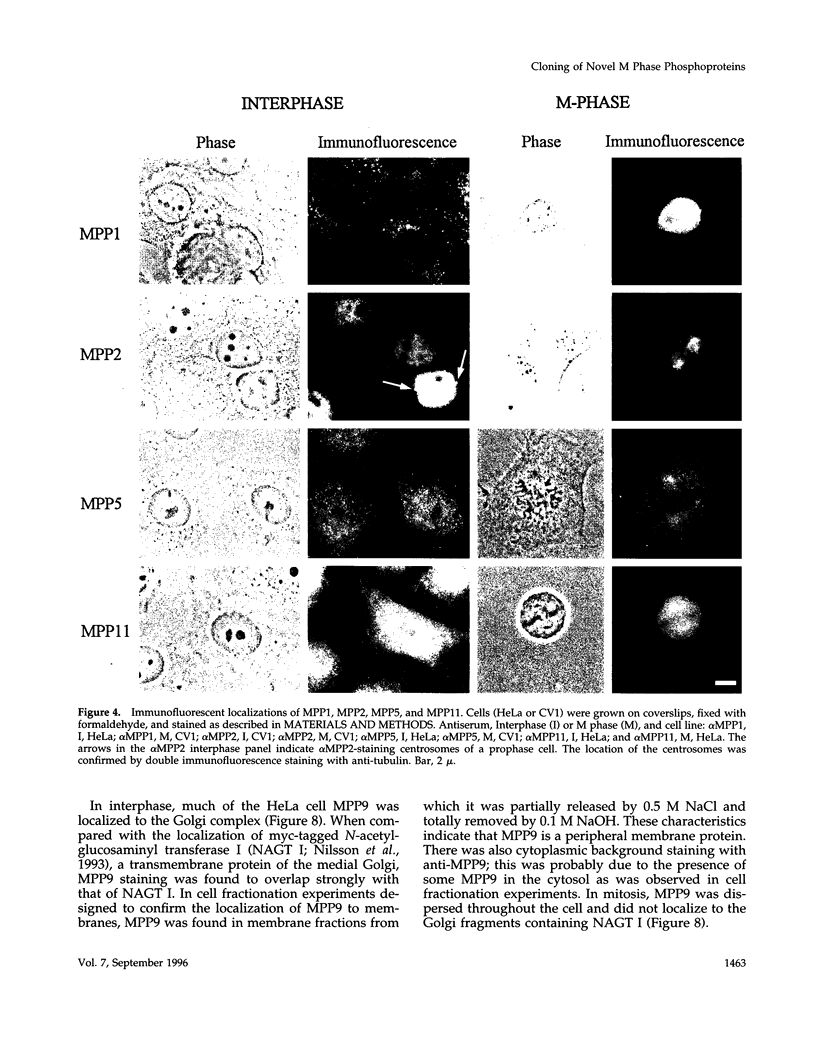
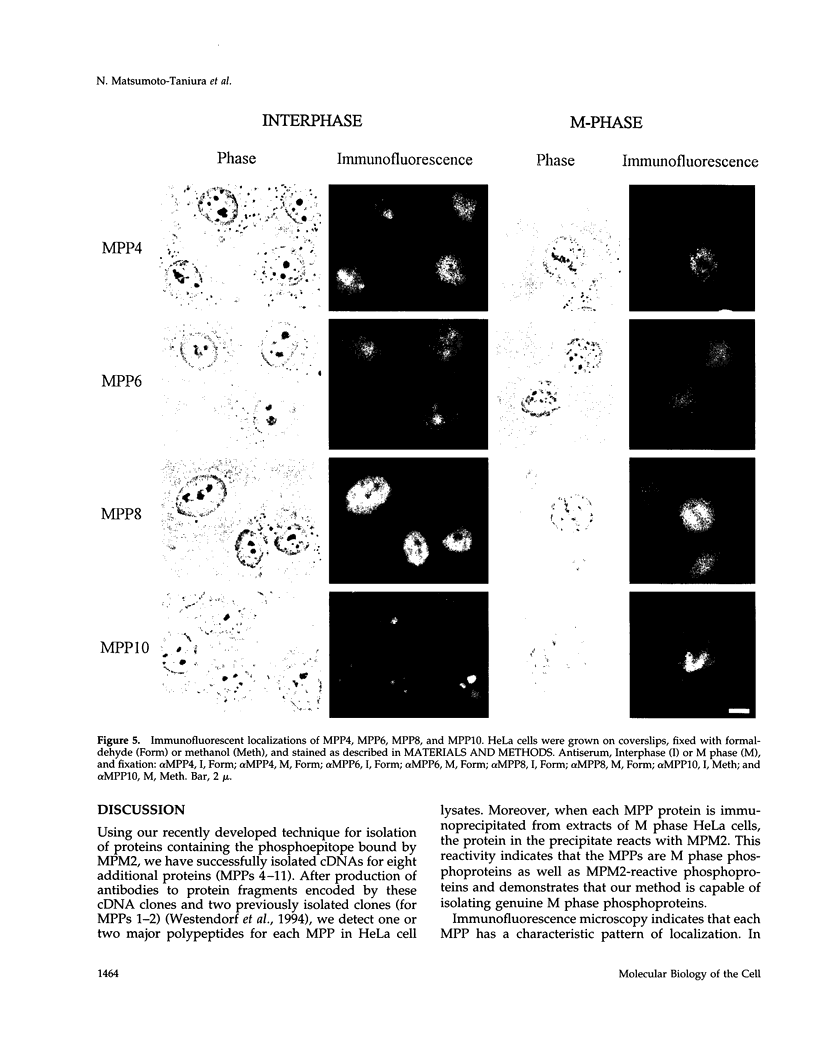
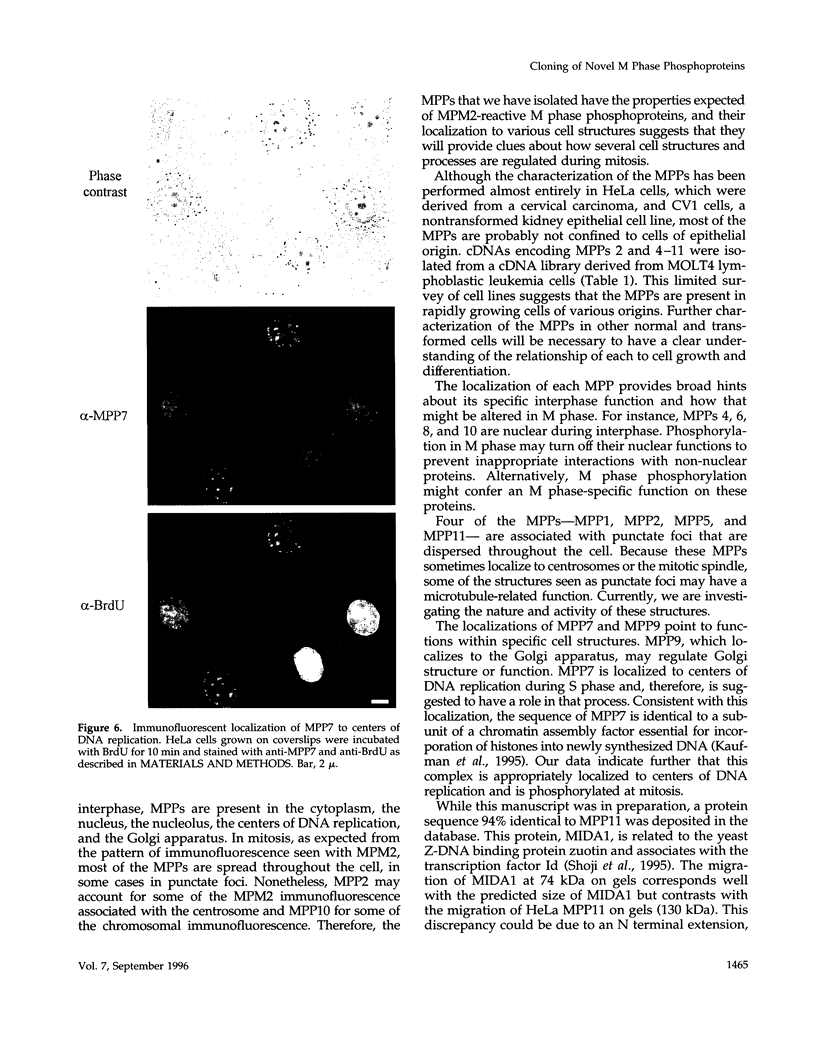
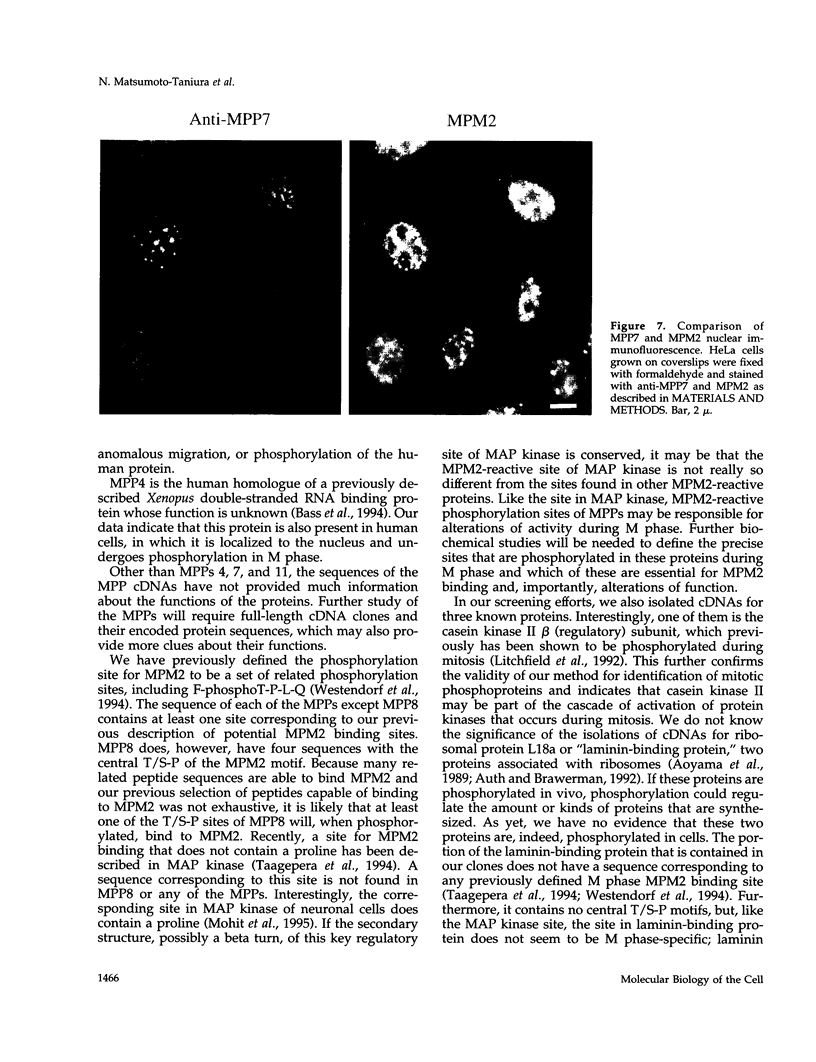
Abstract
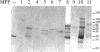
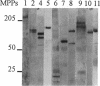
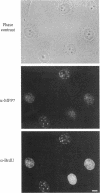
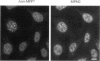
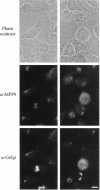
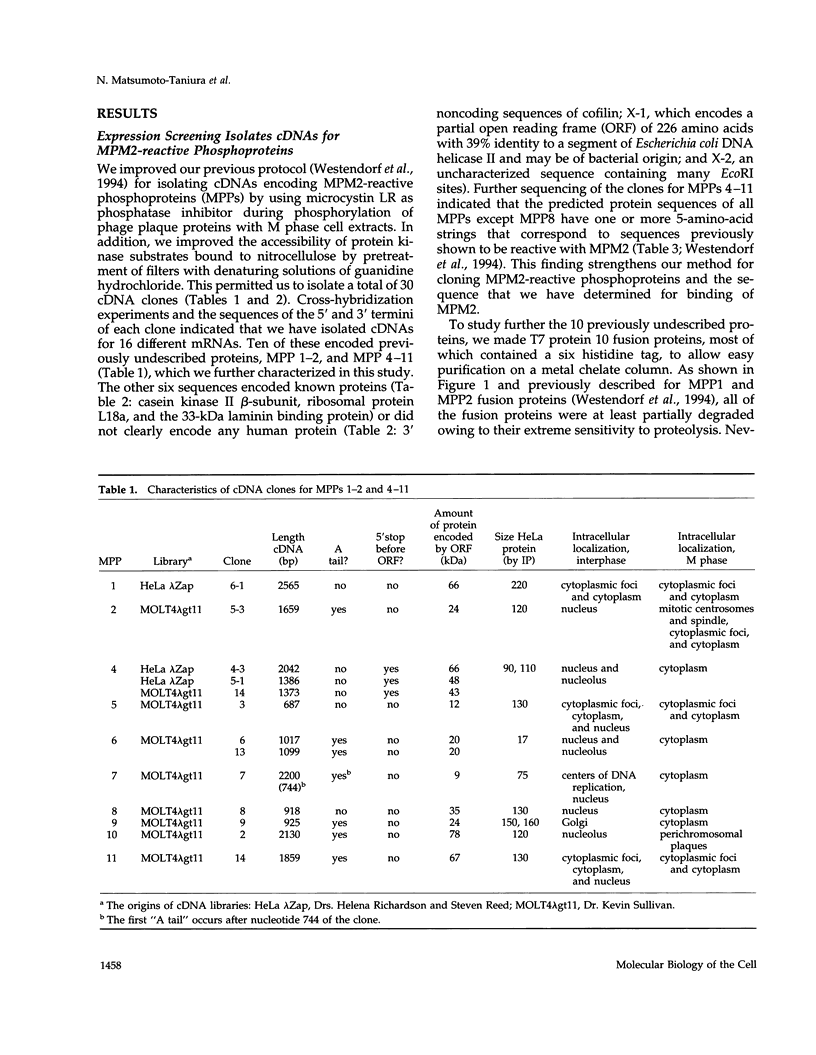
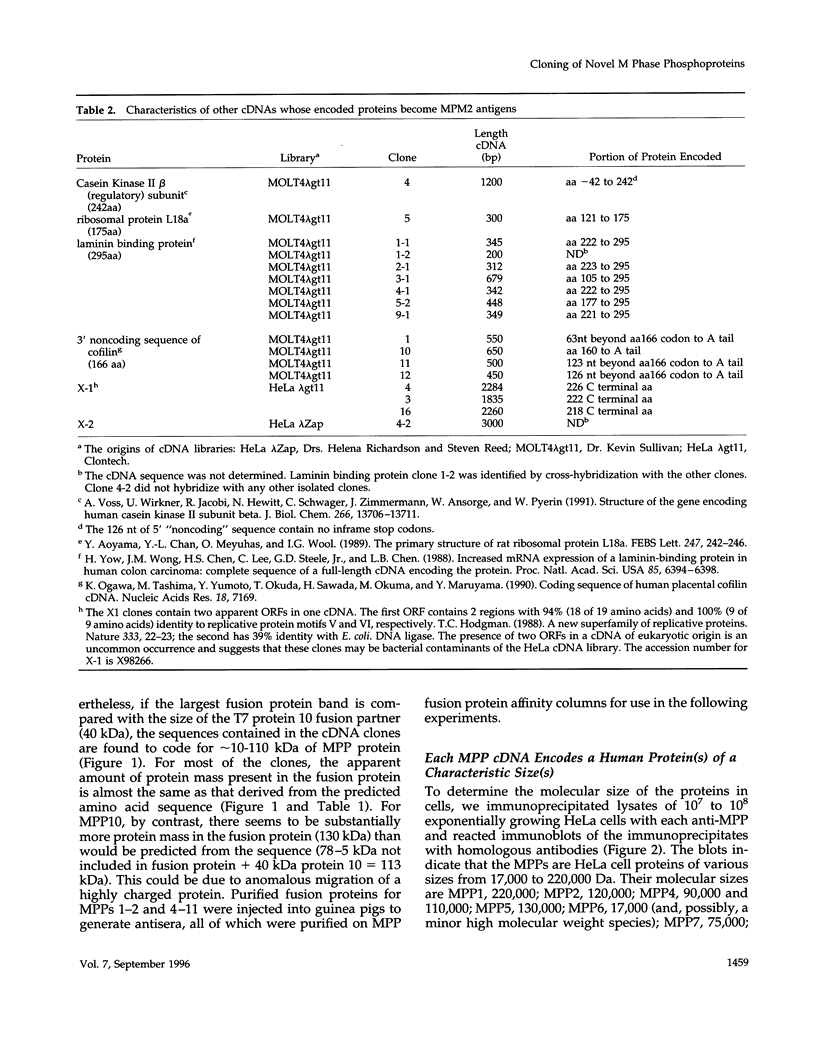
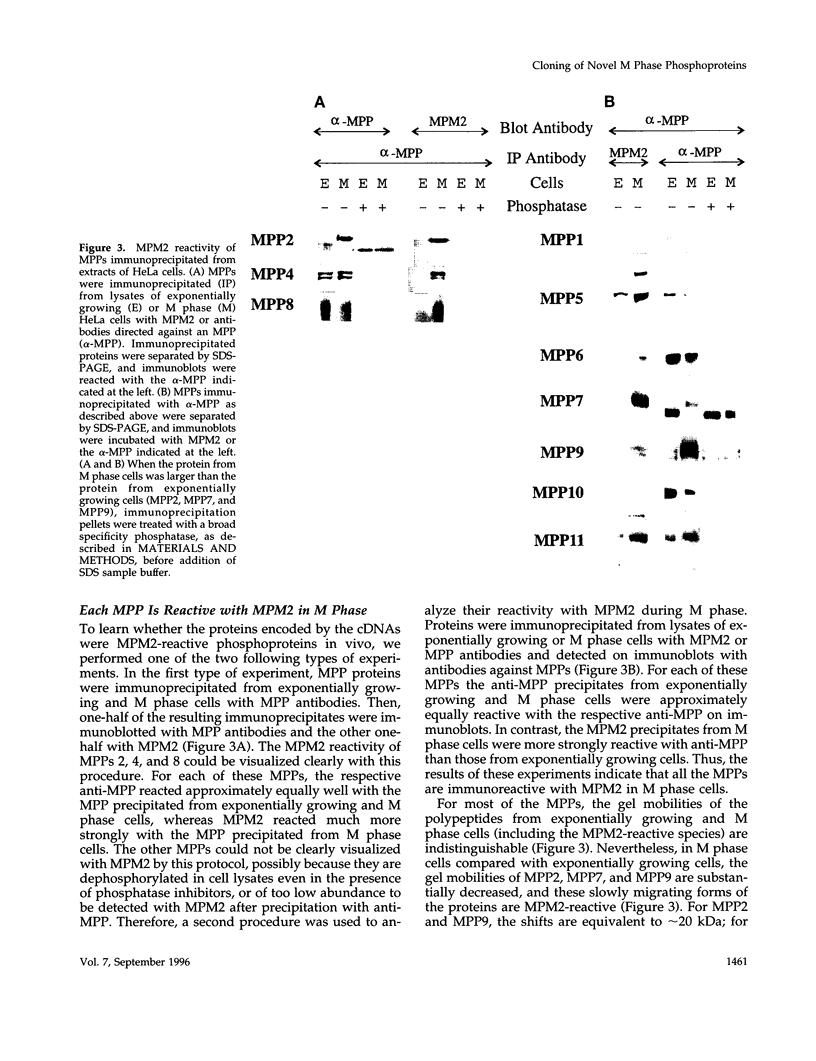
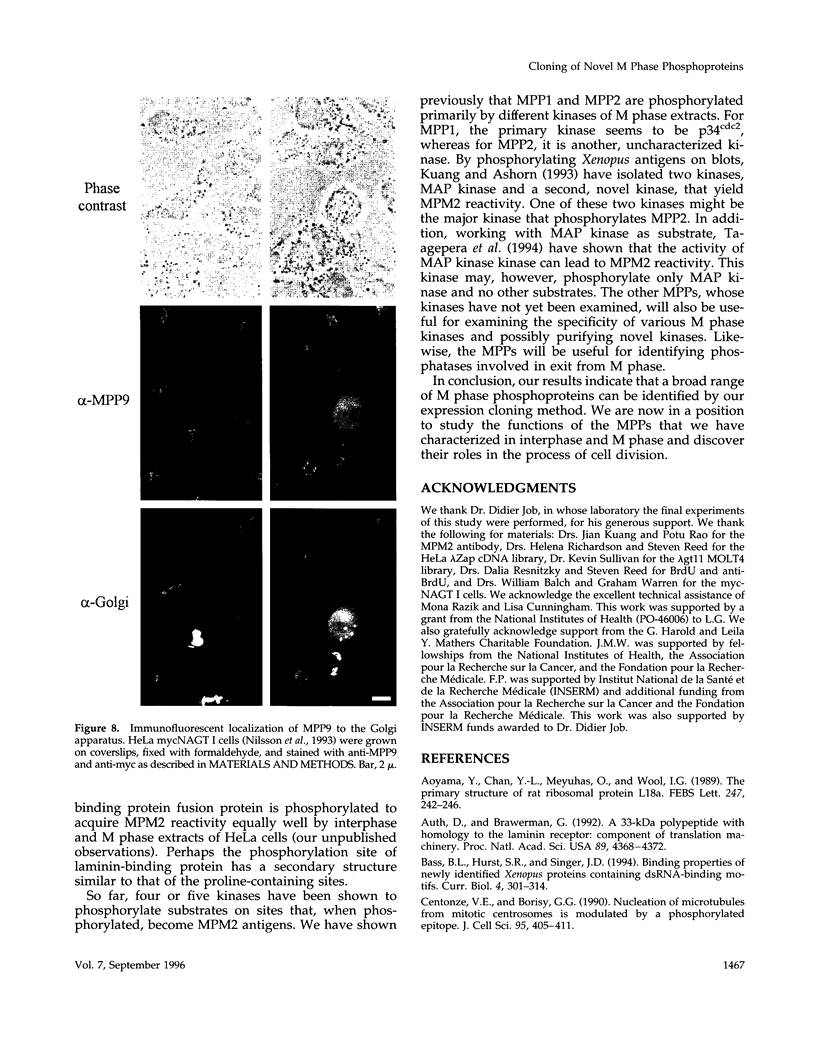
Using an expression cloning technique, we isolated cDNAs for eight M phase phosphoproteins (MPPs 4-11). We then used affinity-purified antibodies to fusion proteins to characterize the intracellular localization and some biochemical properties of these proteins and two others that we identified previously (MPPs 1-2). Each antibody immunoprecipitated one or two protein species of a characteristic size ranging from 17,000 to 220,000 Da. Each MPP, when immunoprecipitated from lysates of M phase cells, was reactive with MPM2, a monoclonal antibody that recognizes a group of related M phase phosphorylation sites, including F-phosphoT-P-L-Q. This reactivity indicated that all the MPPS encoded genuine M phase phosphoproteins. When antibodies to the MPPS were used for immunofluorescence microscopy, each anti-MPP gave a characteristic pattern of localization. In interphase, several of the MPPs were nuclear proteins, whereas others were cytoplasmic or distributed throughout the cell. Three MPPS were strikingly localized to interphase structures: MPP7 to centers of DNA replication, MPP9 to the Golgi complex, and MPP10 to nucleoli. In mitosis, most of the MPPs were distributed throughout the cells. Further studies of the 10 MPPs, most of which are previously undescribed, are expected to provide new understandings of the process of cell division.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama Y., Chan Y. L., Meyuhas O., Wool I. G. The primary structure of rat ribosomal protein L18a. FEBS Lett. 1989 Apr 24;247(2):242–246. doi: 10.1016/0014-5793(89)81344-4. [DOI] [PubMed] [Google Scholar]
- Auth D., Brawerman G. A 33-kDa polypeptide with homology to the laminin receptor: component of translation machinery. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4368–4372. doi: 10.1073/pnas.89.10.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass B. L., Hurst S. R., Singer J. D. Binding properties of newly identified Xenopus proteins containing dsRNA-binding motifs. Curr Biol. 1994 Apr 1;4(4):301–314. doi: 10.1016/s0960-9822(00)00069-5. [DOI] [PubMed] [Google Scholar]
- Centonze V. E., Borisy G. G. Nucleation of microtubules from mitotic centrosomes is modulated by a phosphorylated epitope. J Cell Sci. 1990 Mar;95(Pt 3):405–411. doi: 10.1242/jcs.95.3.405. [DOI] [PubMed] [Google Scholar]
- Coleman T. R., Dunphy W. G. Cdc2 regulatory factors. Curr Opin Cell Biol. 1994 Dec;6(6):877–882. doi: 10.1016/0955-0674(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Davis F. M., Tsao T. Y., Fowler S. K., Rao P. N. Monoclonal antibodies to mitotic cells. Proc Natl Acad Sci U S A. 1983 May;80(10):2926–2930. doi: 10.1073/pnas.80.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draetta G. F. Mammalian G1 cyclins. Curr Opin Cell Biol. 1994 Dec;6(6):842–846. doi: 10.1016/0955-0674(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Draetta G., Luca F., Westendorf J., Brizuela L., Ruderman J., Beach D. Cdc2 protein kinase is complexed with both cyclin A and B: evidence for proteolytic inactivation of MPF. Cell. 1989 Mar 10;56(5):829–838. doi: 10.1016/0092-8674(89)90687-9. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Brizuela L., Beach D., Newport J. The Xenopus cdc2 protein is a component of MPF, a cytoplasmic regulator of mitosis. Cell. 1988 Jul 29;54(3):423–431. doi: 10.1016/0092-8674(88)90205-x. [DOI] [PubMed] [Google Scholar]
- Elledge S. J., Harper J. W. Cdk inhibitors: on the threshold of checkpoints and development. Curr Opin Cell Biol. 1994 Dec;6(6):847–852. doi: 10.1016/0955-0674(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Gautier J., Minshull J., Lohka M., Glotzer M., Hunt T., Maller J. L. Cyclin is a component of maturation-promoting factor from Xenopus. Cell. 1990 Feb 9;60(3):487–494. doi: 10.1016/0092-8674(90)90599-a. [DOI] [PubMed] [Google Scholar]
- Gautier J., Norbury C., Lohka M., Nurse P., Maller J. Purified maturation-promoting factor contains the product of a Xenopus homolog of the fission yeast cell cycle control gene cdc2+. Cell. 1988 Jul 29;54(3):433–439. doi: 10.1016/0092-8674(88)90206-1. [DOI] [PubMed] [Google Scholar]
- Glass C. A., Glass J. R., Taniura H., Hasel K. W., Blevitt J. M., Gerace L. The alpha-helical rod domain of human lamins A and C contains a chromatin binding site. EMBO J. 1993 Nov;12(11):4413–4424. doi: 10.1002/j.1460-2075.1993.tb06126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heichman K. A., Roberts J. M. Rules to replicate by. Cell. 1994 Nov 18;79(4):557–562. doi: 10.1016/0092-8674(94)90541-x. [DOI] [PubMed] [Google Scholar]
- Hirano T., Mitchison T. J. Cell cycle control of higher-order chromatin assembly around naked DNA in vitro. J Cell Biol. 1991 Dec;115(6):1479–1489. doi: 10.1083/jcb.115.6.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Pines J. Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell. 1994 Nov 18;79(4):573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- Karsenti E., Bravo R., Kirschner M. Phosphorylation changes associated with the early cell cycle in Xenopus eggs. Dev Biol. 1987 Feb;119(2):442–453. doi: 10.1016/0012-1606(87)90048-0. [DOI] [PubMed] [Google Scholar]
- Kaufman P. D., Kobayashi R., Kessler N., Stillman B. The p150 and p60 subunits of chromatin assembly factor I: a molecular link between newly synthesized histones and DNA replication. Cell. 1995 Jun 30;81(7):1105–1114. doi: 10.1016/s0092-8674(05)80015-7. [DOI] [PubMed] [Google Scholar]
- King R. W., Jackson P. K., Kirschner M. W. Mitosis in transition. Cell. 1994 Nov 18;79(4):563–571. doi: 10.1016/0092-8674(94)90542-8. [DOI] [PubMed] [Google Scholar]
- Kuang J., Ashorn C. L. At least two kinases phosphorylate the MPM-2 epitope during Xenopus oocyte maturation. J Cell Biol. 1993 Nov;123(4):859–868. doi: 10.1083/jcb.123.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang J., Ashorn C. L., Gonzalez-Kuyvenhoven M., Penkala J. E. cdc25 is one of the MPM-2 antigens involved in the activation of maturation-promoting factor. Mol Biol Cell. 1994 Feb;5(2):135–145. doi: 10.1091/mbc.5.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang J., Zhao J., Wright D. A., Saunders G. F., Rao P. N. Mitosis-specific monoclonal antibody MPM-2 inhibits Xenopus oocyte maturation and depletes maturation-promoting activity. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4982–4986. doi: 10.1073/pnas.86.13.4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé J. C., Capony J. P., Caput D., Cavadore J. C., Derancourt J., Kaghad M., Lelias J. M., Picard A., Dorée M. MPF from starfish oocytes at first meiotic metaphase is a heterodimer containing one molecule of cdc2 and one molecule of cyclin B. EMBO J. 1989 Oct;8(10):3053–3058. doi: 10.1002/j.1460-2075.1989.tb08456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litchfield D. W., Lüscher B., Lozeman F. J., Eisenman R. N., Krebs E. G. Phosphorylation of casein kinase II by p34cdc2 in vitro and at mitosis. J Biol Chem. 1992 Jul 15;267(20):13943–13951. [PubMed] [Google Scholar]
- Lohka M. J., Kyes J. L., Maller J. L. Metaphase protein phosphorylation in Xenopus laevis eggs. Mol Cell Biol. 1987 Feb;7(2):760–768. doi: 10.1128/mcb.7.2.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller J., Wu M., Gerhart J. C. Changes in protein phosphorylation accompanying maturation of Xenopus laevis oocytes. Dev Biol. 1977 Jul 15;58(2):295–312. doi: 10.1016/0012-1606(77)90093-8. [DOI] [PubMed] [Google Scholar]
- Meijer L., Arion D., Golsteyn R., Pines J., Brizuela L., Hunt T., Beach D. Cyclin is a component of the sea urchin egg M-phase specific histone H1 kinase. EMBO J. 1989 Aug;8(8):2275–2282. doi: 10.1002/j.1460-2075.1989.tb08353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohit A. A., Martin J. H., Miller C. A. p493F12 kinase: a novel MAP kinase expressed in a subset of neurons in the human nervous system. Neuron. 1995 Jan;14(1):67–78. doi: 10.1016/0896-6273(95)90241-4. [DOI] [PubMed] [Google Scholar]
- Mueller P. R., Coleman T. R., Dunphy W. G. Cell cycle regulation of a Xenopus Wee1-like kinase. Mol Biol Cell. 1995 Jan;6(1):119–134. doi: 10.1091/mbc.6.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson T., Pypaert M., Hoe M. H., Slusarewicz P., Berger E. G., Warren G. Overlapping distribution of two glycosyltransferases in the Golgi apparatus of HeLa cells. J Cell Biol. 1993 Jan;120(1):5–13. doi: 10.1083/jcb.120.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P. Ordering S phase and M phase in the cell cycle. Cell. 1994 Nov 18;79(4):547–550. doi: 10.1016/0092-8674(94)90539-8. [DOI] [PubMed] [Google Scholar]
- O'Connell M. J., Nurse P. How cells know they are in G1 or G2. Curr Opin Cell Biol. 1994 Dec;6(6):867–871. doi: 10.1016/0955-0674(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Sherr C. J. G1 phase progression: cycling on cue. Cell. 1994 Nov 18;79(4):551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- Shoji W., Inoue T., Yamamoto T., Obinata M. MIDA1, a protein associated with Id, regulates cell growth. J Biol Chem. 1995 Oct 20;270(42):24818–24825. doi: 10.1074/jbc.270.42.24818. [DOI] [PubMed] [Google Scholar]
- Taagepera S., Dent P., Her J. H., Sturgill T. W., Gorbsky G. J. The MPM-2 antibody inhibits mitogen-activated protein kinase activity by binding to an epitope containing phosphothreonine-183. Mol Biol Cell. 1994 Nov;5(11):1243–1251. doi: 10.1091/mbc.5.11.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taagepera S., Rao P. N., Drake F. H., Gorbsky G. J. DNA topoisomerase II alpha is the major chromosome protein recognized by the mitotic phosphoprotein antibody MPM-2. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8407–8411. doi: 10.1073/pnas.90.18.8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandre D. D., Davis F. M., Rao P. N., Borisy G. G. Phosphoproteins are components of mitotic microtubule organizing centers. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4439–4443. doi: 10.1073/pnas.81.14.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandré D. D., Centonze V. E., Peloquin J., Tombes R. M., Borisy G. G. Proteins of the mammalian mitotic spindle: phosphorylation/dephosphorylation of MAP-4 during mitosis. J Cell Sci. 1991 Apr;98(Pt 4):577–588. doi: 10.1242/jcs.98.4.577. [DOI] [PubMed] [Google Scholar]
- Westendorf J. M., Rao P. N., Gerace L. Cloning of cDNAs for M-phase phosphoproteins recognized by the MPM2 monoclonal antibody and determination of the phosphorylated epitope. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):714–718. doi: 10.1073/pnas.91.2.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda Y., Maul G. G. A nucleolar auto-antigen is part of a major chromosomal surface component. Chromosoma. 1990 Apr;99(2):152–160. doi: 10.1007/BF01735332. [DOI] [PubMed] [Google Scholar]
- Ye X. S., Xu G., Pu R. T., Fincher R. R., McGuire S. L., Osmani A. H., Osmani S. A. The NIMA protein kinase is hyperphosphorylated and activated downstream of p34cdc2/cyclin B: coordination of two mitosis promoting kinases. EMBO J. 1995 Mar 1;14(5):986–994. doi: 10.1002/j.1460-2075.1995.tb07079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J. Y., Kuang J., Adlakha R. C., Rao P. N. Threonine phosphorylation is associated with mitosis in HeLa cells. FEBS Lett. 1989 Jun 5;249(2):389–395. doi: 10.1016/0014-5793(89)80665-9. [DOI] [PubMed] [Google Scholar]