Abstract
Vitamin C (ascorbate) has important antioxidant functions that can help protect against oxidative stress in the brain and damage associated with neurodegenerative disorders such as Alzheimer’s disease. When administered parenterally ascorbate can bypass saturable uptake mechanisms in the gut and thus higher tissue concentrations can be achieved than by oral administration. In the present study we show that ascorbate (125 mg/kg) administered intraperitoneally (i.p.) 1-hour before testing, partially attenuated scopolamine-induced (1 mg/kg i.p.) cognitive deficits in Morris water maze performance in young mice. Cumulative search error, but not escape latency nor path length, was significantly improved during acquisition in ascorbate plus scopolamine-treated mice although performance did not equal that of control mice. During the probe trial, scopolamine led to increased search error and chance level of time spent in the platform quadrant, whereas mice pre-treated with ascorbate prior to scopolamine did not differ from control mice on these measures. Ascorbate had no effect on unimpaired, control mice and neither did it reduce the peripheral, activity-increasing effects of scopolamine. Ascorbate alone increased acetylcholinesterase activity in the medial forebrain area but had no effect in cortex or striatum. This change, and its action against the amnestic effects of the muscarinic antagonist scopolamine, suggest that ascorbate may be acting in part via altered cholinergic signaling. However, further investigation is necessary to isolate the cognition-enhancing effects of ascorbate.
Keywords: Ascorbic acid, Vitamin C, Y maze, water maze, learning and memory, acetylcholinesterase, antioxidant
1. Introduction
Vitamin C (ascorbic acid) is of vital importance in the brain, which will sequester it at the expense of other organs in conditions of low ascorbate availability [28]. In fact, in guinea pigs dying of scurvy, when other organs are almost devoid of ascorbic acid, brain levels did not decrease below about 25 % of normal [19]. In the first written study of scurvy, British naval surgeon Dr. James Lind reported his surprise in finding that “the brains of these poor people were always sound and entire (p.245)” [30]. Ascorbate is a powerful antioxidant and thus may help prevent oxidative stress and related damage in a number of neurodegenerative diseases including Alzheimer’s disease [15, 17]. Furthermore, ascorbate is critical for the synthesis of norepinephrine from dopamine, is involved in regulation of dopamine and glutamate transmission [43] and is also involved in release of acetylecholine from vesicles [27] and thus has also been proposed to have important neuromodulatory functions [44].
We have recently shown that ascorbate (125 mg/kg) administered via intraperitoneal (i.p.) injection reversed some of the learning and memory deficits in aged (12 month) and very old (24 month) APP/PSEN1 transgenic mice, a mouse model of Alzheimer’s disease [16]. Twelve consecutive days of daily treatments did not cause any changes in oxidative stress, amyloid plaque level or stored ascorbate levels in brain or liver, measured 24 hours following the final treatment. We hypothesized that the effect of treatments was mediated via a short-term pharmacological-like action of ascorbate on neurotransmission. The muscarinic antagonist scopolamine causes short-term learning and memory deficits and has been used to provide a model of pharmacologically-induced dementia [47, 53]. When administered parenterally, ascorbate improved cognition against age- and scopolamine-induced amnesia in a passive avoidance task, a memory test in the elevated plus maze, and a habituation test in light-dark activity chambers [9, 39, 49]. The most common form of treatment for Alzheimer’s disease involves acetylcholinesterase inhibitors (AChEIs) which work in degenerating cholinergic systems by keeping acetylcholine active for longer periods at the synapse. It has been proposed that ascorbate may have AChEI properties [10] although this has not been confirmed. Blueberry extract, another antioxidant compound, administered i.p. for 7 days has also been found to inhibit acetylcholinesterase (AChE) activity and enhance cognitive function in mice [38]. Longer (7 weeks) dietary treatment with antioxidant polyphenols found in tea also reduced AChE activity and attenuated scopolamine-induced amnesia in mice [23]. The present investigation was, therefore, conducted to seek confirmation as to whether ascorbate may act as a modulator of the cholinergic system or AChEI in young wild-type mice.
In the present study young wild-type mice were treated with scopolamine to induce memory deficits in the Morris water maze. Ascorbate treatment (i.p.) was investigated for its ability to reverse these deficits. Mice were also assessed for effects of drug on anxiety and activity to ascertain whether ascorbate mediated the cognitive effects of scopolamine, and to confirm that these could be dissociated from any non-cognitive peripheral effects such as increased locomotor activity. Following behavioral testing, mice were given one final treatment of ascorbate and/or scopolamine and brain tissue, liver, and serum were assessed for any short-term changes in ascorbate level, oxidative stress or AChE activity resulting from the treatments.
2. Materials and Methods
Subjects
The current study was performed to further elucidate data and hypotheses obtained in APP/PSEN1 transgenic mice, a model of Alzheimer’s disease [16]. The experiments were, therefore, conducted in B6C3F1/J mice, which form the background strain for the APP/PSEN1 mouse line. Twelve male and 12 female B6C3F1/J mice were obtained at 6 weeks of age from The Jackson Laboratory (Bar Harbor, ME). Animals were housed in single-sex cages in groups of four in a light- and temperature-controlled environment on a 12-h light/12-h dark schedule with free access to food and water for the duration of the experiment. Behavioral testing began when mice were aged 12 weeks. A further 12 12-week-old mice were used for visible platform testing in the water-maze and a final 20 mice were used for additional neurochemistry assays as described below but were not used for behavioral testing. All procedures were approved by the Vanderbilt University Institutional Animal Care and Use Committee and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Groups
For behavioral testing, four test groups each comprised six animals. Equal numbers of male and female mice were used in each group. Groups received the vehicle alone (CON), ascorbate alone (AA), scopolamine alone (SCOP), or ascorbate and scopolamine (AA-SCOP). For additional biochemical assays, a further 20 mice were divided equally among the four groups with approximately equal numbers and male and female mice in each group. Visible platform testing was conducted with only six CON and six SCOP mice.
Drugs
Ascorbate solutions (125 mg/kg) were prepared immediately before administration in deionized water and adjusted to pH 7. Solutions were kept in the dark in aluminum-foil covered containers and administered within 30 minutes of preparation in order to minimize the effects of oxidation. Scopolamine hydrochloride (S-1013) was obtained from Sigma-Aldrich (St Louis, MO, USA) and dissolved in 0.9 % physiological saline to a final dose of 1 mg/kg. Ascorbate or the vehicle was administered i.p. 60 min. before each daily behavioral testing session, including the water maze probe trial. Scopolamine was administered i.p. 30 min. prior to behavioral testing. Administration volume for both drugs was 10 ml/kg.
Behavioral procedures
Differences in anxiety and locomotor activity can negatively impact on ability to perform complex learning tasks such as the water maze. We therefore first tested mice in the zero maze and locomotor activity chambers to provide a more complete behavioral phenotype in order to interpret correctly data from Y-maze and water maze tests.
Locomotor activity
Locomotor activity was assessed in commercially-available activity monitors (ENV-510; MED Associates, Georgia, VT), as previously described [52]. Activity was automatically recorded by the breaking of infrared beams as the mouse explored the chamber, and analyzed using a Windows-based computer. Each session lasted 30 min, and the chambers were cleaned with a 10% alcohol solution between each mouse.
Zero maze
Anxiety was assessed using a standard Zero maze (San Diego Instruments, CA). A flat, plastic ring (inner edge diam. 51 cm, outer edge diam. 61 cm) was divided into four alternating sections, two of which had 15 cm high walls (closed sections) and 2 of which had just a 1 cm lip to prevent mice falling from the maze (open sections). The apparatus was raised 61 cm from the ground. Mice were released in the center of one of the open sections and permitted to explore the maze for 5 min. Sessions were filmed from above and scored by a trained experimenter for time spent in open and closed sections, and number of transitions between sections.
Y-maze
Spontaneous alternation behavior was tested in a standard Y-maze made of clear acrylic tubing, as previously described [18]. The number and sequence of arm entries made during a 5-min. session were recorded. Alternations were defined as an entry into each arm within three consecutive arm choices (e.g. ABC or BAC). Percent alternation was calculated as the number of alternations divided by the number of total arm entries minus two.
Water maze
Hidden-platform testing was conducted in a 107-cm diam. pool with a circular acrylic platform (10 cm diam.) submerged 1 cm below the surface of the water, as previously described [16, 18]. Mice were given four acquisition trials per day for 9 days in a massed fashion, i.e., each mouse completed all four trials before the next mouse began its trials. The water maze was located in the centre of a room with distinct, visual cues fixed to the walls that were clearly visible from the pool. These extra-maze cues remained stationary throughout acquisition and probe test sessions. Sessions were captured by an overhead camera and analyzed in real time using an NIH Image macro on a Macintosh computer written specifically for the water maze task [31, 32]. Escape latency and path length to reach the hidden platform were recorded during acquisition. Time spent swimming in the periphery (within 8 cm from edge of pool) can indicate increased anxiety and is a non-cognitive behavior that can adversely affect learning and the measurement of learning in the water maze [56]. Swim speed can be a confounding factor for assessing escape latency and path length does not provide any information about where the mouse is swimming in relation to the platform. Therefore, water maze acquisition can also be assessed using cumulative search error (distance from the platform during daily acquisition sessions) calculated as a daily average from each of the four training trials on that day. This can be a more informative measure because it takes into account trial time and proximity to the platform.
Twenty-four hours following target acquisition a 60-s probe trial was conducted. The time spent in the target versus non-target quadrants and the average distance from the platform location (search error [6, 14]) were the primary dependent measures derived from the probe trial. Swim speed and peripheral swimming were also assessed during the probe trial.
An additional 12 mice were tested on a cued-platform version of the water maze (as previously described [16]) following treatment with either saline or scopolamine (1 mg/kg) in order to establish whether scopolamine had a detrimental effect on visual acuity as has previously been demonstrated in mice at higher doses of scopolamine [45]. Ascorbate has not been found to affect visual acuity in the water maze [16]. In the cued-platform version of the water maze task the platform is visible above the surface of the water, which is not rendered opaque prior to testing, and is also marked with a clearly-visible black ball suspended above the target. Mice received four trials per day for 3 days. The platform was located in a one of four different positions in each daily trial, and the order of platform locations was different each day.
Neurochemical procedures
Tissue acquisition
Animals were anaesthetized using isoflurane and then quickly sacrificed by decapitation. Trunk blood was collected, stored on wet ice for 1 hour, and subsequently centrifuged at 4 °C at 13 000 rpm for 20 minutes. Serum was collected from each sample for measurement of ascorbate levels. Liver samples were removed and weighed and kept at −80 °C until assayed. Brains were quickly removed and placed in an ice-cold mouse brain matrix with 1 mm coronal section slice intervals (Zivic instruments, Pittsburgh, PA). The first blade was placed at the optic chiasm, two slices were taken anterior and two slices taken posterior to this point. The four segments were then separated and cooled further on the blade using dry ice. A 1 mm diameter tissue biopsy punch was then used to remove selected core samples from each section (8 cortex, 3 striatum, 2 medial forebrain bundle (MFB)) with reference to a stereotaxic mouse brain atlas [40]. Punch samples were stored at −80 °C until needed for determination of AChE activity. Remaining unsliced tissue included frontal cortex and cerebellum and was used for analysis of ascorbate, malondialdehyde (MDA) and glutathione (GSH).
Ascorbate measurement
Ascorbate levels were measured in cortex, liver and blood serum. Concentrations were measured by ion pair HPLC and electrochemical detection as previously described [16, 37].
Acetylcholinesterase activity
Acetylcholinesterase activity was measured using a colorimetric assay based on the methods of Ellman [12]. The protocol was modified for use with 96 well microtiterplates as previously described [16].
Malondialdehyde (MDA)
MDA was quantified to detect any short-term changes in lipid peroxidation following treatments using methods based on [46] but modified for use with 96 well microtiter plates. Small, weighed tissue samples were homogenized in 1 ml 5% TCA solution and serum (50 µl) was treated with 300 µl of ice cold 5% TCA. Samples were vortexed and then centrifuged at 13 000 rpm at room temperature for 5 min. The sample (300 µl) was reacted with the same volume of 20 mM thiobarbituric acid (TBA) for 35 minutes in a water bath at 95 °C, followed by 10 minutes at 4 °C. An MDA standard was also prepared with 3 µl of 1 mMol MDA combined with 300 µl of 5 % TCA and both mixed with 300 µl TBA. The standards were placed in the water bath and refrigerator at the same time as the samples. A standard curve was prepared from dilutions of this solution. Sample or standard (250 µl) was added to separate wells of a 96-well microtiterplate. Malondialdehyde was then specifically measured on a Spectramax M5 microplate reader (Molecular Devices, USA) with an excitation wavelength of 515 and an emission wavelength of 553. Duplicate samples were assayed for both standards and samples.
Glutahione (GSH)
GSH was measured as an additional indication of change in oxidative mechanisms following treatments. Total glutathione (GSH plus oxidized form glutathione disulfide (GSSG)) was measured according to the methods of [42] using a spectrophotometric/microplate reader assay method. Briefly, weighed samples were homogenized in 0.5 ml of homogenization buffer; liver in ice-cold 5% metaphosphoric acid and brain in 0.6% sulfosalicylic acid–Triton-X solution. Samples were centrifuged and the supernatant diluted 1 in 4 with homogenization fluid. 20 µl of sample or GSH standard was added to separate wells of a 96-well microtiterplate. 120 µl of DTNB and Glutathione reductase solution was added to the sample and after allowing 30 s for conversion of GSSG to GSH 60 µl of β-NADPH was added and GSH was then specifically measured on a Spectramax M5 microplate reader (Molecular Devices, USA) at 412 nm using a kinetic measurement program that took one reading every 30 s for 2 ½ min.
Statistics
All data were analyzed using SPSS 16.0 for Windows. There were no independent effects of sex so data were collapsed into treatment groups for further analyses. For single dependent variables in the Y-maze, Zero maze, water maze probe trial, and neurochemical assays univariate ANOVAs were conducted with treatment group as the between-groups factor. Locomotor activity and water maze acquisition measures of escape latency, path length, swim speed, and cumulative search error were analyzed using repeated measures ANOVA (RMANOVA) with Time or Session as the repeated factors and the same between-groups factor of treatment group. For water maze probe trial analysis, preferential swimming in the probe trial was assessed using a t-test against chance performance of 25 % (15 s) in the target quadrant with analyses conducted separately for each group. In the Y-maze, percent alternation was also assessed against chance alternation performance (of 50 %) using a one-sample t-test within each group. Following a significant omnibus ANOVA, Bonferroni post-hoc comparisons were conducted. The comparisons of interest in the present experiment were the effect of each treatment compared to the control mice, and scopolamine-treated versus ascorbate and scopolamine-treated mice.
3. Results
Locomotor activity
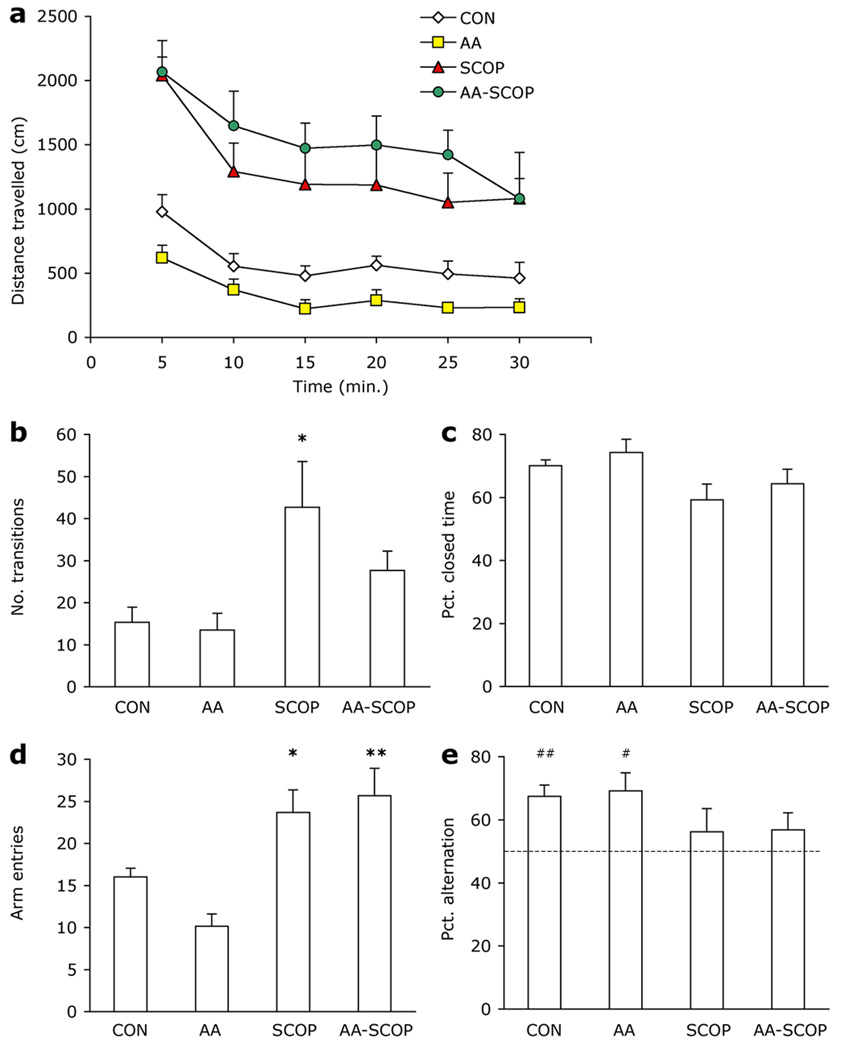
Scopolamine increased mouse locomotor activity [F3, 20 = 12.305, p<.001; Fig. 1a]. Overall SCOP and AA-SCOP mice traveled further than CON and AA mice (ps<.05). Despite differences in overall activity levels, all mice habituated to the activity chambers over the course of the 30-min. session [Time F5, 100 = 31.152, p<.001, Time × Group F5, 100 = 1.841, p<.039]. Group differences were significant at each 5-min time point (ps<.05) and the decrease in activity across time was also significant in each group (ps<.05) although rate of decrease differed among the groups. Moving speed did not differ among the treatment groups [F3, 20 = 2.434, p=.095].
Figure 1. Anxiety and activity.
Scopolamine injections led to increased activity in all three dry-land tasks but did not affect alternation behavior or anxiety. (a) Distance traveled during 30-min. locomotor activity session. (b) Transitions between the open and closed areas of the Zero maze and (c) percent time spent in closed areas of the Zero maze. (d) Number of arm entries and (e) percent alternations in the Y-maze. Chance performance of 50 % alternation marked by dotted line (---). AA mice did not differ from CON mice on any of the above measures. Data represent group means ± 1 S.E.M. Different from CON *p<.05; **p<.01. Different from chance alternation #p<.05, ##p<.01.
Zero maze
Scopolamine also increased activity in the Zero maze as shown by the group differences in number of transitions [F3, 20 = 4.308, p=.017; Fig. 1b], however, only the SCOP group differed significantly from CON mice (p=.045). Anxiety in the zero maze, assessed via percent time spent in closed areas of the maze, was normal (aprox. 60–75 %) in all mice and did not differ among groups [F3, 20 = 2.578, p=.082; Fig. 1c].
Y maze
An overall effect of treatment in the Y-maze arm entries [F3, 20 = 9.765, p≤.001; Fig. 1d] indicated that SCOP and AA-SCOP mice made more arm entries than CON mice (ps<.05). The differences in activity level did not affect alternation behavior. Percent alternation was aprox. 10 % lower in scopolamine-treated mice but there was no significant difference among the groups [F3, 20 = 1.45, p=.258; Fig. 1e]. However, when each group’s performance was compared to chance alternation level of 50 % a different pattern emerged. Both CON and AA groups alternated above chance [CON t(5) = 4.863, p=.005; AA t(5) = 3.356, p=.02], but neither scopolamine-treated group alternated at above-chance levels [SCOP t(5) = .835, p=.442; AA-SCOP t(5) = 1.25, p=.267].
Water maze
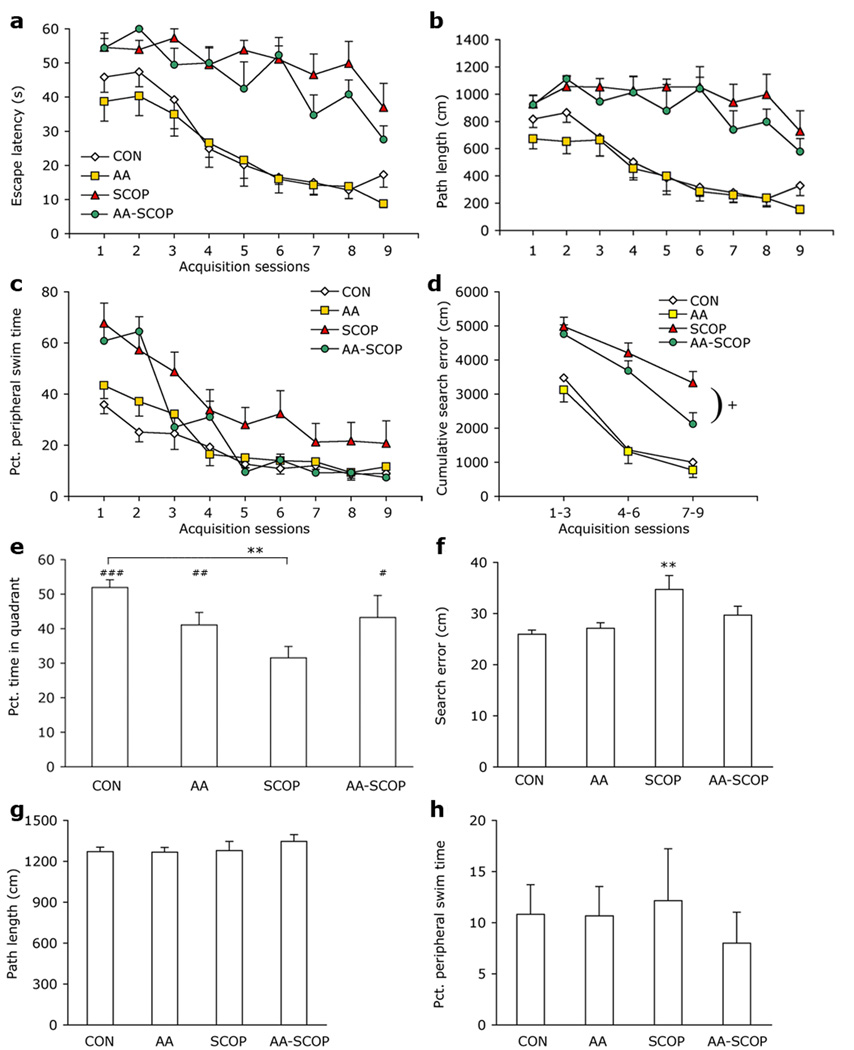
Escape latency and path length decreased across the 9 days of training in all four groups [escape latency F8, 160 = 20.835, p<.001, Fig. 2a; path length F8, 160 = 15.108, p<.001, Fig. 2b]. There was a main effect of group and Group × Session interaction effects for both measures [Fs >1.815, ps<.016]. SCOP and AA-SCOP mice took longer and traveled farther to locate the platform than did CON mice (ps<.001) and the AA-SCOP treatment performed no better than SCOP alone mice on either measure (ps>.24). Nevertheless, some improvement was seen in each of the four groups on both measures (ps<.05) indicating that even scopolamine-treated mice learned something about the location of the platform and the rules governing escape from the maze. Time spent swimming in the periphery of the maze decreased across acquisition sessions for all groups [F8, 160 = 77.489, p<.001; Fig. 2c], however, peripheral swimming differed among the groups [Group F3, 20 = 4.406, p=.016; Session × Group F24, 160 = 4.042 p<.001]. Across the whole of water maze acquisition only the SCOP group spent significantly more time in the periphery than CON mice (p<.001). AA-SCOP mice did not differ from CON mice (p=.151), but neither did they spend significantly less time than SCOP mice in the periphery (p=.066). Reduction in peripheral swim time across sessions was seen in all four groups (ps<.05). SCOP and AA-SCOP groups had higher peripheral swim times than controls from the first test session (CON 35.9 %; AA 43.4 %; SCOP 67.7 %; AA-SCOP 60.8 %). However, by the final day of testing, AA-SCOP mice had reached the same low levels as CON and AA groups.
Figure 2. Attenuation of scopolamine-induced deficit in water maze.
Scopolamine treatment led to impaired learning and memory in the acquisition and probe phases of water maze testing but these deficits were significantly attenuated when ascorbate treatments were given 30 min. prior to scopolamine. (a) Water maze escape latencies and (b) path lengths across nine acquisition sessions. (c) Percent time spent in the periphery of the maze during acquisition sessions. (d) Cumulative search error during acquisition. (e) Percent time spent in platform quadrant during the 60 sec. probe trial to assess platform preference. (f) Search error during probe trial. (g) Path length and (h) peripheral swim time did not differ among groups during the probe trial. Data represent group means ± 1 S.E.M. Different from SCOP +p<.05; different from CON **p<.01; platform quadrant different from chance performance of 25 % of time in platform quadrant #p<.05, ##p<.01, ###p<.001.
The measure of cumulative search error combines information from each of the three measures described above. Data were collapsed into 3-day bins for clarity. Cumulative search error decreased across sessions indicating improvement in all groups [F2, 40 = 90.801, p<.001; Fig. 2d], but performance differed among the groups [Group F3, 20 = 18.543, p<.001; Session × Group F6, 40 = 2.422 p=.043]. On days 1–3, SCOP and AA-SCOP groups did not differ from each other (p=.60) and both had a greater cumulative search error than CON mice (ps<.002). However, by days 7–9 although both SCOP (p<.001) and AA-SCOP (p<.03) mice still performed more poorly than CON mice, AA-SCOP had significantly lower cumulative search error than SCOP-alone mice (p<.023). These data represent a partial rescue of the scopolamine-induced learning deficit by ascorbate. Performance of AA and CON mice did not differ at any point during acquisition trials (ps>.41). Swim speed did not vary among the groups during water maze acquisition trials [Fs < 3.371, ps > .10].
Preferentially swimming in the quadrant that previously contained the platform indicates memory for the former platform location. Preferential swimming was seen in CON, AA, and AA-SCOP, who each spent significantly more than 25 % of the time (15 s) swimming in the platform quadrant but the same was not true for the SCOP group [CON t(5) =12.24, p<.001; AA t(5) = 4.46, p=.007; SCOP t(5) =1.96, p=.108; AA-SCOP t(5) =2.83, p=.037; Fig. 2e]. The proportion of the probe trial that mice spent swimming in the platform quadrant also differed among the groups [F3, 20 = 3.996, p=.022; Fig. 2e]. SCOP mice spent significantly less time swimming in the platform quadrant than CON mice (p=.003). AA-SCOP mice spent slightly more time swimming in the platform quadrant than SCOP mice. Although this difference was not significant (p=.063), neither did they differ significantly from CON mice (ps=.156). AA and CON mice did not differ (p=.083). Similarly, search error during the probe trial differed according to group [F3, 20 = 4.86, p=.011; Fig. 2f]. Only SCOP mice had a significantly greater search error than CON mice (p=.002). AA and AA-SCOP mice did not differ from CON mice (ps>.145), although neither was AA-SCOP search error significantly smaller than that of SCOP mice. Given that activity levels were greater in scopolamine treated mice in the land-based tasks described above, and peripheral swimming was greater in scopolamine-treated mice during water maze acquisition, it was important to establish that these factors did not differ greatly among the groups during the probe trial in order to be able to attribute the differences found to cognitive ability. Path length during the probe trial did not differ among the groups [F3, 20 = .581, p=.635; Fig 2g]. Peripheral swimming during the probe trial was also similar among groups [F3, 20 = .237, p=.870; Fig. 2h]. Group means ranged from 8 to 12.5 %, which are similar to those found on the final day of training (7.4 to 20.8 %) and indicate that mice were not simply circling at the pool perimeter, but were more likely actively searching for the platform. Swim speed was similar among the groups during the probe trial (CON 21.2 cm/s, AA 21.2 cm/s, SCOP 21.3 cm/s, AA-SCOP 22.4 cm/s) [F3, 20 = .579, p=.636]. Performance on the visible platform version of the water maze was not adversely affected by scopolamine treatment. Neither escape latency nor path length varied between the groups across the 3 days of testing [Fs<.892, ps>.367].
Neurochemical data
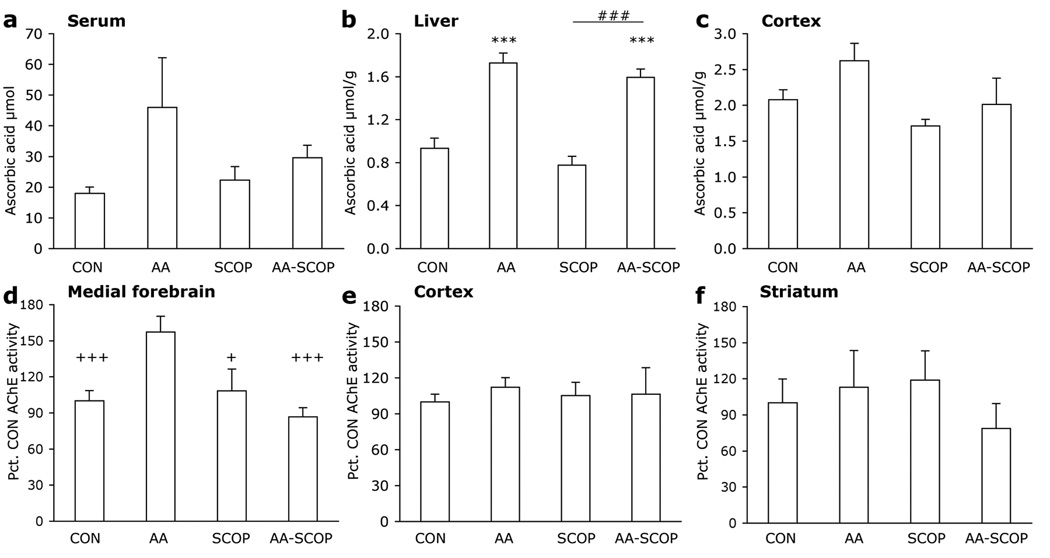
Following behavioral testing mice were given one further treatment prior to sacrifice. Times of sacrifice were 30 min. following scopolamine injections or 60 min. following ascorbate injections to correspond with the timing of behavioral tests. Scopolamine had no effect on ascorbate levels in serum, liver or brain. Ascorbate treatments led to a small, non-significant increase in serum ascorbate [F3, 18 = 1.735, p<.196; Fig. 3a]. The same pattern was reflected in the liver [F3, 20 = 29.171, p<.001; Fig. 3b], where ascorbate levels were significantly greater in AA and AA-SCOP mice than CON or SCOP mice (ps<.001). None of the treatment groups differed from CON mice in ascorbate levels in the cortex. Although there was a significant omnibus ANOVA on brain ascorbate levels [F3, 20 = 3.121, p=.049; Fig. 3c], Bonferroni post hoc tests showed no significant differences for any of the comparisons of interest (CON vs all other treatment groups, AA-SCOP vs SCOP ps>.413).
Figure 3. Ascorbate treatments increase ascorbate levels and AChE activity.
Ascorbate levels in (a) serum, (b) liver and (c) cortex following ascorbate and scopolamine treatments. AChE levels in (d) medial forebrain, (e) cortex and (f) striatum following treatments. Data represent group means + 1 S.E.M. Different from CON ***p<.001; SCOP different from AA-SCOP mice ##p<.01; different from AA mice +p<.05, +++p<.001.
Large but non-significant increases were seen in AChE activity the MFB area but not in cortex or striatum with AA. To confirm the trend the treatments were repeated in an additional 5 mice per group (in MFB area and cortex only). Data from both experiments were combined and expressed as percentage of wild-type enzyme activity. In the MFB area significantly greater AChE levels were seen in AA mice than each of the other three treatment groups [F3, 40 = 7.961, p<.001; Fig. 3d]. Ascorbate treatments did not affect AChE activity in the cortex or striatum [Fs <.364, ps>.78; Figs. 3e–f].
There were no short-term changes in MDA in serum, liver or cortex as a result of treatments [Fs<1.803, ps>.17]. GSH measurements were also unchanged in the treatment groups compared to CON mice [Fs<1.487, ps>.237].
4. Discussion
The primary finding of the present study was that systemic AA treatment attenuated scopolamine-induced memory deficits in the water maze. Although it is still not clear by what mechanism this rescue occurred, we provide evidence that implicates AA modulation of the cholinergic system.
In the water maze, SCOP mice swam for longer and traveled farther before locating the platform and these measures were not improved in the AA-SCOP group. However, cumulative search error showed that AA-SCOP mice demonstrated improved specificity of search during acquisition over SCOP mice. Thus, although both of these groups may have taken a similar amount of time to locate the platform, the AA-SCOP group swam closer to the platform during their search, indicating enhanced learning about the location of the platform. This result was supported by data from the probe trial during which CON, AA and AA-SCOP mice all showed a preference for swimming in the platform quadrant over non-target quadrants. In contrast, the SCOP mice spent equal amounts of time swimming in each quadrant. Search error (mean distance form the platform location) was also significantly greater in SCOP mice compared to CON mice, but not significantly different in AA-SCOP mice. Path length and peripheral swim time did not differ among the groups during the probe trial and thus we can attribute the deficits seen in the SCOP mice, and the improvement brought about by ascorbate treatment prior to scopolamine administration, to cognitive function and not to increased locomotion or other non-cognitive side-effects. These data mimic those described previously in which daily ascorbate treatments improved water maze performance in aged and very old APP/PSEN1 transgenic mice [16].
Although scopolamine led to increased thigmotaxis in the water maze and wall-hugging behavior is often thought to be an indicator of anxiety [56], scopolamine did not increase anxiety behavior in the zero maze. None of the treatment groups differed on this task in terms of time spent in the closed zones (next to a high wall) of the maze, which indicates anxiety in this task [50]. This result implies that the thigmotaxis observed in the present study was not solely a result of anxiety and instead may be related to a lack of procedural learning, i.e. that the mouse must leave the relative safe area near the wall and move toward the centre of the pool to locate the platform. All mice began on day one with similarly high percentage of time spent near the wall but CON and AA groups quickly learned to swim in more central zones in order to locate the escape platform. Although AA has previously been reported to reduce fearfulness in quail chicks [21] it is more likely that the effect on thigmotaxis observed in the present study was related to procedural learning ability rather than anxiety, as demonstrated by the AA-SCOP mice gradually decreasing in thigmotaxic behavior across acquisition trials in combination with reduced escape latency and search error.
Despite a 10 % reduction in percent alternation in scopolamine-treated mice, there was not a significant difference among the groups according to the ANOVA conducted on percent alternation for each group. These data do not perfectly conform to other reports of scopolamine treatments in mice in this task which typically show approximately 20 % drop in alternation with scopolamine [1, 22, 24], although other studies have shown lower percentage decrease following scopolamine treatment similar to that reported here [26]. Such differences may be influenced by lab-to-lab differences including maze type (e.g. open versus enclosed arms), testing room, and mouse strain. Although the effect was smaller than anticipated, scopolamine-treated mice were indeed impaired compared to the control and ascorbate only-treated mice, with only these latter two groups alternating at significantly greater than chance levels. There was no improvement attributable to the ascorbate pre-treatment in the Y-maze. These data mirror results previously reported by our lab in APP/PSEN1 transgenic mice in which water maze but not Y-maze performance was improved by AA treatment [16]. The Y-maze is a 1-trial task whereas the water maze involves several days of training. It may be, therefore, that ascorbate treatments have a cumulative effect, or the amelioratory effect may be specific to the type of memory being tested, short-term spatial working memory versus long-term spatial reference memory. Although the Y-maze is used to measure spatial working memory it differs in many respects from the water maze in that the mouse does not need to learn any rules, and the exploration is limited to a restricted space. Further investigation is required to clarify these possibilities.
Scopolamine increased activity in the Y-maze, Zero-maze and locomotor activity chambers and this effect was not attenuated by concomitant treatment with ascorbate. Despite increased activity, alternation behavior, anxiety levels, and habituation were all normal in these tasks even in scopolamine-treated mice. Mice that received ascorbate treatment alone did not differ from control mice. This suggests that although ascorbate can mediate the effects of scopolamine, age and APP/PSEN1 genotype (present study and [3, 9, 16]) it does not impact normal functioning in healthy, young mice.
The ascorbate treatments increased circulating ascorbate regardless of scopolamine treatment. There was no significant increase in serum ascorbate, but in the liver, ascorbate treatment led to a two-fold increase in detected ascorbate. Serum ascorbate levels are typically more variable due to factors such as fluid intake and traces of haemolysis in the samples. Furthermore, any surge in serum ascorbate levels had likely peaked prior to the 1 hour time point when mice were sacrificed [8] and already been transferred to other tissues (e.g. liver). Ascorbate levels did not increase significantly in the brain following i.p. administration. Ascorbate does not cross the blood brain barrier and instead must be transported via the SVCT2 transporter at the choroid plexus [2]. This allows much higher concentrations of ascorbate to accumulate in brain than are found in blood, against a concentration gradient, but it is a saturable and rate-limited process that regulates intake despite any sudden changes in blood [17]. Nevertheless, there may have been small, acute, changes in extracellular fluid that were not detected by our assay, but that still led to down-stream changes in neurotransmitter signaling and the improvements in cognitive ability in the AA-SCOP group. Thus the absence of a detectable rise in stored brain ascorbate does not preclude its having reached sites of action in the brain. Some notice should also be paid to the fact that only a single dose of ascorbate was tested in the present study (125 mg/kg). It is likely that higher doses may be tolerated without the ascorbate assuming pro-oxidant properties and a more complete rescue of the scopolamine-induced deficits may be possible, or even modulation of the cognitive abilities of young mice in the absence of scopolamine. This proposition remains to be tested.
Although neither MDA nor GSH levels were altered by experimental treatments in the present study, scopolamine has previously been shown to increase oxidative stress [13] and thus it is a very real possibility that the antioxidant function of ascorbate also contributes to its cognitive benefits. There may have been small, undetected changes in other oxidative stress markers, or it is also possible that following the many days of treatments and training, adaptive mechanisms were triggered in order to maintain a homeostatic balance. Both ascorbate [9, 39] and a wide range of other antioxidant substances have also been beneficial to cognitive ability against scopolamine [13, 20, 36] and also against age and low antioxidant dietary challenges [3, 51, 54]. Despite this range of promising data, the exact mechanisms of the effects are still unclear. Among the explanations for potential antioxidant action on cognition are protection of hippocampal synaptic plasticity and long-term potentiation [25, 48, 55], and permitting neurotransmission via membrane fluidity [35], or reducing oxidative damage leading to cell death.
Following ascorbate administration, AChE activity increased in AA mice in the cholinergic-rich MFB (and surrounding areas) that projects to the neocortex, which is implicated in attention and learning [11, 33]. This effect was not widespread across other brain areas. Improved function in this area could have led to the AA-SCOP mice being able to learn more about their environment than SCOP mice through enhanced attentional function. Although still plausible, this explanation is rendered somewhat less convincing by the lack of a significant difference in AChE activity between SCOP and CON mice. Most treatments for Alzheimer’s disease rely on AChE inhibitors. Ascorbate (60 mg/kg i.p.) has previously been observed to inhibit AChE activity in a manner similar to metrifonate, a known AChE inhibitor [10]. In contrast, in another study scopolamine administration (i.p.) decreased acetylcholinesterase activity and vitamin C alone or in combination with vitamin E reversed this effect when administered orally, although the authors did not describe the effect of C alone without scopolamine [29]. In the present study we found no effect of scopolamine on AChE and an ascorbate-induced increase, and this effect was limited to a specific area of the brain. Based on the current data it seems unlikely therefore that the ascorbate could be used as an AChE inhibitor to rival currently available drugs. Nevertheless, ascorbate was effective against the amnestic effects of the muscarinic antagonist scopolamine, and thus it is likely that cholinergic function was modulated in some way. The studies described above, and the neurochemical and behavioral data presented here, though mixed, suggest that there is some interaction between ascorbate and the cholinergic system. It is possible, for example, that there was an overall upregulation of cholinergic function including AChE activity, resulting from a cumulative effect of 14 days of ascorbate treatments. Without further data it is not possible to form a firm conclusion as to the mechanism of the effect of ascorbate on cognition from these data. Any effect on the cholinergic system makes promising the idea that ascorbate could add to potential therapies for Alzheimer’s disease but more information is needed concerning the exact mechanism.
There are several other likely lines of investigation that should also be followed. Firstly, ascorbate is an essential cofactor in the synthesis of norepinephrine from dopamine and thus catecholamine function may be modulated by ascorbate treatments. Although MDA and GSH were not affected in the current study, this does not mean that more localized oxidative stress reactions were not affected. Neurotransmitter uptake and receptor affinity can be adversely affected by oxidative stress at the synapse [4, 5] and thus increased extracellular ascorbate could provide short-term antioxidant protection at the synapse. A final important function of ascorbate is a heteroexchange with glutamate. Reduced ability of neurons to re-uptake glutamate from the synaptic cleft can lead to excess glutamate and neurotoxicity. Administration of glutamate to striatum, hippocampus and other forebrain areas and cortical projections leads to release of ascorbate from neurons and thus it has been argued that ascorbate protects against glutamatergic neurotoxicity [7, 34, 41].
5. Conclusion
Scopolamine-induced learning deficits in the water maze were partially reversed by ascorbate. The effects of ascorbate were restricted to cognitive performance and did not decrease scopolamine-induced activity in the land-based mazes. The data presented above support the notion that ascorbate has neuromodulatory and cognitive-enhancing properties. Whereas changes in the cholinergic system offer a possible mechanism for the observed effects, further research is required to fully elucidate this relationship or to establish the alternative mechanisms.
Acknowledgements
This work was supported by a grant from the National Institutes of Health (AG023138). All behavioral testing was conducted using the facilities in the Vanderbilt Murine Neurobehavioral Laboratory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson R, Higgins GA. Absence of central cholinergic deficits in ApoE knockout mice. Psychopharmacology. 1997;132:135–144. doi: 10.1007/s002130050329. (Berl) [DOI] [PubMed] [Google Scholar]
- 2.Angelow S, Haselbach M, Galla HJ. Functional characterisation of the active ascorbic acid transport into cerebrospinal fluid using primary cultured choroid plexus cells. Brain Res. 2003;988:105–113. doi: 10.1016/s0006-8993(03)03350-x. [DOI] [PubMed] [Google Scholar]
- 3.Arzi A, Hemmati AA, Razian A. Effect of vitamins C and E on cognitive function in mouse. Pharmacol Res. 2004;49:249–252. doi: 10.1016/j.phrs.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Berman SB, Hastings TG. Inhibition of glutamate transport in synaptosomes by dopamine oxidation and reactive oxygen species. J Neurochem. 1997;69:1185–1195. doi: 10.1046/j.1471-4159.1997.69031185.x. [DOI] [PubMed] [Google Scholar]
- 5.Berman SB, Zigmond MJ, Hastings TG. Modification of dopamine transporter function: effect of reactive oxygen species and dopamine. J Neurochem. 1996;67:593–600. doi: 10.1046/j.1471-4159.1996.67020593.x. [DOI] [PubMed] [Google Scholar]
- 6.Bernardo A, McCord M, Troen AM, Allison JD, McDonald MP. Impaired spatial memory in APP-overexpressing mice on a homocysteinemia-inducing diet. Neurobiol Aging. 2007;28:1195–1205. doi: 10.1016/j.neurobiolaging.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 7.Cammack J, Ghasemzadeh B, Adams RN. The pharmacological profile of glutamate-evoked ascorbic acid efflux measured by in vivo electrochemistry. Brain Res. 1991;565:17–22. doi: 10.1016/0006-8993(91)91731-f. [DOI] [PubMed] [Google Scholar]
- 8.Chen Q, Espey MG, Sun AY, Lee JH, Krishna MC, Shacter E, Choyke PL, Pooput C, Kirk KL, Buettner GR, Levine M. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci U S A. 2007;104:8749–8754. doi: 10.1073/pnas.0702854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Angelis L, Furlan C. The effects of ascorbic acid and oxiracetam on scopolamine-induced amnesia in a habituation test in aged mice. Neurobiol Learn Mem. 1995;64:119–124. doi: 10.1006/nlme.1995.1050. [DOI] [PubMed] [Google Scholar]
- 10.Dhingra D, Parle M, Kulkarni SK. Comparative brain cholinesterase-inhibiting activity of Glycyrrhiza glabra, Myristica fragrans, ascorbic acid, and metrifonate in mice. J Med Food. 2006;9:281–283. doi: 10.1089/jmf.2006.9.281. [DOI] [PubMed] [Google Scholar]
- 11.Dunnett SB, Everitt BJ, Robbins TW. The basal forebrain-cortical cholinergic system: interpreting the functional consequences of excitotoxic lesions. Trends Neurosci. 1991;14:494–501. doi: 10.1016/0166-2236(91)90061-x. [DOI] [PubMed] [Google Scholar]
- 12.Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 13.Fan Y, Hu J, Li J, Yang Z, Xin X, Wang J, Ding J, Geng M. Effect of acidic oligosaccharide sugar chain on scopolamine-induced memory impairment in rats and its related mechanisms. Neurosci Lett. 2005;374:222–226. doi: 10.1016/j.neulet.2004.10.063. [DOI] [PubMed] [Google Scholar]
- 14.Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- 15.Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 16.Harrison FE, Hosseini AH, McDonald MP, May JM. Vitamin C reduces spatial learning deficits in middle-aged and very old APP/PSEN1 transgenic and wild-type mice. Pharmacol Biochem Behav. 2009;93:443–450. doi: 10.1016/j.pbb.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrison FE, May JM. Vitamin C function in the brain: vital role of the ascorbate transporter SVCT2. Free Radic Biol Med. 2009 doi: 10.1016/j.freeradbiomed.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison FE, Yu SS, Van Den Bossche KL, Li L, May JM, McDonald MP. Elevated oxidative stress and sensorimotor deficits but normal cognition in mice that cannot synthesize ascorbic acid. J Neurochem. 2008;106:1198–1208. doi: 10.1111/j.1471-4159.2008.05469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes RE, Hurley RJ, Jones PR. The retention of ascorbic acid by guinea-pig tissues. Br J Nutr. 1971;26:433–438. doi: 10.1079/bjn19710048. [DOI] [PubMed] [Google Scholar]
- 20.Jeong EJ, Ma CJ, Lee KY, Kim SH, Sung SH, Kim YC. KD-501, a standardized extract of Scrophularia buergeriana has both cognitive-enhancing and antioxidant activities in mice given scopolamine. J Ethnopharmacol. 2009;121:98–105. doi: 10.1016/j.jep.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Jones RB, Satterlee DG, Cadd GG. Timidity in Japanese quail: effects of vitamin C and divergent selection for adrenocortical response. Physiol Behav. 1999;67:117–120. doi: 10.1016/s0031-9384(99)00039-6. [DOI] [PubMed] [Google Scholar]
- 22.Kim DH, Hung TM, Bae KH, Jung JW, Lee S, Yoon BH, Cheong JH, Ko KH, Ryu JH. Gomisin A improves scopolamine-induced memory impairment in mice. Eur J Pharmacol. 2006;542:129–135. doi: 10.1016/j.ejphar.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 23.Kim HK, Kim M, Kim S, Kim M, Chung JH. Effects of green tea polyphenol on cognitive and acetylcholinesterase activities. Biosci Biotechnol Biochem. 2004;68:1977–1979. doi: 10.1271/bbb.68.1977. [DOI] [PubMed] [Google Scholar]
- 24.Kim MJ, Choi SJ, Lim ST, Kim HK, Kim YJ, Yoon HG, Shin DH. Zeatin supplement improves scopolamine-induced memory impairment in mice. Biosci Biotechnol Biochem. 2008;72:577–581. doi: 10.1271/bbb.70480. [DOI] [PubMed] [Google Scholar]
- 25.Kim SU, Jin MH, Kim YS, Lee SH, Cho YS, Cho KJ, Lee KS, Kim YI, Kim GW, Kim JM, Lee TH, Lee YH, Shong M, Kim HC, Chang KT, Yu DY, Lee DS. Peroxiredoxin II preserves cognitive function against age-linked hippocampal oxidative damage. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 26.Kokkinidis L, Anisman H. Interaction between cholinergic and catecholaminergic agents in a spontaneous alternation task. Psychopharmacology. 1976;48:261–270. doi: 10.1007/BF00496859. (Berl) [DOI] [PubMed] [Google Scholar]
- 27.Kuo CH, Hata F, Yoshida H, Yamatodani A, Wada H. Effect of ascorbic acid on release of acetylcholine from synaptic vesicles prepared from different species of animals and release of noradrenaline from synaptic vesicles of rat brain. Life Sci. 1979;24:911–915. doi: 10.1016/0024-3205(79)90341-2. [DOI] [PubMed] [Google Scholar]
- 28.Kuo CH, Yonehara N, Yoshida H. Subcellular ascorbic acid in scorbutic guinea pig brain. J Nutr Sci Vitaminol. 1979;25:9–13. doi: 10.3177/jnsv.25.9. (Tokyo) [DOI] [PubMed] [Google Scholar]
- 29.Lee L, Kang SA, Lee HO, Lee BH, Jung IK, Lee JE, Hoe YS. Effect of supplementation of vitamin E and vitamin C on brain acetylcholinesterase activity and neurotransmitter levels in rats treated with scopolamine, an inducer of dementia. J Nutr Sci Vitaminol. 2001;47:323–328. doi: 10.3177/jnsv.47.323. (Tokyo) [DOI] [PubMed] [Google Scholar]
- 30.Lind J (1772) A treatise on the scurvy In three parts. … Together with a critical and chronological view of what has been published on the subject. By James Lind. In: printed for S. Crowder, D. Wilson and G. Nicholls, T. Cadell, T. Becket and Co. G. Pearch, and W. Woodfall, London, p xiv,[2],559,[551]p.
- 31.Miyakawa T, Yamada M, Duttaroy A, Wess J. Hyperactivity and intact hippocampus-dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. J Neurosci. 2001;21:5239–5250. doi: 10.1523/JNEUROSCI.21-14-05239.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyakawa T, Yared E, Pak JH, Huang FL, Huang KP, Crawley JN. Neurogranin null mutant mice display performance deficits on spatial learning tasks with anxiety related components. Hippocampus. 2001;11:763–775. doi: 10.1002/hipo.1092. [DOI] [PubMed] [Google Scholar]
- 33.Muir JL, Dunnett SB, Robbins TW, Everitt BJ. Attentional functions of the forebrain cholinergic systems: effects of intraventricular hemicholinium, physostigmine, basal forebrain lesions and intracortical grafts on a multiple-choice serial reaction time task. Exp Brain Res. 1992;89:611–622. doi: 10.1007/BF00229886. [DOI] [PubMed] [Google Scholar]
- 34.O'Neill RD, Fillenz M, Sundstrom L, Rawlins JN. Voltammetrically monitored brain ascorbate as an index of excitatory amino acid release in the unrestrained rat. Neurosci Lett. 1984;52:227–233. doi: 10.1016/0304-3940(84)90166-6. [DOI] [PubMed] [Google Scholar]
- 35.Omoi NO, Arai M, Saito M, Takatsu H, Shibata A, Fukuzawa K, Sato K, Abe K, Fukui K, Urano S. Influence of oxidative stress on fusion of pre-synaptic plasma membranes of the rat brain with phosphatidyl choline liposomes, and protective effect of vitamin E. J Nutr Sci Vitaminol. 2006;52:248–255. doi: 10.3177/jnsv.52.248. (Tokyo) [DOI] [PubMed] [Google Scholar]
- 36.Orhan I, Aslan M. Appraisal of scopolamine-induced antiamnesic effect in mice and in vitro antiacetylcholinesterase and antioxidant activities of some traditionally used Lamiaceae plants. J Ethnopharmacol. 2009;122:327–332. doi: 10.1016/j.jep.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 37.Pachla LA, Kissinger PT. Analysis of ascorbic acid by liquid chromatography with amperometric detection. Methods Enzymol. 1979;62:15–24. doi: 10.1016/0076-6879(79)62183-3. [DOI] [PubMed] [Google Scholar]
- 38.Papandreou MA, Dimakopoulou A, Linardaki ZI, Cordopatis P, Klimis-Zacas D, Margarity M, Lamari FN. Effect of a polyphenol-rich wild blueberry extract on cognitive performance of mice, brain antioxidant markers and acetylcholinesterase activity. Behav Brain Res. 2009;198:352–358. doi: 10.1016/j.bbr.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 39.Parle M, Dhingra D. Ascorbic Acid: a promising memory-enhancer in mice. J Pharmacol Sci. 2003;93:129–135. doi: 10.1254/jphs.93.129. [DOI] [PubMed] [Google Scholar]
- 40.Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. San Diego, CA: Academic; 2001. [Google Scholar]
- 41.Pierce RC, Rebec GV. Intraneostriatal administration of glutamate antagonists increases behavioral activation and decreases neostriatal ascorbate via nondopaminergic mechanisms. J Neurosci. 1993;13:4272–4280. doi: 10.1523/JNEUROSCI.13-10-04272.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2006;1:3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- 43.Rebec GV, Pierce RC. A vitamin as neuromodulator: ascorbate release into the extracellular fluid of the brain regulates dopaminergic and glutamatergic transmission. Prog Neurobiol. 1994;43:537–565. doi: 10.1016/0301-0082(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 44.Rice ME. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000;23:209–216. doi: 10.1016/s0166-2236(99)01543-x. [DOI] [PubMed] [Google Scholar]
- 45.Robinson L, Harbaran D, Riedel G. Visual acuity in the water maze: sensitivity to muscarinic receptor blockade in rats and mice. Behav Brain Res. 2004;151:277–286. doi: 10.1016/j.bbr.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 46.Sabharwal AK, May JM. alpha-Lipoic acid and ascorbate prevent LDL oxidation and oxidant stress in endothelial cells. Mol Cell Biochem. 2008;309:125–132. doi: 10.1007/s11010-007-9650-z. [DOI] [PubMed] [Google Scholar]
- 47.Sarter M, Bruno JP. Cognitive functions of cortical acetylcholine: toward a unifying hypothesis. Brain Res Brain Res Rev. 1997;23:28–46. doi: 10.1016/s0165-0173(96)00009-4. [DOI] [PubMed] [Google Scholar]
- 48.Serrano F, Klann E. Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Res Rev. 2004;3:431–443. doi: 10.1016/j.arr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Shahidi S, Komaki A, Mahmoodi M, Atrvash N, Ghodrati M. Ascorbic acid supplementation could affect passive avoidance learning and memory in rat. Brain Res Bull. 2008;76:109–113. doi: 10.1016/j.brainresbull.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 50.Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterisation of the elevated "zero-maze" as an animal model of anxiety. Psychopharmacology. 1994;116:56–64. doi: 10.1007/BF02244871. (Berl) [DOI] [PubMed] [Google Scholar]
- 51.Shukitt-Hale B, Kalt W, Carey AN, Vinqvist-Tymchuk M, McDonald J, Joseph JA. Plum juice, but not dried plum powder, is effective in mitigating cognitive deficits in aged rats. Nutrition. 2009;25:567–573. doi: 10.1016/j.nut.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 52.Siesser WB, Zhao J, Miller LR, Cheng SY, McDonald MP. Transgenic mice expressing a human mutant beta1 thyroid receptor are hyperactive, impulsive, and inattentive. Genes Brain Behav. 2006;5:282–297. doi: 10.1111/j.1601-183X.2005.00161.x. [DOI] [PubMed] [Google Scholar]
- 53.Sunderland T, Tariot PN, Weingartner H, Murphy DL, Newhouse PA, Mueller EA, Cohen RM. Pharmacologic modelling of Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry. 1986;10:599–610. doi: 10.1016/0278-5846(86)90030-8. [DOI] [PubMed] [Google Scholar]
- 54.Tchantchou F, Chan A, Kifle L, Ortiz D, Shea TB. Apple juice concentrate prevents oxidative damage and impaired maze performance in aged mice. J Alzheimers Dis. 2005;8:283–287. doi: 10.3233/jad-2005-8306. [DOI] [PubMed] [Google Scholar]
- 55.Wang HL, Chen XT, Yin ST, Liu J, Tang ML, Wu CY, Ruan DY. Opposite effects of alpha-lipoic acid on antioxidation and long-term potentiation in control and chronically lead-exposed rats. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:303–310. doi: 10.1007/s00210-008-0307-6. [DOI] [PubMed] [Google Scholar]
- 56.Wolfer DP, Stagljar-Bozicevic M, Errington ML, Lipp HP. Spatial Memory and Learning in Transgenic Mice: Fact or Artifact? News Physiol Sci. 1998;13:118–123. doi: 10.1152/physiologyonline.1998.13.3.118. [DOI] [PubMed] [Google Scholar]