Abstract
We cloned and characterized a rice gene OsBIABP1 encoding an AMP-binding protein. The full-length cDNA of OsBIABP1 is 1912-bp long and is predicted to encode a 558-aa protein. OsBIABP1 contains a typical AMP-binding signature motif and shows high similarity to members of AMP-binding protein family. OsBIABP1 is expressed in stems, leaves and flowers of rice plants, but is not expressed, or expressed at a very low level, in rice roots. The expression of OsBIABP1 was induced by some defense-related signal molecules, e.g., salicylic acid (SA), benzothiadiazole, jasmonic acid (JA), and 1-amino cyclopropane-1-carboxylic acid, which mediate SA- and JA/ethylene (ET)-dependent defense signaling pathways, respectively. Furthermore, the expression of OsBIABP1 is activated by the infection of Magnaporthe oryzae, and the induced expression is quicker and stronger during early stages of pathogenesis in incompatible interaction than that in compatible interaction between rice and M. oryzae. Our results suggest that OsBIABP1 may be a defense-related AMP-binding protein that is involved in the regulation of defense response through SA and/or JA/ET signaling pathways.
Keywords: Rice, Oryza sativa L., Magnaporthe oryzae, Defense response, OsBIABP1, AMP-binding protein
INTRODUCTION
Plants defend themselves against potential pathogens by activating a battery of defense mechanisms, in which thousands of defense genes are coordinately expressed. Plant responses in compatible and incompatible interactions between Arabidopsis and Pseudomonas syringae are qualitatively similar but quantitatively different soon after infection, and the amplitude of the responses in the compatible interaction increases later (Tao et al., 2003). Of all defense mechanisms, hypersensitive response (HR) often occurs at the infection site to restrict spreading of the invading pathogens from primary infection site to other parts of the plants. Occurrence of an HR usually is accompanied by systemic activation of defense responses throughout the whole plants, resulting in systemic acquired resistance (Durrant and Dong, 2004; Grant and Lamb, 2006). Recent biochemical, genetic and genomic studies in Arabidopsis have revealed that different signaling pathways are responsible to regulate the activation of disease resistance responses, among which salicylic acid (SA)- and jasmonic acid (JA)/ethylene (ET)-dependent signaling pathways are well documented (Beckers and Spoel, 2006; Wasternack, 2007; Kachroo and Kachroo, 2007). The SA- and JA/ET-dependent signaling pathways are believed to play pivotal roles in regulating active defense responses against biotrophic and necrotrophic pathogens, respectively (Glazebrook, 2005).
Disease resistance signaling pathways in rice share some common features with those in dicot plants including Arabidopsis (Song and Goodman, 2001). OsNPR1, a homolog of Arabidopsis NPR1/NIM1, also defines a disease resistance pathway similar to the NPR1-mediated signaling pathway in Arabidopsis, because the overexpression of Arabidopsis NPR1 or rice endogenous OsNPR1 in transgenic rice resulted in enhanced disease resistance and activation of defense gene expression (Chern et al., 2001; 2005; Fitzgerald et al., 2004; Yuan et al., 2007). Meanwhile, JA has also been demonstrated to play important roles in rice defense response against pathogen infection (Mei et al., 2006; Yara et al., 2007; Qiu et al., 2007), probably coordinately operating with the SA-dependent signaling pathway (Qiu et al., 2007).
Previously, we demonstrated that benzothiadiazole (BTH), a structural analog of SA, can induce disease resistance response against rice blast disease (Magnaporthe oryzae), sheath blight disease (Rhizoctonia solani), and leaf bacterial blight disease (Xanthomonas oryzae pv. oryzae) (Ge et al., 1999; Song et al., 2001; Zhang et al., 2004). To study the molecular mechanism of the BTH-induced disease resistance in rice, we isolated and identified over 200 differentially expressed genes through suppression subtractive hybridization (Song and Goodman, 2002a). Recently, our further studies on some selected differentially expressed genes have revealed that genes encoding mitogen-activated protein kinases (OsBIMK1 and OsBIMK2), protein phosphatase 2C (OsBIPP2C1), homeodomain and ERF transcriptional factors (OsBIHD1 and OsBIERF1~4), phosphoinositide-specific phospholipase C (OsPI-PLC1), F-box protein (OsDRF1), diacylglycerol kinase (OsBIDK1), zinc finger protein (OsBIRF1), DEAD-box RNA helicase (OsBIRH1), and serine carboxypeptidase-like protein (OsBISCPL1) are involved in disease resistance responses and other abiotic stress (Song and Goodman, 2002a; 2002b; Luo et al., 2005a; 2005b; Hu et al., 2006; Cao et al., 2006a; 2006b; 2008; Song et al., 2006; Zhang et al., 2008; Liu et al., 2008a; 2008b; Li et al., 2008). In this study, we cloned and characterized a rice AMP-binding protein gene, OsBIABP1, and found that OsBIABP1 gene can be induced by SA, BTH, JA and 1-amino cyclopropane-1-carboxylic acid (ACC), some well-known defense-related signaling molecules, and by infection with M. oryzae, causal agent of the blast disease. Our preliminary results suggest that OsBIABP1 may be involved in regulating rice disease resistance response through mediating different signaling pathways.
MATERIALS AND METHODS
Growth of rice plants, chemical treatments, and pathogen inoculation
Rice cultivar Yuanfengzao (Oryza sativa L. subsp. indica) and a pair of isogenic lines (H8R and H8S) were used in this study. Rice seedlings were grown in field soil packed in plastic pots (8 cm in diameter and 10 cm in height, 10 seedlings/pot) under 14 h light/10 h dark at 27/22 °C (day/night) in a growth room. Samples for tissue-specific expression were harvested from mature rice plants grown in a greenhouse with natural sunlight, frozen immediately in liquid nitrogen, and stored at −80 °C until use.
Three-week-old seedlings of cv. Yuanfengzao were treated by spraying with solutions of 0.3 mmol/L BTH (Novartis Crop Protection Inc., USA), 1.0 mmol/L SA (pH 6.5) (Sigma, St. Lousi, USA), 100 μmol/L JA (Sigma, St. Lousi, USA) or 100 μmol/L ACC (Sigma, St. Lousi, USA), respectively. JA and ACC were dissolved in 0.1% (v/v) ethanol. Control seedlings were treated in the same way as spraying with 0.1% (v/v) ethanol or distilled sterilized water. Three-week-old seedlings of H8R and H8S were inoculated by leaf spraying with spore suspension (5×105 spores/ml in 0.05% (v/v) Tween-20) of M. oryzae (race ZB1, strain 85-14B1). The inoculated seedlings were kept at 100% relative humidity in dark for 36 h and then returned to the growth chamber under 14 h light/10 h dark at 27/22 °C (day/night). Leaf samples were collected at different time points and stored at −80 °C until use.
Cloning of OsBIABP1 cDNA and bioinformatics analysis
Differentially expressed cDNA clone BNHN-n7 contained a 284-bp insert, which showed a high level of similarity to putative AMP-binding protein (ABP) in plants. The 5′- and 3′-end fragments were amplified using phage DNA prepared from a rice cDNA library as template in order to obtain information of the full-length cDNA sequence of this putative ABP gene. The sequence-specific primers used were BNHN-n7-1F (CATGGACAAGCTCGGCGCCA) and BNHN-n7-1R (CTGGCTAGATGCCGCTACA). The 5′-end fragment was amplified with BNHN-n7-1R and a vector primer T3-2 (CCTGCAGGTCGACACTAGTG), while the 3′-end fragment was amplified with BNHN-n7-1F, and a vector primer T7-2 (CCACCCGGGTGGAAAATCGA). A pair of gene-specific primers, BNHN-n7-2F (AAGAGCTCATGGACAAGCTCGGC, a SacI site is underlined) and BNHN-n7-2R (CCCTCGAGCCGCTACATTTTACT, an XhoI site is underlined), was then used for the amplification of the entire open reading frame (ORF) sequence. All PCR products were purified and cloned into pUCm-T vector (Sangon, Shanghai, China). Plasmid containing the complete ORF sequence was designated pUCmT-BNHN-n7-1.
DNA sequencing was performed on both strands of the plasmids using a MegaBACE 1000 DNA Analysis System (Amersham Biosciences, UK). Similarity searches were carried out using BLAST programs at the National Center for Biotechnology Information (NCBI) GenBank database (http://www.ncbi.nlm.nih.gov/BLAST/). Sequence alignments and phylogenetic tree analysis were conducted using ClustalW method using DNAstar software (LaserGene, Madison, WI, USA).
RNA extraction and reverse transcription-polymerase chain reaction analysis of gene expression
Total RNA was extracted with Trizol reagent (Invitrogen, Shanghai, China) following the manufacturer’s instruction. Samples of total RNA (0.5 μg) were reverse-transcribed using SuperScript III Kit (Invitrogen, Shanghai, China), followed by polymerase chain reaction (PCR) reaction using 1 μl of the RT product and 10 pmol of each primer in a total volume of 25 μl. The amplification conditions were as follows: 95 °C 3 min, 23~40 cycles of 94 °C for 15 s, 55 °C for 30 s, and 72 °C for 1 min. PCR reactions were performed 23 cycles for ACTIN1 gene, 40 cycles for tissue-specific expression, and 32 cycles for induction of OsBIABP1 expression, and the PCR products were electrophoresed on a 1.2% (w/v) agarose gel. OsBIABP1-specific primers used are OsBIABP1-rt-1F (GAGGCGATCGGCTTCGAGGTC) and OsBIABP1-rt-1R (GTGGACGTCTTCGGTAACTCGTC). Rice ACTIN1 gene (accession No. X16280) was used as a control with primers of ACTIN1-1F (ACTGTCCCCATCTATGAAGGA) and ACTIN1-1R (CTGCTGGAATGTGCTGAGAGA).
RESULTS
Cloning of OsBIAMP1 gene cDNA
In our previous studies, over 200 differentially expressed cDNA clones associated with BTH-induced defense response were identified (Song and Goodman, 2002a). BLAST searching revealed that a differentially expressed cDNA clone, BNHN-n7, containing a 284-bp insert, showed high similarity to genes encoding plant ABPs, indicating that the insert in BNHN-n7 may be a part of a rice ABP gene. To obtain this putative ABP gene, the 5′- and 3′-end sequences were amplified and a full-length cDNA was assembled. Because clone BNHN-n7 was identified originally from a suppression subtractive hybridization library constructed by subtracting cDNAs from BTH-induced rice leaf tissue with cDNAs from uninoculated control leaf tissue (Song and Goodman, 2002a), this gene was thus designated as OsBIABP1 for Oryza sativa L. BTH-induced AMP-binding protein 1.
The full-length cDNA of OsBIABP1 is 1912-bp long, which contains a predicted ORF of 1677 bp, and 5′- and 3′-UTR sequences of 87 and 148 bp, respectively. Database searches identified a full-length cDNA, AK106615, isolated from Oryza sativa L. subsp. Japonica by KOME project (http://cdna01.dna.affrc.go.jp/cDNA/), which has one nucleotide difference from OsBIABP1 cDNA. However, this nucleotide difference does not cause amino acid difference in predicted proteins. The OsBIABP1 gene corresponds to locus Os04g57850 as predicted by TIGR Rice Genome Annotation. OsBIABP1 is localized at chromosome 3 of the rice genome and consists of 2 exons and 1 intron.
Characterization of OsBIAMP1 protein
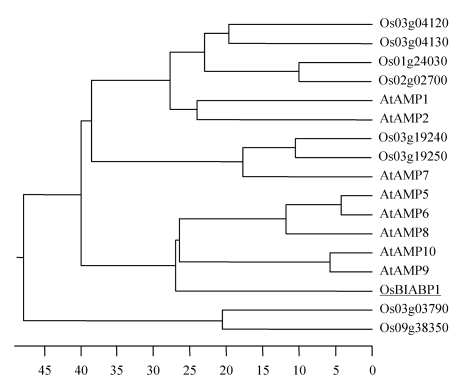
The 1677-bp ORF of the OsBIABP1 gene is predicted to encode a 558-aa protein with a calculated molecular weight of 60.4 kD and an isoelectric point of 7.87 (Fig.1). BLAST searches at the NCBI GenBank database (http://www.ncbi.nlm.nih.gov/) indicate that OsBIABP1 contains a highly conserved region (from Leu-17 to Met-544), often seen in acyl-CoA synthetases (PRK08162, CaiC) that are involved in lipid/secondary metabolites biosynthesis, transport, and catabolism. Further domain search using PROSITE program at ExPASy server (http://au.expasy.org/) identified a conserved AMP-binding signature motif sequence LNYTSGTTSAPK (Fig.1). Similarity searches and phylogenetic tree analysis revealed that OsBIABP1 has high levels of similarity to Atabidopsis AtAMPs, specifically to AtAMP5, AtAMP6, and AtAMP8~10, giving 58.5%~64.5% identity at amino acid sequence level (Fig.2). OsBIABP1 and these Arabidopsis AMPs form a small group in the phylogenetic tree (Fig.2).
Fig.1.
Comparison of OsBIABP1 with Arabidopsis AtAMP5 (At5g16370) and AtAMP9 (At1g21540)
Sequences were aligned using ClustalW program with DNAstar software. The conserved AMP-binding signature motif is indicated by a solid line on the sequences
Fig.2.
Phylogenetic tree analysis of OsBIABP1 with ABPs from rice and Arabidopsis
Sequence alignment and phylogenetic tree were constructed using ClustalW program in DNAstar software. The ABP proteins used for alignment are Arabidopsis thaliana AtAMP1 (At1g20560), AtAMP2 (At2g17650), AtAMP5 (At5g16370), AtAMP6 (At5g16340), AtAMP7 (At3g 16910), AtAMP8 (At1g75960), AtAMP9 (At1g21540), and AtAMP10 (At1g21530); Oryza sativa L. Os03g04120, Os03g04130, Os01g24030, Os02g02700, Os03g19240, Os03g19250, Os03g03790, Os09g38350
Tissue-specific expression of OsBIABP1 in rice
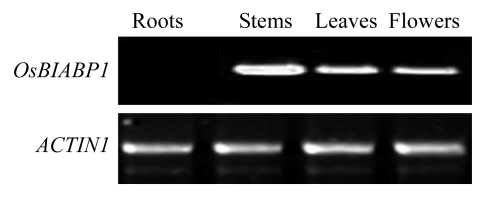
Tissue-specific expression of OsBIABP1 in rice plants grown under normal growth condition was examined by semi-quantitative RT-PCR. To avoid the risk to amplify homologous genes, downstream reverse primer was designed to be located in the UTR region at the 3′-end to improve specificity in PCR amplification. The specificity of the RT-PCR primers was further confirmed by sequencing of the PCR products. Under normal growth conditions, expression levels of OsBIABP1 in different tissues of rice plants were very low, so we could not detect any product using 30~35 cycles of PCR. When RT-PCRs were increased to 40 cycles, a single band was observed. As shown in Fig.3, transcript of OsBIABP1 was detected in stems, leaves, and flowers, but not in root samples. OsBIABP1 was expressed at relatively high levels in stems as compared with those in leaves and flowers (Fig.3). This result suggests that OsBIABP1 is differentially expressed in a tissue-specific manner in rice.
Fig.3.
Expression of OsBIABP1 in different tissues of rice plants grown under normal growth condition
Rice plants were grown in soil and samples from different tissues and organs were collected for analysis of gene expression. RT-PCR was performed 40 cycles for OsBIABP1 using gene-specific primers and 23 cycles for ACTIN1 used as a control
Induced expression of OsBIABP1 by defense-related signaling molecules
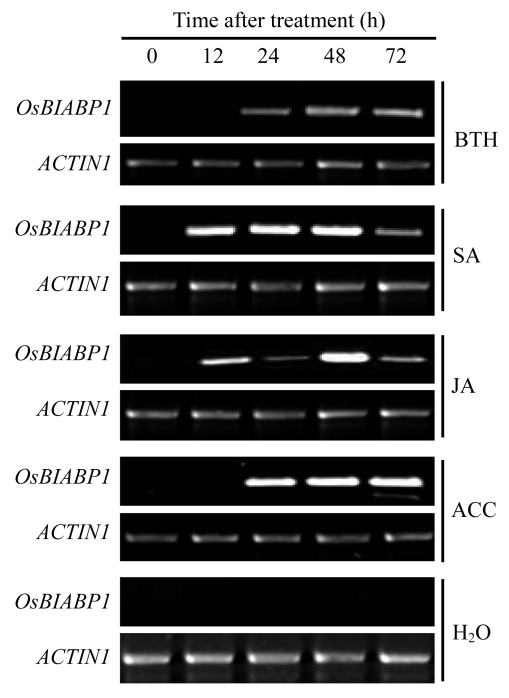
The clone BNHN-n7 appeared to be differentially expressed in rice leaf tissue after treatment with BTH, indicating that OsBIABP1 might be induced by BTH. We therefore first checked whether the expression of OsBIABP1 in rice seedlings was induced by BTH. As expected, the induced expression of OsBIABP1 was observed 24 h after treatment, peaked at 48 h, and maintained a relatively high level until 72 h after BTH treatment (Fig.4). We also analyzed whether the expression of OsBIABP1 gene was induced by other different defense-related signal molecules including SA, JA, and ACC. Treatments with SA, JA or ACC all induced OsBIRH1 expression in rice seedlings (Fig.4). SA treatment led to a rapid induced expression of OsBIABP1 within 12 h and maintained to a relatively high level during 12~48 h after treatment (Fig.4). In JA-treated seedlings, the expression level of OsBIABP1 increased significantly at 12 h, decreased slightly at 24 h, and increased again at 48 h after treatment (Fig.4). Similar to the kinetic in BTH-treated seedlings, ACC treatment induced markedly expression of OsBIABP1 at 24 h, and a high level of expression was maintained during 24~72 h in rice seedlings (Fig.4). However, no significant expression of OsBIABP1 was observed in water-treated control seedlings during the experiment period (Fig.4). These results suggest that OsBIABP1 is responsible to multiple defense signal molecules and therefore might be involved in different defense signaling pathways.
Fig.4.
Induced expression of OsBIABP1 in rice seedlings in response to treatments with different defense-related signal molecules
Three-week-old seedlings were treated by spraying with 0.3 mmol/L BTH, 1.0 mmol/L SA, 100 μmol/L JA, 100 μmol/L ACC or water, and leaf samples were collected at each time point after treatment. RT-PCR was performed 36 cycles for OsBIABP1 using gene-specific primers and 23 cycles for ACTIN1 used as a control
Expression patterns of OsBIABP1 in rice-M. oryzae interactions
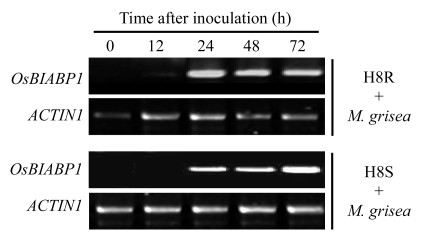
Expression patterns of OsBIABP1 in incompatible and compatible interactions between rice and the blast fungus, M. oryzae, were also analyzed. In these experiments, a pair of isogenic lines, H8R and H8S, was used, in which disease responses of H8R and H8S seedlings to the tested strain of M. oryzae represent an incompatible interaction and a compatible interaction, respectively. Disease symptom usually was seen on three-week-old seedlings of H8R and H8S three days after inoculation with the fungus. As shown in Fig.5, the expression of OsBIABP1 was induced in rice seedlings after inoculation with M. oryzae; however, the expression kinetics showed different patterns. In incompatible interaction of H8R seedlings with the blast fungus, the expression of OsBIABP1 was detected at 12 h, peaked at 24 h, and maintained a relatively high level during 24~72 h after inoculation (Fig.5). By contrast, in compatible interaction of H8S seedlings with M. oryzae, significant expression of OsBIABP1 was observed at 24 h, increased gradually, and peaked at 72 h after inoculation (Fig.5). These results indicate that the expression of OsBIABP1 is activated by pathogen, and that the induced expression is much quicker and stronger during early stages of pathogenesis in incompatible interaction than in compatible interaction between rice and M. oryzae.
Fig.5.
Induced expression of OsBIABP1 in interactions between rice and Magnaporthe oryzae
Three-week-old rice seedlings of H8R and H8S were inoculated with M. oryzae and leaf samples were collected at each time point after inoculation. RT-PCR was performed 36 cycles for OsBIABP1 using gene-specific primers and 23 cycles for ACTIN1 used as a control
DISCUSSION
The AMP-binding proteins comprise a large family, whose members show diverse biochemical activities/functions, such as peptide antibiotic synthetases and siderophores, polyketide synthetases, 4-coumarate-CoA ligases, acetyl-CoA synthetases (ACSs), and acyl-CoA synthetases in plants (Shockey et al., 2000). In Arabidopsis, at least 44 genes encoding proteins containing the AMP-binding signature motif have been identified (Shockey et al., 2000; 2002). ACSs are the important members in this family because ACSs catalyze biochemically lipid metabolism, whose products have been shown to play crucial roles in a number of plant growth and development processes (Hayshi et al., 2002; Fulda et al., 2002; Schnurr et al., 2000; 2002). OsBIABP1 protein contains a conserved ACS domain and a characteristic AMP-binding signature motif sequence. However, results from phylogenetic tree analysis revealed that OsBIABP1 is much closer to Arabidopsis AMPBPs than to ACSs. It is therefore likely that OsBIABP1 is a member of AMPBPs rather than an ACS in rice. Biochemical activity for most members of the AMPBPs remains unclear. Thus, revealing if OsBIABP1 has ACS activity or not and exploring its novel biochemical activity will be the future challenges.
Analyses of expression patterns have revealed that Arabidopsis AMP binding protein (AMPBP) genes are expressed in a tissue-specific manner. The expression patterns of the AMPBP genes varied widely in different tissues with a high degree of overlap, indicating the complexity of function of this family in Arabidopsis (Shockey et al., 2000; 2002; Schnurr et al., 2000). Pepper CaSIG4 encoding an ACS, rapeseed ACS6 and another ACS gene, were also shown to be expressed in specific tissues, e.g., CaSIG4 in flowers and rapeseed ACS6 in lipogenic tissues, respectively (Fulda et al., 1997; Pongdontri and Hills, 2001; Lee et al., 2001). We found that OsBIABP1 is expressed mainly in aboveground stems, leaves, and flowers, indicating that OsBIABP1 may function in rice growth and development of aboveground parts.
It was recently demonstrated that the AMP-binding proteins, including ACSs, play roles in plant adaption to environment stress. The Arabidopsis ACN1, encoding a peroxisome-localized ACS, is involved in acetate utilization by activating exogenous acetate to the coenzyme A form for entry into the glyoxylate cycle (Turner et al., 2005). The pepper CaSIG4, encoding a plasma membrane-localized ACS, was induced rapidly in leaves after SA treatment and infection with Xanthomonas campestris pv. vesicatoria, suggesting a role in defense response (Lee et al., 2001). Expression of OsBIABP1 can be induced by SA, JA, and ACC, which mediate different plant defense signaling pathways, the SA-dependent pathway and the JA/ET pathway, respectively (Glazebrook, 2005). Importantly, pathogen-induced expression of OsBIABP1 is much quicker and stronger during early stages of pathogenesis in incompatible interaction than in compatible interaction between rice and M. oryzae. These inducible features indicate that OsBIABP1 has a function in defense response through mediating SA- and JA/ET-dependent defense signaling pathways in rice. This is partially supported by recent observations that some defense-related factors can coordinate both SA- and JA-dependent signaling pathways to regulate disease resistance responses (Mei et al., 2006; Qiu et al., 2007).
Lipid metabolism is a complex, highly regulated process that plays essential roles in normal plant growth and development as well as in response to environmental stress. Some classes of AMP-binding proteins, e.g., ACSs and long-chain ACS (LACSs), are directly or indirectly involved in fatty acid metabolism (Hayshi et al., 2002; Fulda et al., 2002; Schnurr et al., 2002; Weimar et al., 2002), which may yield important signaling molecules including the well-known JA. It has been recently shown that fatty acid metabolism-derived signaling plays a critical role in regulation of defense responses against pathogen infection in Arabidopsis (Kachroo et al., 2003; 2004; 2008; Chandra-Shekara et al., 2007). Characterization of OsBIABP1 may provide an opportunity to explore the function of fatty acid metabolism in rice disease resistance responses. Further studies with knockout/knockdown mutants and/or overexpression in transgenic plants will be helpful in understanding of the biological function and the mechanism of OsBIABP1 in rice growth/development and environmental stress responses.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 30771399), the High-Tech Research and Development Program (863) of China (No. 2007AA10Z140) and the Research Fund for the Doctoral Program of Higher Education of the Ministry of Education of China (No. 20070335111)
References
- 1.Beckers GJ, Spoel SH. Fine-tuning plant defence signalling: salicylate versus jasmonate. Plant Biol. 2006;8(1):1–10. doi: 10.1055/s-2005-872705. [DOI] [PubMed] [Google Scholar]
- 2.Cao Y, Song F, Goodman RM, Zheng Z. Molecular characterization of four rice ethylene-responsive element binding protein genes and their expressions in response to biotic and abiotic stresses. J Plant Physiol. 2006;163(11):1167–1178. doi: 10.1016/j.jplph.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Cao Y, Wu Y, Zheng Z, Song F. Overexpression of the rice EREBP-like gene OsBIERF3 enhances disease resistance and salt tolerance in transgenic tobacco. Physiol Mol Plant Pathol. 2006;67(3-5):202–211. doi: 10.1016/j.pmpp.2006.01.004. [DOI] [Google Scholar]
- 4.Cao Y, Yang Y, Zhang H, Li D, Zheng Z, Song F. Overexpression of a rice defense-related F-box protein gene OsDRF1 in tobacco improves disease resistance through potentiation of defense gene expression. Physiol Plant. 2008;134(3):440–452. doi: 10.1111/j.1399-3054.2008.01149.x. [DOI] [PubMed] [Google Scholar]
- 5.Chandra-Shekara AC, Venugopal SC, Barman SR, et al. Plastidial fatty acid levels regulate resistance gene-dependent defense signaling in Arabidopsis. Proc Natl Acad Sci USA. 2007;104(17):7277–7282. doi: 10.1073/pnas.0609259104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chern MS, Fitzgerald HA, Yadav RC, Canlas PE, Dong X, Ronald PC. Evidence for a disease-resistance pathway in rice similar to the NPR1-mediated signaling pathway in Arabidopsis . Plant J. 2001;27(2):101–113. doi: 10.1046/j.1365-313x.2001.01070.x. [DOI] [PubMed] [Google Scholar]
- 7.Chern MS, Fitzgerald HA, Canlas PE, Navarre DA, Ronald PC. Overexpression of a rice NPR1 homolog leads to constitutive activation of defense response and hypersensitivity to light. Mol Plant-Microbe Interact. 2005;18(6):511–520. doi: 10.1094/MPMI-18-0511. [DOI] [PubMed] [Google Scholar]
- 8.Durrant WE, Dong X. Systemic acquired resistance. Annu Rev Phytopathol. 2004;42(1):185–209. doi: 10.1146/annurev.phyto.42.040803.140421. [DOI] [PubMed] [Google Scholar]
- 9.Fitzgerald HA, Chern MS, Navarre R, Ronald PC. Overexpression of (At)NPR1 in rice leads to a BTH- and environment-induced lesion-mimic/cell death phenotype. Mol Plant-Microbe Interact. 2004;17(2):140–151. doi: 10.1094/MPMI.2004.17.2.140. [DOI] [PubMed] [Google Scholar]
- 10.Fulda M, Heinz E, Wolter FP. Brassica napus cDNAs encoding fatty acyl-CoA synthetase. Plant Mol Biol. 1997;33(5):911–922. doi: 10.1023/A:1005780529307. [DOI] [PubMed] [Google Scholar]
- 11.Fulda M, Shockey JM, Werber M, Wolter FP, Heinz E. Two long-chain acyl-CoA synthetases from Arabidopsis thaliana involved in peroxisomal fatty acid β-oxidation. Plant J. 2002;32(1):93–103. doi: 10.1046/j.1365-313X.2002.01405.x. [DOI] [PubMed] [Google Scholar]
- 12.Ge X, Song F, Zheng Z. Systemic acquired resistance to Magnaporthe grisea in rice induced by BTH. Acta Agric Zhejiangensis. 1999;11(4):311–314. (in Chinese) [Google Scholar]
- 13.Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol. 2005;43(1):205–227. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- 14.Grant M, Lamb C. Systemic immunity. Curr Opin Plant Biol. 2006;9(4):414–420. doi: 10.1016/j.pbi.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Hayshi H, de Bellis L, Hayshi Y, Nito K, Kato A, Hayashi M, Hara-Nishimura L, Nishimura M. Molecular characterization of an Arabidopsis acyl-coenzyme A synthetases localized on glyoxysomal membranes. Plant Physiol. 2002;130(4):2019–2026. doi: 10.1104/pp.012955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu X, Song F, Zheng Z. Molecular characterization and expression analysis of a rice protein phosphatase 2C gene, OsBIPP2C1, and overexpression in transgenic tobacco conferred enhanced disease resistance and abiotic tolerance. Physiol Plant. 2006;127(2):225–236. doi: 10.1111/j.1399-3054.2006.00671.x. [DOI] [Google Scholar]
- 17.Kachroo A, Kachroo P. Salicylic acid-, jasmonic acid- and ethylene-mediated regulation of plant defense signaling. Genet Eng. 2007;28:55–83. doi: 10.1007/978-0-387-34504-8_4. [DOI] [PubMed] [Google Scholar]
- 18.Kachroo A, Lapchyk L, Fukushige H, Hildebrand D, Klessig D, Kachroo P. Plastidial fatty acid signaling modulates salicylic acid- and jasmonic acid-mediated defense pathways in the Arabidopsis ssi2 mutant. Plant Cell. 2003;15(12):2952–2965. doi: 10.1105/tpc.017301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kachroo A, Venugopal SC, Lapchyk L, et al. Oleic acid levels regulated by glycerolipid metabolism modulate defense gene expression in Arabidopsis . Proc Natl Acad Sci USA. 2004;101(14):5152–5157. doi: 10.1073/pnas.0401315101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kachroo A, Fu DQ, Havens W, Navarre D, Kachroo P, Ghabrial SA. An oleic acid-mediated pathway induces constitutive defense signaling and enhanced resistance to multiple pathogens in soybean. Mol Plant-Microbe Interact. 2008;21(5):564–575. doi: 10.1094/MPMI-21-5-0564. [DOI] [PubMed] [Google Scholar]
- 21.Lee SJ, Suh MC, Kim S, Kwon JK, Kim M, Paek KH, Choi D, Kim BD. Molecular cloning of a novel pathogen-inducible cDNA encoding a putative acyl-CoA synthetase from Capsicum annuum L. Plant Mol Biol. 2001;46(6):661–671. doi: 10.1023/A:1011677028605. [DOI] [PubMed] [Google Scholar]
- 22.Li D, Liu H, Zhang H, Wang X, Song F. OsBIRH1, a DEAD-box RNA helicase with functions in modulating defence responses against pathogen infection and oxidative stress. J Exp Bot. 2008;59(8):2133–2146. doi: 10.1093/jxb/ern072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu H, Wang X, Zhang H, Yang Y, Ge X, Song F. A rice serine carboxypeptidase-like gene OsBISCPL1 is involved in regulation of defense responses against biotic and oxidative stress. Gene. 2008;420(1):57–65. doi: 10.1016/j.gene.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Zhang H, Yang Y, Li G, Yang Y, Wang X, Basnayake BM, Li D, Song F. Functional analysis reveals pleiotropic effects of rice RING-H2 finger protein gene OsBIRF1 on regulation of growth and defense responses against abiotic and biotic stresses. Plant Mol Biol. 2008;68(1-2):17–30. doi: 10.1007/s11103-008-9349-x. [DOI] [PubMed] [Google Scholar]
- 25.Luo H, Song F, Goodman RM, Zheng Z. Up-regulation of OsBIHD1, a rice gene encoding BELL homeodomain transcriptional factor, in disease resistance responses. Plant Biol. 2005;7(5):459–568. doi: 10.1055/s-2005-865851. [DOI] [PubMed] [Google Scholar]
- 26.Luo H, Song F, Zheng Z. Overexpression in transgenic tobacco reveals different roles for the rice homeodomain gene OsBIHD1 in biotic and abiotic stress responses. J Exp Bot. 2005;56(420):2673–2682. doi: 10.1093/jxb/eri260. [DOI] [PubMed] [Google Scholar]
- 27.Mei C, Qi M, Sheng G, Yang Y. Inducible overexpression of a rice allene oxide synthase gene increases the endogenous jasmonic acid level, PR gene expression, and host resistance to fungal infection. Mol Plant-Microbe Interact. 2006;19(10):1127–1137. doi: 10.1094/MPMI-19-1127. [DOI] [PubMed] [Google Scholar]
- 28.Pongdontri P, Hills M. Characterization of a novel plant acyl-coA synthetase that is expressed in lipogenic tissues of Brassica napus L. Plant Mol Biol. 2001;47(6):717–726. doi: 10.1023/A:1013652014744. [DOI] [PubMed] [Google Scholar]
- 29.Qiu D, Xiao J, Ding X, Xiong M, Cai M, Cao Y, Li X, Xu C, Wang S. OsWRKY13 mediates rice disease resistance by regulating defense-related genes in salicylate- and jasmonate-dependent signaling. Mol Plant-Microbe Interact. 2007;20(5):492–499. doi: 10.1094/MPMI-20-5-0492. [DOI] [PubMed] [Google Scholar]
- 30.Schnurr JA, Shockey J, Browse J. Characterization of an acyl-CoA synthetase from Arabidopsis thaliana . Biochem Soc Trans. 2000;28(6):957–958. doi: 10.1042/BST0280957. [DOI] [PubMed] [Google Scholar]
- 31.Schnurr JA, Shockey JM, de Boer G, Browse JA. Fatty acid export from the chloroplast. Molecular characterization of a major plastidial acyl-coenzayme A synthetase from Arabidopsis . Plant Physiol. 2002;129(4):1700–1709. doi: 10.1104/pp.003251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shockey J, Schnurr J, Browse J. Characterization of the AMP-binding protein gene family in Arabidopsis thaliana: will the real acyl-CoA synthetases please stand up? Biochem Soc Trans. 2000;28(6):955–957. doi: 10.1042/BST0280955. [DOI] [PubMed] [Google Scholar]
- 33.Shockey JM, Fulda MS, Browse JA. Arabidopsis contains nine long-chain Acyl-Coenzyme A synthetase genes that participate in fatty acid and glycerolipid metabolism. Plant Physiol. 2002;129(4):1710–1722. doi: 10.1104/pp.003269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song D, Song F, Zheng Z. A novel rice MAPK gene, OsBIMK2, is involved in disease resistance responses. Plant Biol. 2006;8(5):587–596. doi: 10.1055/s-2006-924149. [DOI] [PubMed] [Google Scholar]
- 35.Song F, Goodman RM. Molecular biology of disease resistance in rice. Physiol Mol Plant Pathol. 2001;59(1):1–11. doi: 10.1006/pmpp.2001.0353. [DOI] [Google Scholar]
- 36.Song F, Goodman RM. OsBIMK1, a rice MAP kinase gene involved in disease resistance responses. Planta. 2002;215(6):997–1005. doi: 10.1007/s00425-002-0794-5. [DOI] [PubMed] [Google Scholar]
- 37.Song FM, Goodman RM. Molecular cloning and characterization of a rice phosphoinositide-specific phospholipase C gene, OsPI-PLC1 that is activated in systemic acquired resistance. Physiol Mol Plant Pathol. 2002;61(1):31–40. doi: 10.1006/pmpp.2002.0414. [DOI] [Google Scholar]
- 38.Song F, Ge X, Zheng Z, Xie Y. Benzothiadiazole induces systemic acquired resistance in rice against bacterial leaf blight. Chin J Rice Sci. 2001;15(4):323–326. (in Chinese) [Google Scholar]
- 39.Tao Y, Xie Z, Chen W, Glazebrook J, Chang HS, Han B, Zhu T, Zou G, Katagiri F. Quantitative nature of Arabidopsis responses during compatible and incompatible interactions with the bacterial pathogen Pseudomonas syringae . Plant Cell. 2003;15(2):317–330. doi: 10.1105/tpc.007591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turner JE, Greville K, Murphy EC, Hooks MA. Characterization of Arabidopsis fluoroacetate-resistant mutants reveals the principal mechanism of acetate activation for entry into the glyoxylate cycle. J Biol Chem. 2005;280(4):2780–2787. doi: 10.1074/jbc.M407291200. [DOI] [PubMed] [Google Scholar]
- 41.Wasternack C. Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot. 2007;100(4):681–697. doi: 10.1093/aob/mcm079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weimar JD, Dirusso CC, Dilio R, Black PN. Functional role of fatty acyl-Coenzyme A synthetase in the transmembrane movement and activation of exogenous long-chain fatty acids. J Biol Chem. 2002;277(33):29369–29376. doi: 10.1074/jbc.M107022200. [DOI] [PubMed] [Google Scholar]
- 43.Yara A, Yaeno T, Hasegawa M, Seto H, Montillet JL, Kusumi K, Seo S, Iba K. Disease resistance against Magnaporthe grisea is enhanced in transgenic rice with suppression of omega-3 fatty acid desaturases. Plant Cell Physiol. 2007;48(9):1263–1274. doi: 10.1093/pcp/pcm107. [DOI] [PubMed] [Google Scholar]
- 44.Yuan Y, Zhong S, Li Q, Zhu Z, Lou Y, Wang L, Wang J, Wang M, Li Q, Yang D, et al. Functional analysis of rice NPR1-like genes reveals that OsNPR1/NH1 is the rice orthologue conferring disease resistance with enhanced herbivore susceptibility. Plant Biotechnol J. 2007;5(2):313–324. doi: 10.1111/j.1467-7652.2007.00243.x. [DOI] [PubMed] [Google Scholar]
- 45.Zhang W, Ge X, Song F, Zheng Z. Systemic acquired resistance of rice against sheath blight disease induced by benzothiadiazole. Acta Phytophylacica Sin. 2004;30(2):171–176. (in Chinese) [Google Scholar]
- 46.Zhang W, Chen J, Zhang H, Song F. Overexpression of a rice diacylglycerol kinase gene OsBIDK1 enhances disease resistance in transgenic tobacco. Mol Cells. 2008;26(3):258–264. [PubMed] [Google Scholar]