Abstract
Background
Duodenal cancer is the leading cause of cancer death in familial adenomatous polyposis (FAP) after colorectal cancer. The lifetime risk of developing duodenal cancer is 4-10%. Current treatment guidelines recommend endoscopic surveillance with a prophylactic pancreaticoduodenectomy (PD) in advanced duodenal polyposis, defined using the Spigelman staging system. Because no clinical trials have assessed this recommendation, a modeling approach was employed to evaluate the cost-effectiveness of various treatment strategies.
Methods
A Markov model was constructed to estimate the life expectancy and cost of three different strategies: PD at Spigelman stage III, PD at Spigelman stage IV, and PD at cancer diagnosis. A cohort of 30 year-old FAP patients with total colectomies was simulated until age 80. The analysis was from a societal perspective. Extensive sensitivity analysis was performed to assess the impact of model uncertainty on results.
Results
At all stages of polyposis and all ages under 80, prophylactic surgery at Spigelman stage IV resulted in the greatest life expectancy. Surgery at stage IV was more effective and more expensive than surgery at cancer diagnosis, with an incremental cost of $3,200 per quality-adjusted life year gained. Surgery at stage III was not a viable option. The results were robust to wide variation in model parameters, but were sensitive to the post-PD quality of life score.
Conclusions
Prophylactic PD at stage IV duodenal polyposis in FAP is a cost-effective approach that results in greater life expectancy than surgery at either stage III or cancer diagnosis.
Keywords: cost-effectiveness, duodenal cancer, duodenal polyposis, familial adenomatous polyposis, pancreaticoduodenectomy
Introduction
Familial adenomatous polyposis (FAP) is an autosomal dominant disease resulting from a defect in the adenomatous polyposis coli (APC) gene (1). Hundreds of premalignant adenomas develop in the colon and rectum, conferring an almost 100% lifetime risk of colorectal cancer. A prophylactic colectomy is recommended in early adulthood to prevent the development of colorectal cancer.
FAP is also associated with a number of extracolonic manifestations, including osteomas, epidermoid cysts, dental abnormalities, hypertrophy of the retinal pigment epithelium, desmoid tumors, adenomas of the upper gastrointestinal tract, and a number of malignancies (2). One of the most important of these is duodenal polyposis (3). Individuals with FAP have nearly a 100% lifetime risk of developing duodenal polyposis (4-5). Duodenal adenomas have a similar biology to colorectal adenomas and are thought to progress to cancer via an analogous adenoma-carcinoma sequence (2-3). While the risk of developing duodenal cancer with FAP is 100 - 330 times that without FAP (6-7), the absolute lifetime risk is 4 - 10% (8-9). Nevertheless, duodenal cancer is the second leading cause of cancer death in individuals with FAP after colorectal cancer (10-14).
The degree of duodenal polyposis can be tracked by endoscopy with biopsy and quantified using the Spigelman staging scale (15). The Spigelman staging scale gives a separate score for the number, size, histology, and degree of dysplasia of the duodenal polyps. The sum of these scores is converted into a stage rating from 0 to IV, with stage 0 corresponding to no polyposis and stage IV corresponding to severe polyposis. The risk of developing cancer increases with increasing Spigelman stage (16). Currently, endoscopic screening is recommended every 5 years to 6 months, with the frequency depending on the Spigelman stage (16-17).
The most effective intervention for reducing the risk of developing duodenal cancer is a prophylactic pancreaticoduodenectomy (PD). PD is a major operation with substantial morbidity and mortality. When deciding whether or not to undergo prophylactic surgery, patients with FAP and duodenal polyposis must balance potential risks and benefits. If surgery is pursued too aggressively, the patient risks surgical mortality and morbidity when cancer might not have developed. If surgery is not pursued aggressively enough, the patient risks the development of a preventable cancer.
Although the overall prevalence of FAP, estimated at 6,000 - 7,400 families in the United States, makes duodenal polyposis a rare condition, the significant morbidity these individuals face and their high rate of resource utilization make management of this condition a greater public health concern than might first be thought (18). To date, no clinical trial has been performed to determine at what degree of polyposis, if any, prophylactic surgery should be recommended. Such a trial would be difficult to perform, as duodenal cancer in FAP is a relatively rare disorder with a slow pathogenesis. We constructed a decision-analytic model to synthesize data from observational studies and used the model to evaluate the health and economic outcomes associated with three surgical management strategies for patients with FAP and duodenal polyposis.
Methods
Overview
We constructed a Markov cohort model to evaluate the costs, life years, and quality-adjusted life years (QALYs) associated with three surgical management strategies for duodenal polyposis in FAP. The model simulates the natural history of duodenal polyposis and routine endoscopic screening in a cohort of 30-year-old individuals with FAP. Superimposed on this model is a mechanism of surgical intervention that can interrupt the natural history.
Cost-effectiveness analysis was performed, with the incremental cost-effectiveness ratio (ICER) calculated as the change in total cost over the change in total effectiveness between two strategies. If a strategy is both more costly and less effective than another strategy, the first strategy is said to dominate the second strategy. Although there is no explicit willingness-to-pay (WTP) threshold for medical interventions in the U.S., we used a threshold of $80,000 per quality-adjusted life year (QALY) to determine cost-effectiveness. This represents the ICER for hemodialysis, a widely cited benchmark for WTP decisions, adjusted to 2007 U.S. dollars (19).
Our analysis followed the guidelines of the Panel on Cost-effectiveness in Health in Medicine (20). The analysis was performed from a societal perspective. All future costs and QALYs were discounted at a 3% annual rate and costs were expressed in 2007 U.S. dollars. Reported life years were undiscounted. The model was constructed using commercially available software (TreeAge Pro 2008 Suite Release 1.2, Treeage Software, Williamstown, MA).
Model Design
The model structure is shown in Figure 1. The initial cohort consisted of individuals with FAP at age 30 who had undergone a total colectomy and were considered to be at no risk for developing colorectal cancer. During each (1-month) cycle of the model, patients could remain in the same disease state or progress to a more advanced disease state. The clinically perceived disease state was based on endoscopic and pathologic findings, and was tracked separately from the underlying biological state. The perceived polyposis state advanced only with endoscopic screening. All individuals in the model underwent endoscopic screening with biopsy as per current screening recommendations (16-17).
Figure 1. Model schematic.
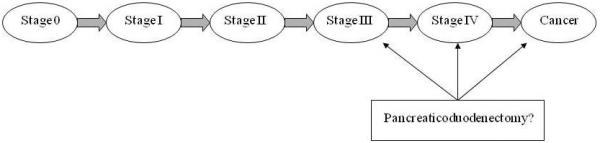
The model begins with a cohort of age 30 individuals with familial adenomatous polyposis and a total colectomy. They progress linearly through stages until they die or reach age 80. Perceived disease state is clinically tracked by endoscopies with biopsy, while the true disease state corresponds to the underlying biological disease progression. Surgery can be offered at stage III, stage IV, or cancer, depending on the management strategy. Individuals may die from surgical complications, duodenal cancer, or from other causes. Stages are based on Spigelman criteria.
For the purposes of the model, the onset of cancer was defined as the time when cancer could be detected by endoscopy with biopsy. Once cancer developed, patients could present symptomatically to undergo an endoscopy in addition to their regularly scheduled endoscopic screening. All patients diagnosed with duodenal cancer received standard therapeutic and palliative care (21). The model was run until each individual died or reached age 80.
Three management strategies were evaluated: 1) PD upon diagnosis with stage III polyposis, 2) PD upon diagnosis with stage IV polyposis, and 3) PD only upon cancer diagnosis.
Model Inputs
Model input parameters and the values used for the base-case scenario and sensitivity analysis are summarized in Table 1. Estimates for the base-case scenario were derived from the literature.
Table 1.
Model Inputs and Ranges for Sensitivity Analysis
| Variable | Base Case | Sensitivity Analysis Range | Reference(s) |
|---|---|---|---|
| Polyposis distribution and progression | |||
| Stage distribution at age 30 (%) | See methods | ||
| Stage 0 | 80 | 100/60 | |
| Stage I | 11 | 0/18 | |
| Stage II | 9 | 0/10 | |
| Stage III | 0 | 0/10 | |
| Stage IV | 0 | 0/2 | |
| Cancer | 0 | 0/0 | |
| Transition probabilities per month (%) | |||
| Stages 0 to IV | 0.46 | 50%-200% × BC | See methods |
| Stage IV to Cancer | 0.37 | 50%-200% × BC | |
| Endoscopy characteristics | |||
| Screening frequency | (16-17) | ||
| Stage 0 | 5 years | ||
| Stage I | 5 years | ||
| Stage II | 3 years | ||
| Stage III | 1 year | ||
| Stage IV | 6 months | ||
| Complication rate | 1.6:100,000 | 50% - 200% × BC | (27-28) |
| False negative rate (%) | 29 | 0-60 | (26) |
| Symptomatic cancer presentation (%) | 50 | 0-100 | (23, 29) |
| Pancreaticoduodenectomy characteristics | |||
| Eligible for curative surgery (%) | 25-75 | (30) | |
| Age 30-39 | 50 | ||
| 40-49 | 66 | ||
| 50-59 | 64 | ||
| 60-69 | 60 | ||
| 70-79 | 55 | ||
| 80+ | 33 | ||
| Perioperative mortality (%) | 5 | 0-10 | (32-33) |
| Cancer mortality per month | |||
| Age-related | 1.6 × US life table | 1-2.1 × US life table | (12, 35) |
| Undiagnosed cancer (%) | 0.11 | 0-1000% × BC | (30) |
| Post-curative surgery (%) | 50% - 200% × BC | (34) | |
| 0-7 months | 1.8 | ||
| 8-41 months | 0.89 | ||
| 42-86 months | 0.41 | ||
| 87 + months | 0 | ||
| Post-palliative surgery (%) | 50% - 200% × BC | (34) | |
| 0-12 months | 12 | ||
| 13 + months | 5.7 | ||
| Outcome adjustments | |||
| Utility reductions | |||
| Endoscopy | -0.3 * 1 day | 0 - 0.5 | (47) |
| Endoscopy complication | -0.3 * 1 week | 0 - 0.5 | (47) |
| Pancreaticoduodenectomy | -0.3 * 4 weeks | 0 - 0.5 | (40) |
| Quality of life adjustment factors | |||
| Well | 1 | (48) | |
| Age 30-39 | 0.91 | ||
| Age 40-49 | 0.88 | ||
| Age 50-59 | 0.85 | ||
| Age 60-69 | 0.83 | ||
| Age 70-79 | 0.79 | ||
| Age 80+ | 0.75 | ||
| Post pancreaticoduodenectomy | 0.98 | 0.8 - 1 | See methods |
| Cancer | 0.47 | 0.25 - 1 | (49-50) |
| Discount rate (%) | 3 | 0 - 5 | (51) |
| Costs ($) | |||
| Cancer care | 67,565 | 50% - 200% × BC | (52) |
| Endoscopy | 903 | 50% - 200% × BC | (53) |
| Endoscopy complication | 9,355 | 50% - 200% × BC | (54) |
| Pancreaticoduodenectomy | 30,568 | 50% - 200% × BC | (40) |
| Post pancreaticoduodenectomy | 159 | 50% - 200% × BC | See methods |
| Day's wages | 147 | 50% - 200% × BC | US Bureau of Labor |
Disease Progression
We derived estimates for the stage distribution at age 30 and the transition probabilities between different stages of polyposis and cancer from the published literature. Studies estimating the cumulative risk for duodenal cancer range from a low of 3-4% at age 70 to a high of 10% at age 60 (8-9). We calibrated our model to a 4.9% cumulative risk of cancer at age 62 (see appendix for details).
Endoscopic Screening
We assumed that all patients would undergo screening endoscopy with biopsy as recommended by the American Society for Gastrointestinal Endoscopy and recent publication: endoscopy with biopsy every 5, 3, 3, 1, and 0.5 years in stages 0-IV respectively (16-17). Reports of endoscopy false negative rates for polyposis staging and cancer diagnosis range from 20% to 56% (2, 8, 22-26). We selected a false negative rate of 29% from a representative study for the base-case scenario (26). A false negative endoscopy resulted in a perceived stage one stage lower than the biological stage. We assumed that the perceived stage could not be greater than the biological stage and that the perceived stage would never decrease. The frequency of endoscopy complications requiring surgery was estimated from the literature (27-28).
Fifty percent of cancer diagnoses are made after a patient presents symptomatically rather than at a scheduled screening endoscopy(8, 23, 29). To reflect this in the model, following the development of cancer, patients had a linearly increasing risk of presenting with symptoms leading to an endoscopy. The rate of symptom development was adjusted so that 50% of cancers were diagnosed following symptomatic presentation.
Surgery
PD was used prophylactically in stage III and stage IV individuals, as well as therapeutically in cancer patients who were candidates for curative surgery. All stage III and IV patients were assumed to be surgical candidates for a PD if it was part of the management strategy. For cancer patients, surgical candidacy was a function of age based on operability data for duodenal cancer from the Surveillance Epidemiology and End Results (SEER) database (30). Overall, 54% of patients were candidates for curative surgery. Individuals with inoperable cancer received palliative care. Stage III and stage IV patients who received a PD, as well as cancer patients surviving to 5 years after surgery, were considered to have no risk of future duodenal cancer.
PD perioperative mortality ranges from 1-9%, with high volume being associated with a lower mortality rate (17). We used 5% for our base-case analysis to represent typical hospital results and enhance generalizability (31-34).
Cancer Mortality
FAP increases the risk for developing a number of conditions in addition to colorectal and duodenal cancers. These include neoplastic lesions such as desmoid, brain, pancreatic, and thyroid tumors as well as non-neoplastic lesions. To account for this, we adjusted the age-related risk of death from the 2004 U.S. life table upwards by a factor of 1.6 based on a study of FAP relative mortality after excluding colorectal and duodenal cancers (12, 35). Survival curves following curative surgery and palliative care were derived from the literature (34). (See appendix for further details.)
Outcome Adjustments
Standard utility adjustments were made using published values. (See appendix for details.) We modeled long-term quality of life after a PD by assuming 15% of the surgical population would develop diabetes due to the surgery (36-38), resulting in an overall post-PD quality of life score of 0.98 (39). A onetime 30% utility penalty for six weeks modeled short-term PD complications and perioperative recovery (40).
Costs
All costs were derived from published estimates adjusted to 2007 U.S. dollars using the medical care component of the Consumer Price Index (US Bureau of Labor Statistics, 2007). Patient time costs, calculated from the mean daily wage based on a 7.5 hour work day, were included in the cost of procedures (US Bureau of Labor). (See appendix for details.)
Model Validation
To demonstrate model validity, we compared model outputs to independent data sets not used in its construction or calibration. (See appendix for model validation methodology and results.)
Analyses
The model was analyzed as a Markov cohort simulation using the base-case estimates. Primary outcomes included lifetime cost, life years and QALYs, from which we calculated ICERs comparing the three strategies. Secondary outcomes included the number of endoscopies and surgeries, lifetime risk of cancer, and causes of death. One-way sensitivity analysis was performed to examine how assumptions about model parameters influenced results.
Results
Base-Case Analysis
The results of the base-case analysis are summarized in Table 2. PD at stage IV was the most cost-effective strategy, with an ICER of $3,200/QALY compared to PD at cancer diagnosis. PD at stage IV was both more effective and less expensive than PD at stage III, which was therefore considered to be dominated.
Table 2.
Base-case results for a cohort of 30-year-olds with FAP
| Outcome | PD at Cancer | PD at Stage III | PD at Stage IV |
|---|---|---|---|
| Cost (U.S. $) | 12,500 | 17,900 | 13,100 |
| QALYs | 20.02 | 20.13 | 20.21 |
| ICER (U.S. $/QALY) | - | Dominated | 3,200 |
| Life years (undiscounted years) | 42.81 | 43.72 | 43.72 |
| Cancers diagnosed (% of cohort) | 11.7 | 0.3 | 1.1 |
| Procedures | |||
| Endoscopies (per person) | 16.5 | 7.6 | 11.6 |
| Surgery (% of cohort) | 7.0 | 43.0 | 25.7 |
| Deaths (% of cohort) | |||
| Surgery | 0.4 | 2.2 | 1.3 |
| Cancer | 7.2 | 0.2 | 0.7 |
- = reference strategy; Dominated = both less effective and more costly than another strategy; ICER = incremental cost-effectiveness ratio; PD = pancreaticoduodenectomy; QALY = discounted quality-adjusted life years; U.S. $ = discounted 2007 U.S. dollars.
Compared to surgery at the time of cancer diagnosis, pursuing a strategy of PD at stage IV in a hypothetical cohort of 10,000 individuals at age 30 would prevent 1,060 cancers, 650 cancer deaths, and 49,000 endoscopies. On the other hand, an additional 1,870 PD surgeries would be performed, leading to 90 additional perioperative deaths. Overall, 9,100 years of life would be saved.
Sensitivity Analysis
An extensive sensitivity analysis was performed (Table 3). The model was robust to a wide range of changes in parameter estimates; in almost all cases, PD at stage IV was cost-effective relative to PD at cancer diagnosis and dominated PD at stage III. The model was not sensitive to initial stage distribution, resectability, perioperative PD mortality, palliative care mortality, curative care mortality, age-related mortality, or mortality from undiagnosed cancer.
Table 3.
Results of sensitivity analysis
| PD at Stage III |
PD at Stage IV |
PD at Cancer |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Cost | QALY | ICER | Cost | QALY | ICER | Cost | QALY | ICER |
| Polyposis distribution and progression | |||||||||
| Stage distribution at age 30 (% Stg 0/I/II/III/IV/Cancer) | |||||||||
| 100/0/0/0/0/0 | 14,000 | 20.20 | Dominated | 10,300 | 20.26 | 6,400 | 9,700 | 20.17 | - |
| 60/18/10/10/2/0 | 26,800 | 19.96 | Dominated | 19,700 | 20.08 | 700 | 19,400 | 19.59 | - |
| Transition probabilities | |||||||||
| Stages 0 to IV | |||||||||
| 50% × BC | 9,800 | 20.26 | Dominated | 7,200 | 20.30 | 3,900 | 2,000 | 20.26 | - |
| 200% × BC | 32,800 | 19.85 | Dominated | 27,400 | 19.92 | 1,000 | 26,800 | 19.27 | - |
| Stages IV to cancer | |||||||||
| 50% × BC | 17,900 | 20.14 | Dominated | 13,000 | 20.23 | 25,300 | 11,200 | 20.16 | - |
| 200% × BC | 18,000 | 20.12 | Dominated | 13,300 | 20.18 | - | 14,100 | 19.88 | Dominated |
| Pancreaticoduodenectomy characteristics | |||||||||
| Eligible for curative surgery (%) | |||||||||
| 25 | 17,900 | 20.13 | Dominated | 13,000 | 20.20 | 5,500 | 11,700 | 19.97 | - |
| 75 | 17,900 | 20.14 | Dominated | 13,100 | 20.21 | 1,800 | 12.800 | 20.06 | - |
| Perioperative mortality (%) | |||||||||
| 0 | 18,200 | 20.26 | Dominated | 13,200 | 20.27 | 3,000 | 12,500 | 20.04 | - |
| 10 | 17,600 | 20.01 | Dominated | 12,900 | 20.15 | 3,600 | 12,500 | 20.03 | - |
| Cancer mortality | |||||||||
| Age-related | |||||||||
| 1 × US life table | 19,100 | 20.90 | Dominated | 14,100 | 20.98 | 2,600 | 13,500 | 20.77 | - |
| 2.1 × US life table | 17,000 | 19.57 | Dominated | 12,400 | 19.64 | 3,700 | 11,700 | 19.48 | - |
| Post-curative surgery | |||||||||
| 50% × BC | 17,900 | 20.14 | Dominated | 13,100 | 20.22 | 3,300 | 12,600 | 20.07 | - |
| 200% × BC | 17,900 | 20.13 | Dominated | 13,100 | 20.21 | 3,100 | 12,400 | 19.99 | - |
| Post-palliative surgery | |||||||||
| 50% × BC | 17,900 | 20.13 | Dominated | 13,100 | 20.21 | 3,300 | 12,600 | 20.04 | - |
| 200% × BC | 17,900 | 20.13 | Dominated | 13,100 | 20.21 | 3,100 | 12,400 | 20.03 | - |
| Outcome adjustments | |||||||||
| Quality of life adjustment factors | |||||||||
| Post pancreaticoduodenectomy | |||||||||
| 0.8 | 17,900 | 19.69 | Dominated | 13,100 | 20.01 | Dominated | 12,500 | 20.01 | - |
| 1 | 17,900 | 20.18 | Dominated | 13,100 | 20.23 | 2,900 | 12,500 | 20.04 | - |
| Cancer | |||||||||
| 0.25 | 17,900 | 20.13 | Dominated | 13,100 | 20.21 | 2,900 | 12,500 | 20.02 | - |
| 1 | 17,900 | 20.14 | Dominated | 13,100 | 20.22 | 4,000 | 12,500 | 20.08 | - |
| Discount rate (%) | |||||||||
| 0 | 38,600 | 35.18 | Dominated | 29,400 | 35.35 | 900 | 28,900 | 34.81 | - |
| 5 | 11,700 | 15.07 | Dominated | 8,300 | 15.12 | 5,600 | 7,800 | 15.03 | - |
| Costs | |||||||||
| Cancer care | |||||||||
| 50% × BC | 17,900 | 20.13 | Dominated | 12,900 | 20.21 | 10,900 | 11,000 | 20.04 | - |
| 200% × BC | 18,000 | 20.13 | Dominated | 13,400 | 20.21 | - | 15,500 | 20.04 | Dominated |
| Endoscopy | |||||||||
| 50% × BC | 16,000 | 20.13 | Dominated | 10,400 | 20.21 | 8,000 | 9,000 | 20.04 | - |
| 200% × BC | 21,700 | 20.13 | Dominated | 18,400 | 20.21 | - | 19,500 | 20.04 | Dominated |
| Pancreaticoduodenectomy | |||||||||
| 50% × BC | 14,700 | 20.13 | Dominated | 11,400 | 20.21 | - | 12,100 | 20.04 | Dominated |
| 200% × BC | 24,300 | 20.13 | Dominated | 16,400 | 20.21 | 17,500 | 13,300 | 20.04 | - |
| Post pancreaticoduodenectomy | |||||||||
| 50% × BC | 15,100 | 20.13 | Dominated | 11,800 | 20.21 | - | 12,300 | 20.04 | Dominated |
| 200% × BC | 23,600 | 20.13 | Dominated | 15,600 | 20.21 | 15,700 | 12,900 | 20.04 | - |
- = reference strategy; BC = base-case scenario; Dominated = both less effective and more costly than another strategy; ICER = incremental cost-effectiveness ratio (U.S. $/QALY gained); PD = pancreaticoduodenectomy; QALY = discounted quality-adjusted life years; U.S. $ = discounted 2007 U.S. dollars.
There is considerable variability in literature reports of lifetime duodenal cancer risk. The transition rates between stages 0-IV and IV-cancer were varied independently as well as simultaneously. At lifetime cancer risks of over 50%—well in excess of plausible values—PD at stage IV dominated PD at stage III and PD at cancer diagnosis. At a lifetime cancer risk of under 1%—well under all published estimates—the ICER of PD at stage IV relative to surgery at the time of cancer diagnosis was less than $50,000/QALY gained.
Wide variation in the discount rate and all utility reductions, quality-of-life adjustments, and costs did not change the optimal strategy. One exception was the long-term quality-of-life following a PD. Using a WTP threshold of $80,000/QALY, PD at cancer diagnosis dominated both PD at stage III and stage IV if quality of life after PD was lower than 0.83.
To account for possible heterogeneity in optimal management strategies according to individual patient characteristics, we performed a subgroup analysis (see appendix for methodology and results). Regardless of the cohort's initial age or stage, PD at stage IV maximized life expectancy. We also performed a multiway sensitivity analysis to address simultaneous uncertainty in multiple variables (see appendix for methodology and results). In 99% of the multiway sensitivity trials performed, PD at stage IV was a cost-effective management strategy compared to surgery at cancer diagnosis (ICER < $80,000/QALY).
Discussion
The results of our analysis suggest that PD at stage IV duodenal polyposis in patients with FAP is both an effective and cost-effective management strategy compared to PD at cancer diagnosis or stage III polyposis. Once stage IV polyposis has been diagnosed, PD mortality and morbidity is substantially less than the mortality and morbidity from future cancers. Surgery at stage IV would prevent more than 90% of duodenal cancers. By decreasing the length of time spent in stage IV, which has frequent endoscopies, the average total number of endoscopies would decrease by almost 5 per person. The cost savings from performing fewer endoscopies and reducing the number of cancers would partially offset the increase in surgical costs. While the number of individuals affected by duodenal polyposis is small in absolute terms, the results from the model highlight the large increase in life expectancy at very low marginal cost of recommending surgery at stage IV versus surgery at cancer diagnosis, making the choice of management strategy an important public health concern for this population.
PD at stage III was dominated by PD at stage IV. However, PD at stage IV resulted in almost 1.1% of the model cohort developing duodenal cancer. Surgery at stage III would further reduce the lifetime risk of cancer to 0.3%. This benefit, however, comes at the cost of 43% of the cohort undergoing PD. The high number of surgeries would increase costs, and due to perioperative mortality and post-surgery morbidity, decrease QALYs relative to PD at stage IV.
Our findings were generally insensitive to wide variations in model parameter estimates. The model was sensitive, however, to post-PD quality of life. A number of studies have measured the quality of life following a PD. Patients have equivalent quality of life scores before surgery and at one year follow surgery, and compared to control groups, report only mildly lower quality of life overall (41-44). This supports a post-PD quality of life utility substantially above the threshold of 0.83 found by sensitivity analysis.
Vasen et al. previously constructed a simple decision analysis model of duodenal cancer in FAP, finding that endoscopic surveillance increased life expectancy (9). Our model was constructed to answer the question of at what stage surgery should be recommended, assuming that endoscopic surveillance is occurring. Our analysis employed a Markov model to explicitly model the underlying disease natural history and treatment states, whereas Vasen et al. used a decision tree approach and did not include a cost-effectiveness analysis.
A limitation of our study, as in any modeling study, is uncertainty in the parameter estimates. Our parameter estimates were based on data from multiple sources with heterogeneous study design and populations. Future studies of FAP natural history and treatment outcomes can better inform these parameter estimates. However, the relative insensitivity of our results to a wide range of parameter estimates supports the model's conclusions.
All disease models are a simplification of reality. The best efforts were made to construct a comprehensive model that accurately reflects clinical realities. A number of simplifying assumptions, however, were needed to make the model more understandable and transparent, and to account for the availability of clinical data. We assumed that transitions between stages 0 - IV occurred at a constant rate. Although there is no underlying biological rationale that this should be the case, the literature supports this assumption as a first approximation (5, 16, 45-46). Our model does not explicitly model transition rates as a function of age. Although such an approach might better approximate the underlying pathogenesis, sufficient clinical data were not available. Thus, at extremes of age, our model predictions may be less accurate. Disease regression is biologically supported, but there is a lack of sufficient data to quantify its effect. We felt that including it in the model would increase complexity and decrease transparency without much benefit. Additionally, disease progression transitions rates were calibrated to empiric data tracking disease progression in aggregate; because of this, our model implicitly includes the possibility of disease regression in its transition rates. While not everyone in stage III and stage IV is a surgical candidate, and prophylactic PD does not reduce risk of future duodenal cancer to zero, these assumptions simplify the model. Finally, we assumed perfect adherence to recommended screening protocols. If patients do not undergo screening at suggested intervals (assuming that results in cancer being diagnosed at more advanced stages), this assumption may bias the model towards delaying surgical intervention.
Several new treatment modalities for duodenal polyposis are currently being studied, including photodynamic therapy, thermal ablation, and argon plasma coagulation. The use of non-steroidal anti-inflammatory drugs in chemoprevention is also being examined. At present, however, the long-term outcomes of these approaches are unknown and further study is needed to assess efficacy. These potential treatments could be valuable additions to the model in the future.
In conclusion, prophylactic PD at stage IV duodenal polyposis in patients with FAP is a cost-effective approach that results in greater life expectancy than surgery at either stage III or cancer diagnosis. Effective clinical decision-making requires considering this recommendation within the context of each patient's unique history and preferences to create an individually appropriate management strategy.
Supplementary Material
Acknowledgements
Presented in part as an oral presentation at the Digestive Disease Week, New Orleans, 2004. We gratefully acknowledge the assistance from Lauren E. Cipriano.
Funding: This work was supported by grants from the American Society for Endoscopic Research Outcomes and Effectiveness [NIH/NCI K07CA107060 to CH] and by fellowships from the Office of Enrichment Programs and the Pasteur Program, both at Harvard Medical School [to WHG].
Footnotes
Conflicts of Interest: There are no conflicts of interest.
References
- 1.Cruz-Correa M, Giardiello FM. Familial adenomatous polyposis. Gastrointest Endosc. 2003;58:885–94. doi: 10.1016/s0016-5107(03)02336-8. [DOI] [PubMed] [Google Scholar]
- 2.Kadmon M, Tandara A, Herfarth C. Duodenal adenomatosis in familial adenomatous polyposis coli. A review of the literature and results from the Heidelberg Polyposis Register. Int J Colorectal Dis. 2001;16:63–75. doi: 10.1007/s003840100290. [DOI] [PubMed] [Google Scholar]
- 3.Brosens LA, Keller JJ, Offerhaus GJ, Goggins M, Giardiello FM. Prevention and management of duodenal polyps in familial adenomatous polyposis. Gut. 2005;54:1034–43. doi: 10.1136/gut.2004.053843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bulow S, Bjork J, Christensen IJ, et al. Duodenal adenomatosis in familial adenomatous polyposis. Gut. 2004;53:381–6. doi: 10.1136/gut.2003.027771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heiskanen I, Kellokumpu I, Jarvinen H. Management of duodenal adenomas in 98 patients with familial adenomatous polyposis. Endoscopy. 1999;31:412–6. doi: 10.1055/s-1999-41. [DOI] [PubMed] [Google Scholar]
- 6.Offerhaus GJ, Giardiello FM, Krush AJ, et al. The risk of upper gastrointestinal cancer in familial adenomatous polyposis. Gastroenterology. 1992;102:1980–2. doi: 10.1016/0016-5085(92)90322-p. [DOI] [PubMed] [Google Scholar]
- 7.Pauli RM, Pauli ME, Hall JG. Gardner syndrome and periampullary malignancy. Am J Med Genet. 1980;6:205–19. doi: 10.1002/ajmg.1320060305. [DOI] [PubMed] [Google Scholar]
- 8.Bjork J, Akerbrant H, Iselius L, et al. Periampullary adenomas and adenocarcinomas in familial adenomatous polyposis: cumulative risks and APC gene mutations. Gastroenterology. 2001;121:1127–35. doi: 10.1053/gast.2001.28707. [DOI] [PubMed] [Google Scholar]
- 9.Vasen HF, Bulow S, Myrhoj T, et al. Decision analysis in the management of duodenal adenomatosis in familial adenomatous polyposis. Gut. 1997;40:716–9. doi: 10.1136/gut.40.6.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belchetz LA, Berk T, Bapat BV, Cohen Z, Gallinger S. Changing causes of mortality in patients with familial adenomatous polyposis. Dis Colon Rectum. 1996;39:384–7. doi: 10.1007/BF02054051. [DOI] [PubMed] [Google Scholar]
- 11.Galle TS, Juel K, Bulow S. Causes of death in familial adenomatous polyposis. Scand J Gastroenterol. 1999;34:808–12. doi: 10.1080/003655299750025741. [DOI] [PubMed] [Google Scholar]
- 12.Nugent KP, Spigelman AD, Phillips RK. Life expectancy after colectomy and ileorectal anastomosis for familial adenomatous polyposis. Dis Colon Rectum. 1993;36:1059–62. doi: 10.1007/BF02047300. [DOI] [PubMed] [Google Scholar]
- 13.Jagelman DG, DeCosse JJ, Bussey HJ. Upper gastrointestinal cancer in familial adenomatous polyposis. Lancet. 1988;1:1149–51. doi: 10.1016/s0140-6736(88)91962-9. [DOI] [PubMed] [Google Scholar]
- 14.Arvanitis ML, Jagelman DG, Fazio VW, Lavery IC, McGannon E. Mortality in patients with familial adenomatous polyposis. Dis Colon Rectum. 1990;33:639–42. doi: 10.1007/BF02150736. [DOI] [PubMed] [Google Scholar]
- 15.Spigelman AD, Williams CB, Talbot IC, Domizio P, Phillips RK. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet. 1989;2:783–5. doi: 10.1016/s0140-6736(89)90840-4. [DOI] [PubMed] [Google Scholar]
- 16.Groves CJ, Saunders BP, Spigelman AD, Phillips RK. Duodenal cancer in patients with familial adenomatous polyposis (FAP): results of a 10 year prospective study. Gut. 2002;50:636–41. doi: 10.1136/gut.50.5.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirota WK, Zuckerman MJ, Adler DG, et al. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc. 2006;63:570–80. doi: 10.1016/j.gie.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Church J, Kiringoda R, LaGuardia L. Inherited colorectal cancer registries in the United States. Dis Colon Rectum. 2004;47:674–8. doi: 10.1007/s10350-003-0115-5. [DOI] [PubMed] [Google Scholar]
- 19.Winkelmayer WC, Weinstein MC, Mittleman MA, Glynn RJ, Pliskin JS. Health economic evaluations: the special case of end-stage renal disease treatment. Med Decis Making. 2002;22:417–30. doi: 10.1177/027298902236927. [DOI] [PubMed] [Google Scholar]
- 20.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. Jama. 1996;276:1253–8. [PubMed] [Google Scholar]
- 21.Cusack J. Diagnosis and management of small bowel neoplasms. In: Savarese D, editor. UpToDate. UpToDate; Wellesley, MA: 2008. [Google Scholar]
- 22.Cahen DL, Fockens P, de Wit LT, Offerhaus GJ, Obertop H, Gouma DJ. Local resection or pancreaticoduodenectomy for villous adenoma of the ampulla of Vater diagnosed before operation. Br J Surg. 1997;84:948–51. doi: 10.1002/bjs.1800840711. [DOI] [PubMed] [Google Scholar]
- 23.de Vos tot Nederveen Cappel WH, Jarvinen HJ, Bjork J, Berk T, Griffioen G, Vasen HF. Worldwide survey among polyposis registries of surgical management of severe duodenal adenomatosis in familial adenomatous polyposis. Br J Surg. 2003;90:705–10. doi: 10.1002/bjs.4094. [DOI] [PubMed] [Google Scholar]
- 24.Gallagher MC, Shankar A, Groves CJ, Russell RC, Phillips RK. Pylorus-preserving pancreaticoduodenectomy for advanced duodenal disease in familial adenomatous polyposis. Br J Surg. 2004;91:1157–64. doi: 10.1002/bjs.4527. [DOI] [PubMed] [Google Scholar]
- 25.Ryan DP, Schapiro RH, Warshaw AL. Villous tumors of the duodenum. Ann Surg. 1986;203:301–6. doi: 10.1097/00000658-198603000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galandiuk S, Hermann RE, Jagelman DG, Fazio VW, Sivak MV. Villous tumors of the duodenum. Ann Surg. 1988;207:234–9. doi: 10.1097/00000658-198803000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silvis SE, Nebel O, Rogers G, Sugawa C, Mandelstam P. Endoscopic complications. Results of the 1974 American Society for Gastrointestinal Endoscopy Survey. Jama. 1976;235:928–30. doi: 10.1001/jama.235.9.928. [DOI] [PubMed] [Google Scholar]
- 28.Pennock JL, Oyer PE, Reitz BA, et al. Cardiac transplantation in perspective for the future. Survival, complications, rehabilitation, and cost. J Thorac Cardiovasc Surg. 1982;83:168–77. [PubMed] [Google Scholar]
- 29.Ruo L, Coit DG, Brennan MF, Guillem JG. Long-term follow-up of patients with familial adenomatous polyposis undergoing pancreaticoduodenal surgery. J Gastrointest Surg. 2002;6:671–5. doi: 10.1016/s1091-255x(02)00045-8. [DOI] [PubMed] [Google Scholar]
- 30.Surveillance Epidemiology and End Results (SEER) Program Public-Use Data. National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/data/. Published April 2008. Accessed July 2008. [Google Scholar]
- 31.Barnes SA, Lillemoe KD, Kaufman HS, et al. Pancreaticoduodenectomy for benign disease. Am J Surg. 1996;171:131–4. doi: 10.1016/s0002-9610(99)80087-7. discussion 4-5. [DOI] [PubMed] [Google Scholar]
- 32.Gallagher MC, Phillips RK, Bulow S. Surveillance and management of upper gastrointestinal disease in Familial Adenomatous Polyposis. Fam Cancer. 2006;5:263–73. doi: 10.1007/s10689-005-5668-0. [DOI] [PubMed] [Google Scholar]
- 33.Yeo CJ, Sohn TA, Cameron JL, Hruban RH, Lillemoe KD, Pitt HA. Periampullary adenocarcinoma: analysis of 5-year survivors. Ann Surg. 1998;227:821–31. doi: 10.1097/00000658-199806000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howe JR, Klimstra DS, Moccia RD, Conlon KC, Brennan MF. Factors predictive of survival in ampullary carcinoma. Ann Surg. 1998;228:87–94. doi: 10.1097/00000658-199807000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Center for Health Statistics . U.S. Life Table 2004: National Vital Statistics Report. Washington: DNCfHS: 2007. [Google Scholar]
- 36.Ishikawa O, Ohigashi H, Eguchi H, et al. Long-term follow-up of glucose tolerance function after pancreaticoduodenectomy: comparison between pancreaticogastrostomy and pancreaticojejunostomy. Surgery. 2004;136:617–23. doi: 10.1016/j.surg.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Slezak LA, Andersen DK. Pancreatic resection: effects on glucose metabolism. World J Surg. 2001;25:452–60. doi: 10.1007/s002680020337. [DOI] [PubMed] [Google Scholar]
- 38.Farkas G, Leindler L, Daroczi M, Farkas G., Jr. Prospective randomised comparison of organ-preserving pancreatic head resection with pylorus-preserving pancreaticoduodenectomy. Langenbecks Arch Surg. 2006;391:338–42. doi: 10.1007/s00423-006-0051-7. [DOI] [PubMed] [Google Scholar]
- 39.Rubenstein JH, Scheiman JM, Anderson MA. A clinical and economic evaluation of endoscopic ultrasound for patients at risk for familial pancreatic adenocarcinoma. Pancreatology. 2007;7:514–25. doi: 10.1159/000108969. [DOI] [PubMed] [Google Scholar]
- 40.McMahon PM, Halpern EF, Fernandez-del Castillo C, Clark JW, Gazelle GS. Pancreatic cancer: cost-effectiveness of imaging technologies for assessing resectability. Radiology. 2001;221:93–106. doi: 10.1148/radiol.2211001656. [DOI] [PubMed] [Google Scholar]
- 41.Warnick SJ, Jr., Velanovich V. Correlation of patient-derived utility values and quality of life after pancreaticoduodenectomy for pancreatic cancer. J Am Coll Surg. 2006;202:906–11. doi: 10.1016/j.jamcollsurg.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 42.Muller MW, Dahmen R, Koninger J, et al. Is there an advantage in performing a pancreas-preserving total duodenectomy in duodenal adenomatosis? Am J Surg. 2008;195:741–8. doi: 10.1016/j.amjsurg.2007.08.061. [DOI] [PubMed] [Google Scholar]
- 43.Ohtsuka T, Yamaguchi K, Chijiiwa K, Kinukawa N, Tanaka M. Quality of life after pylorus-preserving pancreatoduodenectomy. Am J Surg. 2001;182:230–6. doi: 10.1016/s0002-9610(01)00709-7. [DOI] [PubMed] [Google Scholar]
- 44.Huang JJ, Yeo CJ, Sohn TA, et al. Quality of life and outcomes after pancreaticoduodenectomy. Ann Surg. 2000;231:890–8. doi: 10.1097/00000658-200006000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saurin JC, Gutknecht C, Napoleon B, et al. Surveillance of duodenal adenomas in familial adenomatous polyposis reveals high cumulative risk of advanced disease. J Clin Oncol. 2004;22:493–8. doi: 10.1200/JCO.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 46.Moozar KL, Madlensky L, Berk T, Gallinger S. Slow progression of periampullary neoplasia in familial adenomatous polyposis. J Gastrointest Surg. 2002;6:831–7. doi: 10.1016/s1091-255x(02)00062-8. discussion 7. [DOI] [PubMed] [Google Scholar]
- 47.Provenzale D, Schmitt C, Wong JB. Barrett's esophagus: a new look at surveillance based on emerging estimates of cancer risk. Am J Gastroenterol. 1999;94:2043–53. doi: 10.1111/j.1572-0241.1999.01276.x. [DOI] [PubMed] [Google Scholar]
- 48.Hanmer J, Lawrence WF, Anderson JP, Kaplan RM, Fryback DG. Report of nationally representative values for the noninstitutionalized US adult population for 7 health-related quality-of-life scores. Med Decis Making. 2006;26:391–400. doi: 10.1177/0272989X06290497. [DOI] [PubMed] [Google Scholar]
- 49.Feenstra TL, Hamberg-van Reenen HH, Hoogenveen RT. Rutten-van Molken MP. Cost-effectiveness of face-to-face smoking cessation interventions: a dynamic modeling study. Value Health. 2005;8:178–90. doi: 10.1111/j.1524-4733.2005.04008.x. [DOI] [PubMed] [Google Scholar]
- 50.Rubenstein JH, Vakil N, Inadomi JM. The cost-effectiveness of biomarkers for predicting the development of oesophageal adenocarcinoma. Aliment Pharmacol Ther. 2005;22:135–46. doi: 10.1111/j.1365-2036.2005.02536.x. [DOI] [PubMed] [Google Scholar]
- 51.Russell LB, Gold MR, Siegel JE, Daniels N, Weinstein MC. The role of cost-effectiveness analysis in health and medicine. Panel on Cost-Effectiveness in Health and Medicine. Jama. 1996;276:1172–7. [PubMed] [Google Scholar]
- 52.Yabroff KR, Lamont EB, Mariotto A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;100:630–41. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- 53.Rubenstein JH, Inadomi JM, Brill JV, Eisen GM. Cost utility of screening for Barrett's esophagus with esophageal capsule endoscopy versus conventional upper endoscopy. Clin Gastroenterol Hepatol. 2007;5:312–8. doi: 10.1016/j.cgh.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 54.Hur C, Nishioka NS, Gazelle GS. Cost-effectiveness of aspirin chemoprevention for Barrett's esophagus. J Natl Cancer Inst. 2004;96:316–25. doi: 10.1093/jnci/djh039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


