Abstract
Uterine artery constrictor responses to elevation of intraluminal pressure (myogenic tone) are considerably enhanced in late pregnant rats, although the underlying causes remain unknown. A single uterine horn ligation model was used to differentiate local from systemic influences, and to test the hypothesis that that factors associated with the site of placentation, rather than systemic hormonal changes, are primarily involved in the induction of this adaptive process. Radial uterine arteries were dissected from the gravid and non-gravid uterine horns of late pregnant rats, cannulated and pressurized. Changes in arterial diameter and smooth muscle [Ca2+]i in response to the elevation of intraluminal pressure were studied using intact and endothelium-denuded arteries loaded with the ratiometric Ca2+-sensitive dye fura-2. Elevations of pressure from 10 to 60 and 100 mm Hg resulted in passive arterial distention of arteries from non-gravid horns with a minor change in [Ca2+]i. In contrast, arteries from gravid horns developed myogenic tone associated with a significant elevation in [Ca2+]i. Synchronous oscillations in [Ca2+]i and lumen diameter were frequently observed in vessels from gravid horns. Endothelial denudation augmented tone in the gravid horn but did not uncover myogenic tone in vessels from the non-gravid horn. In summary, pregnancy-associated uterine artery myogenic behavior is due to an upregulation of calcium-handling mechanisms, occurs independently of the endothelium, and is induced by local uteroplacental influences.
Keywords: one-horn pregnant rat, smooth muscle Ca2+, fura-2
INTRODUCTION
Normal human and mammalian pregnancy is a state of a marked cardiovascular adaptation that includes changes in blood pressure, heart rate, stroke volume, and vascular reactivity. Perhaps the most dramatic changes occur in the uterine circulation, and are manifested by vessel growth and remodeling, as well as significant changes in vasomotor function.1–11
Vasoconstriction, in response to an elevation in intraluminal pressure, is a fundamental property of resistance blood vessels and is termed myogenic tone. This response to pressure or stretch is intrinsic to vascular smooth muscle cells (SMCs) and a well-established physiological phenomenon.12–16 Pressure-induced tone contributes to vascular regional resistance of small arteries and arterioles and serves to protect capillaries from excessive blood pressure.13 Elevation of SMC cytoplasmic [Ca2+]i (due to Ca2+ influx into cells and Ca2+ release from internal stores) as well as Ca2+ sensitization of the contractile process are two major mechanisms of pressure-induced vasoconstriction.14–21
In late gestation, pressure-induced tone of vessels from mesenteric and renal circulations of rodents is significantly reduced, most often due to augmented endothelial vasodilatory influences.10, 22, 23 Pressure-induced vasoconstriction has been described in uterine arteries from pregnant animals, and it is most evident in smaller radial arteries.6, 9, 10, 21, 24–26 Myometrial arteries from pregnant women can also develop myogenic tone in response to pressure elevation27. Studies from several different laboratories, including our own, demonstrated that myogenic tone of small uterine arteries of the rat is significantly enhanced in late gestation.6, 21, 26 Although we recently reported diminished activity of SMC delayed rectifier potassium channels (which results in enhanced depolarization) to be an underlying mechanism for enhanced tone during gestation, the factor or factors that induce this adaptation are not known.21
Pregnancy is also associated with significant expansive growth of uterine vessels, and our recent study demonstrated unequivocally that local (uteroplacental) rather than systemic (endocrine) influences play the dominant role in the structural remodeling of both large and small uterine arteries.3, 11, 28, 29 In this study, we hypothesized that changes in arterial structure (remodeling) and function (reactivity) may be linked, such that local uteroplacental factors associated with vessel enlargement would also induce phenotypic changes in the cells within the vascular wall, e.g. myogenic tone and reactivity in SMCs. This may be due to direct (altered cellular handling of calcium) or indirect (increased wall tension due to an increase in lumen diameter leading to depolarization) effects of remodeling on the cells of the vascular wall. The alternative hypothesis is that changes in myogenic behavior result from the altered humoral (endocrine) milieu of pregnancy and would therefore not be expected to be site-specific.
The objective of this study was to differentiate between systemic and local influences in effecting myogenic tone by using a surgical ligation model in which implantation is restricted to one of two rat uterine horns. If differences were apparent (and they were), secondary objectives were to: (1) determine whether augmented myogenic tone is due to increased elevation in smooth muscle [Ca2+]i; and (2) define the role of the endothelium in the differential myogenic behavior of uterine arteries from the nongravid versus gravid uterine horn.
Our data demonstrate that late pregnancy is associated with a striking enhancement of uterine artery myogenic reactivity that is due to local uteroplacental influences, occurs independently of the endothelium, and can be attributed to an upregulation of SMC calcium handling mechanisms that lead to oscillatory elevations in SMC cytosolic [Ca2+]i.
METHODS
All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85–23, Revised 1996), and the experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Vermont.
Animals and Preparation of Arteries
In this study, we utilized a surgical ligation model in which the implantation was restricted to one of two rat uterine horns.28 Fifteen virgin female rats had one-sided uterine horn ligation surgery, accomplished by tying off the oviduct with non-resorbable suture at 8 weeks of age by Charles River Laboratories. Animals were received at our facility at 9 weeks of age with staples approximating a cranial-caudal incision in the right flank. Staples were removed 7 days post-op and the rats were acclimated for at least one week before breeding.
Surgically ligated rats were bred overnight in isolated pairs using metabolic cages at the University of Vermont Small Animal Facility. If a seminal plug was observed on the following morning, that day was designated day 1 of pregnancy. Pregnancy was confirmed by veterinary technicians by observation and/or palpation on days 11–16. Post-mortem evaluation confirmed an empty right uterine horn and a silk ligature in the upper portion of the oviduct.
Single-horn late pregnant rats were used on day 20/22 of pregnancy at 13 to17 weeks of age. They were anesthetized by an intraperitoneal injection of Nembutal (50mg/kg) and killed by decapitation. The abdominal wall was transected and the entire uterus and uterine vasculature were rapidly removed and pinned in a dissecting dish filled with aerated cold physiological salt solution (PSS: see Solutions and drugs for composition).
Second-order uterine radial arteries were identified within the mesometrial arcade and dissected from the nongravid and gravid horns. Only uteroplacental arteries (radial arteries feeding the placenta) were dissected from the vasculature of pregnant uteri. Arterial segments were cannulated from both ends in the arteriograph and continuously superfused at 3 ml/min with aerated (10% O2, 5% CO2, and 85% N2) PSS at 37°C. Initial intraluminal pressure was set at 10 mm Hg using a servo-null pressure system (Living Systems Instrumentation, Burlington, VT). All experiments were performed under no intraluminal flow conditions.
In a separate set of experiments, the endothelium was removed by infusing air into one end of a cannulated vessel for 5 to 6 minutes, followed by gentle and brief (5 seconds) perfusion with regular PSS before pressurization of the artery. The effectiveness of this denudation procedure was confirmed by the preservation of normal constrictor reactivity to phenylephrine, combined with the absence of a dilatory response to acetylcholine.
Measurement of Smooth Muscle Cell [Ca2+]i in Pressurized Arteries
After an equilibration period of 20 min at 37°C at 10 mm Hg and measurement of background fluorescence, SMCs within the wall of arteries pressurized at 10 mm Hg were selectively loaded with 5 μmol/L fura-2. An arterial segment was incubated extraluminally in fura-2 AM loading solution at room temperature in the dark for 60 min under no-perfusion conditions. Extraluminal incubation of pressurized arteries with fura-2 AM solution does not result in the loading of endothelial cells with fura 2 most likely due to the presence of elastic lamina in the wall of uterine arteries preventing the diffusion of fura 2 from smooth muscle to the endothelial layer. Fura-2-loaded arteries were then continuously superfused at 3 mL/minute with aerated PSS at 37°C. Ratiometric measurements of fura-2 fluorescence were performed using a photomultiplier system (IonOptix Inc, Milton, MA). Background-corrected ratios of 510 nm emission were obtained at a sampling rate of 5 Hz from arteries alternately excited at 340 and 380 nm. Lumen diameter was simultaneously monitored using the SoftEdge Acquisition Subsystem (IonOptix). All experimental protocols were started following an additional 15 to 20 minutes equilibration period at 10 mm Hg to allow intracellular de-esterification of fura-2 AM.
After the equilibration period, corresponding levels of [Ca2+]i and diameters were recorded for 5 minutes at 10 mm Hg, and intraluminal pressure was then elevated to 60 and 100 mm Hg. Changes in arterial diameter and levels of SMC [Ca2+]i were monitored until a stabilization of myogenic constriction occurred (typically 10 minutes for each level of pressure). Papaverine (100 μmol/L) and diltiazem (10 μmol/L) were applied at the end of each experiment, and the arterial diameter was recorded at 10, 60, and 100 mm Hg from the maximally dilated artery. The degree of myogenic tone at any given level of intraluminal pressure was expressed as the percentage reduction from a fully relaxed diameter.
Solutions and Drugs
The physiological salt solution (PSS) contained: 119 mmol/L NaCl, 4.7 mmol/L KCl, 24.0 mmol/L NaHCO3, 1.2 mmol/L KH2PO4, 1.6 mmol/L CaCl2, 1.2 mmol/L MgSO4, 0.023 mmol/L EDTA, and 11.0 mmol/L glucose, pH = 7.4. For the fura-2 calibration procedure, we used a solution of the following composition: 140 mmol/L KCl, 20 mmol/L NaCl, 5 mmol/L HEPES, 5 mmol/L EGTA, 1 mmol/L MgCl2, 5 μmol/L nigericin and 10 μmol/L ionomycin, pH = 7.1.
All chemicals were purchased from Sigma Chemical Co (St. Louis, MO) with the exception of ionomycin and nigericin, which were obtained from Calbiochem (La Jolla, CA). Fura-2 AM and pluronic acid were purchased from Invitrogen (Carlsbad, CA). Fura-2 AM was dissolved in dehydrated DMSO as a 1 mmol/L stock solution, frozen in small aliquots and used within one week of preparation. Papaverine was dissolved in deionized water and used the same day only. Diltiazem was prepared as a 10 mmol/L stock solution in deionized water and kept refrigerated until use (1–2 weeks). Ionomycin and nigericin were prepared as 10 mmol/L stock solutions in methanol and kept at −20°C until use.
Calculations and Statistical Analysis
SMC [Ca2+]i was calculated using the following equation30: [Ca2+]i = Kdβ (R − Rmin)/(Rmax − R), where R is the experimentally measured ratio (340/380 nm) of fluorescence intensities, Rmin is a ratio in the absence of [Ca2+]i and Rmax is a ratio at Ca2+-saturated fura 2 conditions, β is a ratio of the fluorescence intensities at 380 nm excitation wavelength at Rmin and Rmax. Rmin, Rmax and β were determined by an in situ calibration procedure from the arteries treated with nigericin (5 μmol/L) and ionomycin (10 μmol/L). Calibration was performed on a group of vessels loaded extraluminally with fura-2 (n = 4). These values were then pooled and used to convert the ratio values into a [Ca2+]i. The Kd (the dissociation constant for fura-2) was 282 nM, as determined by using in situ titration of Ca2+ in fura-2 loaded small arteries.31
Arterial diameter, pressure and ratio values were simultaneously recorded using an IonOptix data acquisition program and imported into Sigma Plot and Sigma Stat programs for graphical representation, calculations, and statistical analysis.
Data are expressed as means ± SEM, where each n = number of arterial segments studied. Only one artery was used from nongravid or gravid uteri of a late pregnant rat. A paired or unpaired Student’s t-test or two way repeated measures analysis of variance (ANOVA) were used to determine the significance of differences between sets of data, with P < .05 considered significant.
RESULTS
Effects of Gestation and Surgical Restriction of Placentation on Vessel Dimensions, and on the Development of Myogenic Tone in Uterine Radial Arteries
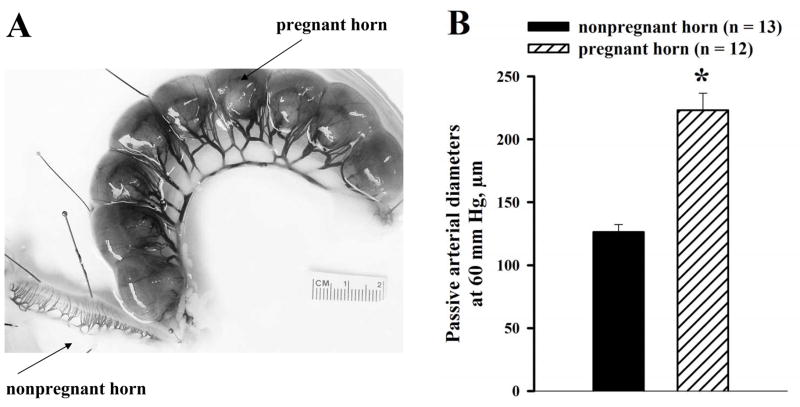
In single-horn pregnant rats, late pregnancy was associated with significant expansive remodeling of the uterine vasculature in the gravid versus nongravid horn, as described in an earlier study,28 and shown in the photograph in Figure 1A. The passive diameters of cannulated radial arteries (measured at 60 mm Hg in the presence of 10 μmol/L diltiazem and 100 μmol/L papaverine) were 77% larger in gravid (223 ± 14 μm, n = 12) versus nongravid horns (126 ± 6 μm, n = 13, Figure 1B).
Figure 1.
A, Photograph showing the uterus of a late pregnant (20 day) rat that underwent surgical ligation of one uterine horn several weeks prior to breeding. Most of the picture is taken up by the pregnant horn that contained 9 pups. The nonpregnant horn can be seen in the lower left corner, along with the mesometrial arcade containing arteries and veins that perfuse the uterine corpus. Note difference in the size of the vasculature due to the predominance of local influences on remodeling, as previously described.28 B, Summary graph demonstrating a significant difference in the passive diameters of uterine radial arteries from nonpregnant versus pregnant horns of late pregnant rats. All vessels were pressurized to 60 mm Hg and maximally dilated with 10 μmol/L diltiazem and 100 μmol/L papaverine. * Significant difference at P < .05 (unpaired Student’s t-test); numbers in parentheses indicate the number of arteries tested.
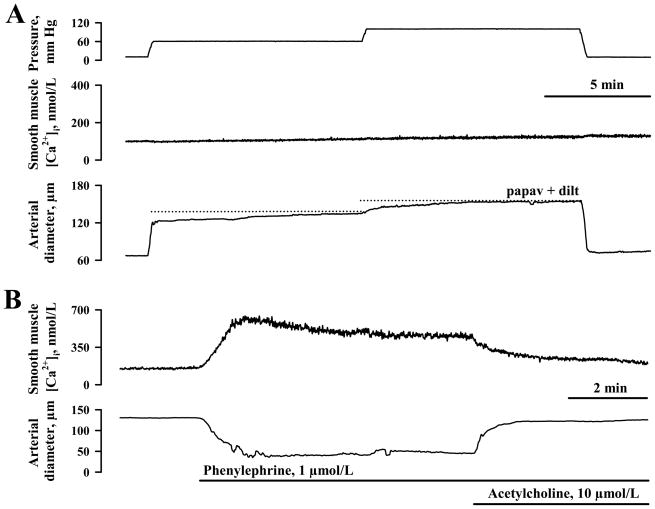
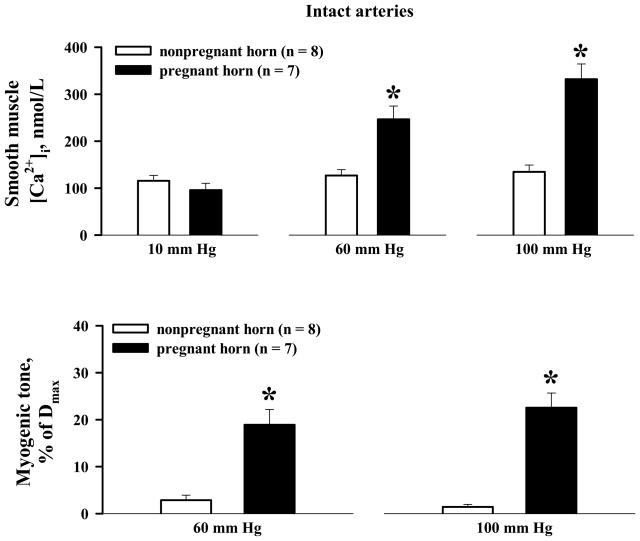
A stepwise elevation of intraluminal pressure from 10 to 60, and from 60 to 100 mm Hg resulted in very minor elevations in SMC [Ca2+]i and little or no vasoconstriction of uterine radial arteries from nongravid horns (Figure 2A). On average, the rise of SMC [Ca2+]i above the basal [Ca2+]i levels was 11 ± 4 nmol/L and 19 ± 7 nmol/L at 60 and 100 mm Hg, respectively (n = 8, Figure 4).
Figure 2.
A, Representative tracings showing the effects of stepwise elevation in intraluminal pressure from 10 to 60 and then to 100 mm Hg on smooth muscle cell (SMC) [Ca2+]i and the diameter of a uterine radial artery from the non-pregnant horn of a late pregnant rat. The dotted lines indicate the maximal diameter of the same artery in the presence of 100 μmol/L papaverine and 10 μmol/L diltiazem. B, Smooth muscle and endothelial integrity of the same artery is evidenced by the presence of constrictor and dilator responses to phenylephrine and ACh, respectively, along with changes in SMC [Ca2+]i.
Figure 4.
Summary graphs showing significant differences in pressure-induced SMC [Ca2+]i responses and associated myogenic tone of uterine arteries from nonpregnant versus pregnant horns of late pregnant rats. No difference was found in the basal levels of SMC [Ca2+]i in arteries from nonpregnant versus pregnant horns at 10 mm Hg. Myogenic tone is expressed as a percentage of the diameter measured in the presence of 100 μmol/L papaverine and 10 μmol/L diltiazem (Dmax). *Significantly different at P < .05 (two-way repeated measures ANOVA); numbers in parentheses indicate the number of arteries tested.
At the same time, these vessels demonstrated a significant elevation in SMC [Ca2+]i (by 200 ± 32 nmol/L) and constriction (to 61 ± 4% of their fully relaxed diameters at 60 mm Hg) in response to phenylephrine. Subsequent application of acetylcholine resulted in a significant reduction in SMC [Ca2+]i (by 150 ± 31 nmol/L) to nearly basal levels that were associated with an almost complete vasodilation of 92 ± 4 % (n = 6), as illustrated for one vessel in Figure 2B.
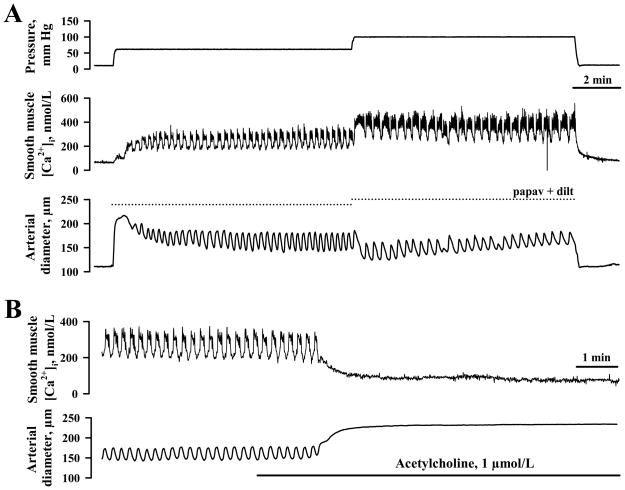
In contrast to vessels from nongravid horns, uteroplacental radial arteries from gravid horns constricted robustly to elevations in pressure (19 ± 3 % and 23 ± 3 % at 60 and 100 mm Hg, respectively). The development of myogenic tone was preceded by a significant increase in SMC [Ca2+]i consisting of a slow [Ca2+]i rise with superimposed fast calcium oscillations that were associated with rhythmic vasoconstrictions (Figure 3A). The averaged increases in cytosolic [Ca2+]i above the basal levels were 151 ± 31 nmol/L and 236 ± 42 nmol/L at 60 and 100 mm Hg, respectively (Figure 4). Application of ACh resulted in full dilatation, demonstrating endothelial integrity of the artery (Figure 3B).
Figure 3.
A, Representative tracings demonstrating the significant elevation in smooth muscle [Ca2+]i and constriction of a uteroplacental radial artery from the pregnant horn of a late pregnant rat in response to stepwise elevation of intraluminal pressure from 10 to 60 and then to 100 mm Hg. The dotted lines indicate the maximal diameter of the same artery in the presence of 100 μmol/L papaverine and 10 μmol/L diltiazem. B, The endothelial integrity of the artery pressurized to 60 mmHg is confirmed by a marked decrease in smooth muscle [Ca2+]i and dilation in response to the application of 1 μmol/L ACh.
Effect of Arterial Denudation on Myogenic Tone, and its Dependence on Extracellular Calcium
Pregnancy is a state of augmented basal production of endothelium-derived dilator factors like nitric oxide (NO) and prostacyclin.2, 4, 5 Therefore, a potential increase in basal vasodilatory effects from the uterine artery endothelium might prevent the development of myogenic tone in arteries from the non-gravid uterine horn. To test this suggestion, we studied pressure-induced responses in uterine arteries after endothelial denudation. The effectiveness of endothelial removal was confirmed by an abolition of dilator responses to ACh.
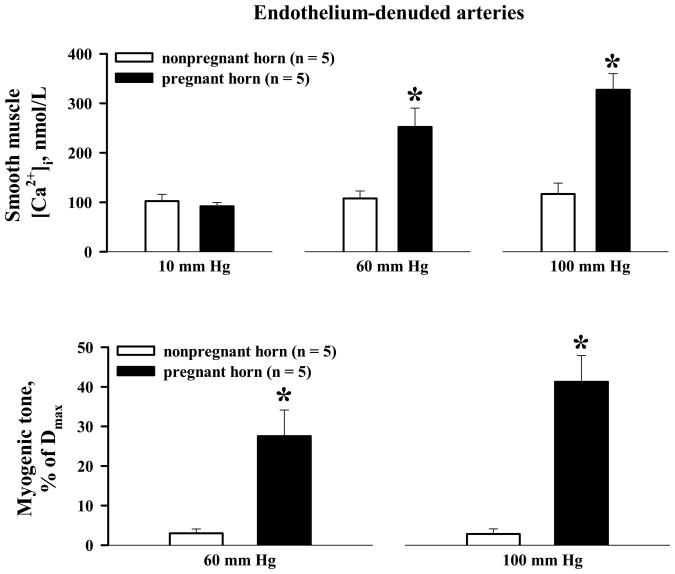
Denuded arteries from nonpregnant horns showed minimal changes in SMC [Ca2+]i in response to pressure elevations from 10 to 60 mm Hg (5 ± 3 nmol/L), and then to 100 mm Hg (14 ± 12 nmol/L, n = 5; Figure 5A), and developed little or no myogenic tone (2 ± 1 and 6 ± 2 % at 60 and 100 mm Hg, respectively; n = 10, Figure 5). Myogenic tone was well maintained in denuded arteries from gravid horns, averaging 28 ± 7 % and 41 ± 7 % at 60 and 100 mm Hg, respectively (n = 5). These constrictor responses were associated with significant increases in SMC [Ca2+]i above basal levels of 160 ± 40 and 236 ± 35 nmol/L at 60 and 100 mm Hg, respectively (n = 5, Figure 5). Denudation was associated with a tendency toward increased myogenic tone that reached statistical significance at 100 mm Hg. The frequency of Ca2+ oscillations was also higher in denuded vs. intact vessels (45 ± 6 osc/min versus 30 ± 3 osc/min at 100 mm Hg, n = 5 and n = 7 respectively; P < .05), although the average increment in SMC [Ca2+]i was not significantly different.
Figure 5.
Summary graphs demonstrating significant differences in pressure-induced smooth muscle [Ca2+]i responses and myogenic tone in endothelium-denuded uterine arteries from nonpregnant versus pregnant horns of late pregnant rats. * Significantly different at P < .05 (two-way repeated measures ANOVA); numbers in parentheses indicate the number of arteries tested.
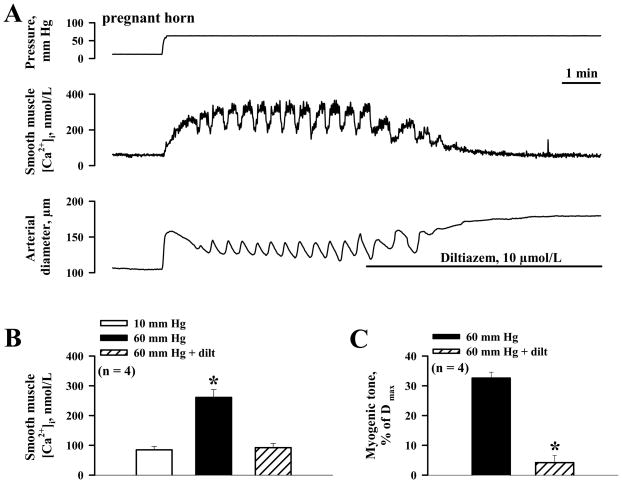
In arteries from gravid horns, both pressure-induced [Ca2+]i oscillatory activity and sustained [Ca2+]i elevations were effectively abolished by diltiazem, an inhibitor of L-type Ca2+ channels (Figure 6A and B); treatment with diltiazem also abolished the myogenic tone (Figure 6C), confirming its dependence on the influx of extracellular calcium, as reported in other vessel types.13, 21, 31
Figure 6.
A, Representative tracings showing inhibition of pressure-induced SMC [Ca2+]i elevation and myogenic tone by 10 μmol/L diltiazem. B, Summary graph demonstrating the effects of diltiazem on the level of SMC [Ca2+]i in response to pressure elevation from 10 to 60 mm Hg. *Significantly different from SMC [Ca2+] levels at 10 mm Hg (paired Student’s t-test). C, Bar graph summarizing the effects of diltiazem on myogenic tone induced by pressure elevation from 10 to 60 mm Hg. Myogenic tone is expressed as a percentage of the fully dilated diameters (Dmax) of each artery (in the presence of a combination of papaverine and diltiazem). *Significantly different from myogenic tone before application of diltiazem at P < 0.05 (paired Student’s t-test). On B and C, Numbers in parentheses indicate the number of arteries tested.
DISCUSSION
This study, using a rat model, clearly demonstrates that the development of myogenic behavior in uterine arteries from pregnant animals is the result of local rather than systemic influences. These have not been identified, and could be direct (paracrine actions of uteroplacental signals, for example), indirect (e.g., increased wall tension within the vascular wall secondary to expansive remodeling), or a combination thereof. The paracrine molecular signaling concept brings up the question of route of delivery – that is, how do secreted signals affect the behavior of arteries that are remote from, and upstream of their location? Venoarterial exchange is one possibility. Its existence in vivo has been documented in the utero-ovarian circulation as a mechanism of luteolysis in a number of species, and molecules secreted from the placenta, or from the paraplacental and myometrial tissues would be present in highest concentrations in the venous outflow. However, the importance of this mechanism has not been established in the setting of gestational remodeling.11
More likely, placentation and vasodilation lead to increased flow and expansive remodeling secondary to elevated shear stress in maternal upstream arteries such as the ones used for this study. The state of the cells within the remodeled wall is not known, but the pattern of gestational uterine vascular remodeling is generally one of increased diameter with an unchanged wall thickness.11 As noted in results, the passive lumen diameters of radial arteries taken from parallel anatomical locations in either horn were significantly (>75%) wider on the pregnant side. By the law of LaPlace (tension = pressure × radius), wall tension and stress would both increase, as might intravascular pressure due to a reduction in upstream resistance. The physical force of increased circumferential stretch would result in vascular SMC depolarization and calcium entry.14, 17, 19, 31, 32 In support of this concept, we recently found that vessels from pregnant animals treated with an inhibitor of endothelial nitric oxide synthase (eNOS) do not remodel and do not exhibit myogenic tone (unpublished observation).
Pregnancy is normally associated with some reduction in systemic blood pressure, therefore, the actual change in wall tension and stress would be dependent upon both factors (intravascular pressure and radius). The development of tone would result in a smaller lumen and a thicker wall, and thus likely reduce wall tension, as well as wall, media and cell stress. In a previous study, we found that different levels of tone in individual vessels within the cerebral circulation could be related to the normalization of media stress.33 Although a similar mechanism may operate in the uterine circulation, there are no published data to support or refute this concept, and it is therefore purely hypothetical.
Like vessels from nonpregnant animals, similar radial arteries taken from nonpregnant horns did not constrict in response to pressure elevation. The functional integrity of these vessels was confirmed by normal reactivity to phenylephrine and acetylcholine, associated with expected changes in SMC [Ca2+]i (Figure 2B). Because pregnancy is a state of increased endothelial vasodilatory influence, increased production of relaxing factors by endothelial cells in the non-gravid horn due to systemic hormonal influences could have been responsible for the absence of myogenic tone in these vessels. The fact that endothelial denudation did not potentiate their pressure-induced responses (Figure 6), effectively eliminates this possibility, and again suggests that changes in physical forces, or vascular SMC phenotype secondary to remodeling are responsible.
Endothelial denudation of arteries from pregnant horns resulted in a significant enhancement of myogenic tone in response to pressure elevation to 100 mm Hg. Although removal of endothelium negates both its inhibitory and excitatory effects on underlying SMCs, the inhibitory influence prevails in late pregnancy and moderates myogenic tone of uteroplacental arteries due to release of vasodilator factors. Nitric oxide may be a contributing factor as in our previous study myogenic tone of uterine arteries from late pregnant rats was significantly enhanced after blockade of NO production with N-nitro-arginine (L-NNA).24 The averaged [Ca2+]i increment associated with myogenic tone was not significantly different between intact and denuded vessels. These data suggest that tonic release of endothelium-derived vasodilator(s) may inhibit the Ca2+ sensitivity of the contractile process and in this way affect the myogenic tone of uterine arteries in late pregnancy. In addition, an increase in frequency of [Ca2+]i oscillations without change in average [Ca2+]i levels (such as seen in this study) may also contribute to enhanced myogenic tone after arterial denudation.
The results also indicate that the striking difference in myogenic behavior of uterine arteries from gravid versus nongravid horns is due to an augmented SMC [Ca2+]i response. The typical pattern of pressure-induced [Ca2+]i responses in myogenic vessels consisted of Ca2+ oscillations superimposed upon a sustained elevation in [Ca2+]i, and vasoconstriction. Both pressure-induced calcium activity and myogenic tone were abolished by diltiazem, implicating Ca2+ influx through L-type Ca2+ channels as the primary pathway for Ca2+ entry being responsible for the development of myogenic tone. Therefore, the mechanisms underlying enhanced myogenic tone in uterine arteries on the site of placentation are similar to those determined in our previous study for uterine arteries from regular late pregnant rats.21
Earlier, we demonstrated that late pregnancy is associated with a decreased function of voltage-gated potassium channels (Kv) in smooth muscle cells from uteroplacental arteries, which results in enhanced pressure-induced depolarization, calcium entry and SMC excitability.21 Decreased Kv channel function not only underlies a pregnancy-enhanced myogenic tone but also predicts an increase in constrictor reactivity of uterine arteries to depolarizing agonists. Indeed, previous studies have demonstrated that uterine arteries from pregnant rats became more responsive to adrenergic stimulation.7
The remarkable growth of the uterine vasculature in late pregnancy is well documented and is due to both hypertrophy and hyperplasia of smooth muscle cells. 29, 34, 35 It is well known that cellular growth is associated with augmented Ca2+ cell signaling that in part is due to depolarization of SMCs through mechanisms linked to a suppression of Kv channel expression and function.36–39 Other mechanisms - such as enhanced Ca2+ entry secondary to upregulation of TRP channel expression - may also be involved.40
The present study confirmed previously published findings that in late pregnancy uterine arteries supplying the hemochorial placenta of rodents can develop a sustained myogenic tone in response to pressure elevation.6, 21, 24, 26, 27, 41 It is known that the maternal compartment of the hemochorial placenta operates under relatively low levels of pressure (10 – 20 mm Hg) as is necessary for avoiding compression of fetal villi and maintaining an adequate maternal-fetal exchange.3 The development of myogenic tone may therefore contribute to an increase in uterine vascular resistance and serve as an adaptive protective mechanism that maintains normal pressure in the intraplacental compartment.
A significant enhancement of uterine artery myogenic tone and adrenergic sensitivity in late pregnancy might also be essential for minimizing blood loss during parturition. Vessels used in this study were taken from 20 day pregnant rats, that is approximately 48 hours prior to term. Although vascular adaptations associated with the last stage of pregnancy have not been characterized to any extent, the induction of myogenic tone and heightened adrenergic sensitivity could both act to limit uteroplacental blood flow and possible hemorrhage during parturition.
In summary, late pregnancy is associated with a striking enhancement of uterine artery reactivity to intraluminal pressure due to an up-regulation of cellular mechanisms that lead to an oscillating elevation in SMC cytosolic [Ca2+]i. These adaptive changes in myogenic behavior are governed by local uteroplacental influences, may be linked to the process of structural remodeling, and are induced by the modulation of intrinsic calcium handling properties of vascular smooth muscle cells independently of endothelial effects.
Acknowledgments
This work was supported by NIH HL073895 and HL079253 to George Osol, and HL067250 and HL088245 to Natalia Gokina.
REFERENCIES
- 1.Poston L, McCarthy AL, Ritter JM. Control of vascular resistance in the maternal and feto-placental arterial beds. Pharmacol Ther. 1995;65(2):215–239. doi: 10.1016/0163-7258(94)00064-a. [DOI] [PubMed] [Google Scholar]
- 2.Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. Am J PhysiolRegul Integr Comp Physiol. 1997;272(2 pt 2):R441–R463. doi: 10.1152/ajpregu.1997.272.2.R441. [DOI] [PubMed] [Google Scholar]
- 3.Moll W. Structure adaptation and blood flow control in the uterine arterial system after hemochorial placentation. Eur J Obstet Gynecol Reprod Biol. 2003;110 (Suppl 1):S19–S27. doi: 10.1016/s0301-2115(03)00169-6. [DOI] [PubMed] [Google Scholar]
- 4.Magness RR, Rosenfeld CR, Hassan A, Shaul PW. Endothelial vasodilator production by uterine and systemic arteries. I. Effects of ANG II on [PGI]2 and NO in pregnancy. Am J Physiol Heart and Circ Physiol. 1996;270(6 pt 2):H1914–H1923. doi: 10.1152/ajpheart.1996.270.6.H1914. [DOI] [PubMed] [Google Scholar]
- 5.Bird IM, Zhang L, Magness RR. Possible mechanisms underlying pregnancy-induced changes in uterine artery endothelial function. Am J Physiol Regul Integr Comp Physiol. 2003;284(2):R245–R258. doi: 10.1152/ajpregu.00108.2002. [DOI] [PubMed] [Google Scholar]
- 6.Osol G, Cipolla M. Interaction of myogenic and adrenergic mechanisms in isolated, pressurized uterine radial arteries from late-pregnant and nonpregnant rats. Am J Obstet Gynecol. 1993;168(2):697–705. doi: 10.1016/0002-9378(93)90519-o. [DOI] [PubMed] [Google Scholar]
- 7.D’Angelo G, Osol G. Regional variation in resistance artery diameter responses to alpha-adrenergic stimulation during pregnancy. Am J Physiol Heart Circ Physiol. 1993;264(1 pt2):H78–H85. doi: 10.1152/ajpheart.1993.264.1.H78. [DOI] [PubMed] [Google Scholar]
- 8.Xiao D, Pearce WJ, Zhang L. Pregnancy enhances endothelium-dependent relaxation of ovine uterine artery: role of NO and intracellular Ca2+ Am J Physiol Heart Circ Physiol. 2001;281(1):H183–H190. doi: 10.1152/ajpheart.2001.281.1.H183. [DOI] [PubMed] [Google Scholar]
- 9.Gokina NI, Goecks T. Upregulation of endothelial cell Ca2+ signaling contributes to pregnancy-enhanced vasodilation of rat uteroplacental arteries. Am J Physiol Heart Circ Physiol. 2006;290(5):H2124–H2135. doi: 10.1152/ajpheart.00813.2005. [DOI] [PubMed] [Google Scholar]
- 10.Veerareddy S, Cooke CL, Baker PN, Davidge ST. Vascular adaptations to pregnancy in mice: effects on myogenic tone. Am J Physiol Heart Circ Physiol. 2002;283(6):H2226–H2233. doi: 10.1152/ajpheart.00593.2002. [DOI] [PubMed] [Google Scholar]
- 11.Osol G, Mandala M. Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda) 2009;24:58–71. doi: 10.1152/physiol.00033.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schubert R, Mulvany MJ. The myogenic response: established facts and attractive hypotheses. Clin Sci (Lond) 1999;96(4):313–326. [PubMed] [Google Scholar]
- 13.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiological Rev. 1999;79(2):387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- 14.Hill MA, Zou H, Potocnik SJ, Meininger GA, Davis MJ. Invited review: arteriolar smooth muscle mechanotransduction: Ca2+ signaling pathways underlying myogenic reactivity. J Appl Physiol. 2001;91(2):973–983. doi: 10.1152/jappl.2001.91.2.973. [DOI] [PubMed] [Google Scholar]
- 15.Osol G. Mechanotransduction by vascular smooth muscle. J Vasc Res. 1995;32(5):275–292. doi: 10.1159/000159102. [DOI] [PubMed] [Google Scholar]
- 16.Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 2006;21:69–78. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]
- 17.Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256(5056):532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- 18.Cole WC, Chen TT, Clement-Chomienne O. Myogenic regulation of arterial diameter: role of potassium channels with a focus on delayed rectifier potassium current. Can J Physiol Pharmacol. 2005;83(8–9):755–765. doi: 10.1139/y05-082. [DOI] [PubMed] [Google Scholar]
- 19.Osol G, Brekke JF, McElroy-Yaggy K, Gokina NI. Myogenic tone, reactivity, and forced dilatation: a three-phase model of in vitro arterial myogenic behavior. Am J Physiol Heart Circ Physiol. 2002;283(6):H2260–H2267. doi: 10.1152/ajpheart.00634.2002. [DOI] [PubMed] [Google Scholar]
- 20.Schubert R, Lidington D, Bolz SS. The emerging role of Ca2+ sensitivity regulation in promoting myogenic vasoconstriction. Cardiovascular Res. 2008;77(1):8–18. doi: 10.1016/j.cardiores.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 21.Telezhkin V, Goecks T, Bonev AD, Osol G, Gokina NI. Decreased function of voltage-gated potassium channels contributes to augmented myogenic tone of uterine arteries in late pregnancy. Am J Physiol Heart Circ Physiol. 2008;294(1):H272–H284. doi: 10.1152/ajpheart.00216.2007. [DOI] [PubMed] [Google Scholar]
- 22.Novak J, Ramirez RJ, Gandley RE, Sherwood OD, Conrad KP. Myogenic reactivity is reduced in small renal arteries isolated from relaxin-treated rats. Am J Physiol Regul Integr Comp Physiol. 2002;283(2):R349–R355. doi: 10.1152/ajpregu.00635.2001. [DOI] [PubMed] [Google Scholar]
- 23.Meyer MC, Brayden JE, McLaughlin MK. Characteristics of vascular smooth muscle in the maternal resistance circulation during pregnancy in the rat. Am J Obstet Gynecol. 1993;169(6):1510–1516. doi: 10.1016/0002-9378(93)90427-k. [DOI] [PubMed] [Google Scholar]
- 24.Gokina NI, Mandala M, Osol G. Induction of localized differences in rat uterine radial artery behavior and structure during gestation. Am J Obstet Gynecol. 2003;189(5):1489–1493. doi: 10.1067/s0002-9378(03)00623-9. [DOI] [PubMed] [Google Scholar]
- 25.Hilgers RH, Bergaya S, Schiffers PM, et al. Uterine artery structural and functional changes during pregnancy in tissue kallikrein-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23(10):1826–1832. doi: 10.1161/01.ATV.0000090672.07568.60. [DOI] [PubMed] [Google Scholar]
- 26.Veerareddy S, Campbell ME, Williams SJ, Baker PN, Davidge ST. Myogenic reactivity is enhanced in rat radial uterine arteries in a model of maternal undernutrition. Am J Obstet Gynecol. 2004;191(1):334–339. doi: 10.1016/j.ajog.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 27.Kublickiene KR, Cockell AP, Nisell H, Poston L. Role of nitric oxide in the regulation of vascular tone in pressurized and perfused resistance myometrial arteries from term pregnant women. Am J Obstet Gynecol. 1997;177(5):1263–1269. doi: 10.1016/s0002-9378(97)70048-6. [DOI] [PubMed] [Google Scholar]
- 28.Fuller R, Barron C, Mandala M, Gokina N, Osol G. Predominance of local over systemic factors in uterine arterial remodeling during pregnancy. Reprod Sci. 2009;16(5):489–500. doi: 10.1177/1933719108329816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Annibale DJ, Rosenfeld CR, Stull JT, Kamm KE. Protein content and myosin light chain phosphorylation in uterine arteries during pregnancy. Am J Physiol Cell Physiol. 1990;259(3 pt1):C484–C489. doi: 10.1152/ajpcell.1990.259.3.C484. [DOI] [PubMed] [Google Scholar]
- 30.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260(6):3440–3450. [PubMed] [Google Scholar]
- 31.Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol. 1998;508(pt 1):199–209. doi: 10.1111/j.1469-7793.1998.199br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harder DR. Pressure-dependent membrane depolarization in cat middle cerebral artery. Circ Res. 1984;55(2):197–202. doi: 10.1161/01.res.55.2.197. [DOI] [PubMed] [Google Scholar]
- 33.Brekke JF, Gokina NI, Osol G. Vascular smooth muscle cell stress as a determinant of cerebral artery myogenic tone. Am J Physiol Heart Circ Physiol. 2002;283(6):H2210–H2216. doi: 10.1152/ajpheart.00633.2002. [DOI] [PubMed] [Google Scholar]
- 34.Ramsey EM, Chez RA, Doppman JL. Radioangiographic measurement of the internal diameters of the uteroplacental arteries in rhesus monkeys. Am J Obstet Gynecol. 1979;135(2):247–251. doi: 10.1016/0002-9378(79)90353-3. [DOI] [PubMed] [Google Scholar]
- 35.Osol G, Cipolla M. Pregnancy-induced changes in the three-dimensional mechanical properties of pressurized rat uteroplacental (radial) arteries. Am J Obstet Gynecol. 1993;168(1 pt 1):268–274. doi: 10.1016/s0002-9378(12)90924-2. [DOI] [PubMed] [Google Scholar]
- 36.Berridge MJ. Calcium signalling and cell proliferation. Bioessays. 1995;17(6):491–500. doi: 10.1002/bies.950170605. [DOI] [PubMed] [Google Scholar]
- 37.Mandegar M, Fung YC, Huang W, Remillard CV, Rubin LJ, Yuan JX. Cellular and molecular mechanisms of pulmonary vascular remodeling: role in the development of pulmonary hypertension. Microvasc Res. 2004;68(2):75–103. doi: 10.1016/j.mvr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Platoshyn O, Golovina VA, Bailey CL, et al. Sustained membrane depolarization and pulmonary artery smooth muscle cell proliferation. Am J Physiol Cell Physiol. 2000;279(5):C1540–C1549. doi: 10.1152/ajpcell.2000.279.5.C1540. [DOI] [PubMed] [Google Scholar]
- 39.Berk BC. Vascular smooth muscle growth: autocrine growth mechanisms. Physiol Rev. 2001;81(3):999–1030. doi: 10.1152/physrev.2001.81.3.999. [DOI] [PubMed] [Google Scholar]
- 40.Landsberg JW, Yuan JX. Calcium and TRP channels in pulmonary vascular smooth muscle cell proliferation. News Physiol Sci. 2004;19:44–50. doi: 10.1152/nips.01457.2003. [DOI] [PubMed] [Google Scholar]
- 41.Cipolla MJ, Binder ND, Osol G. Myoendometrial versus placental uterine arteries: structural, mechanical, and functional differences in late-pregnant rabbits. Am J Obstet Gynecol. 1997;177(1):215–221. doi: 10.1016/s0002-9378(97)70464-2. [DOI] [PubMed] [Google Scholar]