Abstract
Objective: We compare the cardioprotective effects of anesthetic preconditioning by propofol and/or isoflurane in rats with ischemia-reperfusion injury. Methods: Male adult Wistar rats were subjected to 60 min of anterior descending coronary artery occlusion followed by 120 min of reperfusion. Before the long ischemia, anesthetics were administered twice for 10 min followed by 5 min washout. Isoflurane was inhaled at 1 MAC (0.016) in I group, whereas propofol was inhaled intravenously at 37.5 mg/(kg·h) in P group. A combination of isoflurane and propofol was administered simultaneously in I+P group. Results: In control (without anesthetic preconditioning, C group), remarkable myocardial infarction and apoptosis accompanied by an increased level of cardiac troponin T were noted 120 min after ischemia-reperfusion. As compared to those of control group, I and P groups had comparable cardioprotection. In addition, I+P group shares with I and P groups the comparable cardioprotective effects in terms of myocardial infarction and cardiac troponin T elevation. Conclusion: A combination of isoflurane and propofol produced no additional cardioprotection.
Keywords: Anesthetic, Heart, Propofol, Isoflurane, Apoptosis
INTRODUCTION
Myocardial ischemic injury is a potential perioperative threat. Ischemia possesses two sides of myocardial properties ranging from the cell death on one side to beneficial effects of preconditioning on the other side. Preconditioning agents such as inhalation and intravenous anesthetics induce a pronounced cardioprotection and thus may decrease the deleterious effects of myocardial ischemia in perioperative medicine. Indeed, many human studies (de Hert et al., 2002; 2004; Conzen et al., 2003) and animal experiments (Novalija et al., 1999; Warltier et al., 1988) have demonstrated that different anesthetic regimens provide cardioprotection in cardiac surgery by mimicking ischemic preconditioning.
Propofol (2,6-diisopropylphenol) is a lipid-soluble anesthetic agent that has a chemical structure similar to that of phenol-based free radical scavengers such as vitamin E (Aarts et al., 1995; Kokita et al., 1998). It is commonly used as an intravenous anesthetic in cardiac surgery and has been shown to decrease postischemic myocardial dysfunction (Kokita and Hara, 1996), infarct size (Ebel et al., 1999), and histologic degeneration (Ko et al., 1997).
In the absence of myocardial ischemia, the common inhalation anesthetic isoflurane reduces infarct size in animal studies by causing opening of KATP channels (Kersten et al., 1996; 1997). Before aortic cross-clamping in patients undergoing coronary artery bypass surgery, pretreatment with isoflurane improves cardiopulmonary bypass outcome (Haroun-Bizri et al., 2001). In addition, preconditioning isoflurane may offer some additional cardioprotection in coronary artery bypass graft surgery (Belhomme et al., 1999).
The present work has two purposes. The first is to assess the cardioprotective effects of propofol versus isoflurane preconditioning in rats with myocardial ischemia-reperfusion. The second is to determine whether combined propofol and isoflurane anesthesia is able to provide additional cardioprotection in rats with myocardial ischemia-reperfusion.
MATERIALS AND METHODS
This study was conducted in accordance with our institutional guidelines on the use of live animals for research and the experimental protocol was approved by the Animal Care and Use Committee of our medical center laboratory.
Surgical preparation
Male Wistar rats (Academy of Military Medical Sciences Center for Laboratory Animal, Beijing, China) with body weight of 250~300 g were used. The rats were anesthetized by intraperitoneal administration of urethane (Sigma, St. Louis, Missouri, USA). The left femoral vein was cannulated with a polyethylene (PE50)-tubing for fluid or drug administration. Sodium lactate Ringer’s solution (China Otsuka Pharmaceutical Co., Ltd, Beijing, China) was infused continuously at a rate of 4 ml/h for the entire period of experimentation. Needle electrodes were embedded subcutaneously in a lead II configuration to monitor electrocardiogram (ECG). The rats were artificially ventilated after orotracheal intubations. Arterial pH and P CO2 were maintained within normal physiological limits by adjusting the respiratory rate or tidal volume. A catheter connecting to a pressure transducer for left ventricular pressure reading was placed into the left ventricle through the right carotid artery. A thoracotomy was performed at the left second intercostal space and the heart was suspended in a pericardial cradle. A prominent branch of the left anterior descending (LAD) of coronary artery was identified, and a 5-0–prolene ligature snare was looped around LAD of coronary artery inferior to the left atrial appendage for later occlusion. Coronary occlusion was achieved by tightening the snare. The presence of epicardial cyanosis and ST-segment elevation in the ECG verified myocardial ischemia. Reperfusion was achieved by releasing the snare and was confirmed by visual observation of myocardial reactive hyperemia.
Experimental design
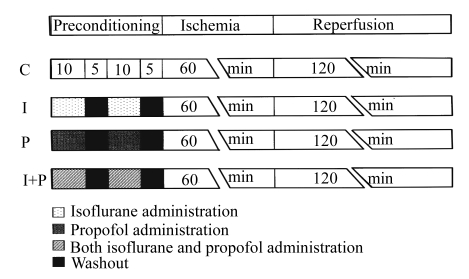
The experimental design is illustrated in Fig.1. A 30-min period was demanded for stabilization after the snare around LAD of coronary artery was performed. All the rats underwent a 60-min LAD occlusion (ischemia) followed by 2 h of reperfusion. Preconditioning was elicited before the onset of myocardial ischemia. Rats were randomly divided into 4 groups. In control (C) group, all rats received 60 min of ischemia followed by 120 min of reperfusion. In I group, all animals received 2 cycles of 10-min inhalation of isoflurane (Abbott Laboratories, Illinois, USA) at concentration of 1.4% (w/v) followed by a 5-min washout, which corresponds to 1.0 minimum alveolar concentration (MAC) (Quasha et al., 1980). The dose of isoflurane was adopted from previous studies (Ludwig et al., 2004; Toller et al., 2000), which used this anesthetic for the purpose of preconditioning. In P group, all rats received an intravenous dose of propofol (37.5 mg/(kg·h), Fresenius Kabi Austria GmbH, Austria) twice for 10 min followed by a 5-min washout, which corresponds to 6 mg/(kg·h) for clinical patients. In I+P group, all rats received 2 cycles of 10-min inhalation of isoflurane and 2 intravenous infusion of propofol for 10 min followed by a 5-min washout.
Fig.1.
Experimental protocols. C: control group without any intervention; I: isoflurane preconditioning group which received isoflurane (1.6%, w/v) twice for 10 min followed by 5 min washout; P: propofol preconditioning group which received propofol 37.5 mg/(kg·h) twice for 10 min followed by 5 min washout; I+P: isoflurane plus propofol preconditioning group which received isoflurane (1.6%, w/v) and propofol 37.5 mg/(kg·h) twice for 10 min followed by 5 min washout simultaneously. All rats underwent 60 min of coronary artery occlusion followed by 2 h of reperfusion
Cardiovascular parameter measurements
All the variables were recorded at the following time points: t 1: baseline (before the preconditioning; 30 min after stabilization); t 2: treatment (before LAD occlusion, 30 min after preconditioning); t 3: 60 min ischemia; t 4: 120 min reperfusion.
Global systolic function was measured in terms of left ventricular systolic pressure (LVSP) and maximum rate of rise of left ventricular pressure dP/dt (dP/dt max). Global left ventricular end-systole was defined as the point of minimum dP/dt (dP/dt min) and left ventricular end-diastole pressure (LVDP) as the beginning of the sharp upslope of the LV dP/dt tracing. Both mean arterial pressure (MAP) and heart rate (HR) were all continuously recorded.
Determination of myocardial infarction
At the end of experiments, the LAD of coronary artery was reoccluded and 2 ml of 0.6% (w/v) Evans blue dye was infused via the left atrial appendage. This maneuver identified the area at risk (AAR) for infarction that remained unstained. Meanwhile, the normally perfused zone was stained in deep blue. The heart was excised quickly and frozen for 1 h at −20 °C. Then the left ventricles separated from the heart were cut into transverse slices of equal thickness (1 mm) and taken photographs. The slices were incubated in 10% (w/v) 2,3,5-triphenyltetrazalium chloride (TTC) for 20 min at 37 °C. These slices were then placed in 10% (w/v) neutral buffered formalin for 48 h to increase the contrast between stained and unattained tissues. Because TTC was hydrogenised by dehydrogenase of viable myocardium in the AAR, it was stained in deep red, whereas the tissue of infarct area appeared pale grey. The slices were photographed again. The infarct area and the AAR were determined by planimetry using Adobe Photoshop 8.0 computer software. Myocardial infarct size was expressed as a percentage of infarct area in the AAR as follows:
| Infarct size (%)=(∑Infarct area/∑AAR)×100%. |
Cardiac troponin T (cTnT) measurement
cTnT measurement was conducted at the following time points: t 1, t 2, t 3 and t 4. Measurement of cTnT was done by the hospital clinical chemistry laboratory, and the analysts were unaware of the conduct of this study.
Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) assay
TUNEL assay was performed using the myocardial sections for histological verification. Color was developed using 3,3-diaminobenzidine tetrachloride (DAB, Sigma, USA). Sections were treated with xylene and ethanol to remove paraffin and for dehydration. They were then washed with phosphate buffered solution (PBS) and incubated in 3% (v/v) H2O2 solution for 20 min. The sections were treated with 5 μg/ml proteinase K for 2 min at room temperature, and re-washed with PBS (1 mol/L, pH 7.4). The sections were then treated with a TUNEL reaction mixture (Roche Mannhein, Germany) at 37 °C for 1 h, and then were washed with distilled water. They were then incubated in anti-fluorescein antibody conjugated with horse-radish peroxidase (HRP) at room temperature for 30 min, washed, and then using the apoptosis detection system fluorescence kit (Promega, WI, USA) according to the manufacturer’s instructions. The nuclei of apoptotic and non-apoptotic cells were counterstained with 0.05% (w/v) DAB as a chromogen. The labeled cells were analyzed by fluorescence microscopy. Each section was evaluated independently without knowledge of the experimental conditions.
Statistical analysis
Statistical analysis was performed using SPSS 12.0 for windows software. Results are expressed as mean±standard deviation (SD). Data of myocardial function over time within each group were analyzed using analysis of variance (ANOVA) with repeated measures on one factor. Differences between groups were analyzed using a one-way ANOVA. If an overall significance among groups was found, least significant difference (LSD) for intergroup comparisons was performed. Changes within and between groups were considered statistically significant with P<0.05.
RESULTS
Hemodynamic parameters
Table 1 summarizes the levels of hemodynamic parameters for the four groups of animals at four time points. All the levels of hemodynamic parameters did not differ among groups throughout the observation interval. There was no significant intra- or intergroup difference in HR, MAP, LVSP, LVDP, dP/dt max, or dP/dt min over time (Table 1).
Table 1.
Indices of cardiac contractile function (mean±SD)
| HR (beat per min) |
MAP (mmHg) |
LVSP (mmHg) |
LVDP (mmHg) |
dP/dtmin (mmHg/ms) |
dP/dtmax (mmHg/ms) |
|||||||||||||||||||
| C | I | P | I+P | C | I | P | I+P | C | I | P | I+P | C | I | P | I+P | C | I | P | I+P | C | I | P | I+P | |
| t1 | 434±29 | 429±26 | 424±24 | 414±32 | 85±8 | 92±11 | 88±10 | 93±11 | 116±7 | 124±5 | 115±14 | 121±13 | 0.5±1.5 | −0.3±2.4 | 0.1±1.9 | 0.7±1.4 | −4.1±0.6 | −4.9±0.5 | −4.2±1.0 | −4.5±0.9 | 6.4±1.0 | 6.3±0.8 | 6.7±1.0 | 6.3±1.2 |
| t2 | 407±59 | 425±40 | 415±27 | 407±24 | 83±8 | 87±6 | 87±15 | 93±14 | 113±15 | 114±12 | 128±11 | 123±7 | 1.2±0.9 | 0.5±1.5 | 0.7±1.7 | 1.2±1.8 | −4.3±1.7 | −4.9±0.6 | −4.8±0.8 | −4.9±0.6 | 5.7±2.0 | 6.4±0.8 | 6.5±1.5 | 6.1±0.7 |
| t3 | 397±62 | 406±41 | 393±32 | 394±24 | 86±8 | 89±18 | 88±13 | 99±12 | 111±16 | 108±15 | 115±9 | 120±8 | 0.8±0.8 | −0.3±1.2 | −0.8±2.6 | 0.2±1.6 | −3.9±1.7 | −4.5±0.8 | −4.6±0.7 | −4.7±0.4 | 5.5±2.1 | 5.8±1.1 | 5.8±1.1 | 6.0±0.6 |
| t4 | 410±45 | 401±43 | 402±31 | 391±35 | 75±18 | 84±12 | 79±12 | 81±13 | 112±8 | 112±15 | 118±14 | 117±14 | 1.2±1.1 | −0.6±1.8 | 0.2±2.6 | 0.9±3.2 | −4.2±1.2 | −4.8±0.8 | −4.8±1.0 | −4.7±0.8 | 5.8±1.4 | 6.0±1.1 | 6.0±1.5 | 5.8±1.1 |
t 1 (baseline)=30 min after stabilization; t 2 (treatment)=30 min after preconditioning or start of coronary artery occlusion; t 3 (1-h ischemia)=1 h after ischemia; t 4 (2-h reperfusion)=2 h after reperfusion
Cardiac troponin T (cTnT) levels
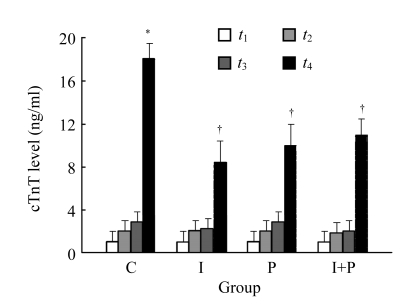
Fig.2 shows the plasma levels of cTnT for the four groups at four time points. In C group, the cTnT values at t 4 were significantly higher than those at t 1 (P<0.01). In addition, the values of plasma cTnT obtained at t 4 for I or P group were significantly lower than those for C group (P<0.01). However, as compared to those of I or P group at t 4, I+P group had insignificant difference (P>0.05). In terms of cTnT, there was an insignificant difference between I-t 4 and P-t 4 group (P>0.05).
Fig.2.
The plasma levels of myocardial troponin (cTnT) for the four groups of animals at the four time points: t 1, 30 min after stabilization; t 2, immediately before the start of coronary artery occlusion; t 3, 60 min after the start of coronary artery occlusion; and t 4, 120 min after the start of reperfusion. Data are mean±SD of 8 rats per group. * P<0.01, compared with C group at t 1; † P<0.01, compared with C group t 4. The assay was performed in duplicate and averaged
Myocardial infarct size and TUNEL-positive cell numbers
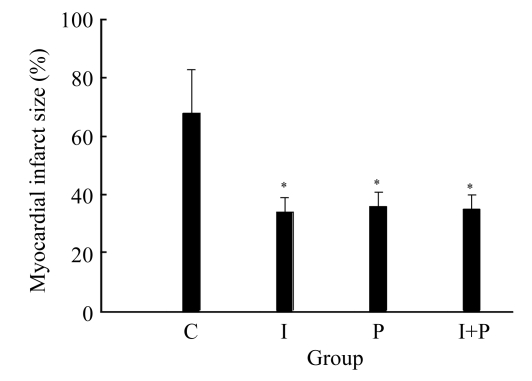
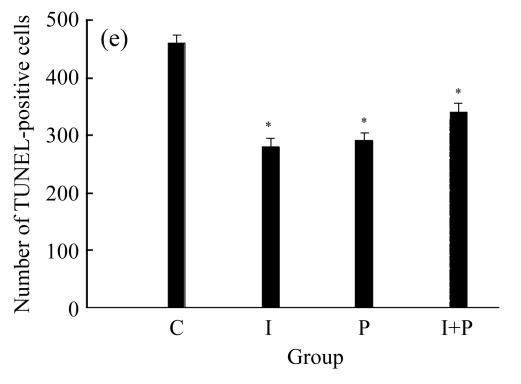
Figs.3 and 4 show the myocardial infarct size and TUNEL-positive cells numbers, respectively, for the four groups obtained at 120 min after the start of reperfusion. It can be seen from these two figures that the values of both myocardial infarct size and TUNEL-positive cells numbers for either I or P group were significantly lower than those of C group (P<0.01). However, the values of both myocardial infarct size and TUNEL-positive cells numbers for I+P group were indifferent from those of I or P group (P>0.05). Again, in terms of myocardial infarct size or TUNEL-positive myocyte numbers, there was an insignificant difference between I and P group (P>0.05).
Fig.3.
The myocardial infarct size in the four groups of animals at the time point of 120 min after the start of reperfusion (t 4). * P<0.01, compared with C group. Data are mean±SD of 8 rats per group
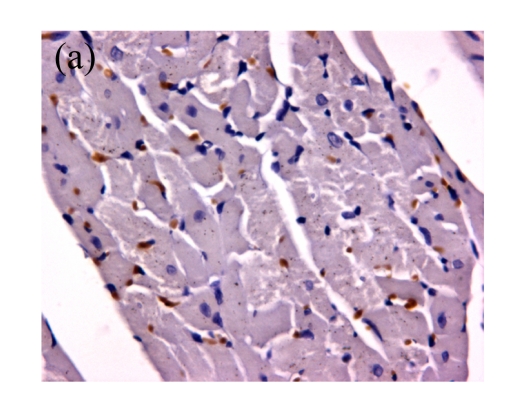
Fig.4.
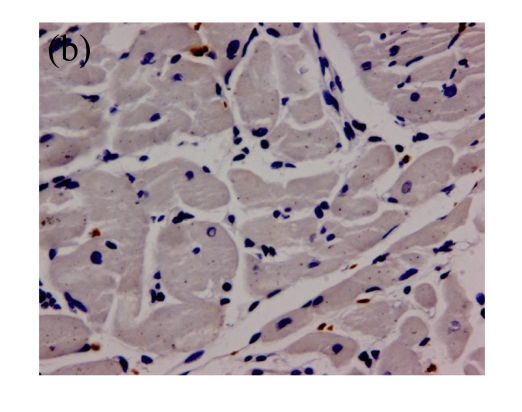
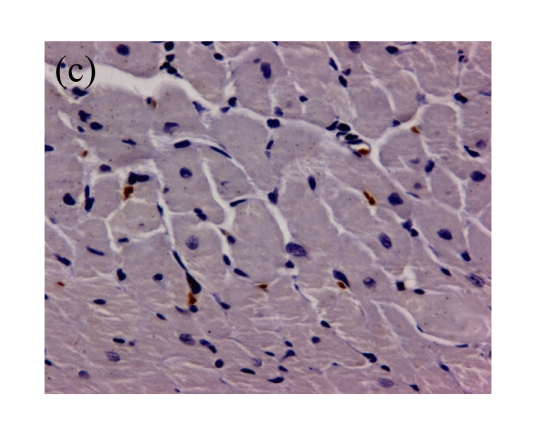
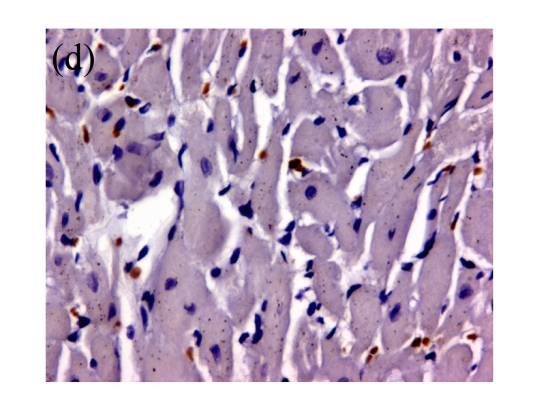
(a)~(d) Representative photomicrographs depicting TUNEL-stained left ventricular tissue sections from (a) a control rat without any intervention (C group), (b) an isoflurane preconditioning rat (I group), (c) a propofol preconditioning rat (P group), and (d) an isoflurane plus propofol preconditioning rat (I+P group). The myocardial TUNEL was performed at the time point of 120 min after the start of reperfusion. (e) Quantitative data are summarized with the number of TUNEL nuclei expressed at 30 fields per section. * P<0.01, compared with C group. Data are mean±SD of 8 rats per group
DISCUSSION
In the present study, we show that propofol-based intravenous regimen or inhalation anesthesia with isoflurane appears to provide equal myocardial protection in myocardial ischemia-reperfusion model when using plasma levels of cTnT, the number of myocardial apoptotic cells, and myocardial infarct size as indices of myocardial damage. Our study confirms the results of several previous studies that pretreatment with isoflurane (Cason et al., 1997; Ismaeil et al., 1999; Piriou et al., 2000; Roscoe et al., 2000) or propofol (Ebel et al., 1999; Ko et al., 1997; Kokita and Hara, 1996) protects the myocardium from a subsequent prolonged ischemia and thus mimics the cardioprotective effects of ischemic preconditioning. In addition, we report that the combined isoflurane and propofol pretreatment does not produce additional cardioprotection during ischemia-reperfusion.
Several mechanisms have been proposed for isoflurane-induced preconditioning. For example, blocking adenosine (A1) receptors abolished isoflurane-induced cardioprotection against myocardial stunning (Kersten et al., 1996) and infarction in rabbits (Ismaeil et al., 1999) and human atrial trabecular muscle (Roscoe et al., 2000). Isoflurane-induced preconditioning was prevented by 5-hydroxydeanoate and gadolinium, suggesting the involvement of mechanogated channels in this phenomenon (Piriou et al., 2000). Administration of KATP channel blocker before isoflurane administration completely blocked the cardioprotection (Tanaka et al., 2003). Isoflurane preconditioning may act via release of small quantities of free radicals to protect myocardium against infarction in rabbits (Mullenheim et al., 2002; Tanaka et al., 2002). Radicals are released from the mitochondria as a consequence of KATP channel opening (Duranteau et al., 1998; Kowaltowski et al., 2001; McPherson and Yao, 2001a). Activation of opiate receptors by morphine leads to activation of mitochondrial KATP channels followed by an increase of intracellular free radical formation (McPherson and Yao, 2001a; 2001b). Furthermore, protein kinase C can be activated by free radicals (Gopalakrishna and Anderson, 1989). Thus, it is likely that protein kinase C is an important factor in the signal transduction cascade of isoflurane preconditioning (Baines et al., 1999).
Other lines of evidence have accumulated to suggest that large quantities of reactive oxygen species released during reperfusion after coronary artery occlusion damage proteins responsible for intracellular homeostasis, produce tissue injury (Ambrosio et al., 1993; Bolli et al., 1988; Zweier et al., 1987), depress contractile function, and increase myocardial infarct size. Propofol, a free radical scavenger, is being used increasingly for cardiac anesthesia and has been known to decrease postischemic myocardial mechanical dysfunction, infarct size, and histological degeneration (Ebel et al., 1999; Ko et al., 1997; Kokita and Hara, 1996). Propofol infusion during the reperfusion period produced a cardioprotective effect and inhibited apoptosis of cardiomyocytes in the ischemia-reperfusion model, with prolonged cold ischemia, in isolated rat hearts (Choi et al., 2007). Propofol also suppresses the activity of neutrophils, and may therefore produce its beneficial effects by reducing free radicals, Ca2+ influx, and neutrophil activity (Scarabelli et al., 2001). It has been shown that levels of systemic cytokines, such as tumor necrosis factor-α, increased after cardiopulmonary bypass (Tomasdottir et al., 2003). It has also been shown that propofol can attenuate apoptosis in tumor necrosis factor-α-induced endothelial cells and enhance the bioavailability of nitric oxide (Luo et al., 2005). Coronary endothelial cell apoptosis precedes myocyte apoptosis (Sayin et al., 2002). Hence, attenuation of vascular endothelial cell apoptosis by propofol could have resulted in reduced cardiomyocytes apoptosis during myocardial ischemia and reperfusion. Other studies have failed to show a protective effect on myocardial function during ischemia and reperfusion (Ebel et al., 1999).
It can be derived from the afore-mentioned descriptions that isoflurane inhalation preconditioning is mainly mediated by activation of KATP channels in coupled with stimulation of adenosine receptors. On the other hand, intravenous propofol preconditioning may be mainly mediated by scavenging free radicals and attenuated systemic inflammation (or overproduction of several pro-inflammatory cytokines).
The present results show that isoflurane and propofol, although having different cardioprotective mechanisms, appear to provide equal myocardial protection in the myocardial ischemia-reperfusion model when using cTnT, and myocardial apoptosis and infarct size as markers of myocardial damage in rats. Furthermore, the current study provides data to show that the combined isoflurane and propofol preconditioning does not produce additional cardioprotection during myocardial ischemia-reperfusion.
In fact, the contention that volatile anesthetic and intravenous anesthetic provide equal cardioprotection during myocardial ischemia-reperfusion in rats is not supported by many other investigators. For example, sevoflurane has been found to provide greater protection of the myocardium than propofol in patients undergoing off-pump coronary artery bypass surgery (Conzen et al., 2003). Sevoflurane shows potential to be superior to propofol in porcine animal model of severe acute ischemia induced by occlusion of the thoracic aorta (Annecke et al., 2007). The discrepancy may be explained by species difference.
The current results show that propofol did not cancel the cardioprotective effects of isoflurane preconditioning. As mentioned in the former section, isoflurane preconditioning may rely on the release of small quantity of free radicals, which may be scavenged by propofol. Propofol cardioprotection is dose-dependent both in the clinics (Xia et al., 2006) and experiments (Xia et al., 2004). It is likely that in the joint isoflurane-propofol group, the protective effects observed may solely come from propofol cardioprotection via its antioxidant property, providing that the dose of propofol used is high enough.
Cardiomyocyte death caused by apoptosis is a primary factor in the pathogenesis of ischemia and reperfusion (van Empel et al., 2005). In the ischemia-reperfusion model described in this paper, only the increased TUNEL-positive cells were observed during ischemia-reperfusion procedure. Other definite sequence of apoptotic events such as caspase-3 activation, mitochondrial damage, or an increasing number of dead cardiac myocytes in response to ischemia and reperfusion was not provided. Nevertheless, as shown in the current study, the increased TUNEL-positive cardiomyocytes were significantly reduced by ischemia and reperfusion preconditioning.
CONCLUSION
Our results demonstrate that isoflurane inhalation or intravenous propofol protects the myocardium from a subsequent prolonged ischemia and mimics the cardioprotective effects of ischemic preconditioning. Isoflurane shares with propofol the same preconditioning potential in preventing myocardial ischemia-reperfusion injury. Furthermore, the combined isoflurane and propofol pretreatment causes no additional preconditioning.
Acknowledgments
We would like to thank Prof. Chih-Chung Wang, Chi Mei Medical Center, Tainan, Taiwan, China for extensive helpful discussion.
References
- 1.Aarts L, van der Hee R, Dekker I, de Jong J, Langemeijer H, Bast A. The widely used anesthetic agent propofol can replace alpha-tocopherol as an antioxidant. FEBS Lett. 1995;357(1):83–85. doi: 10.1016/0014-5793(94)01337-Z. [DOI] [PubMed] [Google Scholar]
- 2.Ambrosio G, Zweier JL, Duilio C, Kuppusamy P, Santoro G, Elia PP, Tritto I, Cirillo P, Condorelli M, Chiariello M. Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. J Biol Chem. 1993;268(25):18532–18541. [PubMed] [Google Scholar]
- 3.Annecke T, Kubitz JC, Kahr S, Hilberath JM, Langer K, Kemming GI, Rehm M, Bittmann I, Conzen PF. Effects of sevoflurane and propofol on ischaemia-reperfusion injury after thoracic-aortic occlusion in pigs. Br J Anaesth. 2007;98(5):581–590. doi: 10.1093/bja/aem049. [DOI] [PubMed] [Google Scholar]
- 4.Baines CP, Cohen MV, Downey JM. Signal transduction in ischemic preconditioning: the role of kinases and mitochondrial K(ATP) channels. J Cardiovasc Electrophysiol. 1999;10(5):741–754. doi: 10.1111/j.1540-8167.1999.tb00251.x. [DOI] [PubMed] [Google Scholar]
- 5.Belhomme D, Peynet J, Louzy M, Launay JM, Kitakaze M, Menasché P. Evidence for preconditioning by isoflurane in coronary artery bypass graft surgery. Circulation. 1999;100(19 Suppl.):11340–11344. doi: 10.1161/01.cir.100.suppl_2.ii-340. [DOI] [PubMed] [Google Scholar]
- 6.Bolli R, Patel BS, Jeroudi MO, Lai EK, McCay PB. Demonstration of free radical generation in “stunned” myocardium of intact dogs with the use of the spin trap alpha-phenyl N-tert-butyl nitrone. J Clin Invest. 1988;82(2):476–485. doi: 10.1172/JCI113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cason BA, Gamperl AK, Slocum RE, Hickey RF. Anesthetic-induced preconditioning: previous administration of isoflurane decreases myocardial infarct size in rabbits. Anesthesiology. 1997;87(5):1182–1190. doi: 10.1097/00000542-199711000-00023. [DOI] [PubMed] [Google Scholar]
- 8.Choi SU, Lee HW, Lim HJ, Yoon SM, Chang SH. The effects of propofol on cardiac function after 4 hours of cold cardioplegia and reperfusion. J Cardiothorac Vasc Anesth. 2007;21(5):678–682. doi: 10.1053/j.jvca.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 9.Conzen PF, Fischer S, Detter C, Peter K. Sevoflurane provides greater protection of the myocardium than propofol in patients undergoing off-pump coronary artery bypass surgery. Anesthesiology. 2003;99(4):826–833. doi: 10.1097/00000542-200310000-00013. [DOI] [PubMed] [Google Scholar]
- 10.de Hert SG, ten Broecke PW, Mertens E, van Sommeren EW, de Blier IG, Stockman BA, Rodrigus IE. Sevoflurane but not propofol preserves myocardial function in coronary surgery patients. Anesthesiology. 2002;97(1):42–49. doi: 10.1097/00000542-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 11.de Hert SG, van der Linden PJ, Cromheecke S, Meeus R, Nelis A, van Reeth V, ten Broecke PW, de Blier IG, Stockman BA, Rodrigus IE. Cardioprotective properties of sevoflurane in patients undergoing coronary surgery with cardiopulmonary bypass are related to the modalities of its administration. Anesthesiology. 2004;101(2):299–310. doi: 10.1097/00000542-200408000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Duranteau J, Chandel NS, Kulisz A, Shao Z, Schumacker PT. Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J Biol Chem. 1998;273(19):11619–11624. doi: 10.1074/jbc.273.19.11619. [DOI] [PubMed] [Google Scholar]
- 13.Ebel D, Schlack W, Comfere T, Preckel B, Thamer V. Effect of propofol on reperfusion injury after regional ischaemia in the isolated rat heart. Br J Anaesth. 1999;83(6):903–908. doi: 10.1093/bja/83.6.903. [DOI] [PubMed] [Google Scholar]
- 14.Gopalakrishna R, Anderson WB. Ca2+- and phospholipid-independent activation of protein kinase C by selective oxidative modification of the regulatory domain. Proc Natl Acad Sci USA. 1989;86(17):6758–6762. doi: 10.1073/pnas.86.17.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haroun-Bizri S, Khoury SS, Chehab IR, Kassas CM, Baraka A. Does isoflurane optimize myocardial protection during cardiopulmonary bypass? J Cardiothorac Vasc Anesth. 2001;15(4):418–421. doi: 10.1053/jcan.2001.24954. [DOI] [PubMed] [Google Scholar]
- 16.Ismaeil MS, Tkachenko I, Gamperl AK, Hickey RF, Cason BA. Mechanisms of isoflurane-induced myocardial preconditioning in rabbits. Anesthesiology. 1999;90(3):812–821. doi: 10.1097/00000542-199903000-00024. [DOI] [PubMed] [Google Scholar]
- 17.Kersten JR, Schmeling TJ, Hettrick DA, Pagel PS, Gross GJ, Warltier DC. Mechanism of myocardial protection by isoflurane. Role of adenosine triphosphate-regulated potassium (KATP) channels. Anesthesiology. 1996;85(4):794–807. doi: 10.1097/00000542-199610000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Kersten JR, Schmeling TJ, Pagel PS, Gross GJ, Warltier DC. Isoflurane mimics ischemic preconditioning via activation of K(ATP) channels: reduction of myocardial infarct size with an acute memory. Anesthesiology. 1997;87(2):361–370. doi: 10.1097/00000542-199708000-00024. [DOI] [PubMed] [Google Scholar]
- 19.Ko SH, Yu CW, Lee SK, Choe H, Chung MJ, Kwak YG, Chae SW, Song HS. Propofol attenuates ischemia-reperfusion injury in the isolated rat heart. Anesth Analg. 1997;85(4):719–724. doi: 10.1097/00000539-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Kokita N, Hara A. Propofol attenuates hydrogen peroxide-induced mechanical and metabolic derangements in the isolated rat heart. Anesthesiology. 1996;84(1):117–127. doi: 10.1097/00000542-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Kokita N, Hara A, Abiko Y, Arakawa J, Hashizume H, Namiki A. Propofol improves functional and metabolic recovery in ischemic reperfused isolated rat hearts. Anesth Analg. 1998;86(2):252–258. doi: 10.1097/00000539-199802000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Kowaltowski AJ, Seetharaman S, Paucek P, Garlid KD. Bioenergetic consequences of opening the ATP-sensitive K(+) channel of heart mitochondria. Am J Physiol Heart Circ Physiol. 2001;280(2):H649–H657. doi: 10.1152/ajpheart.2001.280.2.H649. [DOI] [PubMed] [Google Scholar]
- 23.Ludwig LM, Tanaka K, Eells JT, Weihrauch D, Pagel PS, Kersten JR, Warltier DC. Preconditioning by isoflurane is mediated by reactive oxygen species generated from mitochondrial electron transport chain complex III. Anesth Analg. 2004;99(5):1308–1315. doi: 10.1213/01.ANE.0000134804.09484.5D. [DOI] [PubMed] [Google Scholar]
- 24.Luo T, Xia Z, Ansley DM, Ouyang J, Granville DJ, Li Y, Xia ZY, Zhou QS, Liu XY. Propofol dose-dependently reduces tumor necrosis factor-alpha-induced human umbilical vein endothelial cell apoptosis: effects on Bcl-2 and Bax expression and nitric oxide generation. Anesth Analg. 2005;100(6):1653–1659. doi: 10.1213/01.ANE.0000150945.95254.D8. [DOI] [PubMed] [Google Scholar]
- 25.McPherson BC, Yao Z. Morphine mimics preconditioning via free radical signals and mitochondrial K(ATP) channels in myocytes. Circulation. 2001;103(2):290–295. doi: 10.1161/01.cir.103.2.290. [DOI] [PubMed] [Google Scholar]
- 26.McPherson BC, Yao Z. Signal transduction of opioid-induced cardioprotection in ischemia-reperfusion. Anesthesiology. 2001;94(6):1082–1088. doi: 10.1097/00000542-200106000-00024. [DOI] [PubMed] [Google Scholar]
- 27.Mullenheim J, Ebel D, Frassdorf J, Preckel B, Thamer V, Schlack W. Isoflurane preconditions myocardium against infarction via release of free radicals. Anesthesiology. 2002;96(4):934–940. doi: 10.1097/00000542-200204000-00022. [DOI] [PubMed] [Google Scholar]
- 28.Novalija E, Fujita S, Kampine JP, Stowe DF. Sevoflurane mimics ischemic preconditioning effects on coronary flow and nitric oxide release in isolated hearts. Anesthesiology. 1999;91(3):701–712. doi: 10.1097/00000542-199909000-00023. [DOI] [PubMed] [Google Scholar]
- 29.Piriou V, Chiari P, Knezynski S, Bastien O, Loufoua J, Lehot JJ, Foex P, Annat G, Ovize M. Prevention of isoflurane-induced preconditioning by 5-hydroxydecanoate and gadolinium: possible involvement of mitochondrial adenosine triphosphate-sensitive potassium and stretch-activated channels. Anesthesiology. 2000;93(3):756–764. doi: 10.1097/00000542-200009000-00025. [DOI] [PubMed] [Google Scholar]
- 30.Quasha AL, Eger EI, Tinker JH. Determination and application of MAC. Anesthesiology. 1980;53(4):315–334. doi: 10.1097/00000542-198010000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Roscoe AK, Christensen JD, Lynch III C. Isoflurane, but not halothane, induces protection of human myocardium via adenosine A1 receptors and adenosine triphosphate-sensitive potassium channels. Anesthesiology. 2000;92(6):1692–1701. doi: 10.1097/00000542-200006000-00029. [DOI] [PubMed] [Google Scholar]
- 32.Sayin MM, Ozatamer O, Tasoz R, Kilinc K, Unal N. Propofol attenuates myocardial lipid peroxidation during coronary artery bypass grafting surgery. Br J Anaesth. 2002;89(2):242–246. doi: 10.1093/bja/aef173. [DOI] [PubMed] [Google Scholar]
- 33.Scarabelli T, Stephanou A, Rayment N, Pasini E, Comini L, Curello S, Ferrari R, Knight R, Latchman D. Apoptosis of endothelial cells precedes myocyte cell apoptosis in ischemia/reperfusion injury. Circulation. 2001;104(3):253–256. doi: 10.1161/01.cir.104.3.253. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka K, Weihrauch D, Kehl F, Ludwig LM, LaDisa JFJr, Kersten JR, Pagel PS, Warltier DC. Mechanism of preconditioning by isoflurane in rabbits: a direct role for reactive oxygen species. Anesthesiology. 2002;97(6):1485–1490. doi: 10.1097/00000542-200212000-00021. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka K, Weihrauch D, Ludwig LM, Kersten JR, Pagel PS, Warltier DC. Mitochondrial adenosine triphosphate-regulated potassium channel opening acts as a trigger for isoflurane-induced preconditioning by generating reactive oxygen species. Anesthesiology. 2003;98(4):935–943. doi: 10.1097/00000542-200304000-00021. [DOI] [PubMed] [Google Scholar]
- 36.Toller WG, Kersten JR, Gross ER, Pagel PS, Warltier DC. Isoflurane preconditions myocardium against infarction via activation of inhibitory guanine nucleotide binding proteins. Anesthesiology. 2000;92(5):1400–1407. doi: 10.1097/00000542-200005000-00031. [DOI] [PubMed] [Google Scholar]
- 37.Tomasdottir H, Hjartarson H, Ricksten A, Wasslavik C, Bengtsson A, Ricksten SE. Tumor necrosis factor gene polymorphism is associated with enhanced systemic inflammatory response and increased cardiopulmonary morbidity after cardiac surgery. Anesth Analg. 2003;97(4):944–949. doi: 10.1213/01.ANE.0000078574.76915.11. [DOI] [PubMed] [Google Scholar]
- 38.van Empel VP, Bertrand AT, Hofstra L, Crijns HJ, Doevendans PA, de Windt LJ. Myocyte apoptosis in heart failure. Cardiovasc Res. 2005;67(1):21–29. doi: 10.1016/j.cardiores.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 39.Warltier DC, al Wathiqui MH, Kampine JP, Schmeling WT. Recovery of contractile function of stunned myocardium in chronically instrumented dogs is enhanced by halothane or isoflurane. Anesthesiology. 1988;69(4):552–565. doi: 10.1097/00000542-198810000-00016. [DOI] [PubMed] [Google Scholar]
- 40.Xia Z, Godin DV, Ansley DM. Application of high-dose propofol during ischemia improves postischemic function of rat hearts: effects on tissue antioxidant capacity. Can J Physiol Pharmacol. 2004;82(10):919–926. doi: 10.1139/y04-097. [DOI] [PubMed] [Google Scholar]
- 41.Xia Z, Huang Z, Ansley DM. Large-dose propofol during cardiopulmonary bypass decreases biochemical markers of myocardial injury in coronary surgery patients: a comparison with isoflurane. Anesth Analg. 2006;103(3):527–532. doi: 10.1213/01.ane.0000230612.29452.a6. [DOI] [PubMed] [Google Scholar]
- 42.Zweier JL, Flaherty JT, Weisfeldt ML. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci USA. 1987;84(5):1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]