilson PW, Rich S, Rosenberg IH, Selhub J. Determinants of plasma total homocysteine concentration in the Framingham Offspring cohort. Am J Clin Nutr. 2001;73:613–621. doi: 10.1093/ajcn/73.3.613. [DOI] [PubMed] [Google Scholar]
- 32.Calton BA, Chang SC, Wright ME, et al. History of diabetesmellitus subsequent prostate cancer risk in the NIH-AARPDiet Health Study. Cancer Causes Control. 2007;18:493–503. doi: 10.1007/s10552-007-0126-y. [DOI] [PubMed] [Google Scholar]
- 33.Eckert RE, Schreier U, Drescher P, et al. Regulation of prostatic smooth muscle contractility by intracellular second messengers: implications for the conservative treatment of benign prostatic hyperplasia. Urol Int. 1995;54:6–21. doi: 10.1159/000282685. [DOI] [PubMed] [Google Scholar]


