Abstract
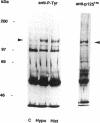
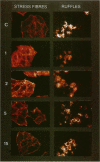
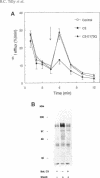
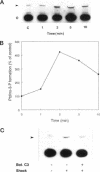
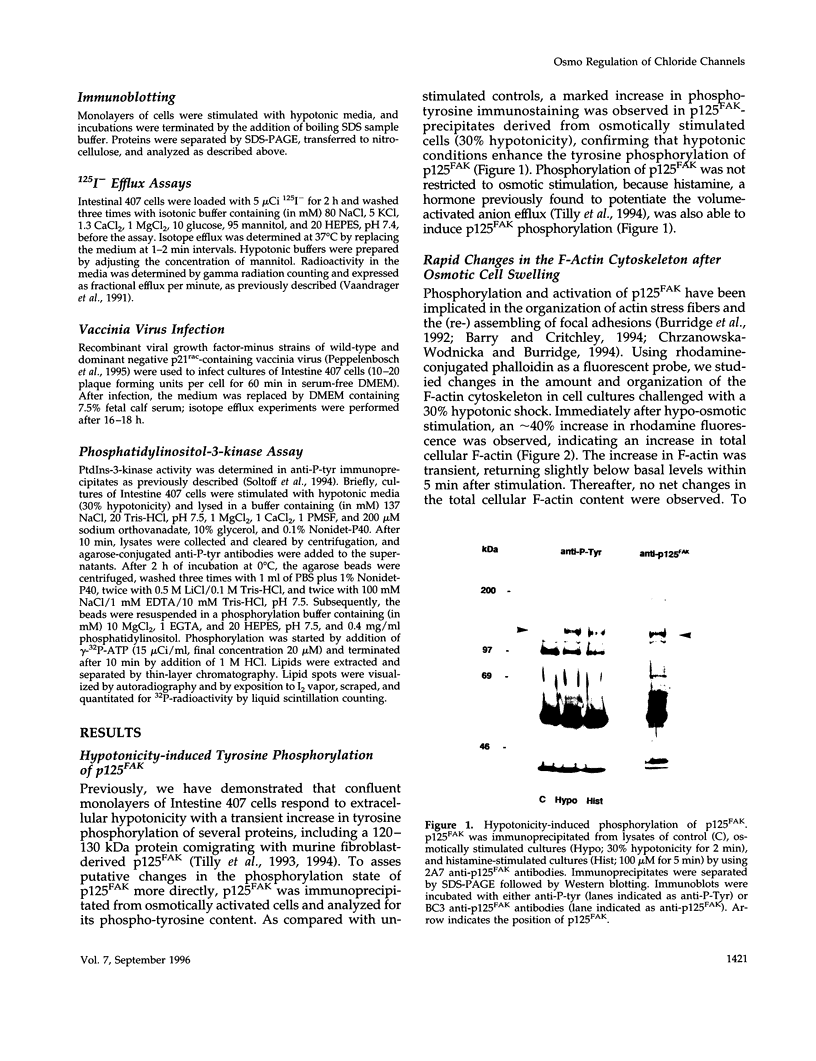
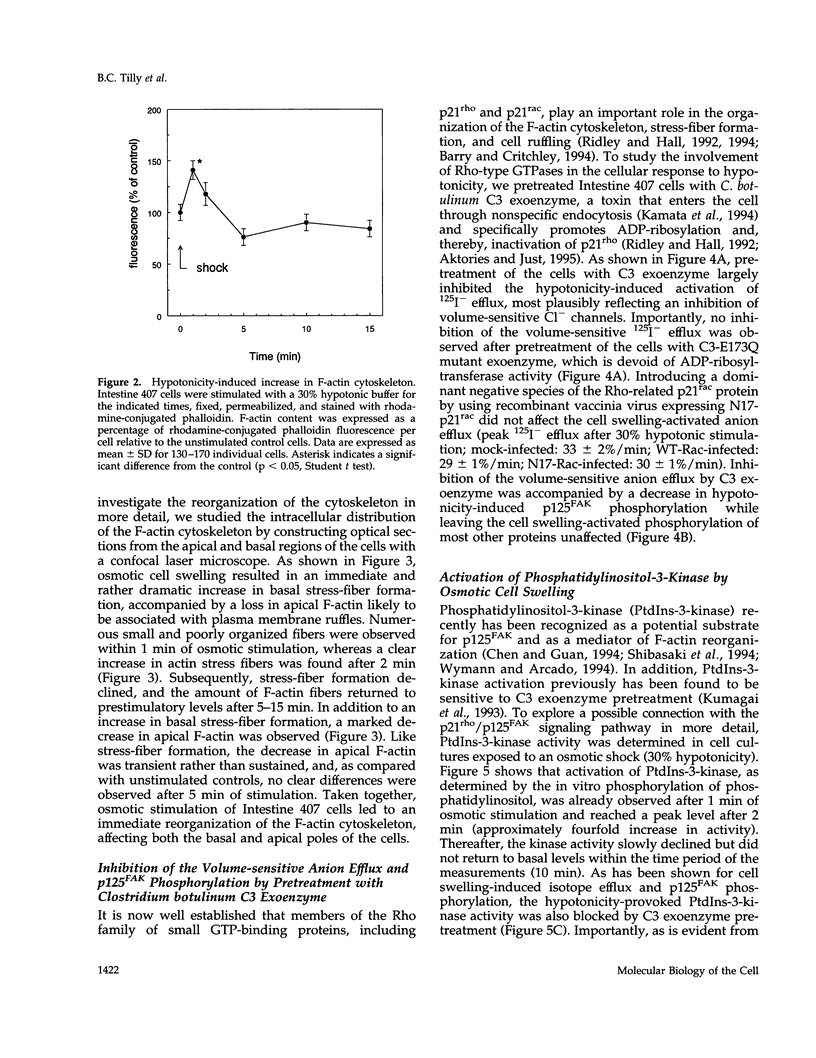
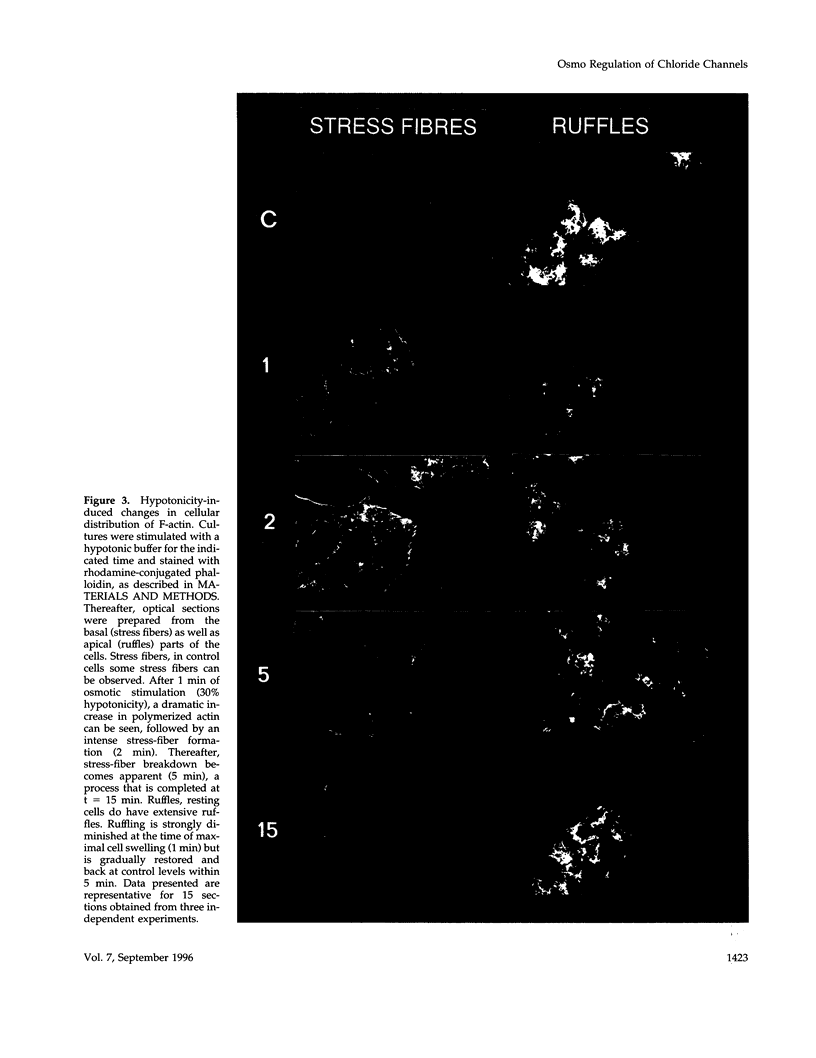
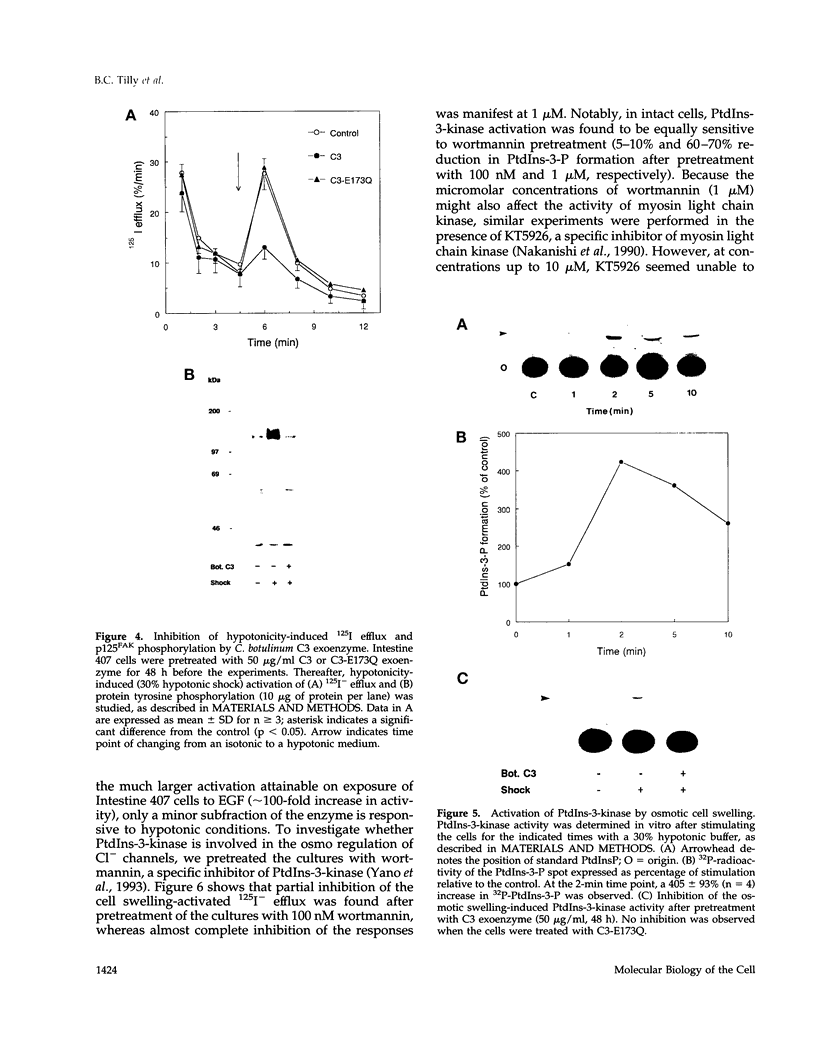
Hypo-osmotic stimulation of human Intestine 407 cells rapidly activated compensatory CL- and K+ conductances that limited excessive cell swelling and, finally, restored the original cell volume. Osmotic cell swelling was accompanied by a rapid and transient reorganization of the F-actin cytoskeleton, affecting both stress fibers as well as apical ruffles. In addition, an increase in total cellular F-actin was observed. Pretreatment of the cells with recombinant Clostridium botulinum C3 exoenzyme, but not with mutant enzyme (C3-E173Q) devoid of ADP-ribosyltransferase activity, greatly reduced the activation of the osmo-sensitive anion efflux, suggesting a role for the ras-related GTPase p21rho. In contrast, introducing dominant negative N17-p21rac into the cells did not affect the volume-sensitive efflux. Cell swelling-induced reorganization of F-actin coincided with a transient, C3 exoenzyme-sensitive tyrosine phosphorylation of p125 focal adhesion kinase (p125FAK) as well as with an increase in phosphatidylinositol-3-kinase (PtdIns-3-kinase) activity. Pretreatment of the cells with wortmannin, a specific inhibitor of PtdIns-3-kinase, largely inhibited the volume-sensitive ion efflux. Taken together, our results indicate the involvement of a p21rho signaling cascade and actin filaments in the activation of volume-sensitive chloride channels.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aktories K., Just I. In vitro ADP-ribosylation of Rho by bacterial ADP-ribosyltransferases. Methods Enzymol. 1995;256:184–195. doi: 10.1016/0076-6879(95)56023-8. [DOI] [PubMed] [Google Scholar]
- Barry S. T., Critchley D. R. The RhoA-dependent assembly of focal adhesions in Swiss 3T3 cells is associated with increased tyrosine phosphorylation and the recruitment of both pp125FAK and protein kinase C-delta to focal adhesions. J Cell Sci. 1994 Jul;107(Pt 7):2033–2045. doi: 10.1242/jcs.107.7.2033. [DOI] [PubMed] [Google Scholar]
- Burridge K., Turner C. E., Romer L. H. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992 Nov;119(4):893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantiello H. F., Prat A. G., Bonventre J. V., Cunningham C. C., Hartwig J. H., Ausiello D. A. Actin-binding protein contributes to cell volume regulatory ion channel activation in melanoma cells. J Biol Chem. 1993 Mar 5;268(7):4596–4599. [PubMed] [Google Scholar]
- Cantiello H. F., Stow J. L., Prat A. G., Ausiello D. A. Actin filaments regulate epithelial Na+ channel activity. Am J Physiol. 1991 Nov;261(5 Pt 1):C882–C888. doi: 10.1152/ajpcell.1991.261.5.C882. [DOI] [PubMed] [Google Scholar]
- Chen H. C., Guan J. L. Association of focal adhesion kinase with its potential substrate phosphatidylinositol 3-kinase. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10148–10152. doi: 10.1073/pnas.91.21.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M., Burridge K. Tyrosine phosphorylation is involved in reorganization of the actin cytoskeleton in response to serum or LPA stimulation. J Cell Sci. 1994 Dec;107(Pt 12):3643–3654. doi: 10.1242/jcs.107.12.3643. [DOI] [PubMed] [Google Scholar]
- Cornet M., Lambert I. H., Hoffmann E. K. Relation between cytoskeleton, hypo-osmotic treatment and volume regulation in Ehrlich ascites tumor cells. J Membr Biol. 1993 Jan;131(1):55–66. doi: 10.1007/BF02258534. [DOI] [PubMed] [Google Scholar]
- Fatherazi S., Izutsu K. T., Wellner R. B., Belton C. M. Hypotonically activated chloride current in HSG cells. J Membr Biol. 1994 Nov;142(2):181–193. doi: 10.1007/BF00234940. [DOI] [PubMed] [Google Scholar]
- Foskett J. K., Spring K. R. Involvement of calcium and cytoskeleton in gallbladder epithelial cell volume regulation. Am J Physiol. 1985 Jan;248(1 Pt 1):C27–C36. doi: 10.1152/ajpcell.1985.248.1.C27. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Foskett J. K. Ionic mechanisms of cell volume regulation in leukocytes. Annu Rev Physiol. 1990;52:399–414. doi: 10.1146/annurev.ph.52.030190.002151. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Smith J. D. Calcium-independent cell volume regulation in human lymphocytes. Inhibition by charybdotoxin. J Gen Physiol. 1990 Jan;95(1):97–120. doi: 10.1085/jgp.95.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallows K. R., Packman C. H., Knauf P. A. Acute cell volume changes in anisotonic media affect F-actin content of HL-60 cells. Am J Physiol. 1991 Dec;261(6 Pt 1):C1154–C1161. doi: 10.1152/ajpcell.1991.261.6.C1154. [DOI] [PubMed] [Google Scholar]
- Hazama A., Okada Y. Ca2+ sensitivity of volume-regulatory K+ and Cl- channels in cultured human epithelial cells. J Physiol. 1988 Aug;402:687–702. doi: 10.1113/jphysiol.1988.sp017229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug T., Koslowsky T., Ecke D., Greger R., Kunzelmann K. Actin-dependent activation of ion conductances in bronchial epithelial cells. Pflugers Arch. 1995 Mar;429(5):682–690. doi: 10.1007/BF00373989. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Lang F. Cell volume in the regulation of hepatic function: a mechanism for metabolic control. Biochim Biophys Acta. 1991 Dec 12;1071(4):331–350. doi: 10.1016/0304-4157(91)90001-d. [DOI] [PubMed] [Google Scholar]
- Häussler U., Rivet-Bastide M., Fahlke C., Müller D., Zachar E., Rüdel R. Role of the cytoskeleton in the regulation of Cl- channels in human embryonic skeletal muscle cells. Pflugers Arch. 1994 Oct;428(3-4):323–330. doi: 10.1007/BF00724514. [DOI] [PubMed] [Google Scholar]
- Jalink K., van Corven E. J., Hengeveld T., Morii N., Narumiya S., Moolenaar W. H. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J Cell Biol. 1994 Aug;126(3):801–810. doi: 10.1083/jcb.126.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata Y., Nishiki T., Matsumura K., Hiroi T., Kozaki S. Morphological effects, rate of incorporation, and the enzymatic action of botulinum ADP-ribosyltransferase, known as C3 exoenzyme, on human neuroblastoma GOTO cells. Microbiol Immunol. 1994;38(6):421–428. doi: 10.1111/j.1348-0421.1994.tb01802.x. [DOI] [PubMed] [Google Scholar]
- Kanner S. B., Reynolds A. B., Vines R. R., Parsons J. T. Monoclonal antibodies to individual tyrosine-phosphorylated protein substrates of oncogene-encoded tyrosine kinases. Proc Natl Acad Sci U S A. 1990 May;87(9):3328–3332. doi: 10.1073/pnas.87.9.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani K., Yonezawa K., Hara K., Ueda H., Kitamura Y., Sakaue H., Ando A., Chavanieu A., Calas B., Grigorescu F. Involvement of phosphoinositide 3-kinase in insulin- or IGF-1-induced membrane ruffling. EMBO J. 1994 May 15;13(10):2313–2321. doi: 10.1002/j.1460-2075.1994.tb06515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai N., Morii N., Fujisawa K., Nemoto Y., Narumiya S. ADP-ribosylation of rho p21 inhibits lysophosphatidic acid-induced protein tyrosine phosphorylation and phosphatidylinositol 3-kinase activation in cultured Swiss 3T3 cells. J Biol Chem. 1993 Nov 25;268(33):24535–24538. [PubMed] [Google Scholar]
- McCarty N. A., O'Neil R. G. Calcium signaling in cell volume regulation. Physiol Rev. 1992 Oct;72(4):1037–1061. doi: 10.1152/physrev.1992.72.4.1037. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Yamada K., Iwahashi K., Kuroda K., Kase H. KT5926, a potent and selective inhibitor of myosin light chain kinase. Mol Pharmacol. 1990 Apr;37(4):482–488. [PubMed] [Google Scholar]
- Nobes C. D., Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995 Apr 7;81(1):53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Peppelenbosch M. P., Qiu R. G., de Vries-Smits A. M., Tertoolen L. G., de Laat S. W., McCormick F., Hall A., Symons M. H., Bos J. L. Rac mediates growth factor-induced arachidonic acid release. Cell. 1995 Jun 16;81(6):849–856. doi: 10.1016/0092-8674(95)90005-5. [DOI] [PubMed] [Google Scholar]
- Rankin S., Morii N., Narumiya S., Rozengurt E. Botulinum C3 exoenzyme blocks the tyrosine phosphorylation of p125FAK and paxillin induced by bombesin and endothelin. FEBS Lett. 1994 Nov 14;354(3):315–319. doi: 10.1016/0014-5793(94)01148-6. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. Signal transduction pathways regulating Rho-mediated stress fibre formation: requirement for a tyrosine kinase. EMBO J. 1994 Jun 1;13(11):2600–2610. doi: 10.1002/j.1460-2075.1994.tb06550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992 Aug 7;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Saito Y., Nemoto Y., Ishizaki T., Watanabe N., Morii N., Narumiya S. Identification of Glu173 as the critical amino acid residue for the ADP-ribosyltransferase activity of Clostridium botulinum C3 exoenzyme. FEBS Lett. 1995 Sep 4;371(2):105–109. doi: 10.1016/0014-5793(95)00851-y. [DOI] [PubMed] [Google Scholar]
- Schaller M. D., Borgman C. A., Cobb B. S., Vines R. R., Reynolds A. B., Parsons J. T. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M. D., Parsons J. T. Focal adhesion kinase: an integrin-linked protein tyrosine kinase. Trends Cell Biol. 1993 Aug;3(8):258–262. doi: 10.1016/0962-8924(93)90053-4. [DOI] [PubMed] [Google Scholar]
- Shibasaki F., Fukami K., Fukui Y., Takenawa T. Phosphatidylinositol 3-kinase binds to alpha-actinin through the p85 subunit. Biochem J. 1994 Sep 1;302(Pt 2):551–557. doi: 10.1042/bj3020551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltoff S. P., Carraway K. L., 3rd, Prigent S. A., Gullick W. G., Cantley L. C. ErbB3 is involved in activation of phosphatidylinositol 3-kinase by epidermal growth factor. Mol Cell Biol. 1994 Jun;14(6):3550–3558. doi: 10.1128/mcb.14.6.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly B. C., Edixhoven M. J., van den Berghe N., Bot A. G., de Jonge H. R. Ca(2+)-mobilizing hormones potentiate hypotonicity-induced activation of ionic conductances in Intestine 407 cells. Am J Physiol. 1994 Nov;267(5 Pt 1):C1271–C1278. doi: 10.1152/ajpcell.1994.267.5.C1271. [DOI] [PubMed] [Google Scholar]
- Tilly B. C., van den Berghe N., Tertoolen L. G., Edixhoven M. J., de Jonge H. R. Protein tyrosine phosphorylation is involved in osmoregulation of ionic conductances. J Biol Chem. 1993 Sep 25;268(27):19919–19922. [PubMed] [Google Scholar]
- Vaandrager A. B., Bajnath R., Groot J. A., Bot A. G., De Jonge H. R. Ca2+ and cAMP activate different chloride efflux pathways in HT-29.cl19A colonic epithelial cell line. Am J Physiol. 1991 Dec;261(6 Pt 1):G958–G965. doi: 10.1152/ajpgi.1991.261.6.G958. [DOI] [PubMed] [Google Scholar]
- Wennström S., Siegbahn A., Yokote K., Arvidsson A. K., Heldin C. H., Mori S., Claesson-Welsh L. Membrane ruffling and chemotaxis transduced by the PDGF beta-receptor require the binding site for phosphatidylinositol 3' kinase. Oncogene. 1994 Feb;9(2):651–660. [PubMed] [Google Scholar]
- Wymann M., Arcaro A. Platelet-derived growth factor-induced phosphatidylinositol 3-kinase activation mediates actin rearrangements in fibroblasts. Biochem J. 1994 Mar 15;298(Pt 3):517–520. doi: 10.1042/bj2980517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano H., Nakanishi S., Kimura K., Hanai N., Saitoh Y., Fukui Y., Nonomura Y., Matsuda Y. Inhibition of histamine secretion by wortmannin through the blockade of phosphatidylinositol 3-kinase in RBL-2H3 cells. J Biol Chem. 1993 Dec 5;268(34):25846–25856. [PubMed] [Google Scholar]
- Zachary I., Rozengurt E. Focal adhesion kinase (p125FAK): a point of convergence in the action of neuropeptides, integrins, and oncogenes. Cell. 1992 Dec 11;71(6):891–894. doi: 10.1016/0092-8674(92)90385-p. [DOI] [PubMed] [Google Scholar]
- Ziyadeh F. N., Mills J. W., Kleinzeller A. Hypotonicity and cell volume regulation in shark rectal gland: role of organic osmolytes and F-actin. Am J Physiol. 1992 Mar;262(3 Pt 2):F468–F479. doi: 10.1152/ajprenal.1992.262.3.F468. [DOI] [PubMed] [Google Scholar]
- al-Habori M. Cell volume and ion transport regulation. Int J Biochem. 1994 Mar;26(3):319–334. doi: 10.1016/0020-711x(94)90052-3. [DOI] [PubMed] [Google Scholar]