Abstract
The inflammatory response is critical for our ability to heal wounds and fight off foreign microorganisms. Uncontrolled inflammation is also at the root of most pathology. Recruitment of leukocytes to the site of inflammation plays a defining role in the inflammatory response, and migration of leukocytes across endothelium is arguably the point of no return of the inflammatory response. Assays to study the transmigration of leukocytes have and will continue to shed light on the regulation of this vital response. Assays of transendothelial migration in vitro allow the controlled observation of this phenomenon as well as experiments to study its regulation. In this chapter we describe in vitro assays of transendothelial migration that have been used successfully in the authors’ laboratories for decades and have proven to be reproducible, reliable, and predictive of the behavior of leukocytes and endothelial cells in models of inflammation in vivo.
Introduction
Leukocyte emigration, a critical step in the inflammatory response, involves a sequential series of leukocyte-endothelial interactions of increasing strength that serve to arrest the leukocyte on the endothelium at the site of inflammation (Butcher, 1991) that it may subsequently enter the tissues. Transendothelial migration, or diapedesis, is the step in leukocyte extravasation in which the leukocyte squeezes in ameboid fashion across the endothelial cell, usually passing between tightly apposing endothelial cells at their borders (Muller, 2003) or under certain conditions, leukocytes pass through the endothelial cell (reviewed in (Rao et al., 2007)). There are many ways to model interactions of leukocytes with endothelial cells in vitro. While none of these perfectly recapitulates conditions of the site of inflammation in vivo, they do permit a reductionist approach that allows direct observation, quantitative analysis, and manipulation in ways that are currently impossible to attain in the living organism. Furthermore, since human leukocytes and endothelial cells are easily prepared, these methods allow direct experimentation with the actual molecules that would be relevant in vivo.
This chapter outlines the assays we have used successfully over the past decades for studying transendothelial migration under static and shear flow conditions. These are by no means the only available methods. However, they have proven reliable, reproducible, and predictive of results later obtained in vivo. We also provide methods for the isolation of leukocytes and preparation of the endothelial cells and substrata for these assays.
Methods for investigating leukocyte transendothelial migration under static or flow conditions in vitro
Transendothelial migration assays carried out under static conditions have become very popular due to the ease with which they can be performed. While the static model described lacks the physiologic variable of fluid shear, it has the advantage that the endothelial cells are attached to a physiologic basement membrane—a factor that significantly affects endothelial phenotype and may be of even greater importance for the diapedesis event itself. It is suitable for testing multiple variables (e.g. blocking antibodies or inhibitors) at a single time and can even be adapted for high throughput screening.
The method described here uses endothelial cells plated on hydrated three-dimensional collagen gels generally in 96-well tray format (Muller and Weigl, 1992) and grown to confluence in normal adult human serum in the absence of endogenous growth factors (See figure 1.) Under these conditions endothelial cells grow slowly and show better growth arrest at confluence, better representing the quiescent endothelium lining postcapillary venules at a site of inflammation than a rapidly proliferating monolayer driven by endothelial growth factors.
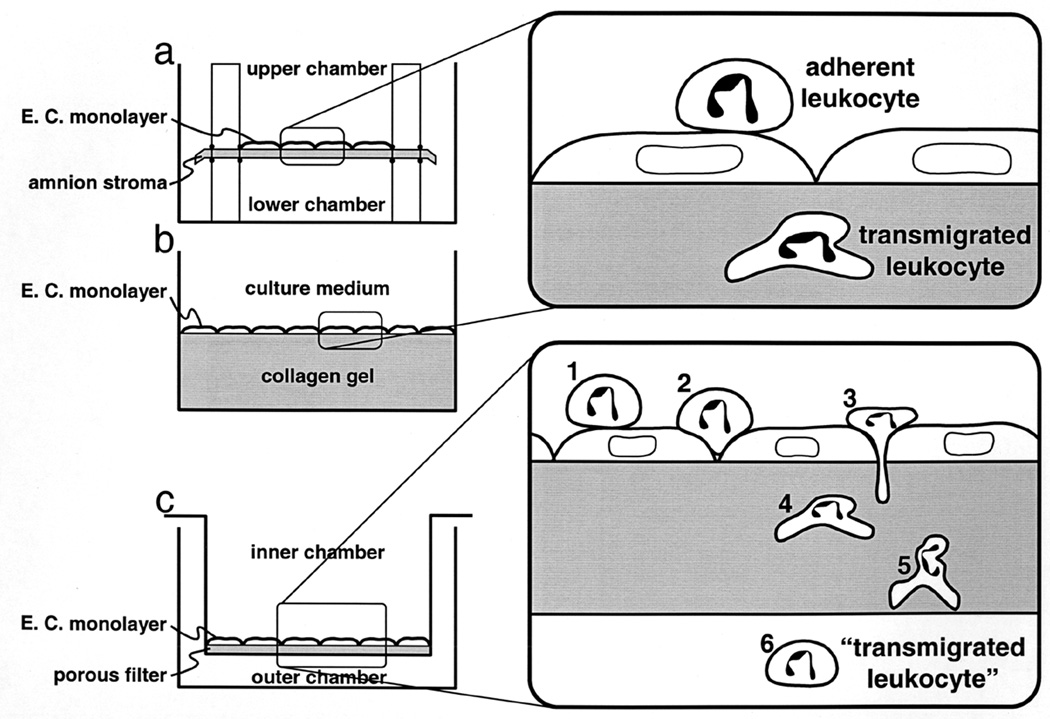
Figure 1.
Schematic diagram of three in vitro assays of transendothelial migration (not drawn to scale.). (a) Endothelial cells (E.C.) cultured on the upper surface of a sheet of amniotic stroma (Furie et al., 1987) that separates two fluid-filled chambers. (b) Endothelial cells cultured on hydrated type I collagen gels overlaid with fibronectin (Muller and Weigl, 1992). Components of the culture medium penetrate into the porous gel. (c) Endothelial cells grown on the upper surface of a porous polycarbonate filter suspended in a larger culture vessel. Culture medium is placed in the inner and outer chambers to reach the apical and basal surfaces of the monolayer, respectively. In methods (a) and (b) adherent leukocytes remaining on the apical surface can be distinguished visually from those that have transmigrated. Procedures have also been devised to strip off any leukocytes remaining bound to the apical surface of the monolayer (Muller and Weigl, 1992). In the filter-chamber method (c), the percentage of the leukocytes added to the upper chamber which appear in the lower chamber is calculated by direct counting. This is technically much easier and faster. However, in order to be counted as “transmigrated,” a leukocyte must 1) attach to the apical surface of the endothelium, 2) migrate to the intercellular junction, 3) diapedese between the endothelial cells, 4) detach from the endothelial cells and penetrate their basal lamina, 5) crawl through the filter itself, and 6) detach from the filter and fall into the chamber below. Reagents that block any of these steps will therefore block the readout of transmigration in this system. (This figure is reproduced from Ref. (Muller, 1996) by permission of R.G. Landes Company.)
A variation of this method has been used to study leukocyte adhesion using fluorescently or radioactively labeled leukocytes (Muller and Weigl, 1992). While this allows rapid assessment of how many leukocytes firmly adhered to the endothelium during the assay, it does not distinguish between those leukocytes that are firmly bound on the apical surface and those that have transmigrated. This is a problem, for example, when assessing the effects of blocking PECAM or CD99 where the arrested leukocytes are firmly adherent but do not migrate across the endothelial monolayer. Thus, we rely on microscopic analysis of the monolayers to distinguish the step in transmigration at which cells are arrested. This is a more tedious analysis, but has allowed us to distinguish that interfering with PECAM homophilic interactions arrests leukocytes at the apical surface of the endothelial cells over the cell borders, whereas blocking heterophilic interaction of leukocyte PECAM domain 6 arrests the leukocyte between the basal surface of the endothelial cell and the basement membrane (Liao et al., 1995), as well as to realize that blocking CD99 interactions arrests leukocytes partway through the endothelial junctions (Lou et al., 2007; Schenkel et al., 2002).
Assays using Transwells® are popular due to their ease of use. These are Boyden chamber-like devices that sit in a tissue culture well. Endothelial cells are grown on top of a porous filter that separates two fluid compartments. Leukocytes are added to the upper chamber and a chemoattractant to the lower chamber. Leukocytes migrate across the endothelial cells, though the filter, and are recovered from the lower chamber and counted. However, due to their cost, analyzing multiple samples simultaneously can be expensive. We have not used this assay extensively because we find that with prolonged culture endothelial cells grow into the pores, complicating analysis of the assay. In addition, the monolayers are difficult to visualize on these filters before fixation and staining. Most relevant, if one is assessing the regulation of transmigration, recovering fewer leukocytes from the lower well does not distinguish among the many potential steps at which the block might occur as described in (Muller, 2001) (See figure 1.)
Cultured endothelial cells produce chemokines and other leukocyte chemoattractants at low levels, even in the absence of activation by inflammatory cytokines (Muller and Weigl, 1992). These accumulate in the culture medium and in the underlying collagen matrix. When the overlying culture medium is washed away the chemoattractants in the gel diffuse out creating a chemotactic gradient for the leukocytes to follow into the collagen gel. Activation of endothelial cells with appropriate proinflammatory cytokines (e.g. IL-1β, TNF-α) or bacterial endotoxin (lipopolysaccharide, LPS) induces the expression of additional adhesion molecules and chemokines that enhance the activation of leukocyte adhesion and decrease the time it will take for a population of leukocytes to migrate across the endothelial monolayer.
By forming collagen gels within cloning cylinders on top of coverslips, the static transmigration assay has been adapted to allow visualization of cells in the acts of adhesion, locomotion, and transmigration (Lou et al., 2007; Schenkel et al., 2004). However, these assays still lack fluid shear, which is particularly important for the initial adhesion step.
Transmigration assays carried out under flow conditions mimic in vivo fluid dynamics within blood vessels of the microcirculation. In these assays transendothelial migration is examined in the context of the multistep adhesion cascade where leukocytes undergo initial attachment and rolling (step 1) and arrest and spreading (step 2) prior to transendothelial migration (step 3) (reviewed in (Butcher, 1991)). Hence, the biochemical and biophysical processes that govern these steps can be fruitfully investigated using in vitro flow chamber assays. The parallel plate flow chamber is the design most frequently used for this purpose and is the design used in the authors lab (Goetz et al., 1999; Lawrence et al., 1987; Luscinskas et al., 1994). A commercially produced flow chamber (GlycoTech Inc, Rockville MD; http://www.glycotech.com/apparatus/parallel.html is manufactured in two configurations. Although we have not used this system, it has been used fruitfully by several laboratories to study leukocyte – endothelial cell interactions (see (Brown and Larson, 2001) and references therein). We have designed and manufactured a parallel plate chamber and recently reported the details of this chamber (Goetz et al., 1999). In our lab’s flow chamber leukocyte – endothelial cell adhesive events are monitored in live time and recorded to video tape or by digital videomicroscopy to a PC for later analysis (Lim et al., 2003). A recent advancement is the use of time lapse digital microscopy in combination with fluorescence microscopy. This has allowed us to investigate of EGFP-tagged molecules transfected into leukocytes or endothelium during the process of leukocyte adhesion and transendothelial migration (Shaw et al., 2001; Shaw et al., 2004).
The most important advantage of the flow assay system is that it mimics in vivo flow and hence allows one to study the distinct steps of leukocyte initial attachment, rolling, arrest, and transmigration. Second, certain cell types such as unfractionated T cells and eosinophils (Cinamon et al., 2001; Cuvelier et al., 2005) transmigrate significantly better in the presence of shear flow and preincubation of the monolayers with apical chemokines. (However, in vitro activated memory T lymphoblasts migrate efficiently under static conditions (Carman et al., 2007).)
Those investigators unable to commit the time and resources to the setup and maintenance of a flow chamber system for studies of transendothelial migration need not be too upset. Everything that we have published using the static transmigration system described here has been borne out in vivo. This includes the role of Mac-1 in locomotion (Phillipson et al., 2006; Schenkel et al., 2004), roles for PECAM (Liao et al., 1997; Liao et al., 1999; Muller et al., 1993) and CD99 (Bixel et al., 2004; Bixel et al., 2007; Schenkel et al., 2002) in transmigration, but not adhesion, and the effects of prolonged (days) culture of transmigrated monocytes in our system on their differentiation into immature dendritic cells and monocytes (Randolph et al., 1998; Randolph et al., 1999). Furthermore, studies on transmigration of myeloid cells show that flow may increase the rate of transmigration quantitatively, but does not change any aspects qualitatively (Cuvelier and Patel, 2001; Kitayama et al., 2000).
In spite of their broad applicability for study of molecular mechanism and biophysical features of transmigration the flow chamber set up described has some disadvantages. First, most flow studies use endothelial cells conditioned in low physiological shear (~2 dynes/cm2) for a very short time (minutes) so that the endothelium has not be pre-conditioned to physiological levels of shear stress. Although studies have shown that junctional complexes are reorganized in the early hours after the application of 10 – 12 dynes/cm2 sheer stress and do not reestablish equilibrium for two days (Noria et al., 1999), no information is available on the status of junctional complexes after short application of low shear stress. Second, there is much evidence to suggest that interactions with the basement membrane are at least as important to the physiology of the endothelium as flow along the apical surface. In the static collagen gels system endothelial cells are grown on a three-dimensional fibrillar matrix, with an endothelial-derived basement membrane. Cells grown on glass coverslips clearly lack this. Third there is a limited number of samples that can be simultaneously tested and complete analysis of rolling speed, adhesion and TEM is time consuming, which reduces the usefulness for large-scale drug screening.
Preparation of Hydrated Collagen Gels for Transmigration Assays
Reference: (Muller et al., 1989)
REAGENTS
Vitrogen 100 (95–98% bovine Type I collagen) from Cohesion Corp., Palo Alto (877) 264-3746
10× Medium 199
0.1N NaOH (sterile filtered)
PROCEDURE
Keep all ingredients cold (4°C). Work in laminar flow hood. Keep the tube in which the reagents are mixed on ice in the hood until you are able to perform all manipulations sufficiently quickly. However, polymerization of collagen gels is not nearly as rapid as solidification of Matrigel®, for example.
-
Mix the ingredients in the following proportions:
8ml Vitrogen
1ml 10× M199 or 10× Dulbecco’s phosphate buffered saline
5ml 0.1N NaOH
Mix well, avoid making air bubbles. The mixture should have a magenta color from the phenol red. The pH is 9–10. (See note 1.)
-
Pipette the desired volume of collagen solution into the culture vessel:
6mm well (96 well microtiter) 50 µl/well 16mm well (24 well cluster) 300 µl/well 30m dish ml/dish 100mm dish ml/dish For smaller volumes automatic repeating pipetters work well. Pipette slowly, since the solution is viscous, to make sure that the proper volume is dispensed. (See notes 2 and 3.)
Place collagen coated dishes in 37° C incubator for about an hour.
The gelled collagen should still be pink from the phenol red and have a whitish tone.
Cover with ≥2 volumes of M199 (1×) or culture medium and let stand overnight in the tissue culture incubator to equilibrate with the gel. In this way the gel and the absorbed medium is at pH 7.4 when the cells are added. (See note 4.)
We routinely coat the gels with human fibronectin (the same as would be used to coat a tissue culture dish) to get somewhat better plating efficiency. Alternatively, let the gels stand in culture medium with human serum for an hour or so before plating the cells. In our experience commercial sources of gelatin contain levels of endotoxin (LPS) too high to use with endothelial cells and leukocytes to avoid unintentional activation.
We grow HUVEC on these gels in M199 + 20% normal adult human serum without added growth factors. HUVEC grow slowly under these conditions. (This is what you want: A model of a stable postcapillary endothelium with a low turnover rate.) For best results don’t expand cultures when transferring to the gels (i.e., split 1:1). We have split 1:2 and 1:3 with good results at times, but inconsistently. For example, resuspend the HUVEC to 3 × 105 cells/ml and plate 100 µl in each well on a 96-well plate. (See note 5.)
NOTES
The optical properties of the gel are better if it polymerizes at an alkaline pH. The phenol red will indicate an alkaline color; this is OK. There is plenty of time to neutralize the gel later. We have tested it extensively, and there are no physiologic or biochemical differences between HUVEC grown on collagen that gels at alkaline pH vs. neutral pH, as long as the gels are allowed to equilibrate with neutral pH solution later, as described below. However, if you wish to incorporate a pH-sensitive bioactive molecule into the collagen prior to polymerization, make up the collagen at pH 7.4 using 1 part (1 ml in the recipe above) NaOH.
If you are plating in larger dishes (e.g. 30 mm dishes or larger), wash the culture vessels with sterile fluid (PBS, Hanks’, etc.) to wet the bottoms and allow for even spreading of the collagen. Aspirate this just before adding collagen.
Most of our experience is with gels made in microtiter wells. We fill the center 60 wells leaving the outer perimeter blank.
The company that sells Vitrogen has turned over many times in the past two decades. While the product appears to be consistent, we have noticed some important differences. In the 1980s and 1990s HUVEC plated on collagen gels after overnight equilibration in M199 grew well. Starting in 2000 we noticed that the gels had to equilibrate for several days in order to get the best monolayers. (Between 4 days and 3 weeks did not seem to make a difference.) Starting with lots received in 2005, we have again been able to get nice monolayers on gels equilibrated only overnight. We recommend that you test this timing when you first try plating your cells. If you allow them to equilibrate for several days or more, we have found that placing water or medium in the bare wells surrounding the ones containing the gels will keep the atmosphere in the tray humid enough so that the outer wells will not dry out.
When growing cells on collagen gels in microtiter trays, visualizing the cells by routine inverted phase contrast microscopy is difficult, since the light bouncing off the sides of the well distorts the optics. Only a small circle of cells in the center of the field will be in good phase relief. Once the monolayer is confluent, however, occasional cells that do not cease dividing will be forced out of the monolayer where they will undergo apoptosis. When we see a few round, granular, apoptotic cells on top of our otherwise smooth monolayer, we can tell that the HUVEC have been confluent for several days and are ready to use in transmigration assays. The apoptotic cells wash off easily and do not interfere with the assays.
Isolation of Peripheral Blood Mononuclear Cells (PBMC)
Reference: (Muller and Weigl, 1992)
This assay is an adaptation of the standard Ficoll-Hypaque procedure optimized to remove platelets that tend to stick to and co-purify with monocytes. It was initially developed for experiments to study the interaction of monocytes with unactivated endothelial cells. Under the conditions of the transmigration assay (see below) lymphocytes do not adhere efficiently to unactivated endothelial monolayers and the few that do adhere do not transmigrate efficiently within the assay time period (20 – 60 minutes). Therefore, the endothelial cells do a better job of purifying monocytes than any man-made purification scheme (Muller and Weigl, 1992). Monocytes can also be purified by negative selection using antibody cocktails and magnetic bead columns; however, one runs the risk of activating them. Unactivated monocytes will not adhere to the sides of the centrifuge tubes (see step 17 below). For more quantitative assessment, we examine CD62L and CD11b expression by flow cytometry. The former should remain high and the latter should not increase during the isolation. A positive control for activation can be obtained by deliberately activating the monocytes with fMLP, etc. for comparison.
REAGENTS
Ficoll-Paque Plus [endotoxin free] (Pharmacia) 10–15 ml per 20 ml blood, room temperature.
Hanks’ Balanced Salt Solution without divalent cations (HBSS)
EDTA (2.7% = 100 mM) pH 7.4 One ml per 10 ml of blood.
Bent, siliconized Pasteur pipettes. (See note 1.)
Hemacytometer
Human serum albumin (HSA), endotoxin-free and suitable for infusion into humans. This can be obtained from a local blood bank or hospital pharmacy.
HBSS + 0.1% human serum albumin (HBSS/HSA) at 4°C.
Medium 199 + 0.1% human serum albumin (M199/HSA) at 4°C.
PROCEDURE
Place 12–15 ml of Ficoll-Paque at the bottom of a 50 ml conical tube. Leave enough room for 40 ml of blood/HBSS. Make one gradient for every 20 ml of blood to be drawn.
Load a syringe with 1 ml EDTA for every 10 ml blood to be drawn. (See note 2) Draw blood into this syringe.
Mix blood/EDTA gently with an equal volume of warm HBSS.
Carefully layer the mixture over the Ficoll retaining a crisp interface. Running the mixture slowly down the side of the tube is a good way to avoid disturbing the interface.
Centrifuge in at 2200 rpm (~ 1,000 × g), 20 minutes, room temperature with the brake OFF. At the end of the spin there should be an upper clear orange layer (plasma + HBSS/HSA), a cloudy interface containing PBMC, a clear band of Ficoll-Paque below that, and a pellet of RBC and PMN at the bottom.
Remove ≥ 12 ml of plasma from the top of the tube. Transfer it to a 15 ml conical tube on ice. (The plasma will be used in a later step for washing the monocytes.)
Rinse the Pasteur pipette several times with HBSS/HSA by pipetting the liquid in and out. Discard the liquid.
Rinse the Pasteur pipette several times with plasma from what is now at the top of the 50 ml conical tube. Discard this liquid.
Blow the air out of the Pasteur pipette and hold the latex bulb shut as you carefully lower the Pasteur pipette to the interface between the plasma and the Ficoll. Do not blow bubbles into the gradient. There will probably be a crust of white cells loosely adherent to the side of the tube along the interface. This is very rich in PBMC. Harvest this first by gradually releasing pressure on the latex bulb and taking in the cells and surrounding liquid. Transfer the cells to a 50 ml tube containing 40 ml of cold HBSS.
Repeat step 9. This time, lower the open end of the pipette to the interface and sweep it along at the level of the interface as you gradually release pressure on the bulb. Aspirate the cells at the interface into the pipette. Transfer them to the tube containing 40 ml of cold HBSS. (See note 3.) Repeat until the interface is mostly free of leukocytes (cloudiness).
Centrifuge the 50 ml tube containing the cells and the 15 ml tube containing the plasma 1500 rpm, 10 min, 4°C. (See note 4.)
Aspriate the supernate from the PBMC pellet. Draw 10 ml of platelet-poor plasma from the 15 ml tube taking care not to disturb the platelet pellet.
Resuspend the PBMC in the 10 ml plasma. Pipette gently, avoiding air bubbles or directly spraying the stream of plasma onto the PBMC.
Transfer 10 – 20 µl to a hemacytometer. Count the cells and assess platelet contamination.
Centrifuge 1,000 rpm, 5 min, 4°C.
Resuspend in 10 ml of cold HBSS/HSA.
Repeat steps 14 – 16 until only rare platelets are seen in the hemacytometer and monocytes appear free of platelets. This usually takes 2 – 3 total washes. Typically some of the leukocytes are lost with each wash. However, this is usually less than 5 – 10% of the total. Larger losses than this on successive washes could be a sign that the monocytes are being activated during the procedure and sticking to tubes or pipettes.
Resuspend to the desired concentration in the desired medium. For the transendothelial migration assay, we resuspend to 2 × 106 cells/ml in M199/HSA.
Assess percentage of monocytes. (See note 5).
NOTES
Bend the thin end of a short siliconized Pasteur pipette to a right angle in a low Bunsen burner flame. Fire polish the tip briefly. This will allow you to harvest PBMC from the interface without collecting fluid from the upper or lower layers. Alternatively, we have used disposable one-piece plastic pipetting bulbs. However, it is harder to keep the tip of these at the interface. In either case, they must be pre-coated with HBSS/HSA and plasma.
Depending on the donor, you will isolate 2 – 4 × 106 PBMC per ml of blood. We find that 20 ml (one gradient) is more than enough to perform the transmigration assay using 60 HUVEC monolayers on microtiter plates. Often we can get enough for 2 such plates (120 assays) from one gradient.
If you run more than one gradient, they can all be combined into the same tube of HBSS at this point.
The PBMC fraction will be considerably contaminated by platelets. This includes platelets adherent to monocytes. To remove them, you will wash in autologous platelet-free plasma containing EDTA in step 15.
There are many ways to assess the percentage of monocytes. We have found that allowing an aliquot of the final suspension to sit on the hemacytometer for about 5 minutes will promote spreading of the monocytes to phase dense cells, while most lymphocytes will remain round and refractile. Then one simply takes the total cell count and large spread cells = monocytes; small round cells = lymphocytes. Obviously, this is not perfect, but when tested head to head with flow cytometry (LeukoGate®; CD14 and CD45) the results were within experimental error. For most donors the percentage of monocytes will range from 20 – 30% of the total PBMC.
Isolation of Peripheral Blood Neutrophils
Reference: (Lou et al., 2007)
For our adhesion and transmigration assays, it is important that the neutrophils (PMN) are activated by contact with the endothelium and not before. Unless care is taken minimize physical disruption or temperature changes to the neutrophil preparation during the isolation procedure, PMN may become activated and background adhesion and transmigration will be high. In this isolation all steps are carried out at room temperature until the transmigration step, when the cocultures are placed in a 37°C tissue culture incubator. Low numbers of remaining red blood cells, which do not interfere with the transmigration assay, are not lysed to avoid osmotic shock to the PMN.
As a test of the status of quiescence of isolated PMN, we sometimes plate endothelial monolayers derived from the same cultures as those used for the transmigration assay directly on plastic 96-well plates and assess PMN adhesion with or without prior activation by TNFα or IL-1β. PMN should not adhere to non-activated endothelial cells, but adhere tightly to activated endothelium.
REAGENTS
Ficoll-Hypaque (ρ = 1.077) Amersham Pharmacia, as for PBMC isolation
Histopaque 1119 (ρ = 1.119) Sigma Aldrich
Pharmaceutical grade heparin solution
Hanks’ Balanced Salt Solution without divalent cations (HBSS)
Medium 199 (M199)
Precoat all surfaces that will come in contact with the PMN with a solution of HBSS + 0.1% HSA (see PBMC isolation protocol). Avoid using glass. All media are at room temperature.
PROCEDURE
Pipette 12 ml Histopaque into a 50 ml conical centrifuge tube.
Carefully overlay 10 ml of Ficoll-Paque.
Draw heparin into a syringe so that there will be 2 units of heparin/ml of blood.
We routinely collect 10 ml of blood for each gradient. At the same time, draw 10 ml of blood into a glass tube to clot for the production of autologous serum.
Dilute heparinized blood 1:1 in HBSS.
Layer it carefully atop the discontinuous gradient.
Centrifuge 2,200 rpm at room temperature for 20 minutes with the brake off.
Harvest neutrophils from the interface between the Ficoll and Histopaque (ρ = 1.077/1.119).
Dilute into 10 ml of HBSS + 0.1% HSA.
Centrifuge 1000 rpm, 5 minutes at room temperature.
Gently resuspend the PMN pellet in 10 ml HBSS + 0.1% HAS.
Repeat step 9.
Resuspend in HBSS + 0.1% HSA + 1% heat inactivated autologous serum to a concentration of 3–5 × 106 PMN/ml.
Allow PMN to “rest” for 20 – 30 minutes at room temperature.
Dilute PMN in M199 + 20% autologous serum to a concentration of 5 × 105 cells/mL for transmigration. Blocking antibodies or other reagents are added at this step.
The transmigration assay is performed as outlined below. The PMN are warmed to 37°C gradually when the culture plate is put into the incubator.
Transendothelial Migration Assay
References: (Muller and Weigl, 1992; Muller et al., 1993)
Resting (not cytokine-activated) monolayers will support adhesion and transmigration of monocytes, but almost no lymphocytes. Therefore, for nonactivated monolayers, the whole PBMC fraction can be applied, yet only monocytes will adhere or transmigrate to any significant extent. Cytokine-activated (IL-1β or TNF-α) or LPS HUVEC monolayers will support adhesion of lymphocytes from the PBMC mixture, but only monocytes will transmigrate to any significant extent for up to several hours. Cytokine-activated monolayers are required to support optimal PMN and T cell adhesion and transmigration.
REAGENTS
Human Umbilical Vein Endothelial Cells (HUVEC) grown to confluence on hydrated collagen gels. (See separate procedure for making gels.) (See note 1.)
Peripheral blood mononuclear cells (PBMC) or neutrophils (PMN). See separate procedures for isolation.
Medium 199 (GIBCO) containing 0.1% human serum albumin (M199/HSA)
Hanks’ Balanced Salt Solution (without divalent cations, HBSS)
Dulbecco’s phosphate buffered saline (DPBS) with calcium and magnesium
10% neutral buffered formalin OR 2.5% glutaraldehyde in 0.1M sodium cacodilate buffer pH 7.4
Wright-Giemsa stain (Several kits are available commercially under names like “Diff-Quik” or “Hema”)
27G needle
Microscope slides and coverslips.
PROCEDURE
-
Resuspend PBMC to a final concentration of 2 × 106/ml in cold M199/HSA. (See note 2.)
Resuspend PMN to a final concentration of 1 × 106/ml in M199/HSA at room temperature.
-
Take HUVEC cultures from the incubator and wash × 3 with warm M199/HSA. This is best done either by aspirating using a Pasteur pipette connected to a vacuum flask or by inverting the plate and shaking gently. In the latter case, blot gently to remove excess liquid from the top of the plate. Refill the wells quickly, ideally using a repeating micropipetter. Do not let the monolayers dry out.
Aspirate and refill the wells by placing the pipette tip at an obtuse angle against the wall of the culture vessel to deflect the force of the stream. Never pipette directly onto the endothelial monolayer surface, as the force may dislodge or damage the endothelial cells. We have also found that when using a repeating pipetter for these washes, placing a small (10–200 µl size) disposable pipette tip on the end of the cartridge results in a narrow stream of fluid that has sufficient force to dislodge cells even when directed against the wall of the culture vessel. Therefore, we recommend against capping the cartridge with a disposable pipette tip.
Aspirate the last wash and add 100 µl of leukocyte suspension to each well. (See note 3.)
Return the culture plate to the tissue culture incubator for the desired time. Use a separate culture plate for each time point.
At the end of the assay, aspirate the remaining supernate. Wash × 3 with warm DPBS (200 µl per wash). (See note 4.)
-
Add fixative (200 µl) to the gels in situ. Fix in formalin for routine microscopy; glutaraldehyde for EM or to obtain firmer (but more brittle) gels. Allow to fix for at least an hour (preferably overnight) before removing monolayers for counting.
If depth of penetration into the collagen is something you will want to assess, fix in glutaraldehyde, as those gels are resistant to collapse under their own weight or that of the coverslip (see steps 11 and 12) preserving better the original dimensions.
Stain the gels with modified Wright Giemsa stain for several minutes by adding stain and washes directly to the monolayers in the wells. If leukocytes have migrated deeply into the collagen gel, it may require over 10 minutes for the stain to penetrate to them.
Rinse gels with distilled water.
Loosen the gels from the well by inserting a 27G needle along the side wall of the well and “rimming” the gel with the needle. Press outward along the wall while doing this to avoid crushing the gel. (See note 5.)
Remove the gels using fine (#5) forceps. Place monolayer side up on a glass slide. Gels can be manipulated by carefully tugging on the edges with fine forceps or the 27G needle. Try to smooth out any visible wrinkles in the gel and restore its original shape.
Cover with a coverslip. Avoid trapping air bubbles.
Examine by light microscopy. Differential Interference Contrast microscopy (DIC/Nomarsky optics) is best to throw the monolayer into relief. By focusing on the endothelial monolayer, one can determine the number of leukocytes attached to the surface of the HUVEC (in focus when the surface of the monolayer is in focus) or transmigrated (out of focus when the surface of the monolayer is in focus, but in focus as one brings the focal plane down below the monolayer. You will be able to see collagen strands around the transmigrated leukocytes.
We routinely count at least 100 cells in the central field of each monolayer for each of six replicate monolayer per condition. If there are not over 100 cells in a single field, cells in multiple fields are counted.
We express the percent transmigration as the number of leukocytes in each field that have transmigrated (i.e. below the HUVEC monolayer) divided by the total number of leukocytes in that field that had adhered (i.e. above and below the HUVEC monolayer.) (See note 6.)
NOTES
It is convenient to grow HUVEC on collagen gels in microtiter wells in a 96-well plate format. We routinely culture cells in the center 60 wells on such a plate, allowing six replicates (columns) of ten experimental conditions (rows) at the same time.
If you plan to add antibodies or other reagents to block or modify transmigration, we have found it best to resuspend the leukocytes to twice this concentration and mix them with an equal volume of antibody or reagent at twice its final concentration. This avoids exposing leukocytes locally (albeit transiently) to high concentrations of reagent that could be activating or have other untoward effects. Stock antibody concentrations are often 100 to 1,000 times the final concentration in the assay.
If testing several variables we find it convenient to test six replicates of each condition, so that all of the wells in a single column receive the same leukocyte sample. Change pipette tips between samples.
When specifically trying to distinguish adhesion to the apical surface by integrins from blockade of transmigration by anti-PECAM reagents, for example, we wash × 2 with warm 1 mM EDTA in HBSS to remove cells bound by divalent cation-dependent interactions. Then we wash × 2 with warm DPBS.
It is good to only stain as many gels as you will count in one sitting, since the stain fades with time. The gels can be restained. Only remove as many gels as you will count on one slide, since they tend to flatten out and dry out once removed from the wells. If they become too dried out or flat, it will become more difficult to distinguish how far into the gel they have migrated.
In the absence of inhibitors, >90% of monocytes or neutrophils that adhere to these monolayers generally transmigrate within 20 – 30 minutes. The time for transmigration per se is rapid (minutes). It takes at least 20 minutes for the population of leukocytes to settle down on the monolayer.
Flow chamber studies
The parallel plate flow chamber use in our laboratory consists of an upper and a lower plate made of stainless steel (see reference (Goetz et al., 1999) for detailed description and Fig 2). The upper plate has an inlet and outlet for inflow and outflow of buffer, a bubble trap (see Note 1), an opening for a temperature probe, and a circular cutout containing a piece of quartz glass. The quartz glass gives efficient passage of a wide spectrum of light, making it possible to perform quantitative fluorescence and bright-field microscopy and it obviously allows monitoring of adhesive events within the flow chamber (Goetz et al., 1997; Shaw et al., 2001; Shaw et al., 2004). The lower plate has a circular cutout where a 25-mm diameter glass coverslip, which is coated with an endothelial cell monolayer or immobilized adhesion proteins (e.g., P- or E-selectins) is placed. A plastic O-ring is placed between the lower plate of the flow chamber and the coverslip to prevent buffer leaking from the flow chamber. A heating plate of the same dimensions as the flow chamber is used to maintain the appropriate temperature. The upper and lower plates of the flow chamber are separated by a silastic gasket (Allied Biomedical; Paso Robles, CA), which defines the flow area (5.0 × 80 mm, and height, 0.25 mm (see Note 2)), within the flow chamber. The position and orientation of the silastic gasket are maintained by locator pins, which are imbedded in the upper plate of the flow chamber. Buffer is drawn through the flow chamber at defined volumetric flow rates with a syringe pump (Harvard Apparatus, model 44; Natick, MA).
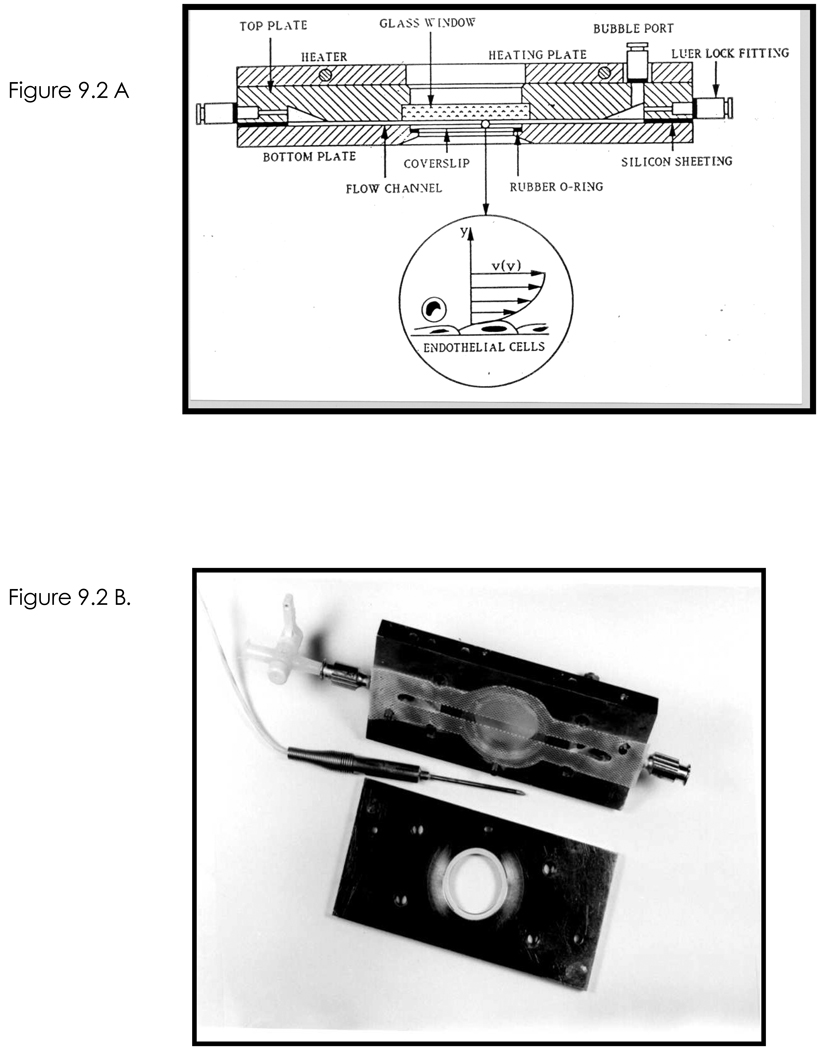
Figure 2.
A. Schematic of the parallel plate flow chamber used to study leukocyte transmigration of cytokine activated HUVEC monolayers under fluid shear stress. (B). Photograph of the top (with gasket in place) and bottom plates that are assembled to create the flow chamber. The temperature probe is placed in the top plate. Reprinted from (Goetz et al., 1999).
Video recording equipment
The flow chamber is mounted on an inverted microscope equipped with 20× and 40× phase or DIC objectives and camera/videoports. Leukocyte attachment, rolling, spreading and migration are best observed at higher magnification (40×). Differential Interference Contrast (DIC or Nomarsky) microscopy is ideal for detecting transmigration of T cells and monocytes. The videoport allows live-time viewing via a black-and-white video camera connected to a PC equipped with a digital recorder. Alternatively the video camera is connected to a black and white monitor and professional grade VCR.
Live cell fluorescence imaging is performed using MetaMorph software (Molecular Devices, Downingtown, PA) to control a digital imaging system coupled to a Nikon model TE2000 inverted microscope (Melville, NY). The details of this system are in reference (Shaw et al., 2004). Briefly, separate excitation and emission filter wheels (Sutter Instrument Co., Novato, CA) combined with a polychroic beamsplitter (Chroma Technology, Brattleboro, VT) to allow for rapid acquisition of different fluorescence channels. Chroma filters for DsRed and YFP are employed for Alexa Fluor-568 (red) and GFP/Alexa Fluor-488 dyes (green), respectively. Transmitted and fluorescent light illumination is controlled by either a Uniblitz electronic shutter (Vincent Associates, Rochester, NY), or a shutter built in the excitation filter wheel. We have had good success with a high-sensitivity cooled CCD camera (ORCA-ER, Hamamatsu Co., Bridgewater, NJ) to acquire images using the MetaMorph software.
Flow assay media
Most physiological cell buffers can be used in the flow assay, however, it is important that the selected buffer contains Ca2+ because some of the adhesion processes, i.e., selectin mediated interactions, are Ca2+ dependent. Our current perfusion media consists of DPBS containing 0.75 mM Ca2+, 0.75 mM Mg2+ and 0.5% human serum albumin (Yang et al., 2006).
Methods
Preparation for the Flow Chamber Assay
Place a finger from a latex glove around microscope objectives for protection from excess flow buffer that causes deposit of salts on microscope components and corrosion of the objectives.
Warm the flow buffer to desired temperature by incubating in water bath for several hours (see Note 3). Preheat the flow chamber to working temperature. Insert the thermal probe into the upper plate and heat upper plate to the desired temperature.
Place the silastic gasket on the underside of the upper plate. The locator pins are used to position the gasket and align the gasket over the quartz glass. Make sure that the gasket is smooth and its width is constant.
Place the O-ring into the bottom plate and insert the coverslip containing either an endothelial cell monolayer or immobilized adhesion molecules on top of the O-ring in the bottom plate. The top plate is then placed over the coverslip and four screws are used to seal the top and bottom plates together. Apply gentle and uniform tightening of these 4 screws to avoid cell damage or cracking of the glass coverslip. Mount the heating plate on top of assembled chamber.
Assemble the inflow and outflow lines to the chamber. Secure outlet to a 50-mL glass syringe pump and set syringe pump to desired flow rate.
Place a videotape in the VCR, and record the dimensions of the screen via a microscope stage micrometer. Record under objective magnifications to be used in the experiments.
Analyses of Leukocyte-Endothelial Adhesion Events
Adhesion: Leukocyte adhesion data are reported as a function of the shear stress at the bottom surface of the flow chamber (see Note 4). Routinely, we perfuse a small volume of leukocytes (106 isolated leukocytes in 100 µL of flow buffer) at 0.5 dynes/cm2 and then once leukocyte attach, the flow rate is increased to 1 dyne/cm2 and buffer alone is perfused for an additional 10 minutes. The number of leukocytes bound per unit area is determined by counting the adherent cells/mm2 in 5 randomly selected microscope fields. Quantification of transendothelial migration using phase contrast or DIC microscopy is as previously described (Rao et al., 2004).
Rolling Velocities. A general feature of leukocyte-endothelial cell interactions under flow is that the adherent leukocytes routinely exhibit rolling behavior in response to the fluid shear flow that is characterized by a low-velocity, high-variance movement (Goetz et al., 1994) (see Note 5). Rolling velocities can be determined either manually (see Note 6) or by automated software (see Note 7). In either case, the goal is to determine the displacement, d, of the leukocyte over a period of time, t (see Note 8). The velocity is then easily calculated by v=d/t.
Approaches to block leukocyte rolling, adhesion or transmigration. A general approach consists of using function blocking mAb to known adhesion molecules or the use of siRNA silencing to knockdown specific molecules in either endothelium or leukocyte. Both or either cell types can be pretreated with blocking mAb for at least 30 min prior to performing the flow study. siRNA knockdown should be optimized for each molecule and cell type.
Notes
Bubble trap: A third port on top of the plate was added in order to facilitate removal of air bubbles that arise during assembly of the chamber.
The gasket we use is Duralistic I Sheeting of a thickness of 0.25 mm. This thickness defines the approximate height of the flow chamber. From the Duralistic I Sheeting, cut a gasket with an inner dimension of 5 mm (width) by 80 mm (length). The width of the flow field should be large relative to the height (e.g., ≥ 20:1 ratio), so that the flow may be approximated as unidirectional and the fluid velocity is spatially dependent only on the distance from the bottom surface of the flow chamber. Observations should be acquired in the middle of the flow chamber, away from the edges. With the assumption of unidirectional, laminar flow, the equation for the shear rate at the bottom surface of the flow chamber is given by γ=6Q/wh2, where Q=the volumetric flow rate, w=the width of the flow field, and h = the height of the flow field (Lawrence et al., 1987). The shear stress, τ, is related to the shear rate, γ, by τ= γμ where μ is the viscosity of the fluid used in the experiments, typically 0.007 poise for a dilute saline solution at 37°C. Thus, τ=6Qµ/wh2.
Our experiments are performed with the flow buffer and flow chamber at 37°C. When the buffer is preincubated in the 37°C heating bath for an hour or less, bubbles in the flow chamber are more common than when the preincubation is done for several hours.
Adhesion under flow involves a balance between the disruptive force and torque exerted on the leukocyte by the flow of the fluid, and a specific adhesive force and torque mediated by complementary receptor pairs on the surface of the adherent leukocyte and the endothelium (Hammer and Apte, 1992). The force and torque exerted by the flow of the fluid, which can be estimated from various theoretical models (Hammer and Apte, 1992), are related to the shear rate, or shear stress, at the leukocyte-endothelium interface. Therefore, in vitro adhesion data are usually reported as a function of the shear stress at the leukocyte-endothelium interface, which is the shear stress at the bottom surface of the flow chamber.
The term low velocity refers to the fact that the leukocytes roll at a velocity routinely <10% of the predicted velocity of a noninteracting leukocyte (Goetz et al., 1994). The theoretical velocity of a noninteracting leukocyte can be estimated from the work of Goldman and colleagues (Goldman et al., 1967). This value depends on the radius of the leukocyte, the shear rate at the fluid coverslip interface, and the distance the leukocyte is from the surface of the coverslip. High variance velocity refers to the fact that the leukocytes do not roll with a constant velocity, but rather accelerate and decelerate as they roll across the monolayer surface (Goetz et al., 1994).
In manual mode, the displacement of the leukocyte can be determined either by recording the experiment with a calibrated reticle in place or by placing a template over the screen during the analysis. One approach is to determine the time required for leukocytes to roll a given distance and calculate the rolling velocity.
Automated image analysis can be done using NIH Image or commercially available software (Ed Marcus Laboratories, Newton, MA).
As leukocytes roll over the endothelium, they may detach from the endothelium and then reattach several cell diameters downstream from the point of detachment. These are usually referred to as skipping leukocytes and are routinely not included in the data set used to determine rolling velocities.
Acknowledgements
The authors wish to thank Jian Shen, C. Forbes Dewey and Peter Morley (Massachusetts Institute of Technology; Cambridge, MA) for the design, fabrication, and maintenance of the flow chambers; Professor Douglas Goetz (Ohio University) for redesign for the flow chamber during his postdoctoral studies in the Luscinskas lab. This work was made possible by grant support from the National Institutes of Health, Bethesda, MD (HL 36028 and HL 53993 to FWL and HL46849, 64774, and 72942 to WAM).
Contributor Information
William A. Muller, Department of Pathology, Northwestern University Feinberg School of Medicine, 303 East Chicago Avenue, Chicago, IL 60611, e-mail: wamuller@northwestern.edu; phone (312) 503-8144; fax (312) 503-8249
F. William Luscinskas, Department of Pathology, Brigham and Women’s Hospital and Harvard Medical School, Center for Excellence in Vascular Biology, 77 Avenue Louis Pasteur, Boston, MA 02115, e-mail: fluscinskas@rics.bwh.harvard.edu; phone (617) 525-4337; fax (617) 525-4333.
References
- Bixel G, Kloep S, Butz S, Petri B, Engelhardt B, Vestweber D. Mouse CD99 participates in T cell recruitment into inflamed skin. Blood. 2004;104:3205–3213. doi: 10.1182/blood-2004-03-1184. [DOI] [PubMed] [Google Scholar]
- Bixel MG, Petri B, Khandoga AG, Khandoga A, Wolburg-Buchholz K, Wolburg H, Marz S, Krombach F, Vestweber D. A CD99-related antigen on endothelial cells mediates neutrophil, but not lymphocyte extravasation in vivo. Blood. 2007 doi: 10.1182/blood-2006-08-043109. [DOI] [PubMed] [Google Scholar]
- Brown DC, Larson RS. Improvements to parallel plate flow chambers to reduce reagent and cellular requirements. BMC Immunol. 2001;2:9. doi: 10.1186/1471-2172-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Carman CV, Sage PT, Sciuto TE, de la Fuente MA, Geha RS, Ochs HD, Dvorak HF, Dvorak AM, Springer TA. Transcellular diapedesis is initiated by invasive podosomes. Immunity. 2007;26:784–797. doi: 10.1016/j.immuni.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinamon G, Shinder V, Alon R. Shear forces promote lymphocyte migration across vascular endothelium bearing apical chemokines. Nature Immunol. 2001;2:515–522. doi: 10.1038/88710. [DOI] [PubMed] [Google Scholar]
- Cuvelier SL, Patel KD. Shear-dependent eosinophil transmigration on interleukin 4-stimulated endothelial cells: A role for endothelium-associated eotaxin-3. J. Exp. Med. 2001;194:1699–1709. doi: 10.1084/jem.194.12.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuvelier SL, Paul S, Shariat N, Colarusso P, Patel KD. Eosinophil adhesion under flow conditions activates mechanosensitive signaling pathways in human endothelial cells. J Exp Med. 2005;202:865–876. doi: 10.1084/jem.20041315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furie MB, Naprstek BL, Silverstein SC. Migration of neutrophils across monolayers of cultured microvascular endothelial cells. An in vitro model of leucocyte extravasation. J Cell Sci. 1987;88(Pt 2):161–175. doi: 10.1242/jcs.88.2.161. [DOI] [PubMed] [Google Scholar]
- Goetz DJ, el-Sabban ME, Pauli BU, Hammer DA. Dynamics of neutrophil rolling over stimulated endothelium in vitro. Biophys J. 1994;66:2202–2209. doi: 10.1016/S0006-3495(94)81016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz DJ, Greif DM, Ding H, Camphausen RT, Howes S, Comess KM, Snapp KR, Kansas GS, Luscinskas FW. Isolated P-selectin glycoprotein ligand-1 dynamic adhesion to P- and E-selectin. J Cell Biol. 1997;137:509–519. doi: 10.1083/jcb.137.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz DJ, Greif DM, Shen J, Luscinskas FW. Cell-cell adhesive interactions in an in vitro flow chamber. Methods Mol Biol. 1999;96:137–145. doi: 10.1385/1-59259-258-9:137. [DOI] [PubMed] [Google Scholar]
- Goldman A, Cox R, Brenner H. Slow viscous motion of a sphere parallel to a plane wall. II Couette Flow. Chem. Eng. Sci. 1967;22:653–660. [Google Scholar]
- Hammer DA, Apte SM. Simulation of cell rolling and adhesion on surfaces in shear flow: general results and analysis of selectin-mediated neutrophil adhesion. Biophys J. 1992;63:35–57. doi: 10.1016/S0006-3495(92)81577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama J, Hidemura A, Saito H, Nagawa H. Shear stress affects migration behavior of polymorphonuclear cells arrested on endothelium. Cell. Immunol. 2000;203:39–46. doi: 10.1006/cimm.2000.1671. [DOI] [PubMed] [Google Scholar]
- Lawrence MB, McIntire LV, Eskin SG. Effect of flow on polymorphonuclear leukocyte/endothelial cell adhesion. Blood. 1987;70:1284–1290. [PubMed] [Google Scholar]
- Liao F, Ali J, Greene T, Muller WA. Soluble domain 1 of platelet-endothelial cell adhesion molecule (PECAM) is sufficient to block transendothelial migration in vitro and in vivo. J Exp Med. 1997;185:1349–1357. doi: 10.1084/jem.185.7.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao F, Huynh HK, Eiroa A, Greene T, Polizzi E, Muller WA. Migration of monocytes across endothelium and passage through extracellular matrix involve separate molecular domains of PECAM-1. J Exp Med. 1995;182:1337–1343. doi: 10.1084/jem.182.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao F, Schenkel AR, Muller WA. Transgenic mice expressing different levels of soluble platelet/endothelial cell adhesion molecule-IgG display distinct inflammatory phenotypes. J Immunol. 1999;163:5640–5648. [PubMed] [Google Scholar]
- Lim YC, Garcia-Cardena G, Allport JR, Zervoglos M, Connolly AJ, Gimbrone MA, Jr, Luscinskas FW. Heterogeneity of endothelial cells from different organ sites in T-cell subset recruitment. Am J Pathol. 2003;162:1591–1601. doi: 10.1016/S0002-9440(10)64293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou O, Alcaide P, Luscinskas FW, Muller WA. CD99 is a key mediator of the transendothelial migration of neutrophils. J. Immunol. 2007;178:1136–1143. doi: 10.4049/jimmunol.178.2.1136. [DOI] [PubMed] [Google Scholar]
- Luscinskas FW, Kansas GS, Ding H, Pizcueta P, Schleiffenbaum BE, Tedder TF, Gimbrone MA., Jr Monocyte rolling, arrest and spreading on IL-4-activated vascular endothelium under flow is mediated via sequential action of L-selectin, beta 1-integrins, and beta 2-integrins. J Cell Biol. 1994;125:1417–1427. doi: 10.1083/jcb.125.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller WA. Transendothelial migration of leukocytes. In: Peltz G, editor. Leukocyte recruitment in inflammatory disease. Austin, TX: R.G. Landis Company; 1996. pp. 3–18. [Google Scholar]
- Muller WA. Migration of leukocytes across endothelial junctions: Some concepts and controversies. Microcirculation. 2001;8:181–193. doi: 10.1038/sj/mn/7800078. [DOI] [PubMed] [Google Scholar]
- Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends in Immunology. 2003;24:326–333. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- Muller WA, Ratti CM, McDonnell SL, Cohn ZA. A human endothelial cell-restricted, externally disposed plasmalemmal protein enriched in intercellular junctions. J Exp Med. 1989;170:399–414. doi: 10.1084/jem.170.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller WA, Weigl S. Monocyte-selective transendothelial migration: Dissection of the binding and transmigration phases by an in vitro assay. J Exp Med. 1992;176:819–828. doi: 10.1084/jem.176.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993;178:449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noria S, Cowan DB, Gotlieb AI, Langille BL. Transient and steady-state effects of shear stress on endothelial cell adherens junctions. Circ Res. 1999;85:504–514. doi: 10.1161/01.res.85.6.504. [DOI] [PubMed] [Google Scholar]
- Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med. 2006;203:2569–2575. doi: 10.1084/jem.20060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph GJ, Beaulieu S, Lebecque S, Steinman RM, Muller WA. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 1998;282:480–483. doi: 10.1126/science.282.5388.480. [DOI] [PubMed] [Google Scholar]
- Randolph GJ, Inaba K, Robbiani DF, Steinman RM, Muller WA. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 1999;11:753–761. doi: 10.1016/s1074-7613(00)80149-1. [DOI] [PubMed] [Google Scholar]
- Rao RM, Betz TV, Lamont DJ, Kim MB, Shaw SK, Froio RM, Baleux F, Arenzana-Seisdedos F, Alon R, Luscinskas FW. Elastase release by transmigrating neutrophils deactivates endothelial-bound SDF-1alpha and attenuates subsequent T lymphocyte transendothelial migration. J Exp Med. 2004;200:713–724. doi: 10.1084/jem.20040499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RM, Yang L, Garcia-Cardena G, Luscinskas FW. Endothelial-dependent mechanisms of leukocyte recruitment to the vascular wall. Circ Res. 2007;101:234–247. doi: 10.1161/CIRCRESAHA.107.151860b. [DOI] [PubMed] [Google Scholar]
- Schenkel AR, Mamdouh Z, Chen X, Liebman RM, Muller WA. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat Immunol. 2002;3:143–150. doi: 10.1038/ni749. [DOI] [PubMed] [Google Scholar]
- Schenkel AR, Mamdouh Z, Muller WA. Locomotion of monocytes on endothelium is a critical step during extravasation. Nat Immunol. 2004;5:393–400. doi: 10.1038/ni1051. [DOI] [PubMed] [Google Scholar]
- Shaw SK, Bamba PS, Perkins BN, Luscinskas FW. Real-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endothelium. J Immunol. 2001;167:2323–2330. doi: 10.4049/jimmunol.167.4.2323. [DOI] [PubMed] [Google Scholar]
- Shaw SK, Ma S, Kim MB, Rao RM, Hartman CU, Froio RM, Yang L, Jones T, Liu Y, Nusrat A, Parkos CA, Luscinskas FW. Coordinated redistribution of leukocyte LFA-1 and endothelial cell ICAM-1 accompany neutrophil transmigration. J Exp Med. 2004;200:1571–1580. doi: 10.1084/jem.20040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Kowalski JR, Zhan X, Thomas SM, Luscinskas FW. Endothelial cell cortactin phosphorylation by Src contributes to polymorphonuclear leukocyte transmigration in vitro. Circ Res. 2006;98:394–402. doi: 10.1161/01.RES.0000201958.59020.1a. [DOI] [PubMed] [Google Scholar]