SUMMARY
Cryopyrin (NALP3) mediates formation of the inflammasome, a protein complex responsible for cleavage of pro-IL-1β to its active form. Mutations in the cryopyrin gene, NLRP3, cause the autoinflammatory disease spectrum: cryopyrin-associated periodic syndromes (CAPS). The central role of IL-1β in CAPS is supported by the remarkable response to IL-1 targeted therapy. We developed two novel Nlrp3 mutant knock-in mouse strains to model CAPS to examine the role of other inflammatory mediators and adaptive immune responses in an innate immune driven disease. These mice had systemic inflammation and poor growth, similar to some human CAPS patients, and demonstrated early mortality, primarily mediated by myeloid cells. Mating these mutant mice to various knock-out backgrounds confirmed the mouse disease phenotype required an intact inflammasome, was only partially dependent on IL-1β, and was independent of T cells. This data suggests CAPS are true inflammasomopathies and provide insight for more common inflammatory disorders.
INTRODUCTION
Cryopyrin is a prototype of the NOD-like receptor (NLR) family, a group of intracellular innate immune proteins thought to act as sensors of pathogen and damage-associated molecular patterns (PAMPs and DAMPs), (Martinon and Tschopp, 2005). Cryopyrin is capable of forming an inflammasome, a macro-molecular complex that directs activation of the IL-1 converting enzyme caspase-1, resulting in cleavage and secretion of the proinflammatory mediator IL-1β, as well as IL-18 and IL-33 (Martinon, 2008; Meylan et al., 2006; Schmitz et al., 2005). Cryopyrin’s importance as a regulator of inflammation was made clear in 2001–2002, when dominantly inherited mutations in NLRP3 (CIAS1), the gene encoding cryopyrin, were proven responsible for a spectrum of inflammatory diseases, including familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome (MWS), and neonatal onset multisystem inflammatory disease (NOMID) now collectively referred to as the cryopyrin-associated periodic syndromes (CAPS) (Aksentijevich et al., 2002; Feldmann et al., 2002; Hoffman et al., 2001).
CAPS represent a continuum in disease severity, with FCAS at the mild end, manifesting cold-induced inflammatory episodes, MWS in the middle with attacks potentially leading to long-term sequelae such as amyloidosis, and NOMID, most severe, with continuous inflammation causing neurological impairment and deforming arthropathy (Aksentijevich et al., 2007). As patients lack high titer autoantibodies and self-reactive T cells, CAPS fall under the rubric of autoinflammation, a class of conditions considered separate from autoimmunity in that pathology is thought to arise from dysregulation of the innate immune system with little contribution from adaptive immunity.
The inflammasome has been implicated indirectly in the pathogenesis of other Mendelian autoinflammatory disorders, such as familial Mediterranean fever (Chae et al., 2003; Stehlik et al., 2003; Yu et al., 2007) and the syndrome of pyogenic arthritis, pyoderma gangrenosum, and acne (Wise et al., 2002). The inflammasome also appears to play a role in complex diseases such as gout and asbestosis, in which monsodium urate crystals and asbestos fibers act as DAMPS to trigger IL-1 mediated inflammation (Dostert et al., 2008; Martinon et al., 2006)
Mutations in cryopyrin are thought to result in inappropriate activation of the inflammasome, leading to excessive IL-1β release and CAPS disease manifestations. Treatment with the IL-1β inhibitors rilonacept or anakinra prevents episodes in MWS and FCAS and resolves most inflammatory signs and symptoms in NOMID (Hoffman et al., 2008). However, there is an emerging body of data suggesting mutations in cryopyrin may have other effects besides increased IL-1β. A recent publication demonstrated CAPS mutations increase necrotic-like cell death in a monocyte cell line in an ASC dependent, caspase-1 independent manner (Willingham et al., 2007). Since necrosis releases proinflammatory mediators normally sequestered in cells, this pathway may also lead to inflammation and provide a possible explanation for why some CAPS patients have incomplete clinical responses to anakinra/rilonacept (Goldbach-Mansky et al., 2006; Hoffman et al., 2008).
Efforts to dissect the role of the inflammasome in CAPS have been hampered by the limitations of working with patient tissue samples, the heterogeneity intrinsic to humans, and the lack of specific inflammasome inhibitors. Although a mouse model of uncontrolled IL-1β signaling is available (the IL-1 receptor antagonist knock-out) (Horai et al., 2000), this model does not address IL-1β-independent functions of the inflammasome. We therefore engineered mutations associated with two specific phenotypes (FCAS and MWS) into mouse Nlrp3 to examine the roles of IL-1β and other cytokines, as well as characterize inflammasome functions beyond the innate immune system.
The results presented here show CAPS mutations lead to severe inflammatory disease in mice dependent on the inflammasome and myeloid lineage. The major physiological consequence of CAPS-associated inflammasome activation is IL-1β-mediated, however other effectors are clearly involved. While a robust T cell response was observed, it was not necessary for disease, demonstrating a marked predominance of innate immunity in the pathogenesis of CAPS inflammasomopathies that may be extended to autoinflammation in general.
RESULTS
Development of Nlrp3A350VneoR/+ and Nlrp3L351PneoR/+ mice
To gain a better understanding of CAPS disease processes, we generated two knock-in mouse strains. The alanine 352 to valine (A352V) and leucine 353 to proline (L353P) mutations were chosen because they are strongly associated with classical MWS and FCAS patients, respectively, and less so in patients with overlap syndromes (Hoffman et al., 2003; Hoffman et al., 2001). Secondly, these mutations affect adjacent amino acids and are predicted to affect a surface involved in inter-domain or inter-protein interactions in the assembled inflammasome (Figure S1 A). The A352 and L353 residues (A350 and L351 in mouse cryopyrin) are conserved in mouse and human cryopyrin, as is the immediate protein environment of these residues in models of the cryopyrin structure (Aksentijevich et al., 2007).
To mutate these residues, we engineered a construct introducing a floxed neomycin resistance cassette into intron 2, directly upstream of alanine 350 and leucine 351. ES cell colonies positive for the A350V or L351P mutations were used to create chimeric mice, which then yielded offspring heterozygous for A350V or L351P, lines Nlrp3A350VneoR/+ and Nlrp3L351PneoR/+ (Figure S1B). Sequence analysis of cDNA from Nlrp3A350VneoR/+ and Nlrp3L351PneoR/+ mice demonstrated lack of expression from the mutated alleles, consistent with transcriptional silencing due to the reverse orientation of the neomycin resistance cassette (neoR) relative to the Nlrp3 gene. We therefore bred mice to various lines expressing Cre recombinase under control of different promoters: zona pelucida 3 (CreZ: recombinase expression prior to completion of the first meiotic division, all cells expressing cryopyrin express A350V/L351P) (Lewandoski et al., 1997) lysozyme (CreL: A350V/L351P expression in myeloid lineage cells only) (Clausen et al., 1999), and tamoxifen inducible, with Cre recombinase fused to the estrogen responsive protein (CreT: A350V/L351P expression induced upon exposure to 4-hydroxytamoxifen) (Hayashi and McMahon, 2002). The mouse lines generated are as follows: Nlrp3A350V/+/CreZ; Nlrp3A350V/+/CreL; Nlrp3A350V/+/CreT and Nlrp3L351P+/CreZ; Nlrp3L351P/+/CreL; and Nlrp3L351P/+/CreT. PCR and cDNA sequencing have shown that the mutant alleles were now being expressed (Figure S1C).
Embryonic or myeloid-specific expression of A350V or L351P results in neonatal or perinatal lethality with severe inflammatory signs
Nlrp3A350V/+/CreZ pups demonstrated an inflammatory phenotype at or by 1–2 days post-birth, developing skin abscesses that gave way to scaling erythema by day 4 (Figure 1A). In addition, these mice exhibited profound growth delay with lack of pigmentation and hair growth observable by day 4. Although Nlrp3 wildtype (CreZ or CreL) mice grew steadily from birth, mutant pups gained weight slowly, and then lost weight before dying between days 2–14 (Figure 1B). Nlrp3A350V/+/CreL pups demonstrated a similar phenotype, but had different mortality kinetics such that 70% of these mice survived until day 7–9 before dying at an increased rate (p<0.0001, Figure 1B).
Figure 1. Expression of L351P is lethal in the perinatal period and expression of A350V causes a severe inflammatory phenotype with death by day fourteen post-birth.
(A) Gross phenotype of Nlrp3A350V/+/CreL, representative pictures. Mutants are depicted at the top, days 2 and 4, and on the left, days 6 and 8. Black arrows denote an abscess (day 2) and scaling erythema (day 8). (B) Survival (top) and growth (bottom) curves for WT (n=86, CreZ shown only), Nlrp3A350V/+/CreZ (n=50), and Nlrp3A350V/+/CreL, (n=40). A log-rank (Mantel-Cox) test comparing Nlrp3A350V/+/CreZ and Nlrp3A350V/+/CreL survival curves yielded a P value <0.0001. Error bars shown for mean daily weights on growth curves are SDev (n=1–17). (C) Nlrp3A350V/+/CreL, day 8, tissue sections from synovium, liver, meninges, and conjunctiva, stained with haematoxylin and eosin at 10X and 40X (insets) magnification. (D) Birth table of pups resulting from CreL+/+ x Nlrp3A350VNeoR/+ or Nlrp3L351PNeoR/+ matings, with 50% of offspring expected to be mutant. Shown: total pups; total mutant pups days 1 and 2 post-birth.
While different from the urticaria-like lesions seen in MWS patients, the cutaneous erythema, abscesses, and scaling observed in pups expressing A350V nevertheless suggested underlying inflammation. Hematoxylin and eosin staining of various tissues showed pronounced leukocytic infiltrates in skin, liver, spleen, joint, sinus, conjunctiva, bone marrow, and tongue, which upon higher magnification were shown to be mainly neutrophilic (Figure 1C and data not shown). There was also evidence of significant necrosis in the gut and kidney that was not associated with inflammation (data not shown), suggesting that the cause of death in these pups is due to multisystem organ failure secondary to inflammation and necrosis. Tissue sections of 7–9 day old Nlrp3A350V/+/CreZ and Nlrp3A350V/+/CreL mice were very similar.
Although the L353P human mutation is considered milder than A352V, only six Nlrp3L351P/+/CreL pups were identified by genotyping and were discovered dead within one day of birth, suggesting they were either still-born, or died soon after birth. Many still-born pups were likely cannibalized before detection. Numbers of total mice born (affected and unaffected) are shown in Figure 1D, first column, and mutant pups observed alive on days 1 and 2 are shown in the adjacent columns. Most Nlrp3A350V/+/CreT and Nlrp3L351P/+/CreT mice survived normally without phenotype, although inflammatory changes in the skin and/or growth delay were noted in some older (>12 weeks) mice, likely due to “leaky” creT activity causing expression of mutant cryopyrin.
Complete blood counts (CBC) of both Nlrp3A350V/+/CreZ and Nlrp3A350V/+/CreL pups showed very similar profiles: the overall white blood cell count was only mildly elevated and pronounced neutrophilia and thrombocytosis were evident (data not shown). Similar hematological and skin findings have been described consistently in CAPS patients (Goldbach-Mansky et al., 2006; Hoffman et al., 2004). Given the similarities in the gross phenotype, pathology, and white blood cell differential of Nlrp3A350V/+/CreZ and Nlrp3A350V/+/CreL, it is clear that the myeloid lineage plays a pivotal role in murine disease. To examine this role more specifically, we decided to focus on the Nlrp3A350V/+/CreL mice and myeloid cells from Nlrp3A350V/+/CreT and Nlrp3L351P/+/CreT mice for further experimentation.
Multiple cytokines are upregulated in Nlrp3A350V/+/CreL serum
To investigate cytokine levels in Nlrp3A350V/+/CreL mice, we performed a Luminex assay on serum obtained 6–8 days post birth. Multiple cytokines, chemokines, and growth factors were significantly elevated in Nlrp3A350V/+/CreL compared to wildtype littermates (Figure 2A), including IL-1β, and IL-6, two cytokines known to have major roles in CAPS inflammation (Goldbach-Mansky et al., 2006). Consistent with pronounced neutrophilia, the growth factor GCSF and neutrophil chemoattractant KC were also upregulated. IL-18, which like IL-1β, is processed to a mature, secreted form by caspase-1, was also increased (Figure 2A). The Th2 lineage cytokines IL-4, IL-9, IL-10, and IL-13 showed varying amounts of upregulation, but the classical Th1 cytokines IFNγ and IL-12 were not significantly different from WT, nor was IL-17 (Figure S2). Serum TNFα was not significantly different in Nlrp3A350V/+/CreL mice compared to WT mice (Figure 2A), a finding consistent with previously described CAPS patients (Hoffman et al., 2004).
Figure 2. Multiple cytokines are upregulated in the serum and skin of Nlrp3A350V/+/CreL mice.
(A) Luminex analysis of serum obtained at days 6–8 from WT and Nlrp3A350V/+/CreL pups, n=10–12 mice, each graph point represents one mouse, mean and SEM are shown. (B) Immunohistochemistry on skin from WT and Nlrp3A350V/+/CreL pups for IL-1β or IL-6 (red stain), and an isotype control followed by haematoxylin staining. Similar staining was observed on sections from Nlrp3A350V/+/CreZ (not shown).
IL1-β and IL-6 are increased in Nlrp3A350V/+/CreL skin
The increase in serum IL-1β and IL-6 raised the question as to whether these cytokines might also be upregulated at the tissue level. Accordingly, we performed immunohistochemistry on inflamed skin sections from Nlrp3A350V/+/CreL pups for IL-1β and IL-6. Increased expression of both cytokines, IL-1β primarily in the dermis, and IL-6 in the epidermis, was noted (Figure 2B). This is consistent with skin biopsies from CAPS patients and implying a role for these cytokines in skin pathology (Hoffman et al., 2004).
Inflammasome function is similar in cells from Nlrp3A350V/+/creT and Nlrp3L351P/+/creT mice and CAPS patients
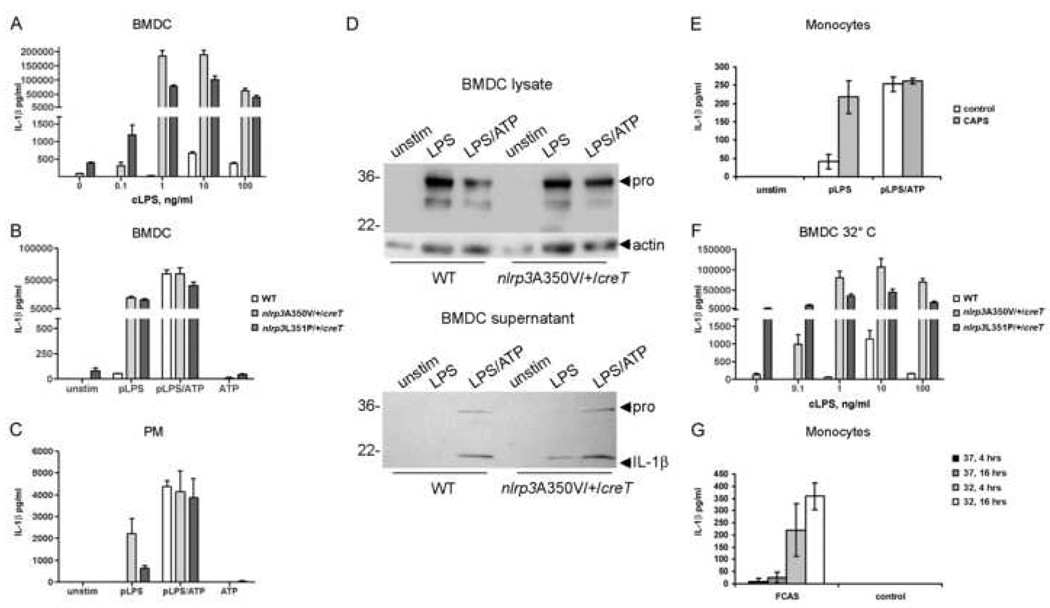
Since the Nlrp3A350V/+/CreZ and Nlrp3A350V/+/CreL mice exhibit such a severe phenotype, we wanted to confirm that inflammasome function in the mice was similar to human CAPS patients. The innate immune stimulant LPS signals through TLR4 on the cell surface and multiple second messengers within the cell to cause upregulation of pro-IL-1β synthesis. Release of mature IL-1β does not occur without exogenously applied ATP, which is likely to be mediated through the ATP-dependent P2X7 ion channel to increase potassium ion efflux (Mariathasan et al., 2006). Mutation-positive CAPS patient cells have been shown to secrete IL-1β in the absence of ATP, perhaps because the CAPS mutation renders cryopyrin constitutively active (Gattorno et al., 2007). To determine whether Nlrp3A350V/+/CreT cells are also capable of secreting IL-lβ without ATP, we stimulated tamoxifen-treated bone marrow-derived dendritic cells (BMDC) from Nlrp3A350V/+/CreT, Nlrp3L351P/+/CreT, and WT adult mice with different LPS preparations and/or ATP.
ELISA analysis of supernatants showed no IL-1β release from WT BMDC at LPS concentrations below 10 ng/ml, while IL-1β was easily detectable from mutant BMDC at 0.1 ng/ml, suggesting that mutant cells are hyperresponsive. Maximal IL-1β release was observed from all BMDCs at 10 ng/ml, however, Nlrp3A350V/+/CreT and Nlrp3L351P/+/CreT BMDC secreted levels of 300 and 150 times, respectively, that of WT BMDC (Figure 3A). The IL-1β secretion observed from WT cells implicated possible ATP contamination in our crude LPS preparation. To further characterize the stimulus requirement in Nlrp3A350V/+/CreT and Nlrp3L351P/+/CreT, we treated BMDC with a highly purified preparation of LPS (100 ng/ml), with and without ATP, and measured IL-1β secretion. Consistent with previous results, WT cells did not secrete appreciable amounts of IL-1β in the absence of ATP, however mutant cells released high levels of IL-1β with LPS alone (p < 0.0001) (Figure 3B). As expected, the addition of ATP resulted in IL-1β release from WT cells, however, we also observed a two to three-fold upregulation in secretion from Nlrp3A350V/+/CreT and Nlrp3L351P/+/CreT cells, (p = 0.008 and 0.004). When this experiment was repeated in Nlrp3A350V/+/CreT and Nlrp3L351P/+/CreT peritoneal macrophages (PM), cells again secreted IL-1β in the absence of ATP (Figure 3C), However, the levels of IL-1β secreted by these cells with or without ATP were much lower than those observed with BMDC, probably due to a lower cell concentration. Interestingly, a small amount of IL-1β was released in the absence of any stimulus from Nlrp3L351P/+/CreT BMDC (Figure 3A and B), but not from PM (Figure 3C).
Figure 3. Myeloid cells from Nlrp3A350V/+/CreT and Nlrp3L351p/+/CreT mice and CAPS patients react similarly to innate stimuli.
Tamoxifen-treated Nlrp3A350V/+/CreT, Nlrp3L351P/+/CreT, and littermate WT BMDC were incubated with varying amounts of crude LPS (A) or pure LPS (100 ng/ml) with and without ATP (5mM) (B). (C) Tamoxifen-treated peritoneal macrophages (PM) were incubated with pure LPS with and without ATP. IL-1β in the supernatants was measured by ELISA. (A–C) n=2–3 mice with 3 wells each / genotype. (D) Western blotting for IL-1β or actin loading control, Nlrp3A350V/+/CreT BMDC lysates (top), and supernatants (bottom). (E) In vitro stimulation of monocytes from 3 CAPS patients and 3 normal human controls with pure LPS with and without ATP. (F) In vitro cold stimulation of WT, Nlrp3A350V/+/CreT, and Nlrp3L351P/+/CreT BMDC, n=2–3. Cells were incubated at 32°C overnight and supernatants were analyzed by ELISA for IL-1β. (G) In vitro cold stimulation of monocytes from 5 FCAS patients and 2 normal human controls, 4 hrs and overnight. Also shown, incubation at 37°C.
IL-1β is first synthesized as a 34 kDa proprotein, which is cleaved by caspase-1 to the 17 kDa mature form. To determine which form is secreted, we performed Western blotting analysis on the Nlrp3A350V/+/CreT cell supernatants used for ELISA. For comparison, we probed lysates of LPS and LPS/ATP-stimulated cells. Nlrp3A350V/+/CreT BMDC secreted primarily mature IL-1β, as well as some pro-IL-1β, whereas proIL-1β predominated in cell lysates (Figure 3D). A small amount of proIL-1β was detected in the supernatants from all cells treated with LPS and ATP, possibly due to cell lysis, as a slight drop in cell viability was observed after incubation with ATP. WT and Nlrp3A350V/+/CreT lysates contained similar amounts of pro-IL-1β, independent of ATP, indicating that ATP is only necessary for secretion of IL-1β and not for synthesis.
As a comparison to stimulations done on murine cells, we also stimulated monocytes from three mutation-positive MWS/NOMID patients, human mutations D303N or R260W (Dode et al., 2002), with LPS, with and without ATP. Patient cells, but not controls, secreted IL-lβ when stimulated with LPS alone, whereas both controls and CAPS secreted in response to LPS and ATP (Figure 3E).
FCAS patients suffer inflammatory attacks upon brief exposure to cold temperatures and peripheral blood monocytes from patients secrete IL-1β spontaneously when incubated at 32°C (Rosengren et al., 2007). To determine if Nlrp3A350V/+/CreT and Nlrp3L351P/+/CreT cells were also stimulated by low temperatures, we incubated BMDC at 32°C and stimulated with varying amounts of crude LPS. Nlrp3L351P/+/CreT spontaneously secreted high levels of IL-1β at 32°C (p = 0.013) (Figure 3F), consistent with monocytes from FCAS patients, with a further upregulation in secretion with LPS. In contrast, incubation at 32°C alone did not result in secretion from Nlrp3A350V/+/CreT cells. These cells secreted as expected when stimulated with LPS, with a trend of higher IL-1β release at 0.1 ng/ml LPS than previously at 37°C (Figure 3A). This suggests that temperature might have an activating effect on these cells as well, although it is not enough to cause secretion in the absence of stimulant. PM from Nlrp3L351P/+/CreT did not secrete spontaneously at 32°C (data not shown), indicating that not all myeloid cell sources in these mice are temperature-responsive. As a comparison, we tested a family of five FCAS patients, human mutation F523C (Aksentijevich et al., 2007), for spontaneous secretion. Similar to cells from mice, patient monocytes robustly secreted IL-1β when incubated at 32°C (Figure 3G).
Disease expression in mice is partially dependant on IL-1β
CAPS patients treated with the IL-1β inhibitors anakinra (Amgen) or rilonacept (Regeneron) have almost complete resolution of symptoms and normalization of serum markers of inflammation (Goldbach-Mansky et al., 2006; Hoffman et al., 2008). Since Nlrp3A350V/+/CreL pups show similar cellular infiltrates, CBCs, and increased serum and tissue levels of IL-1β, we hypothesized that IL-1β inhibition would ameliorate murine disease. We treated Nlrp3A350V/+/CreL pups from birth or soon after with a mouse form of rilonacept, mIL-1 Trap. Pups injected subcutaneously every other day survived on average three days longer than vehicle-injected littermates (P=0.003, Figure 4A), despite doses up to 60 times those used normally in humans. Wildtype littermates given equal doses of mIL-1 Trap grew and gained weight normally, but mIL-1 Trap did not significantly increase Nlrp3A350V/+/CreL growth rate (Figure 4A), nor did it visibly impact skin pathology.
Figure 4. IL-1β is required for murine disease.
(A) Nlrp3A350V/+/CreL and wildtype littermates were treated with subcutaneously injected mIL-1 Trap every other day beginning day 1–2 post-birth. Survival (left) (n=9) and growth (right) (n=2–9 mice), P=0.003 by log-rank (Mantel-Cox) comparison. (B) Survival (left) and growth (right) of Nlrp3A350V/+/CreL/Il-1r+/− and Nlrp3A350V/+/CreL/Il-lr−/−, (n=13–18 for survival and 1–9 for growth). (C) Survival (left) and growth (right) of Nlrp3L351P/+/CreL/Il-1r−/−, (n=33 for survival and 2–12 for growth). Error bars shown for mean daily weights on growth curves are SDev. (D) Luminex analysis of serum from Nlrp3A350V/+/CreL/Il-lr−/−, n=5, compared with Luminex data from WT and Nlrp3A350V/+/CreL in figure 2 (averages and SEM shown only).
Since it is impossible to know how efficiently mIL-1 Trap inhibits endogenous mouse IL-1β in neonate tissues, we decided to assess IL-1β’s role in a more definitive manner. We first mated Nlrp3A350VneoR/+ and Nlrp3L351PneoR/+ mice to Il-1r−/− mice, which do not express the IL-1 receptor (Glaccum et al., 1997), and then bred these mice to the CreL line. Nlrp3A350V/+/CreL/Il-1r−/− mice were essentially protected from neonatal lethality, with improved weight gain compared to Nlrp3A350V/+/CreL and Nlrp3A350/+/CreL/Il-1r+/− mice (Figure 4B). However, growth rates were variable within litters, with some pups indistinguishable from WT and others growing more slowly. Some signs of limited cutaneous inflammation were observed and these were also variable within litters (data not shown).
Although the expression of L351P appears to result in perinatal lethality on an intact Il-1r background, most Nlrp3L351P/+/CreL/Il-1r−/− mice were born with a milder phenotype compared to Nlrp3A350V/+/CreL mice, with less prominent skin or hair findings and improved growth. Additionally, all Nlrp3L351P/+/CreL/Il-1r−/− mice survived at least ten days. Approximately eighty percent of Nlrp3L351P/+/CreL/Il-1r−/− died by 35 days after birth, but the remaining twenty percent were still alive after 75 days (Figure 4C). This variability in survival and phenotype may be due to a mixed genetic background, as the Il-1r−/− mice are not fully backcrossed.
To assess which cytokines are dependent on IL-1R signaling, we performed a Luminex assay on serum from Nlrp3A350V/+/CreL/Il-1r−/−. A significant number of cytokines previously upregulated in Nlrp3A350V/+/CreL on an intact Il-1r background were now at levels roughly equivalent to WT, including KC, GCSF, IL-6, and IL-4 (Figure 4D, WT and Nlrp3A350V/+/CreL means from figure 2A shown for comparison). IL-1β serum levels in Nlrp3A350V/+/CreL/Il-1r−/− did not change, nor did IL-18, presumably because it is also cleaved by caspase-1 and not dependent on activation of the IL-1R.
Nlrp3A350V/+/CreT BMDC drive an inflammatory phenotype
FACS analysis of Nlrp3A350V/+/CreZ showed pronounced neutrophilia consistent with the infiltrates observed in other tissues (figure S3). In addition, expression of T cell activation markers CD69 and CD25, measured by mean fluorescence intensity (MFI), was increased on both CD4+ and CD8+ T cells (figure S3 and data not shown), suggesting an ongoing T cell process which could be mediated by dendritic cells. As a first step, we stimulated WT and Nlrp3A350V/+/CreT BMDC with LPS and examined the expression of MHC class II antigen, CD40 and OX40 ligand (OX40L), molecules known to be involved in APC functions (Chen et al., 1999; Murata et al., 2000; Ni and O'Neill, 1997). Although there was no difference in expression of MHC II and CD40, LPS-activated Nlrp3A350V/+/CreT BMDC expressed higher levels of surface OX40L than WT BMDC, measured by MFI (Figure 5A). Thus, Nlrp3A350V/+/CreT BMDC are primed to direct an inflammatory T cell response.
Figure 5. Nlrp3A350V/+/creT BMDC drive an inflammatory T cell phenotype.
(A) Tamoxifen-treated BMDC from Nlrp3A350V/+/creT and WT mice were treated with LPS, and stained for CD40, OX40L, and MHC class II receptor. (B–I) BMDC were plated with OT II naïve T cells and OVA antigen under Th1, Th2, or Thl7 cell polarizing conditions, or left unpolarized. (B–D) FACS analysis of intracellular IFNγ and IL-17. (E–I) Luminex analysis of culture supernatants for IFNγ, IL-17, IL-5, and IL-4.
To assess this response, we exposed BMDC to naive T cells isolated from an OT II mouse (all T cells are derived from a single clone specific for an ovalbumin peptide (OVA) (Barnden et al., 1998) under various polarizing conditions. When polarized toward a Th1 profile, a significantly higher percentage of T cells incubated with Nlrp3A350V/+/CreT BMDC stained positively for IFNγ at low concentrations of OVA antigen, as measured by MFI, with 31% of cells positive at an antigen concentration of 10 nM compared to 8% of cells incubated with WT BMDC (Figure 5B). This percentage gradually increased to 74%, at a concentration of 1 µM OVA, at which the two strains were about equal. Higher levels of antigen resulted in cell death, likely because IFNγ levels became cytotoxic (data not shown). When polarized toward a Th17 phenotype, a two-fold increase in the number of IL-17-positive T cells cocultured with Nlrp3A350V/+/CreT BMDC was observed at 1 µM antigen concentrations (Figure 5C). Finally, when cells were left untreated, a two-fold increase in IL-17-positive cells was observed from Nlrp3A350V/+/CreT cells at the highest (10 µM) antigen concentration (Figure 5D). Luminex analysis of supernatants was consistent with the FACS results (Figure 5E–G) demonstrating a modest increase in secreted IL-5 from Nlrp3A350V/+/CreT cells polarized to a Th2 phenotype at higher antigen concentrations (Figure 5H). A four-fold increase in secreted IL-4 in unpolarized Nlrp3A350V/+/CreT wells at intermediate antigen concentrations (Figure 5I) was also observed. All told, these results suggest that given the right culture milieu, Nlrp3A350V/+/CreT BMDC can efficiently direct a variety of T cell responses, but when left unpolarized promote a Th17 phenotype.
Murine disease does not require T cells and is strictly dependent on an intact inflammasome with no requirement for wildtype cryopyrin
The elevated numbers of activated T cells in our mice and the robust response from naive T cells stimulated with Nlrp3A350V/+/CreT antigen presenting cells suggested a contributing role for T cells in murine disease. To determine whether T cells play a role in disease pathology, we mated Nlrp3A350VneoR/+ mice with Rag1−/− mice, which contain no mature T or B cells (Mombaerts et al., 1992) and then mated to CreL. Nlrp3A350V/+/CreL/Rag−/− mice died at approximately the same time as Nlrp3A350V/+/CreL on an intact or haplosufficient Rag1 background, with similar cutaneous inflammation and growth delay (Figure 6A, Figure 1B, and data not shown). This data suggests that the observed T cell activation profile is not a major contributing factor to disease expression and mortality.
Figure 6. An intact inflammasome is required for pathology mediated by cryopyrin knock-in mutations, but T and B cells are not. Mutant phenotype does not require wildtype cryopyrin.
Nlrp3A350VneoR/+ were bred with Asc−/−, Rag−/−, and Nlrp3−/−. (A) Survival (top) and growth (bottom) of Nlrp3A350V/+/CreL/Rag−/− (n=l–3 for growth, 6 for survival), Nlrp3A350V/+/CreL/Asc−/− (n=1–3 for growth, 7 for survival), and Nlrp3A350V/−/CreL (n=l–3 for growth, 7 for survival). (B) Survival (top) and growth (bottom) of Nlrp3L351P/+/CreL/Asc−/− (n=13 for survival, 2–3 for growth). Error bars shown for mean daily weights on growth curves are SDev.
The cryopyrin inflammasome also includes caspase-1 and the adaptor proteins, ASC and CARDINAL (Agostini et al., 2004). It is hypothesized that CAPS mutations render the inflammasome constitutively active, independent of PAMP or DAMP sensing, leading to enhanced activation of caspase-1 and over-production of IL-1β. To determine whether the inflammasome is necessary for the phenotype in Nlrp3A350V/+/CreL and perinatal lethality in Nlrp3L351P/+/CreL, we mated Nlrp3A350VneoR/+ and Nlrp3L351PneoR/+ mice to Asc−/− mice (Hoffman et al., 2008; Kanneganti et al., 2006), and then mated to CreL mice. Nlrp3A350V/+/CreL/Asc+/− mice exhibited survival kinetics similar to Nlrp3A350V/+/CreL on an Asc-intact background, and Nlrp3L351P/+/CreL/Asc+/− mice were not observed, suggesting one Asc allele is sufficient for disease (data not shown). However, Nlrp3A350V/+/CreL/Asc−/− and Nlrp3L351P/+/CreL/Asc−/− mice appeared phenotypically normal and grew at a rate comparable to WT siblings (Figure 6A and B). Taken together, these results indicate both cryopyrin mutants exert their effects via the inflammasome.
Molecular models and studies with recombinant protein and transfection systems suggest cryopyrin self-associates (Aksentijevich et al., 2007; Duncan et al., 2007). Since CAPS is dominantly inherited with expression of both WT and mutant alleles in all patients, WT cryopyrin may be necessary for inflammasome oligomerization and disease phenotype. To determine the role of WT cryopyrin in the pathology of Nlrp3A350V/+/CreL mice, we bred Nlrp3A350VneoR/+ to an Nlrp3−/− background (Kanneganti et al., 2006; Sutterwala et al., 2006) and then bred to CreL. The growth rate and survival of mice hemizygous for cryopyrin A350V (Nlrp3A350V/−/CreL) was the same or decreased compared to that of Nlrp3A350V/+/CreL, indicating that WT protein is not required for murine disease (Figure 6A and Figure 1B).
DISCUSSION
We used CAPS mutant knock-in mice expressing MWS and FCAS mutations A350V and L351P to demonstrate a pivotal, but not exclusive, role for IL-1β in the CAPS autoinflammatory disease spectrum. Further, we show that murine disease is a genuine inflammasomopathy solely dependent on the innate immune system and an intact inflammasome. While an adaptive response occurs, it is not an important factor for the early mortality and severe inflammation observed in these mice. Although our mice have a more severe phenotype than MWS and FCAS patients, we nonetheless consider them good models for human disease, as neutrophils are the predominant cell type and IL-1β and IL-6 upregulated in our mice are also upregulated in CAPS patients. At a cellular level, myeloid cells from mice are similar to patient monocytes, as these cell types show hyperresponsiveness to LPS independent of ATP treatment. Additionally, both FCAS patient cells and cells from the Nlrp3L351P/+/CreT line secrete IL-1β when exposed to 32°C.
Although FCAS is generally considered a milder form of CAPS than MWS, expression of L351P resulted in perinatal or presumed intrauterine mortality. The inflammatory phenotype observed in A350V-expressing mice is usually apparent the day of birth, however some pups could not be distinguished from WT littermates the following day, suggesting the phenotype may begin at birth or shortly after. An explanation for why the L351P FCAS mouse phenotype is more severe could be provided by in vitro studies, in which we showed Nlrp3L351P/+/CreT BMDC had increased spontaneous IL-1β secretion compared to Nlrp3A350V/+/CreT, which is also observed in patient monocytes in this study. If this spontaneous secretion occurs in vivo, the inflammatory phenotype may begin before birth. It is also possible that exposure to room temperature at birth could result in perinatal mortality in Nlrp3L351P/+/CreL mice.
IL-1β inhibition treatment generally resolves symptoms and normalizes laboratory evidence of inflammation in most CAPS patients (Goldbach-Mansky et al., 2006; Hoffman et al., 2004; Hoffman et al., 2008), however mouse IL-1 Trap only extended Nlrp3A350V/+/CreL survival a few days, with no improvement in skin findings or growth. No pharmacokinetic information for mIL-1 Trap is available making it possible that drug concentrations in tissues do not reach therapeutic levels. It is also possible that initiation of mIL-1 Trap therapy in the first few days of life is too late. Treatment of pregnant mice did not have any effect on the phenotype of pups (unpublished observation), however it is also not known whether mIL-1 Trap crosses the placenta.
The increased life span of Nlrp3A350V/+/CreL/Il-1r−/− and live birth of Nlrp3L351/+/CreL/Il-1r−/− is definitive proof of IL-1β’s central role in this murine disease, however the residual phenotype in Nlrp3A350V/+/CreL/Il-1r−/− and early mortality in most Nlrp3L351P/+/CreL/Il-1r−/− mice implicate IL-1β-independent pathways. Il-1r−/− prevents IL-1β signaling, but does not affect the release of IL-1β or other caspase-1-activated cytokines, such as IL-18 and IL-33. Serum IL-lβ and IL-18 levels remained elevated in the Nlrp3A350V/+/CreL/Il-1r−/− mice, as expected, but most of the other upregulated cytokines were normalized, although mild increases in others were still evident (unpublished observation), perhaps because these are IL-18/IL-33-dependent. It is possible that treatment with anti-IL-18 or soluble ST2 (IL-33) receptor will rescue the residual phenotype (Kakkar and Lee, 2008).
Crossing onto an Asc−/− background completely rescued the phenotype for both mutations, providing definitive proof that these mutations are true gain-of-function lesions acting through the inflammasome. Studies in overexpression systems suggest high levels of cryopyrin cause spontaneous secretion of IL-1β (Agostini et al., 2004; Dowds et al., 2004) and therefore it may be possible that CAPS manifestations are due to increased expression of cryopyrin. We were unable to measure cryopyrin protein levels endogenously, as the protein is not highly expressed and specific antisera are lacking. Preliminary qRTPCR analysis of cryopyrin mRNA in BMDC did not show a consistent increase in mutants (unpublished observation), although it is possible cryopyrin protein is upregulated without an increase in message. A second hypothesis is that CAPS mutations affect the stability of cryopyrin, perhaps increasing its half-life. However, preliminary kinetic experiments using metabolic labeling to follow tagged, overexpressed protein suggest that the half-life of L353P mutant cryopyrin is shorter than that of normal protein at 37°C.
The mechanism behind the cold-induced IL-1β release in FCAS patient monocytes and nlrp3L351P/+/CreT BMDC is not well-understood. Mechanisms proposed for other cold sensitive phenotypes are temperature induced macromolecular changes that affect protein function or intramolecular interactions (Pain, 1987), or membrane alterations affecting fluidity (Pain, 1987; Thieringer et al., 1998). It is also possible that cold temperatures could affect the expression or stability of inflammasome components. Metabolic labeling assays at 32°C demonstrate an increase in the half life of L353P-mutated cryopyrin, suggesting that lower temperatures stabilize protein that would normally be degraded at 37°C. Further studies are needed to elucidate the mechanisms involved.
Fulminant disease arising from mutant expression in the myeloid lineage alone strongly implies these cells are sufficient for disease. However, subtle differences in survival kinetics between the nlrp3A350V/+/CreL and nlrp3A350V/+/CreZ lines were noted after many litters, indicating non-myeloid cells may impact survival kinetics. Cryopyrin is mainly expressed hematopoietically, suggesting this unidentified cell subset(s) is in the immune lineage, although some expression has been demonstrated in various types of epithelial cells as well (Kummer et al., 2007). An ongoing T cell process in mutant mice is likely, as BMDC were primed to direct a Th17 phenotype in naive T cells. This is perhaps unsurprising, as stimulated BMDC and mutant mouse serum contained high levels of IL-1β, a cytokine shown to be critically important to the Th17 phenotype in humans (Chen and O'Shea, 2008) and in mice (Chung et al., 2009). Our preliminary results using either pharmacological IL-1β blockade or the Il-1r−/− background abrogate the Th17 phenotype directed by Nlrp3A350V/+/CreT BMDC (unpublished observation).
Although intriguing, the physiological relevance of the increased IL-17-positive T cells incubated with Nlrp3A350V/+/CreT BMDC is difficult to ascertain. Although KC, IL-6, and GCSF, all Thl7-associated cytokines (Ouyang et al., 2008), were highly upregulated in serum, IL-17 itself was not. There was also no increase in the skin or spleen of mRNA for IL-17 or IL-22, another Th17-associated cytokine (unpublished observation). The contribution of any T cell response to disease in cryopyrin mutant mice appears to be minimal. Breeding the mutation onto the Rag1−/− background demonstrated that T cells were not necessary for inflammatory signs or mortality, suggesting that it is the myeloid lineage alone and not its downstream effects on the adaptive response that causes murine disease.
In conclusion, we have established mouse models for CAPS, autoinflammatory syndromes arising from gain-of-function mutations in cryopyrin. Although knock-out mouse models for Mendelian autoinflammatory genes have been published, our study is one of the first to specifically examine disease-causing mutations in the context of a knock-in mouse. As a model of inflammasome dysregulation, these mice will be important in elucidating the roles of the other caspase-1-dependent cytokines, as well as testing novel therapeutic compounds. Given the importance of IL-1β and the inflammasome to innate immunity, cryopyrin knock-in mice may be applied to the study of many diseases along the autoinflammatory-autoimmune spectrum.
EXPERIMENTAL PROCEDURES
Targeting plasmid construction
The Nlrp3 knock-in targeting constructs pPNTlox2PNlrp3A350V and pPNTlox2PNlrp3L351P were created as follows. Briefly, 4–7 kb regions directly upstream and downstream of a targeted position in intron 2 were cloned around the neoR antibiotic resistance cassette in plasmid pPNTlox2P (Fig S1B). The alanine 350 to valine and leucine 351 to proline point mutations in the 3’ flank were engineered by site-directed mutagenesis using a Stratagene Quik-change kit.
Knock-in mouse generation
129 SvJ stem cells were electroporated with linearized pPNTlox2PNlrp3A350V or pPNTlox2PNlrp3L351P via an established protocol by the UCSD mouse transgenic core. Colonies heterozygous for A350V or L351P were selected based upon Southern analysis and sequencing. Cre recombinase-mediated excision of the neoR cassette and subsequent expression of the mutant allele was confirmed by PCR and RT-PCR analysis, followed by sequencing. Subsequent mouse genotyping was performed using PCR.
Mouse strains
The following mouse strains were used in this study: FVB/N-Tg(Zp3-Cre)3Mrt/J (embryonic Cre expression; obtained from D. Cleveland (LICR) with permission of G. Martin (UCSF)); B6.129P2-Lyz2tm1(Cre)Ifo/J (myeloid Cre), B6.Cg-Tg(Cre/Esr1)5Amc/J (tamoxifen-inducible Cre), B6;129S1-IL-1r1tm1Roml/J (Il-1r−/−), B6.129S7-Rag1tm1Mom/J (Rag1−/−), C57BL/6-Tg(TcraTcrb)425Cbn/J (OT-II), from Jackson Labs; Asc−/−, Nlrp3−/− (provided by J. Bertin, E. Grant, A. Coyle, and Millennium pharmaceuticals). Mice were cared for in accordance with appropriate institutional guidelines. The experimental protocols were approved by the UCSD Institutional Animal Care and Use Committee.
Peripheral blood analysis
Peripheral blood from 6–9 day old pups was obtained following decapitation. Complete blood counts (CBCs) were performed by the UCSD ACP Diagnostic Laboratory. Serum was analyzed by Luminex assay (Bio-Rad) according to the manufacturer’s instructions.
Histologic analysis
Mouse skin biopsy samples were fixed in 4% paraformaldehyde, embedded in paraffin, sectioned, mounted on slides, deparaffinized and rehydrated before analysis. Sections were subjected to staining with anti-IL-6 (AF-406-NA) at 1:10 and anti-IL-1β (AF-401-NA) at 1:50 (R & D) for tissue cytokine analysis, followed by secondary antibody and haematoxylin staining. (Hoffman et al., 2004)
Treatment with mIL-1 Trap
100–200 ug/g mIL-1 Trap (provided by R. Torres and Regeneron Pharmaceuticals) or diluent was injected subcutaneously in the dorsum of Nlrp3A350V/+CreL pups and wild type littermates every other day beginning within the first 2–3 days of life and continuing until death.
In vitro studies
Bone marrow cells from WT, Nlrp3A350V/+/CreT, and Nlrp3L351P/+/CreT adult mice were plated in DMEM supplemented with 10% fetal calf serum, penicillin/streptomycin, sodium pyruvate, and L-glutamate at 4×l05 cells/well in 96 well culture plates with GMCSF (20 ng/ml) (R and D). After 1 week, cells were exposed to 1 µM 4-hydroxytamoxifen (Sigma) for 48 hrs to stimulate in vitro excision of neoR. For cold exposure, cells were incubated at 32°C overnight and supernatants were collected. For LPS stimulation, cells were cultured either overnight (crude LPS (Calbiochem)) or for 4 hrs (pure LPS (Alexis Biochemicals)), followed by incubation with 5 mM ATP for 30 minutes. Peritoneal macrophages were plated at 5×104 cells/well in 96 well culture plates and incubated overnight, followed by treatment with tamoxifen and stimulation as described above.
Human peripheral blood mononuclear cells were isolated by Ficoll gradient, followed by hypotonic lysis to remove erythrocytes. PBMC were then plated at 105 cells/well in media without serum in a 96 well plate and monocytes were allowed to adhere three hrs at 37°C before the supernatant was replaced with media with 10% FCS. For cold-induced secretion studies, cells were incubated either 4 hrs or overnight at 32 and 37°C. For LPS stimulation, cells were stimulated with 100 ng/ml LPS overnight, followed by treatment with 5 mM ATP for 30 min. Secreted IL-1β was measured by ELISA (R and D). All human subject protocols were approved by the institutional review board of the National Instititute of Arthritis, Musculoskeletal, and Skin Diseases.
OT II T cell and BMDC coculture
To assess surface expression of MHC class II, CD40, and OX40L, BMDC were stimulated 24 hrs with LPS and subjected to FACS analysis as described below. For coculture experiments, WT or Nlrp3A350V/+/CreT tamoxifen-treated BMDC were incubated with a ten-fold excess of OT II naïve CD4+ cells and varying amounts of ovalbumin peptide (Peptides International). Cells were left untreated or were stimulated with various cytokines and anti-cytokine antibodies to direct distinct T helper lineages as follows: Th1: IL-12 and anti IL-4; Th2: IL-4 and anti-IFNγ; Thl7: TGFβ and IL-6 plus anti-IL-2, anti-IL-4, and anti-IFNγ. Antibodies were used at 10 µg/ml and cytokines were used at 5–20 ng/ml. On day 3, cells were re-stimulated with PMA (50 ng/ml), ionomycin (500 ng/ml) and 1:2000 diluted Golgi plug (BD Pharmingen) for 4 hrs, followed by subsequent FACS analysis for intracellular cytokines as below.
Flow cytometry
BMDC, cocultured OT II cells, or splenocytes were prepared, fixed, and stained with PE, FITC, or APC-labeled monoclonal antibodies (BD Pharmingen or EBioscience). Samples were acquired with a FACSCaliber (BD), followed by analysis with FlowJo software.
Statistical analyses
Statistical analyses and graphing were performed in Excel and Graphpad Prism programs using the Student’s T test (Luminex analysis) and the log-rank (Mantel-Cox) test (survival curve comparisons).
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank R. Goldbach-Mansky for providing blood samples from CAPS patients, J. Hillman for luminex analysis, N. Ng and S. Lee for FACS analysis, and J.Y. Cho and S. Aceves for immunohistochemistry support. We also appreciate helpful advice from D. Cleveland, A. Silk, M. McAlonis-Downes, R. Kolodner, A. Laurence, M. Croft, T. Doherty, and D. Broide. The UCSD Cancer Center mouse transgenic and embryonic stem cell core (E. Kothari) and histology core (N. Varki) provided support, and the Ludwig Institute of Cancer Research San Diego branch provided DNA sequencing support. This work was funded by NIH RO1-AI52430 and NIAMS. Dr. Hoffman has received consultant fees from Regeneron and Novartis Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL DATA
Supplemental data include 3 figures.
REFERENCES
- Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- Chae JJ, Komarow HD, Cheng J, Wood G, Raben N, Liu PP, Kastner DL. Targeted disruption of pyrin, the FMF protein, causes heightened sensitivity to endotoxin and a defect in macrophage apoptosis. Mol Cell. 2003;11:591–604. doi: 10.1016/s1097-2765(03)00056-x. [DOI] [PubMed] [Google Scholar]
- Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical Regulation of Early Thl7 Cell Differentiation by Interleukin-1 Signaling. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate Immune Activation Through Nalp3 Inflammasome Sensing of Asbestos and Silica. Science. 2008 doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaccum MB, Stocking KL, Charrier K, Smith JL, Willis CR, Maliszewski C, Livingston DJ, Peschon JJ, Morrissey PJ. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J Immunol. 1997;159:3364–3371. [PubMed] [Google Scholar]
- Hoffman HM, Rosengren S, Boyle DL, Cho JY, Nayar J, Mueller JL, Anderson JP, Wanderer AA, Firestein GS. Prevention of cold-associated acute inflammation in familial cold autoinflammatory syndrome by interleukin-1 receptor antagonist. Lancet. 2004;364:1779–1785. doi: 10.1016/S0140-6736(04)17401-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman HM, Throne ML, Amar NJ, Sebai M, Kivitz AJ, Kavanaugh A, Weinstein SP, Belomestnov P, Yancopoulos GD, Stahl N, Mellis SJ. Efficacy and safety of rilonacept (interleukin-1 Trap) in patients with cryopyrin-associated periodic syndromes: results from two sequential placebo-controlled studies. Arthritis Rheum. 2008;58:2443–2452. doi: 10.1002/art.23687. [DOI] [PubMed] [Google Scholar]
- Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7:827–840. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- Kummer JA, Broekhuizen R, Everett H, Agostini L, Kuijk L, Martinon F, van Bruggen R, Tschopp J. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem. 2007;55:443–452. doi: 10.1369/jhc.6A7101.2006. [DOI] [PubMed] [Google Scholar]
- Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- Martinon F, Tschopp J. NLRs join TLRs as innate sensors of pathogens. Trends Immunol. 2005;26:447–454. doi: 10.1016/j.it.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain RH. Temperature and macromolecular structure and function. Symp Soc Exp Biol. 1987;47:21–33. [PubMed] [Google Scholar]
- Stehlik C, Lee SH, Dorfleutner A, Stassinopoulos A, Sagara J, Reed JC. Apoptosis-associated speck-like protein containing a caspase recruitment domain is a regulator of procaspase-1 activation. J Immunol. 2003;171:6154–6163. doi: 10.4049/jimmunol.171.11.6154. [DOI] [PubMed] [Google Scholar]
- Thieringer HA, Jones PG, Inouye M. Cold shock and adaptation. BioEssays. 1998;20:49–57. doi: 10.1002/(SICI)1521-1878(199801)20:1<49::AID-BIES8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Wise CA, Gillum JD, Seidman CE, Lindor NM, Veile R, Bashiardes S, Lovett M. Mutations in CD2BP1 disrupt binding to PTP PEST and are responsible for PAPA syndrome, an autoinflammatory disorder. Hum Mol Genet. 2002;11:961–969. doi: 10.1093/hmg/11.8.961. [DOI] [PubMed] [Google Scholar]
- Yu JW, Fernandes-Alnemri T, Datta P, Wu J, Juliana C, Solorzano L, McCormick M, Zhang Z, Alnemri ES. Pyrin activates the ASC pyroptosome in response to engagement by autoinflammatory PSTPIP1 mutants. Mol Cell. 2007;28:214–227. doi: 10.1016/j.molcel.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.