Abstract
Dissipation mechanisms of excess photon energy under high-temperature stress were studied in a subtropical forest tree seedling, Ficus concinna. Net CO2 assimilation rate decreased to 16% of the control after 20 d high-temperature stress, and thus the absorption of photon energy exceeded the energy required for CO2 assimilation. The efficiency of excitation energy capture by open photosystem II (PSII) reaction centres (F v′/F m′) at moderate irradiance, photochemical quenching (q P), and the quantum yield of PSII electron transport (Φ PSII) were significantly lower after high-temperature stress. Nevertheless, non-photochemical quenching (q NP) and energy-dependent quenching (q E) were significantly higher under such conditions. The post-irradiation transient of chlorophyll (Chl) fluorescence significantly increased after the turnoff of the actinic light (AL), and this increase was considerably higher in the 39 °C-grown seedlings than in the 30 °C-grown ones. The increased post-irradiation fluorescence points to enhanced cyclic electron transport around PSI under high growth temperature conditions, thus helping to dissipate excess photon energy non-radiatively.
Keywords: Ficus concinna, High-temperature stress, Chlorophyll fluorescence, Photosynthesis, Cyclic electron transport around photosystem I, Dissipation of excitation energy
INTRODUCTION
High temperature adversely affects plant growth and survival in a number of ways. Photosynthesis is one of the most heat-sensitive processes in plants. Inhibition of photosynthesis by high temperature has been studied in many plant species. Heat stress causes a decrease in the photochemical efficiency of photosystem II (PSII) (Berry and Björkman, 1980; Havaux et al., 1996), the depression of the linear electron transport (Berry and Björkman, 1980; Wang et al., 2006), and the decline of the Rubisco activity (Feller et al., 1998; Haldimann and Feller, 2004; Salvucci and Crafts-Brandner, 2004). Gas exchange analysis indicates that the decrease in photosynthesis is associated with the heat-induced internal conductance attenuation and the coupled reduction in CO2 concentration at the sites of carboxylation in spinach (Yamori et al., 2006). However, it is now still a matter of debate how high temperature inhibits photosynthesis.
CO2 assimilation is the major consumer of photon energy absorbed by the antenna pigments and any decrease in the rate of photosynthesis inevitably results in accumulation of excess photon energy under high temperature. Plants have evolved a large variety of mechanisms to protect their photosynthetic apparatus against damage resulting from high temperature. In general, plants prevent the increase of leaf temperature by transpiration (Isoda and Wang, 2002). In addition, heat stress can induce the antioxidant enzymes to overcome the increased oxidative stress (Chaitanya et al., 2002; Guo et al., 2006), or enhance the cyclic electron transport (Bukhov et al., 2000; Wang et al., 2006). In spite of these findings, it remains unclear how heat-treated plants regulate the dissipation of excess photon energy when low CO2 assimilation requires less chemical energy than that provided by the absorbed photons. So far, to our knowledge, little information is available concerning the effects of heat stress on the photosynthetic processes of woody plant leaves. In the present work, we investigated simultaneously chlorophyll (Chl) fluorescence induction to characterize PSII, fluorescence emission, and gas exchange in the total photosynthetic process upon heat stress to explore how heat-treated plants dissipate excess photon energy.
MATERIALS AND METHODS
Plants and growth conditions
Two-year old seedlings of Ficus concinna var. subsessilis were obtained from Zhejiang Subtropical Crop Institute in Wenzhou, China. The seedlings were transplanted to plastic tubes (20.5 cm tall, 18 cm in top diameter) filled with a mixture of 1:1 (v/v) coarse sand and soil and grown in a shaded greenhouse with natural sunlight during the day (maximum of 800 μmol/(m2·s)). Daily maximum and minimum temperatures in the greenhouse were 28 and 20 °C, respectively. About four months later, randomly selected seedlings were placed into two identical growth chambers, one for heat treatment, the other for control. Both growth chambers had identical 14-h photoperiods with a photosynthetic flux density (PFD) of around 400 μmol/(m2·s) at leaf height, at constant relative humidity of approximately 50%. One chamber was kept at 30 °C (day and night) throughout the experimental period as non-stressed control, and the other at 39 °C (day and night) to heat-treat the plants. Plants were fertilized once a week with a half-strength Hoagland solution. In the high-temperature growth chamber, water was regularly supplied during the day to prevent drought stress.
Measurements of gas exchange and chlorophyll fluorescence
Measurements were made on the second fully expanded leaves from the top of four randomly selected seedlings on Day 20 after the treatment. The leaves were developed after the high-temperature treatment. Leaf gas exchange and Chl fluorescence were measured simultaneously using a portable apparatus (LiCor-6400, LiCor Inc., Lincoln Nebraska, USA) equipped with an integrated leaf chamber fluorometer (LI-6400-40, LiCor Inc., Lincoln Nebraska, USA) under ambient CO2 concentrations, 21% O2 and 50% relative humidity. Leaf chamber temperature maintained equilibrium to the ambient temperature, which was equivalent to the treatment temperature. Actinic light (AL, 800 μmol/(m2·s)) supplied with light-emitting diodes (90% red light, 630 nm; 10% blue light, 470 nm) was used to record the steady-state Chl fluorescence level (F s) and the gas exchange parameters. The minimum Chl fluorescence of the open PSII centre (F o) and the maximal Chl fluorescence of the closed PSII centre (F m) were measured after 30 min of dark-adaptation. The measuring light (ML) intensity to determine F o, was 1 μmol/(m2·s) at 630 nm. An 800-ms saturating pulse light (>6000 μmol/(m2·s)) was applied to measure F m after the dark period and also during AL illumination (F m′). The minimal (F o′) and variable (F v′) fluorescence of light-adapted leaves were determined according to Kramer et al.(2004). The variable fluorescence yield (F v) was calculated as F m−F o. The quantum yield of PSII electron transport [Φ PSII=(F m′−F s)/F m′], the efficiency of excitation energy capture by open PSII reaction centres [F v′/F m′=(F m′−F o′)/F m′], photochemical quenching [q P=(F m′−F s)/(F m′−F o′)] and non-photochemical quenching [q NP=F m/F m′−1] were calculated from measured data (Maxwell and Johnson, 2000). The fraction of open PSII reaction centres was estimated as q L=((F m′−F s)F o′)/((F m′−F o′)F s) (Kramer et al., 2004).
The kinetics of q NP formation and relaxation were obtained by measuring the Chl fluorescence on the same leaves as described by Quick and Stitt (1989). AL intensities varied between 0 and 2000 μmol/(m2·s), and the light-saturated pulses were administered at an interval of 60 s. The energy-dependent quenching (q E) was calculated according to Jin et al.(2008).
The post-irradiation transient increase in Chl fluorescence was recorded according to Lu et al.(2008) and Yang et al.(2007) with some modifications. After dark-adaptation of the sample for 30 min, F o and F m were determined as described above. Subsequently, an AL of 300 μmol/(m2·s) was turned on. After 3 min, the AL was turned off and fluorescence yield changes were continuously recorded.
Statistical methods
Statistical treatments of the data were performed by Student’s t-test for comparison of means. For these analyses we used SPSS 10.0 software (SPSS, Chicago, IL, USA) and set the statistical significance level at P<0.05. All experiments were performed at least four times.
RESULTS AND DISCUSSION
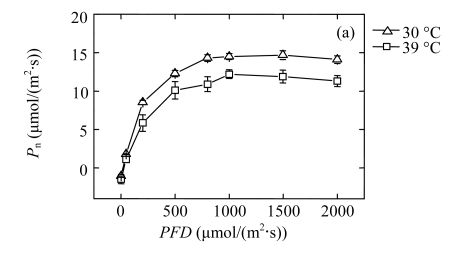
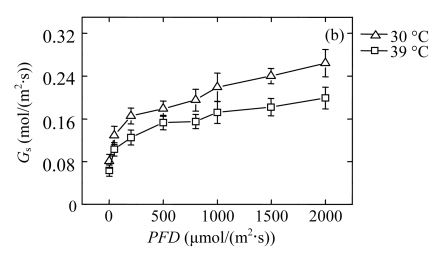
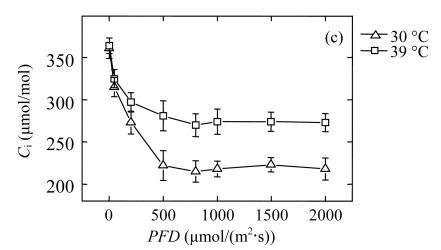
To determine the influence of high temperature on steady-state photosynthesis, we measured the light responses of the net CO2 assimilation rate (P n) in the second fully expanded top leaves of the seedlings (Fig.1a). P n of the 39 °C-plants was decreased significantly as compared with that of the 30 °C-control at PFDs above 200 μmol/(m2·s). At 1000 μmol/(m2·s) light intensity, P n of 39 °C-plants was reduced by about 16%, as compared with that of the 30 °C-control. Similarly, the stomatal conductance (G s) was significantly reduced in 39 °C-plants, decreasing by 22% at the PFD of 1000 μmol/(m2·s) (Fig.1b). Both stomatal and non-stomatal limiting factors affect photosynthesis. In our case, the intercellular CO2 concentrations (C i) of the 39 °C-plants were about 26% higher than those of 30 °C-controls in spite of their lower P n and G s (Fig.1c), which indicates that the reduction of P n of the 39 °C-plants was not due to stomatal factors but a low capacity of CO2-fixation cycle. This result is consistent with previous studies performed by Haldimann and Feller (2005).
Fig.1.
Light responses of (a) the net CO2 assimilation rate (P n), (b) the stomatal conductance (G s), and (c) the intercellular CO2 concentration (C i) determined in the second fully expanded leaves of Ficus concinna seedlings growth at 30 or 39 °C for 20 d
Each data point represents the mean±SE of four independent replicates. Plant grew at 30 °C before high temperature treatment. Measurement temperature was equivalent to the treatment temperature
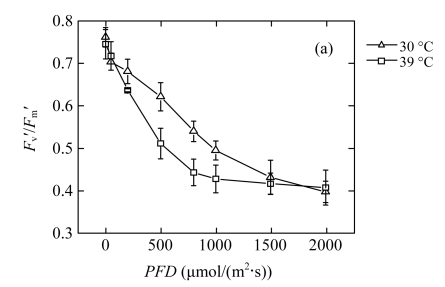
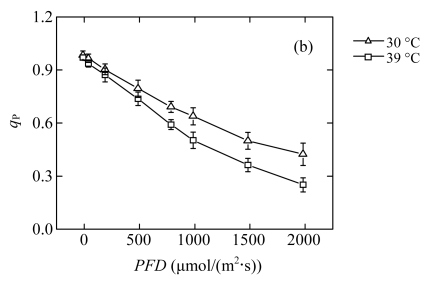
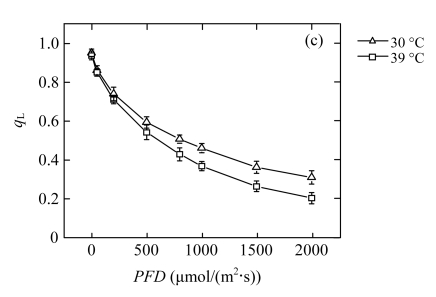
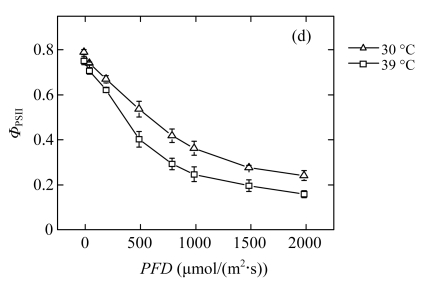
The reduction of P n in high-temperature treated plants results in a lower requirement for excitation energy, and it may in turn inhibit further linear electron transport. The dependence of Chl fluorescence on light intensity was measured in vivo (Fig.2). The quantum yield of PSII electron transport (Φ PSII) at each PFD was lower in the 39 °C-plants than in the 30 °C-plants (Fig.2d), which confirmed that linear electron passing through PSII was reduced. The treated plants did not show photoinhibition, since the dark-adapted maximum quantum yield of the primary photochemistry of PSII (F v/F m) was maintained (data not shown). However, compared with the 30 °C-plants, the 39 °C-plants exhibited significantly lower efficiency of excitation energy capture by open PSII reaction centres (F v′/F m′) at moderate PFDs (Fig.2a). The photochemical quenching (q P) exhibited an approximately linear decrease with increasing light intensity (Fig.2b). The rate of decrease was more rapid in the 39 °C-plants than in the 30 °C-plants. The change trend of the fraction of open PSII reaction centres (q L) was similar to q P in both groups (Fig.2c). The low values of q P and q L indicated more reduced primary quinone (Q A) in PSII and less reduced plastoquinone (PQ) pool (Maxwell and Johnson, 2000; Kramer et al., 2004). At high PFDs, the observed reduction in linear electron transport is due to the decrease of the number of open PSII reaction centres. At moderate PFDs, the decreased PSII efficiency is due to both the increased heat dissipation and more closed centres.
Fig.2.
Light responses of (a) the efficiency of excitation energy capture by open PSII reaction centres (F v′/F m′), (b) the photochemical quenching (q P), (c) the fraction of open PSII reaction centres (q L), and (d) the quantum yield of PSII electron transport (Φ PSII) determined in the second fully expanded leaves of Ficus concinna seedlings grown at 30 or 39 °C for 20 d
Each data point represents the mean±SE of four independent replicates. Plant grew at 30 °C before high temperature treatment. Measurement temperature was equivalent to the treatment temperature
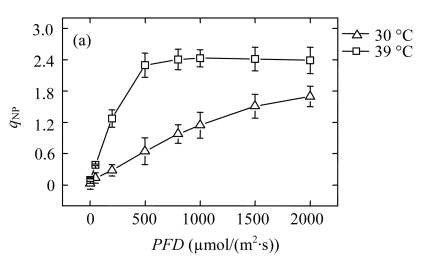
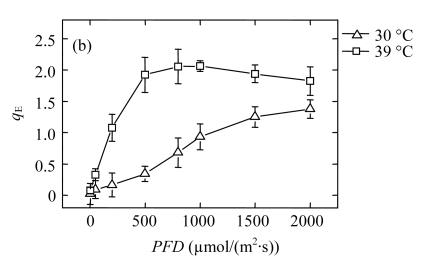
High temperature led to an excess of excitation energy, since the CO2 assimilation was decreased. If not dissipated safely, the excess energy causes damage to the photosynthetic apparatus (Demmig-Adams and Adams III, 1992). Non-radiative dissipation of excitation energy has been suggested to play a crucial role in the response of plants to various adverse environmental stresses (Demmig-Adams and Adams III, 1992). In this study, the q NP in the 39 °C-plants was much higher than that in the 30 °C-plants, especially under moderate AL (Fig.3a), indicating that the increase in q NP was the main pathway to dissipate the excessive energy. We assessed the contributions of the q E components to the total quenching effect using steady-state photosynthesis rates for a variety of AL intensities by dark relaxation kinetics. The change trend of q E was similar to that of q NP in both groups (Fig.3b). Therefore, the increase in q NP is attributable to q E. The q E component is believed to be directly dependent on pH gradient across the thylakoid membrane (Shikanai et al., 2002; Štroch et al., 2004). Formation of a pH gradient associated with the activation of the xanthophyll cycle (Thiele and Krause, 1994) may explain the increase of the q E component. Therefore, we further contemplated what process generates the pH gradient necessary for this q E increase, since the linear electron transport was decreased in the 39 °C-plants.
Fig.3.
Light responses of (a) the non-photochemical quenching (q NP) and (b) the energy-dependent non-photochemical quenching (q E) determined in the second fully expanded leaves of Ficus concinna seedlings grown at 30 °C or 39 °C for 20 d
Each data point represents the mean±SE of four independent replicates. Plant grew at 30 °C before high temperature treatment. Measurement temperature was equivalent to the treatment temperature
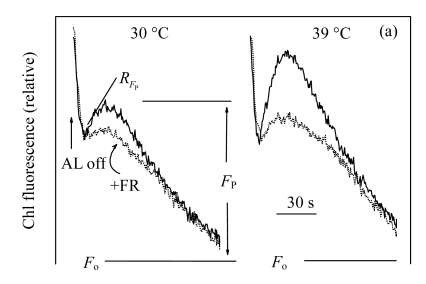
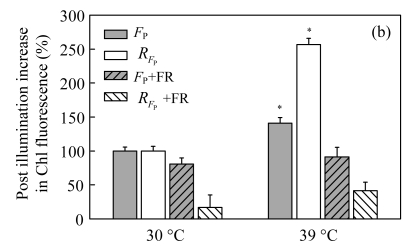
The post-irradiation transient increase of Chl fluorescence is thought to arise from the reduction of PQ by nicotinamide adenine dinucleotide phosphate (NADPH) or other electron donors accumulated in leaves under irradiation (Shikanai et al., 1998; Peltier and Cournac, 2002; Yang et al., 2007; Lu et al., 2008). In higher plants and cyanobacteria, these processes involve mainly cyclic electron flow around PSI (Peltier and Cournac, 2002; Deng et al., 2003; Munné-Bosch et al., 2005; Lu et al., 2008). Figs.4a and 4b illustrates the post-irradiation transient increase of Chl fluorescence in the control and treated plants. The transient increase of Chl fluorescence was more pronounced in the 39 °C-plants than in the 30 °C-plants (Fig.4a). The transient rise was caused by the reduction of PQ, because it was quenched by far-red light (FR) (Figs.4a and 4b). These findings may indicate that high temperature could down-regulate linear electron transport and stimulate the PSI cyclic electron transport. Cyclic electron transport around PSI is solely driven by PSI and generates pH and consequently adenosine triphosphate (ATP) without net accumulation of NAD(P)H (Shikanai, 2007). In higher plants, PSI cyclic electron transport consists of two partly redundant routes of electrons, the NAD(P)H dehydrogenase-dependent and antimycin A-sensitive pathways (Shikanai, 2007). Cyclic electron flow around PSI may be responsible for enhanced proton pumping and is involved in energy dissipation when CO2 availability is reduced (Burrows et al., 1998; Shikanai et al., 2002; Wang et al., 2006).
Fig.4.
Effects of high temperature (39 °C) on the kinetics (a) and the height (b) of post-irradiation transient increase in Chl fluorescence in the leaves of Ficus concinna after 20 d of treatment
AL: actinic light (300 μmol/(m2·s)); F o: minimum Chl fluorescence yield when PSII reaction centres are open; F P: height of post-irradiation fluorescence increase. R FP: value of the slope of the height. The curves indicate the transient increase in Chl fluorescence after 3 min of AL illumination. In another experiment, the far-red light (FR, above 720 nm, 7 μmol/(m2·s)) was turn on 10 s before AL was turned off (dotted line). Each experiment was repeated four times and average values were calculated. Plant grew at 30 °C before high temperature treatment. Measurement temperature was equivalent to the treatment temperature. The initial slope of Chl fluorescence in the control plants is regarded as 100%. The significant level of difference between treated and control seedlings is indicated by an asterisks; * P<0.05
CONCLUSION
It has been suggested that cyclic electron flow around PSI is required to supply sufficient proton motive force to initiate energy-dependent excitation quenching (q E) (Yang et al., 2007; Jin et al., 2008). Our results show that cyclic electron flow around PSI plays an important role in the production of pH gradient across the thylakoid membrane (ΔpH) that leads to the effective dissipation of excess excitation energy under high temperature conditions. Therefore, cyclic electron transport around PSI is an important photoprotective mechanism for adapting the photosynthetic apparatus to high temperature.
Footnotes
Project supported by the Natural Science Foundation of Zhejiang Province, China (No. Y3090276), the Major Program of Science and Technology Department of Zhejiang Province, China (No. 2007C12023), and the Scientific Research Foundation for PhD of Zhejiang Forestry University, China (No. 2007FR047)
References
- 1.Berry JA, Björkman O. Photosynthetic response and adaptation to temperature in higher plants. Annual Review of Plant Physiology. 1980;31(1):491–543. doi: 10.1146/annurev.pp.31.060180.002423. [DOI] [Google Scholar]
- 2.Bukhov NG, Samson G, Carpentier R. Nonphotosynthetic reduction of the intersystem electron transport chain of chloroplasts following heat stress. Steady-state rate. Photochemistry and Photobiology. 2000;72(3):351–357. doi: 10.1562/0031-8655(2000)072<0351:NROTIE>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Burrows PA, Sazanov LA, Svab Z, Maliga P, Nixon PJ. Identification of a functional respiratory complex in chloroplasts through analysis of tobacco mutants containing disrupted plastid ndh genes. The EMBO Journal. 1998;17(4):868–876. doi: 10.1093/emboj/17.4.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaitanya KV, Sundar D, Masilamani S, Ramachandra RA. Variation in heat stress-induced antioxidant enzyme activities among three mulberry cultivars. Plant Growth Regulation. 2002;36(2):175–180. doi: 10.1023/A:1015092628374. [DOI] [Google Scholar]
- 5.Demmig-Adams B, Adams III WW. Photoprotection and other responses of plants to high light stress. Annual Review of Plant Physiology and Plant Molecular Biology. 1992;43(1):599–626. doi: 10.1146/annurev.pp.43.060192.003123. [DOI] [Google Scholar]
- 6.Deng Y, Ye J, Mi H. Effects of low CO2 on NAD(P)H dehydrogenase, a mediator of cyclic electron transport around photosystem I in the cyanobacterium Synechocystis PCC6803. Plant and Cell Physiology. 2003;44(5):534–540. doi: 10.1093/pcp/pcg067. [DOI] [PubMed] [Google Scholar]
- 7.Feller U, Crafts-Brandner SJ, Salvucci ME. Moderately high temperatures inhibit ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) activase-mediated activation of Rubisco. Plant Physiology. 1998;116(2):539–546. doi: 10.1104/pp.116.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo YP, Zhou HF, Zhang LC. Photosynthetic characteristics and protective mechanisms against photooxidation during high temperature stress in two citrus species. Scientia Horticulturae. 2006;108(3):260–267. doi: 10.1016/j.scienta.2006.01.029. [DOI] [Google Scholar]
- 9.Haldimann P, Feller U. Inhibition of photosynthesis by high temperature in oak (Quercus pubescens L.) leaves grown under natural conditions closely correlates with a reversible heat-dependent reduction of the activation state of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Cell and Environment. 2004;27(9):1169–1183. doi: 10.1111/j.1365-3040.2004.01222.x. [DOI] [Google Scholar]
- 10.Haldimann P, Feller U. Growth at moderately elevated temperature alters the physiological response of the photosynthetic apparatus to heat stress in pea (Pisum sativum L.) leaves. Plant Cell and Environment. 2005;28(3):302–317. doi: 10.1111/j.1365-3040.2005.01289.x. [DOI] [Google Scholar]
- 11.Havaux M, Tardy F, Ravenel J, Chanu D, Parot P. Thylakoid membrane stability to heat stress studied by flash spectroscopic measurements of the electrochromic shift in intact potato leaves: influence of the xanthophylls content. Plant Cell and Environment. 1996;19(12):1359–1368. doi: 10.1111/j.1365-3040.1996.tb00014.x. [DOI] [Google Scholar]
- 12.Isoda A, Wang P. Leaf temperature and transpiration of field grown cotton and soybean under arid and humid conditions. Plant Production Science. 2002;5(3):224–228. [Google Scholar]
- 13.Jin SH, Wang D, Zhu FY, Li XQ, Sun JW, Jiang DA. Up-regulation of cyclic electron flow and down-regulation of linear electron flow in antisense-rca mutant rice. Photosynthetica. 2008;46(4):506–510. doi: 10.1007/s11099-008-0086-9. [DOI] [Google Scholar]
- 14.Kramer DM, Johnson G, Kiirats O, Edwards GE. New fluorescence parameters for the determination of Q A redox state and excitation energy fluxes. Photosynthesis Research. 2004;79(2):209–218. doi: 10.1023/B:PRES.0000015391.99477.0d. [DOI] [PubMed] [Google Scholar]
- 15.Lu KX, Yang Y, He Y, Jiang DA. Induction of cyclic electron flow around photosystem 1 and state transition are correlated with salt tolerance in soybean. Photosynthetica. 2008;46(1):10–16. doi: 10.1007/s11099-008-0003-2. [DOI] [Google Scholar]
- 16.Maxwell K, Johnson GN. Chlorophyll fluorescence: a practical guide. Journal of Experimental Botany. 2000;51(345):659–668. doi: 10.1093/jexbot/51.345.659. [DOI] [PubMed] [Google Scholar]
- 17.Munné-Bosch S, Shikanai T, Asada K. Enhanced ferredoxin-dependent cyclic electron flow around photosystem I and α-tocopherol quinone accumulation in water-stressed ndhB-inactivated tobacco mutants. Planta. 2005;222(3):502–511. doi: 10.1007/s00425-005-1548-y. [DOI] [PubMed] [Google Scholar]
- 18.Peltier G, Cournac L. Chlororespiration. Annual Review of Plant Biology. 2002;53(1):523–550. doi: 10.1146/annurev.arplant.53.100301.135242. [DOI] [PubMed] [Google Scholar]
- 19.Quick WP, Stitt M. An examination of factors contributing to non-photochemical quenching of chlorophyll fluorescence in barley leaves. Biochimica et Biophysica Acta (BBA)-Bioenergetics. 1989;977(3):287–296. doi: 10.1016/S0005-2728(89)80082-9. [DOI] [Google Scholar]
- 20.Salvucci ME, Crafts-Brandner SJ. Relationship between the heat tolerance of photosynthesis and the thermal stability of rubisco activase in plants from contrasting thermal environments. Plant Physiology. 2004;134(4):1460–1470. doi: 10.1104/pp.103.038323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shikanai T. Cyclic electron transport around photosystem I: genetic approaches. Annual Review of Plant Biology. 2007;58(1):199–217. doi: 10.1146/annurev.arplant.58.091406.110525. [DOI] [PubMed] [Google Scholar]
- 22.Shikanai T, Endo T, Hashimoto T, Yamada Y, Asada K, Yokota A. Directed disruption of the tobacco ndhB gene impairs cyclic electron flow around photosystem I. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(16):9705–9709. doi: 10.1073/pnas.95.16.9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shikanai T, Munekage Y, Kimura K. Regulation of proton-to-electron stoichiometry in photosynthetic electron transport: physiological function in photoprotection. Journal of Plant Research. 2002;115(1):3–10. doi: 10.1007/s102650200001. [DOI] [PubMed] [Google Scholar]
- 24.Štroch M, Špunda V, Kurasová I. Non-radiative dissipation of absorbed excitation energy within photosynthetic apparatus of higher plants. Photosynthetica. 2004;42(3):323–337. doi: 10.1023/B:PHOT.0000046149.97220.18. [DOI] [Google Scholar]
- 25.Thiele A, Krause GH. Xanthophyll cycle and thermal energy dissipation in photosystem II: relationship between zeaxanthin formation, energy-dependent quenching and photoinhibition. Journal of Plant Physiology. 1994;144(3):324–332. [Google Scholar]
- 26.Wang P, Duan W, Takabayashi A, Endo T, Shikanai T, Ye JY, Mi HL. Chloroplastic NAD(P)H dehydrogenase in tobacco leaves functions in alleviation of oxidative damage caused by temperature stress. Plant Physiology. 2006;141(2):465–474. doi: 10.1104/pp.105.070490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamori W, Noguchi K, Hanba YT, Terashima I. Effects of internal conductance on the temperature dependence of the photosynthetic rate in spinach leaves from contrasting growth temperatures. Plant and Cell Physiology. 2006;47(8):1069–1080. doi: 10.1093/pcp/pcj077. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Yan CQ, Cao BH, Xu HX, Chen JP, Jiang DA. Some photosynthetic responses to salinity resistance are transferred into the somatic hybrid descendants from the wild soybean Glycine cyrtoloba ACC547. Physiologia Plantarum. 2007;129(3):658–669. doi: 10.1111/j.1399-3054.2006.00853.x. [DOI] [Google Scholar]