Abstract
The control of hair growth in the adult mammalian coat is a fascinating topic which has just begun to be explored with molecular genetic tools. Complex hair cycle domains and regenerative hair waves are present in normal adult (> 2 month) mice, but more apparent in mutants with cyclic alopecia phenotypes. Each hair cycle domain consists of initiation site(s), a propagating wave and boundaries. By analyzing the dynamics of hair growth, time required for regeneration after plucking, in situ hybridization and reporter activity, we showed that there is oscillation of intra-follicular Wnt signaling which is synchronous with hair cycling, and there is oscillation of dermal bone morphogenetic protein (BMP) signaling which is asynchronous with hair cycling. The interactions of these two rhythms lead to the recognition of refractory and competent phases in the telogen, and autonomous and propagating phases in the anagen. Boundaries form when propagating anagen waves reach follicles which are in refractory telogen. Experiments showed that Krt14-Nog mice have shortened refractory telogen and simplified wave dynamics. Krt14-Nog skin grafts exhibit non-autonomous interactions with surrounding host skin. Implantation of BMP coated beads into competent telogen skin prevents hair wave propagation around the bead. Thus, we have developed a new molecular understanding of the classic early concepts of inhibitory “chalone”, suggesting that stem cells within the hair follicle micro-environment, or other organs, are subject to a higher level of macro-environmental regulation. Such a novel understanding has important implications in the field of regenerative medicine. The unexpected links with Bmp2 expression in subcutaneous adipocytes has implications for systems biology and Evo-Devo.
Keywords: stem cell, hair cycle, biological rhythm, skin appendage, complexity, pattern formation
Introduction
Hair follicle is fascinating because it is an organ that goes through regeneration in the adult under physiological condition. Hair follicles go through anagen (growing phase), catagen (destructive phase), telogen (resting phase) and exogen (the time hair filaments dislodge) stages (Fig. 1). The length of each phase and the total length of one hair cycle vary and presumably is under control of a hair cycle clock as well as some systemic factors (Stenn and Paus, 2001). Most recent works attempting to study molecular biology of hair cycling have been focused on a single hair follicle (Paus and Foitzik, 2004; Fuchs et al., 2001). In this review, we will focus on the behavior of a population of hair follicles.
Fig. 1. Growth of individual hairs vs. growth of hairs in populations.
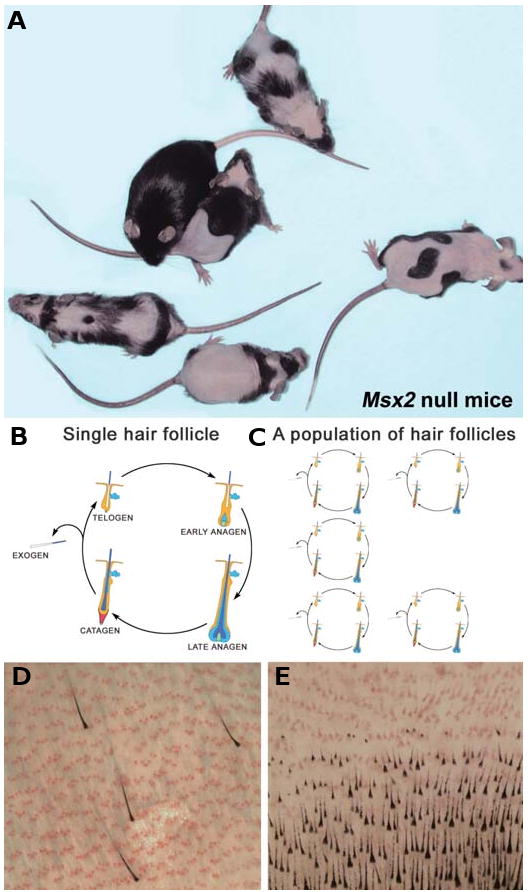
(A) Within one mouse cage each adult Msx2 null mouse displays a different pattern of hair growth. Hair growth patterns can be easily monitored in Msx2 null mice due to the cyclic alopecia phenotype. (B,C) Schematic illustration of hair cycling in one single hair follicle (B) versus cycling of a population of hair follicles (C). (D,E) In mice pelage hair follicles can sometime cycle individually (D), yet most of the time they cycle as coordinated waves (E). Anagen hair follicles are black. The position of telogen hair follicles is marked with sebaceous glands stained in red with Oil Red.
A population of functional units, each capable of oscillating through several functional states (such as neural activity or regenerative cycling), may cycle autonomously and randomly, simultaneously and synchronously, or coordinated to form transient clusters and waves (Fig. 1). However, it has been difficult to visualize and analyze these patterned changes in vivo and to decipher their underlying mechanisms. On the skin of the mouse, hair cycling appears to be coordinated and patterned. This allows us to visualize macroscopic changes in hair growth states clearly on a manageable time scale. Hair cycling patterns are complex, resemble geometric shapes, and change over time. While present in wild type mice, they are most obvious in mutant mice with the cyclic alopecia phenotype (Fig. 2; Ma et al., 2003; Militzer, 2001; Uyttendaele et al., 2004), and in the traveling strips on a special strain of nude mice (Suzuki et al., 2003).
Fig. 2. Long term follow up of changing hair cycle domains on dorsal skin of the same mouse.
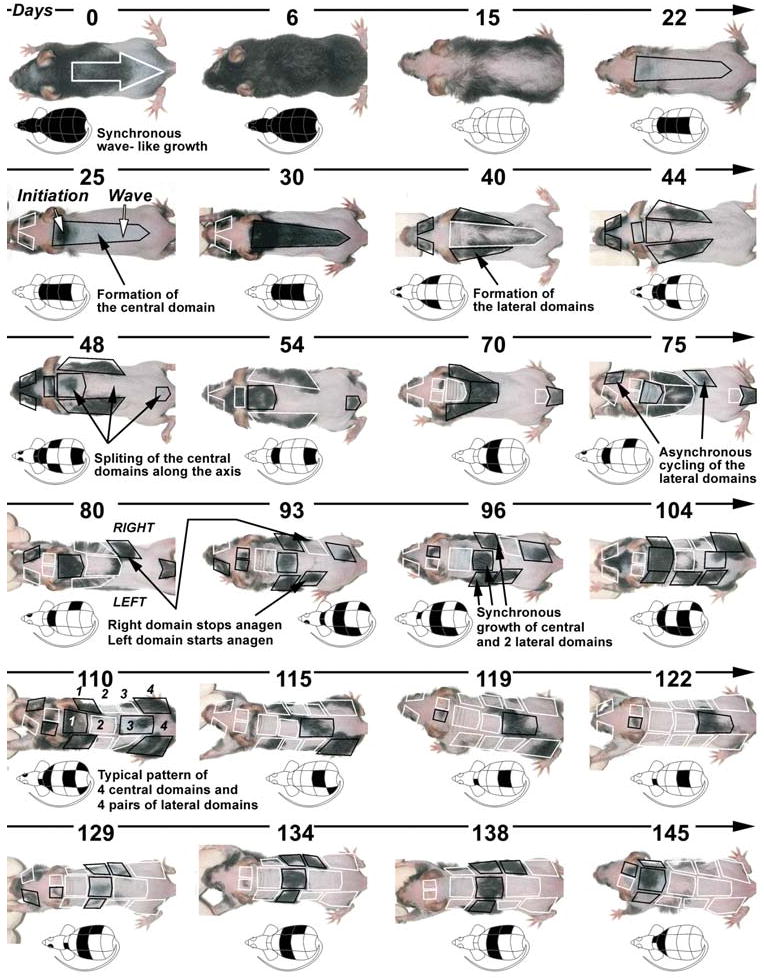
Changes in the coat of a single Msx2 null mouse with cyclic alopecia were followed over 145 days. Pictures were taken every 2-3 days and selective ones are shown here. Next to each picture there is a schematic drawing of the hair growth pattern. Key patterning events are annotated. From Plikus et al., 2008.
Patterned hair growth was first observed in classical studies on wild type rats, mice, and other mammals (Durward and Rundall, 1949). Early observations in rats have described hair growth patterns as successive waves of anagen periodically spreading from the ventral side of the body to the dorsal side, over the trunk, and progressively decreasing in width with age (Butcher, 1936). The periodic nature of these hair growth waves, or regenerative waves, was thought to be inherent and was largely attributed to some genetic, yet unidentified mechanism (Ebling and Johnson, 1961). Conversely, it was demonstrated that the so-called inherent rhythms of hair growth can be modulated by systemic factors, such as steroid hormones (Butcher, 1936; Ebling and Johnson, 1961).
Early experiments with steroid hormones led to the concept of ≪telogen refractivity≫ (Ebling and Johnson, 1961; Johnson, 1958a; Johnson, 1958b). Telogen refractivity was defined by the fact that a systemic influence can induce new anagen only at a particular time, and that there is a time period following anagen during which “the systemic stimulus is unable to exert an effect” (Durward and Rundall, 1949; Ebling and Johnson, 1961). However, many of these clues were not pursued and the knowledge remains very fragmented. Particularly, there is a lack of updated cellular and molecular studies to identify the mechanism of ≪telogen refractivity≫. Although, it was postulated that substances which inhibit anagen development, the so-called ≪chalone(s)≫, may be present in telogen skin (Ebling and Johnson, 1961; Paus et al., 1990), the hypothesis remains untested and undeveloped.
In recent studies (Plikus and Chuong, 2008; Plikus et al., 2008), we analyzed the mechanism of hair cycle domain formation and the phenomenon of changing patterns of multiple hair cycle domains (Fig. 2). To search for molecular candidates, we began by looking for pathways that suppress hair growth. In our expression studies on whole mount skin strip preparations including entire hair cycle domains, we observed an unexpected oscillatory expression of Bmp2 in the inter-follicular dermis. Bmp signaling was previously shown to play pivotal roles in hair follicle development (reviewed in Botchkarev and Sharov, 2004; Botchkarev et al., 1999, 2002, 2003; Plikus et al., 2004), differentiation of matrix cells in postnatal hair follicles (Kulessa et al., 2000; Kobielak et al., 2003; Yuhki et al., 2004), as well as catagen regression (Botchkarev, 2003; Andl et al., 2004; Guha et al., 2004). Additionally, delivery of extraneous noggin by intracutaneous implantation of noggin-soaked beads was able to decrease Bmp signaling activity and initiate anagen (Botchkarev et al., 2001).
In this paper, we review recent works in this topic. A systematic approach which includes dynamic expression profiling, experimentally induced hair regeneration, transplantation, and analyses of large populations of hair follicles and inter-follicular dermis in several transgenic mouse lines was used to achieve the appreciation of a novel higher level of hair cycle control which was neglected before. To this end, we are able to demonstrate that telogen should be further divided into refractory (not responsive to anagen stimuli) and competent (responsive) phases, and anagen should be further divided into propagating (able to stimulate anagen entry within adjacent competent telogen follicles; continues up to anagen IV) and autonomous phases (not able to stimulate other follicles; Fig. 12).
Fig. 12. Functional phases of the hair cycle.
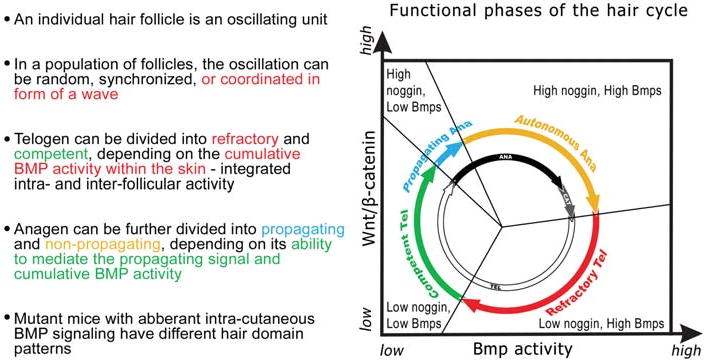
Schematic representation of classical hair cycle stages (inner black and white circle; modified from Stenn and Paus, 2001) and the new functional phases revealed in our studies (colored outer circle). Based on the ability of anagen follicles (gray) to send out signals to induce adjacent hair follicles (by reduction of Bmp activity) in anagen I - IV, not anagen V and later (black), we propose to name these sub-phases propagating anagen (blue) and autonomous anagen (yellow). Based on the ability of telogen follicles (white) to respond or not respond to regenerative signals, we name these sub-phases refractory telogen (red) and competent telogen (green). Modified from Plikus et al., 2008.
Hair cycle domains form because groups of neighboring hair follicles cycle in coordination with each other, but are out-of-phase with follicles which lie outside their neighborhood. Waves spread because anagen follicles in the propagating phase send activators into the inter-follicular macro-environment to facilitate the anagen entry of adjacent competent telogen follicles (Fig. 3 A,B, Fig. 13). Boundaries form because telogen follicles in the refractory phase can not respond to this activation. Using a novel Bmp responsive element (Brugger et al., 2004), we found that integrated levels of Bmp activity oscillate with the refractory and competence statuses of the dermal environment. These findings led us to propose that in addition to short distance micro-environmental control (Blanpain et al., 2004) the activation of hair follicle stem cells is also subjected to long distance macro-environmental control in the dermis.
Fig. 3. Regenerative hair wave and hair cycle domains.
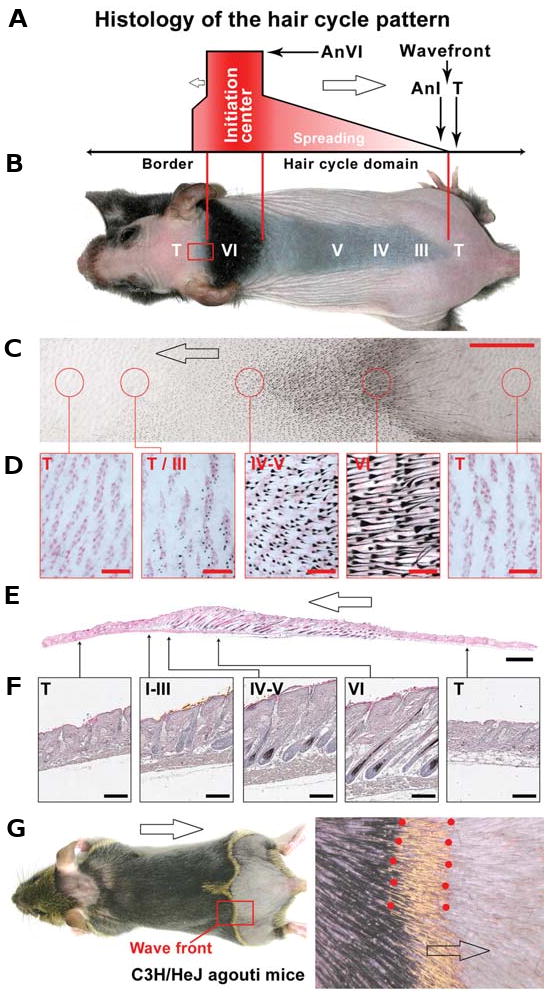
(A) Schematically, a hair cycle domain is composed of an initiation center, a spreading wave, and a boundary. Blank arrows, direction of the spreading waves. An, anagen; T, telogen. Roman numerals show anagen stages in accordance to Muller-Rover et al., 2001. (B) On the mouse back, the hair cycle domain is spreading both cephalically and caudally. T, telogen. (C,D) View of inverted skin clearly shows hair cycle stages. Areas encircled in C are enlarged in (D). (E,F) Longitudinal sections of the skin corresponding to the whole mount skin strip preparation in panel 3C is shown. Note the difference in the thickness of the skin stripe at the different hair cycle stages. Also see Suzuki et al., 2003. (G) In C3H/HeJ agouti mice, the hair fiber pigmentation is yellowish in the distal (eumelanin pigmentation) but black in the more proximal region (pheomelanin pigmentation). This helps us visualize the molting line (flanked by red dots), or the wave front of the hair cycle domain. Modified from Plikus and Chuong, 2008. Size bars: (C) 5 mm; (D) 500 μm; (E) 1 mm; (F) 200 μm.
Fig. 13. Schematic illustration of the relationship between macroenvironment and micro-environment of hair follicles.
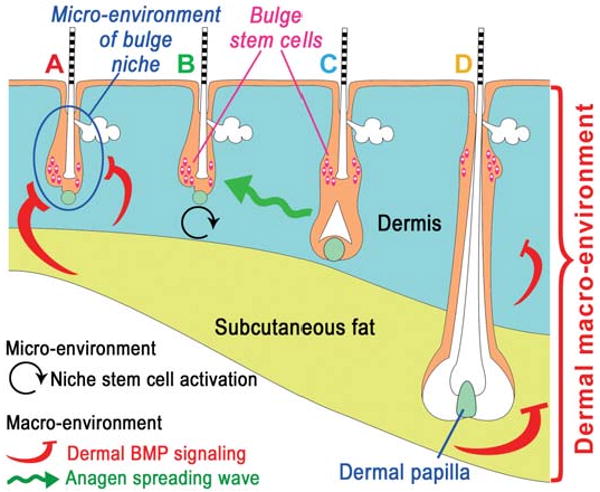
The inter-follicular dermal macroenvironment includes dermis, subcutaneous fat and adjacent follicles. Anagen stimulating (black and green) or inhibitory (red) activities are depicted with colored arrows. Follicles are in different stages: A, refractory telogen; B, competent telogen; C, propagating anagen; D, autonomous anagen follicles. Blue circle in A, intra-follicular micro-environment. Color codes are similar to Fig. 12. From Plikus et al., 2008.
Complex hair cycle domain patterns form because of the juxtaposition of coordinated growth of hair follicles within the domain and discordant growth of hair follicles between domains
Changes in skin pigmentation observed upon hair clipping and cyclic alopecia reveal complex and dynamic hair cycle domains (Fig. 1A). They are termed ≪hair cycle domains≫ because they are based on hair cycle stages which are out of phase in different domains (Fig. 3 A,B). Analysis of a single hair cycle domain reveals a continuum of hair cycle stages and coordinated anagen re-entry (Suzuki et al., 2003, Fig. 3 C-F). Multiple domains form on the skin surface because there are boundaries over which regenerative hair waves can not pass. In a single hair cycle domain, we observe three principal components: an initiation site, a spreading wave, and a boundary (Fig. 3 A,B). Within a domain, the region where follicles reenter anagen first represents the ≪initiation site≫. During growth, the domain expands. On the expanding margin of the domain, there are hair follicles with a continuum of hair cycle stages from anagen I to VI, constituting the “spreading wave”. The advancing anagen I / telogen interface forms the ≪wave front≫ (Fig. 3 C-G). However, sometimes the telogen regions are responsive and sometimes they are resistant to the spreading wave. A ≪boundary≫ forms, if these regions are resistant. A search to understand the reason for this change in responsiveness led us to identify ≪refractory≫ and ≪competent≫ telogen statuses (below).
Domain boundaries are unstable, as they can shift from one cycle to the next. These boundaries are not anatomical, but rather reflect a spatio-temporal sequence of stem cell activation within hair follicles during anagen entry which can shift stochastically. The dynamic changes of hair cycle domains are not limited to a particular mouse strain. We have also observed these patterns in other inbred or non-inbred mice and rats (Plikus and Chuong, 2008).
The telogen can be divided into refractory and competent phases
Since telogen skin does not always respond to the anagen spreading wave, it is logical to postulate that there are refractory and competent states within telogen. To further analyze this ≪refractory≫ status, we performed a 6 to 12 month follow-up study measuring the length of hair cycle stages in living mice. The average anagen length is 12.7 +/- 0.97 days for dorsal skin (n=18) and 7.6 +/- 0.66 days for ventral skin (n=28). In contrast to the low degree of variability in anagen length, telogen length is 59.6 +/-18.7 days for dorsal skin (n=22) and 40.3 +/- 10.3 days for ventral skin (n=30). Telogen shows a great range of variability: from 28 to 88 days for the dorsal skin and from 25 to 78 days for the ventral skin. Interestingly, there is a minimal period of 25-28 days, suggesting that new anagen can not start until this time elapses (Plikus et al., 2008). During this minimal period, hair follicles remain refractory to anagen stimulatory signals. After that, hair follicles become competent and are ready to re-enter anagen once sufficient activators of anagen are present. Thus it is reasonable to divide telogen into an early refractory phase (minimal required telogen, ∼25-28 days in length) and a late competent phase which can vary in length.
We performed experiments to test the hypothesis that the juxtaposition of asynchronous hair cycle stages can lead to the formation of a domain boundary. By locally applying cyclosporine A, a known anagen inducing agent (Maurer et al., 1997), to telogen skin we caused ectopic anagen growth within the treated site. Cyclosporine A induced hair follicles in anagen to cycle 13 days ahead of the neighboring follicle population. By the time a new anagen spreading wave propagated over the dorsal skin, the cyclosporine A-induced hair patch had already re-entered early telogen and became refractory to stimulation, thus forming a new hair cycle domain (Plikus et al., 2008).
We can estimate whether the telogen skin region is in a refractory or competent phase by counting the days elapsed since the beginning of telogen. Club hair plucking is known to stimulate anagen re-entry (Collins, 1918). Different numbers of hairs were plucked to gauge the responses semi-quantitatively. We plucked either 50 or 200 club hairs from early/refractory and late/competent telogen skin and then recorded the number of days taken for new hair filaments to emerge from the plucked follicles (n=16). When 200 club hairs are plucked the competent telogen follicles respond fast (∼9 days). It will take 14 days for a response if 50 club hairs are plucked. Hence in wild type skin, the anagen inducing signal appears to be concentration dependent. On the other hand, plucking from refractory regions requires a significantly longer response time: plucking of 200 hairs requires a minimum of 28 days and plucking of 50 hairs requires even longer (Plikus et al., 2008). Thus the refractory/competent status of a telogen hair follicle judged by hair plucking is consistent with those estimated from analyzing the real time changes of hair cycle domains.
The anagen can be further divided into propagating and autonomous phases
We observed that a wave can propagate in all directions independent of the body axis or the hair follicle orientation. Whenever early anagen hair follicles (anagen I-IV) encounter regions of competent telogen hair follicles, the wave front spreads (Fig. 3 A-F). However, it was observed that late anagen hair follicles (anagen V, VI) can not induce anagen re-entry even when they are next to a competent telogen region. This can be observed when the anagen spreading wave forms a boundary with the refractory telogen hair follicles. Over time, refractory follicles enter the competent phase; and the early anagen follicles on the other side of the boundary also progress into anagen V and VI. Despite these changes, the now competent telogen hair follicles remain quiescent because the late anagen follicles do not send out propagating signals (Plikus et al., 2008). This helps to stabilize domain boundaries. Based on these results, we divide anagen into an earlier propagating phase (anagen I-IV) which can induce adjacent competent telogen follicles to regenerate, and a later autonomous phase which cannot induce adjacent follicles to regenerate even if they are in a competent phase. Old boundaries break only when competent telogen follicles happen to be in phase with adjacent propagating anagen follicles.
Bmp expression level oscillates out of phase with hair cycling: high in autonomous anagen / refractory telogen, low in competent telogen / propagating anagen
What is the molecular basis of the refractory and competent telogen phases? It is likely to involve inhibitors or activators within and/or around telogen hair follicles. We screened several candidate signaling pathways (not shown) and have identified Bmp pathway members to be the likely candidates. We have studied expression of Bmp2, Bmp4 and noggin using in situ hybridization and Bmp4-lacZ and Nog-lacZ (Kulessa et al., 2000) expression visualized with X-gal staining.
We did whole mount in situ hybridization for Bmp2 on longitudinal skin strips spanning the entire hair cycle domain, with the propagating wave front shown on the left and the boundary on the right (Fig. 4 A-C. Plikus et al., 2008). These skin strip preparations are particularly useful since the temporal hair cycle stages are laid out along them in a spatial order. Bmp2 expression intensifies within the spreading wave and reaches its highest level in anagen V-VI. Bmp2 expression remains high in early telogen, but it dwindles down in late telogen, corresponding to the competent telogen phase. Much of this fluctuating Bmp2 expression appears to be in the mesenchymal cells of the dermis surrounding hair follicles. Unexpectedly we found that Bmp2 transcripts are primarily produced by the subcutaneous adipocytes, as judged by the double staining with Sudan red. After Bmp2 expression subsides during competent telogen (Fig. 4B) it re-appears again in the mesenchymal cells surrounding anagen III hair follicles (Fig. 4 B,C).
Fig. 4. Spatial and temporal changes of Bmp2 expression in the regenerating hair wave.
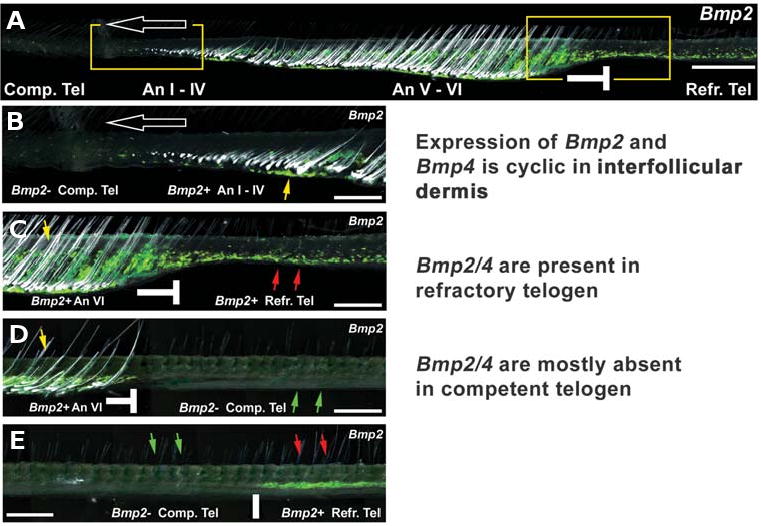
In situ hybridization of Bmp2. From Plikus et al. (2008). (A-E) Dynamic expression of Bmp2 in a spreading hair cycle domain. (A) Different hair cycle stages are spread spatially on a longitudinal skin strip. In situ hybridization for Bmp2 (green). Blank arrows, the direction of the spreading waves; —| sign, boundary between anagen (An, which was competent telogen) and refractory telogen (Tel). Two boxed regions showing wave front and boundary are enlarged in panels (B,C). Also note the change of skin thickness. (B,C) Bmp2 is negative in the wave front region, including competent telogen and propagating anagen. Extrafollicular Bmp2 starts to appear around anagen IV and its expression becomes stronger in anagen VI (yellow arrows). Extrafollicular Bmp2 persists into the refractory telogen stage (red arrows). (D) Progressive changes of (C). Entering late telogen, the region to the right of the boundary becomes Bmp2-negative (green arrows) and competent to enter anagen. However, anagen VI (yellow arrow) follicles do not send out spreading signals and the boundary remains stable. (E) A telogen skin strip shows Bmp2 expression during early and refractory phases (red arrows), but lack thereof during late and competent phase (green arrows). Scale bars, (A) 1 mm; (B-E) 500 μm. From Plikus et al., 2008.
Several other genes were examined in the strip of hair cycle domain (Fig. 5; Plikus et al., 2008, supplement). Similarly, we observed overall Bmp4-lacZ activity to be high in refractory telogen and low in competent telogen. In contrast to Bmp2, Bmp4-lacZ is expressed within, as well as outside the hair follicles. During refractory telogen Bmp4-lacZ is abundantly expressed in the dermal papillae and secondary hair germs of the hair follicles. It is also expressed in the epidermis, dermal fibroblasts and arrector pili muscle of the interfollicular skin. Upon transition into competent telogen, these Bmp4-lacZ expression sites disappear. Most prominently, Bmp4-lacZ expression disappears from the dermal papilla, seemingly to unlock it, allowing it to respond to regeneration signals when they arise. Bmp4 in situ hybridization results are similar to that of Bmp4-lacZ transgene activity.
Fig. 5. The temporal oscillation and spatial distribution of Bmp signaling pathway members throughout the hair cycle is summarized.
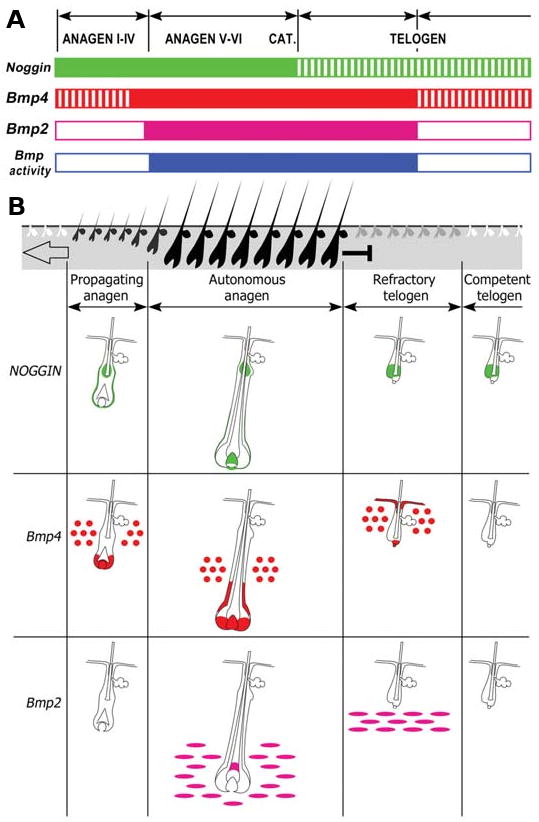
(A) Temporal expression of Bmp4, Bmp2, noggin and a putative Bmp activity reporter (see Fig. 8). Solid area, strong expression; striped areas, expression is lost from some but not all sites. (B) Schematic summary of multiple expression sites of noggin, Bmp4 and Bmp2 during functional phases of the hair cycle. From Plikus et al., 2008.
The Bmp antagonist noggin was also examined using Nog-lacZ expression (Fig. 5, Plikus et al., 2008). While noggin is expressed in the bulge epithelia throughout the hair cycle, its mesenchymal expression oscillates. Nog-lacZ is present in the dermal papilla (Botchkarev et al., 1999) and basal stalk of late anagen VI follicles, but is absent from the inter-follicular adipocytes at all stages examined. During anagen progression, Nog-lacZ first appears throughout the dermal sheath in anagen II. As hair follicles progressively elongate during anagen IIIb-IV, expression of Nog-lacZ becomes restricted to the proximal segment of the dermal sheath and to the basal stalk. By anagen VI, the proximal dermal sheath expresses Nog-lacZ very faintly and strong expression finally localizes within the dermal papilla and the basal stalk. The loss of Nog-lacZ in the dermal papilla coincides with the end of anagen, seemingly to signal the end of the dermal papilla's support for hair growth. In both refractory and competent telogen, Nog-lacZ is absent in the mesenchyme but remains positive in the bulge.
The periodic appearance of multiple hair cycle domains on the mouse skin can be appreciated from a whole mount in situ of Bmp2 (Fig. 6).
Fig. 6. Correlation of refractory telogen and Bmp2 expression.
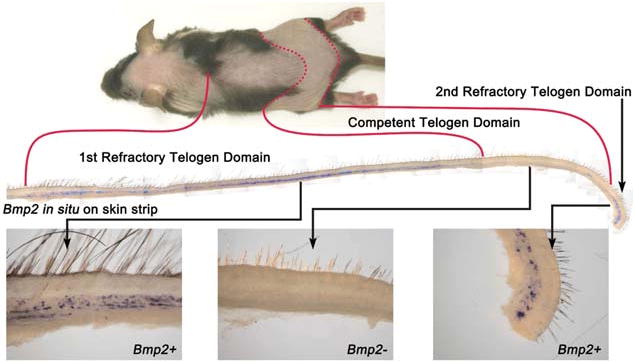
A long skin strip spanning two hair cycle domains shows two Bmp2-expressing segments which also correspond to the refractory telogen region demonstrated by observation.
Attempting to define Bmp activity with 52bpMsx2-hspLacZ transgene activity
Although some expression studies have been done on the level of a single hair follicle, here we have focused on expression dynamics within the entire hair cycle domain including intra- and inter-follicular compartments. To study Bmp activity we use a novel 52bpMsx2-hsplacZ transgene which was reported to act as a faithful Bmp-responsive element (Brugger et al., 2004).
The Bmp pathway is complex and its activity can be modulated by the changing levels of other Bmp antagonists, Bmp receptors, Smads, or even cross-talk between other pathways (Botchkarev, 2003). Therefore we further analyzed 52bpMsx2-hsplacZ trans-gene activity (Fig. 7 A-F), which acts as a faithful Bmp-responsive element, reporting the integrated Bmp activity (Brugger et al., 2004). During refractory telogen, 52bpMsx2-hsplacZ is expressed at high levels in hair follicles (Fig. 7 B,D,G). In competent telogen follicles, the level is minimal (Fig. 7 C,E,G). 52bpMsx2-hsplacZ activity comes back gradually starting at anagen II and increasing toward late anagen.
Fig. 7. Spatial and temporal changes of putative Bmp reporter activity in the regenerating hair wave.
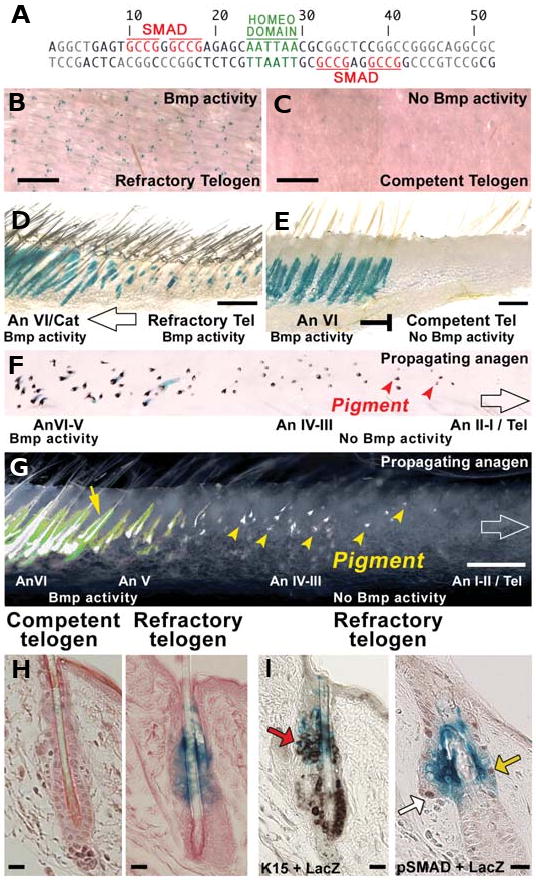
(A) 52bp Msx2 promoter region contains both Smad and homeodomain consensus sites. (B-G) Summed putative Bmp activity may be estimated in vivo using 52bpMsx2-hsplacZ transgene expression. However, this 52 bp also contain a homeobox binding site which can affect Bmp reporter activity. (F,G) Positive trans-gene expression is seen starting from late anagen. Early anagen hair follicles (red arrowheads on F; yellow arrowheads on G) do not show 52bpMsx2-hsplacZ transgene expression. Expression of 52bpMsx2-hsplacZ transgene during telogen is dynamic: it is expressed during early telogen (refractory phase; B,D), but absent during late telogen (competent phase; C,E). (H) Putative Bmp reporter activity is high in refractory but low in competent telogen follicles. (I) Left panel: Putative Bmp reporter activity (blue color) and keratin 15 immuno-localization (brown) positive cells partially overlap (red arrow). Right panel: Similarly, some Bmp reporter activity (blue color) and pSMAD positive cells (brown) overlap (yellow arrow), yet others are positive for either Bmp activity or pSMAD (white arrow) only.
Conventionally, pSMAD immunostaining assay is used to localize canonical Bmp signaling-responsive cells. However, the fact that pSMAD mediated activation of downstream targets of Bmp signaling is heavily modulated by other transcription factors (Brugger et. al., 2004; Rice et al., 2003) raises the issue of reliability of pSMAD immunostaining assay in localizing sites of active Bmp response in target tissues. Indeed, upon expression analysis we found that the extent of spatial overlap between pSMAD and activity of 52bp Msx2-hspLacZ in telogen hair follicles is small and that 52bp Msx2-hspLacZ is largely active in keratin 15-negative cells (Fig. 8I). The nature of this discrepancy is not fully understood. The 52bp fragment of the Msx2 promoter employed here contains both GCCG (pSMAD binding) sites, as well as TTAATT (homeodomain containing protein binding) sites (Brugger et. al., 2004). Availability of GCCG sites for pSMAD binding only is not sufficient to elicit a Bmp response in vivo. Mutation of the TTAATT site abolishes the Bmp response even when all pSMAD binding sites are present (Brugger et. al., 2004).
Fig. 8. Diversity of hair cycle domain patterns in transgenic mice.
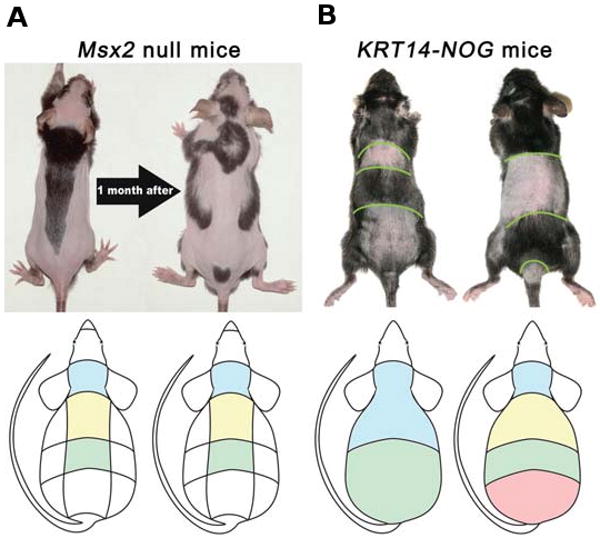
(A) Msx2 null mice form more complex and dynamic patterns than control mice. (B) Krt14-Nog mice form simplified patterns without lateral and central domains. They also do not display stable domain borders, but rather show continuous advancement of regenerating transverse waves. From Plikus et al., 2008.
As described in Brugger et. al., 2004, the Bmp response of the 52bp Msx2-hspLacZ reporter construct is elicited in a tissue- and stage-restricted manner during mouse embryogenesis. We expect that the tissue- and time-restrictive response should continue into adulthood. The nature of such a restrictive Bmp response should be elucidated separately in each case. Collectively, these facts underline the complexity of the Bmp response in target tissues that goes beyond a simple nuclear pSMAD readout. The 52bp Msx2-hspLacZ construct should become very instrumental in dissecting tissue- and time-specific Bmp signaling in vivo.
Global down-regulation of Bmp signaling in the skin of Krt14-Nog mice shortens refractory telogen and alters hair cycle wave patterns
To test the functional role of Bmp activity during refractory telogen, we should be able to confer competence by down-regulating Bmp signaling. We did this by over-expressing ectopic noggin under a keratin 14 promoter in the Krt14-Nog mouse (named K14-Noggin mice in Plikus et al., 2004). Keratin 14 expression areas include the interfollicular epidermis and the outer root sheath of the hair follicle. A cross of Krt14-Nog and 52bp Msx2-hsplacZ mice produced mice with an effective down-regulation of Bmp signaling activity in early telogen hair follicles, as judged by the premature loss of 52bp Msx2-hsplacZ transgene expression. At the functional level, telogen length in dorsal Krt14-Nog hair follicles was dramatically reduced (7.9 +/- 11 days (n=71)) with a minimal length of only 6 days (vs. 28 days in WT) and a maximal length of only 11 days (vs. 88 days in WT). At the same time, the average anagen length remained essentially unchanged (12.3 +/- 1 days (n=71) vs. 12.7 +/- 0.97 days (n=18) in WT). It appears that in Krt14-Nog mice the refractory telogen is reduced to 6 days. Similar changes are found in the ventral skin (Plikus et al., 2008).
We hypothesize that the shortened telogen in Krt14-Nog mice does not allow stochastic initiation events to accumulate. These mice have a dramatically shorter refractory telogen stage and much faster anagen re-entry. The hair follicles engage in continuous wave-like cycling. Since competent telogen is too short to allow the establishment of inter-domain boundaries, they have a much simpler domain pattern (Fig. 8, Plikus et al., 2008). In a special strain of nude mice, similar conditions occur and the hair forming waves are observed as traveling stripes (Suzuki et al., 2003).
We further tested the response of Krt14-Nog hair follicles to hair plucking. The differences in response during early vs. late telogen and upon 50 vs. 200 hair plucking observed in WT mice are eliminated in Krt14-Nog mice. In all cases, plucked Krt14-Nog hair follicles required only 6-7 days to re-enter anagen (Plikus et al., 2008). This is consistent with our hypothesis that a lower inhibitor activity (caused by noggin here) allows stem cells to reach the threshold of activation faster.
When a transplanted Krt14-Nog skin graft is surrounded by the host skin macro-environment, telogen behavior reflects the non-autonomous equilibrium of Bmp activity
To test whether hair cycle behavior changes in Krt14-Nog mice are caused by environmental changes in Bmp signaling or by permanent intrinsic changes to the hair follicles, we transplanted a small graft of Krt14-Nog skin containing about 100 hair follicles onto the dorsal skin of an adult SCID mouse (Fig. 9A). The effects of donor - host interactions are felt in both the host and donor. At times, hair cycling in transplanted Krt14-Nog hair follicles is affected by the surrounding host skin. Donor transgenic follicles remain in telogen for a longer period and can respond to the anagen activating wave originating from the host. At other times, groups of Krt14-Nog hair follicles retain a higher propensity for spontaneous anagen initiation, resulting in hair growth which is out of phase with the surrounding host skin. When the host skin is in refractory telogen, the hair growth is confined to the donor graft. When the host skin is in competent telogen, a halo of hair growth is induced (Plikus et al., 2008).
Fig. 9. (Left). Illustration of transplantation experiment.
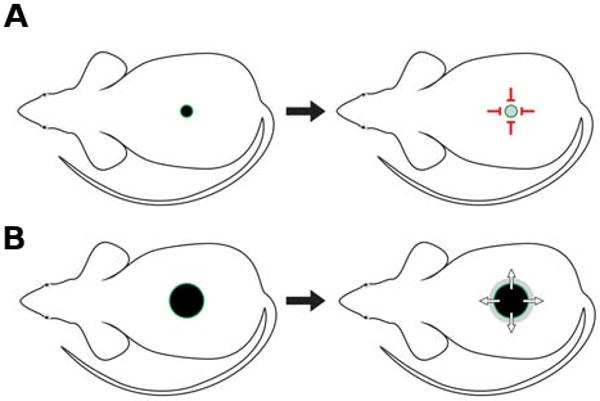
Transplanting Krt14-Nog skin to SCID mice allowed us to study the interaction between skins with either normal or high levels of noggin. (A) When small transplants were used, the refractory telogen phase in Krt14-Nog skin can be partially restored by the surrounding host skin. Red stop signals represent refractory signals that can affect the donor skin. (B) When bigger transplants were used, Krt14-Nog skin can initiate propagation waves spreading in the host skin which is in a competent telogen phase, or to ≪neutralize≫ the refractory telogen region of the host up to 2.8 mm deep. Green arrows represent the spreading signals. The experiments suggest the interactions are non-autonomous (Plikus et al., 2008). Whether this effect is mediated through direct diffusion or an indirect relay remains to be determined.
Transplantation of a small graft of Krt14-Nog; 52bp Msx2-hsplacZ skin onto an adult SCID mouse gave similar results. Examination of LacZ expression showed that the early loss of Bmp signaling activity in early telogen hair follicles within the transgenic skin is restored (Fig. 10B). Transplantation of a 52bp Msx2-hsplacZ skin graft of a similar size was used as a control.
Fig. 10. (Right). Altered activity of putative Bmp reporter in Krt14-Nog mice.
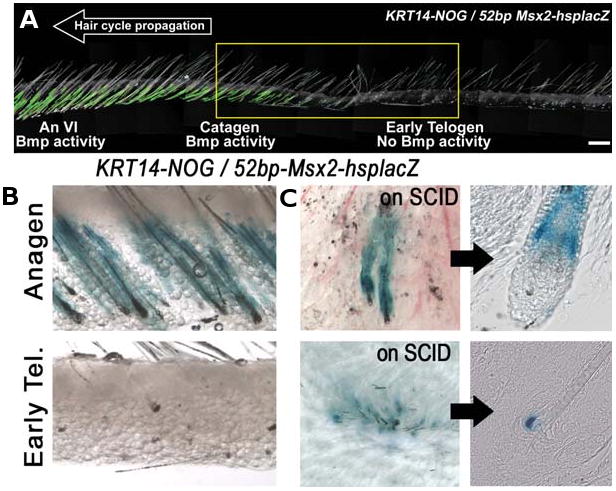
(A) Premature loss of putative Bmp activity during early telogen in Krt14-Nog / 52bp Msx2-hsplacZ mice. (B) Restored 52bp Msx2-hsplacZ transgene activity in small Krt14-Nog skin flaps transplanted on SCID mice. In the Krt14-Nog / 52bp Msx2-hsplacZ skin, there is no Bmp activity. (C) Upon transplantation however, putative Bmp activity is restored in the telogen follicles. From Plikus et al., 2008.
A long term follow up study of the hair cycle behavior showed that Krt14-Nog hair follicles surrounded by the SCID skin now spend an average of 38 days in telogen (vs 7.9 days as part of the original Krt14-Nog skin; Plikus et al., 2008). In addition, the range of telogen lengths for Krt14-Nog hair follicles expands from very narrow (6 to 11 days) to a much wider time period (11 to greater than 100 days). These numbers are comparable to the transplanted control (C57BL/6J) (Plikus et al., 2008).
To further explore this effect, we next transplanted a larger Krt14-Nog skin graft (>1cm in diameter) onto an adult SCID mouse (Fig. 9B). It is now more difficult for the host to control the donor skin behavior. We observed that the transplanted Krt14-Nog hair follicles continue to re-enter anagen after only a short period of telogen. Influenced by the donor, host telogen hair follicles around the perimeter of a Krt14-Nog skin graft acquire early competence (after only 11 days in telogen) and re-enter anagen together with the Krt14-Nog hair follicles. These hair follicles gain competence despite the fact that the rest of the SCID hair follicles further away from the Krt14-Nog transplant remain in refractory telogen (Plikus et al., 2008. The width of this rim is about 2.8 mm, reflecting the influence of noggin signaling.
Thus this transplantation data shows that the hair cycle behavior of both Krt14-Nog and SCID hair follicles can be modulated through changes in the balance of Bmp signaling. These changes are non-autonomous and are reversible. The increase of refractory telogen length in small grafts of Krt14-Nog skin was achieved by “diluting” the strength of the transgenic noggin upon transplantation onto the host skin. We believe that shortening of refractory telogen within a narrow rim of host hair follicles immediately adjacent to the transplant was achieved due to the “leak” of noggin activity from the transplanted Krt14-Nog skin.
Local delivery of BMP protein can convert competent telogen status to refractory
To test further whether BMP protein can indeed confer refractory status, we used recombinant human BMP4-soaked beads and injected them into the skin as described (Botchkarev et al., 1999). We chose the site in front of propagating wave for injection. Interestingly, BMP proteins caused hair propagation wave to go around them. In the future cycling, this would become a new domain. This is not due to physical hindrance of the bead, as albumin-soaked beads do not have this effect (Fig. 11). These results suggest that BMP protein can convert competent telogen status to refractory (Plikus et al., 2008).
Fig. 11. BMP protein can convert competent telogen status to refractory.
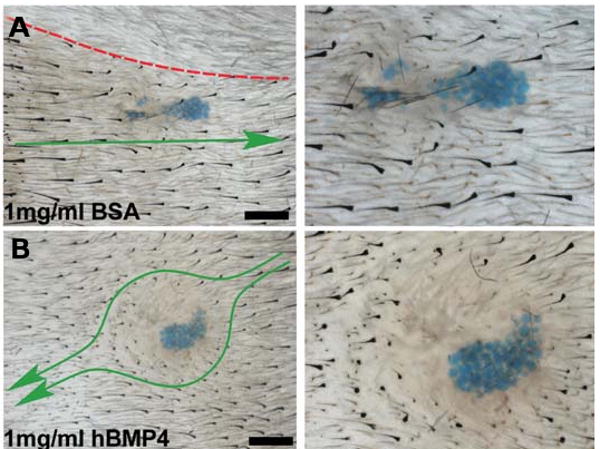
(A) Human BMP4-soaked beads caused hair propagation wave (green arrowed curve) to go around them. (B) Albumin does not have this effect. Red broken line, domain border. From Plikus et al., 2008.
Macro-environmental factors regulate the hair follicle micro-environment and stem cell activation
In populations of hair follicles, individual hair follicles can regenerate in different modes: 1) The random autonomous cycling of each follicle (Fig. 1 B,D), as seen in the human scalp (Van Steensel et al., 2000) and mouse vibrissa (reviewed in Plikus et al., 2006), 2) synchronous cycling as seen in the winter fur growth of a mink, 3) systemic endocrine signals, such as during prolactin regulated cycling (Rose et al., 1995). This implies that while there is a hair cycle clock within each follicle that can regulate stem cell activation via short-distance signaling within the niche (Fuchs et al., 2004; Blanpain et al., 2004), it can also be reset by a systemic signal. Patterned coordinated hair growth on the mouse skin implies the existence of inter-follicular coupling which is mediated by some local medium-range environmental factors (Figs. 1 C,E, 12 and 13). Here we study the production and activities of such factors. Surprisingly, we find that changing levels of Bmp activity can define, although not exclusively, such patterned hair growth behavior.
We define the macro-environment as the inter-follicular environment surrounding a specific hair follicle (Fig. 13). Our experiments demonstrate that complex and dynamic expression of ligands, Bmp2 and Bmp4 and their antagonist, noggin, within this macro-environment (Figs. 5 and 12) contributes to global changes of Bmp signaling activity in the skin and impacts coordinated hair cycle behavior. Previous research has demonstrated that telogen-to-anagen transitions can be promoted by the addition of the extraneous Bmp antagonist, noggin, to the skin (Botchkarev et al., 2001). Expression of endogenous noggin in dermal papillae was shown to start in early anagen hair follicles. This is accompanied by down-regulation of Bmpr-IA in the secondary hair germ which ensures that newly initiated anagen will proceed to completion.
Our data sheds new light on the complex role that Bmp signaling plays within the skin macro-environment in controlling the hair cycle. First, global down-regulation of Bmp2 and Bmp4 expression in the skin is responsible for the transition from refractory to competent telogen. Refractory telogen is characterized by multiple extra- and intrafollicular sites of Bmp2 and Bmp4 expression sites (Fig. 5). Together they create a continuously high Bmp activity in the macro-environment surrounding telogen hair follicles. Domains of high Bmp signaling can be clearly visualized by whole mount staining. They form distinguishable boundaries with domains in competent telogen that are mostly void of Bmp2 and Bmp4 expression sites. Our results suggest that Bmp2 and Bmp4 act synergistically during the refractory phase and disappear around the time when the region gains competence, creating a favorable macro-environment for successful anagen initiation. Secondly, further reduction of Bmp signaling is essential to spread propagating anagen onto competent telogen hair follicles. Bmp2 and Bmp4 remain low at the wavefront of an anagen spreading wave. In addition, high levels of noggin start to be expressed throughout the dermal sheath of early anagen hair follicles synergizing with reduced Bmp ligands. Thirdly, a quick reappearance of nearly all Bmp2 and Bmp4 expression sites and disappearance of noggin expression from most of the dermal sheath during anagen V-VI terminates the propagating stages of anagen and contributes to border stabilization of the hair cycle domains in autonomous anagen.
The finding that Bmp2 transcripts oscillate in subcutaneous adipocytes comes as a surprise. However it seems reasonable, considering that one function of subcutaneous fat is insulation. This adipose - hair growth interaction will open up new understanding in endothermy homeostasis and skin response to environmental and seasonal changes. One issue remaining to be explored is the control of Bmp2 oscillation, which appears to be under a different control mechanism than the hair cycle clock.
In the mouse, the first cycle is more or less synchronized throughout the entire dorsum. However, during consecutive cycles one big dorsal domain fractionates into smaller and smaller domains (Plikus and Chuong, 2008). In adult female mice with multiple fractionated domains, pregnancy mediated by systematic factors (presumably involving hormones, such as prolactin; Fraser and Nay, 1953; Craven et al., 2006), promotes an unusually extended telogen. In such mice the entire dorsum resets into one big domain. If mice are kept from getting pregnant again, fractionation of the domains recurs (our unpublished data). In many transgenic mouse studies to date, dorsal skin from 2-4 week old pups is used as a standard for anagen, catagen, and telogen hair follicles. This is generally correct for mice that are about one month old. However, in adult mice (> 2 months) domains fractionate and one has to account for the complex patterning described here in designing experiments or analyzing results. For example, if a reagent is tested for its potency to alter stem cell behavior, it is important to know whether the macro-environment is at the refractory or competent phase.
Here we discuss the consequence of the effect of oscillation of Bmp signaling in the dermal macro-environment on hair cycling. Another interesting and critical issue is the control of the oscillation of Bmp signaling in the dermal macro-environment. Is it a clock intrinsic to the dermis? Is it dependent on the hair cycle clock? Or both, in the sense that there is a dermal clock but it is modulated by the hair cycle rhythm. What are the molecular bases of these clocks or the interacting signals? Future study will be aimed to answer these questions.
Conclusion
We have learned that the activation of stem cells can be regulated on different hierarchical levels. Anagen re-entry requires the following conditions to be met:
Stem cells have to be competent. In skin, this means that bulge stem cells and their immediate micro-environmental niche have to be competent (Blanpain et al., 2004; Cotsarelis, 2006).
The macro-environment has to be permissive, i.e., inhibitors have to be cleared before activation can occur. Although Bmp activity is a summation of activities of multiple agonists, antagonists, etc., it appears to fulfill the definition of previously predicted telogen inhibitors or chalones (Ebling and Johnson, 1961; Paus et al., 1990).
The level of activator(s) has to be higher than the threshold required to trigger regeneration. This can be achieved via propagating influences from neighboring follicles, or stochastic self-activation based on the original hair cycle clock intrinsic to each follicle (Paus and Foitzik, 2004).
In summary, this project was motivated by the complex hair cycle domains observed on Msx2 null mouse skin (Ma et al., 2002). Integration of long-term careful observations of hair follicle populations, use of transgenic mice, experimental manipulations, and mathematical modeling allowed us to develop the preliminary observation of ≪telogen refractivity≫ into this new level of understanding (Figs. 12 and 13). Such novel concepts have important implications and applications in the activation of stem cells for regeneration. The unexpected link to adipocytes and macro-environmental control are likely to launch new areas of studies at a systematic level and in the Evo-Devo of integument biology. Finally, we expect that the model presented here can be adopted to explain other biological phenomena.
Acknowledgments
We sincerely thank many colleagues for their very helpful discussions when we presented the work in progress in Montagna Symposium on the Biology of Skin in 2006 and in North American Hair Research Society meeting in 2007. This work would not have achieved its current status without their input. This study is supported by grants from NIAMS to CMC and RBW, and NIA to CMC. Plikus is a postdoctoral scholar of California Institute of Regenerative Medicine. We thank Dr. Baker, Maini, and all members of the Chuong Laboratory for discussion. We thank publishers for the kind to use the same or modified version of Figs. 2, 4, 5, 8, 11, 12,13 (Nature) and Fig. 3. (J. Investigative Dermatology).
Abbreviations in this paper
- BMP
bone morphogenetic protein
References
- Andl T, Ahn K, Kairo A, Chu EY, Wine-Lee L, Reddy ST, Croft NJ, Cebra-Thomas JA, Metzger D, Chambon P, Lyons KM, Mishina Y, Seykora JT, Crenshaw EB, Millar SE. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131:2257–2268. doi: 10.1242/dev.01125. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Botchkareva NV, Roth W, Nakamura M, Chen LH, Herzog W, Lindner G, Mcmahon JA, Peters C, Lauster R, Mcmahon AP, Paus R. Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nat Cell Biol. 1999;1:158–164. doi: 10.1038/11078. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Botchkareva NV, Nakamura M, Huber O, Funa K, Lauster R, Paus R, Gilchrest BA. Noggin is required for induction of the hair follicle growth phase in postnatal skin. FASEB J. 2001;5:2205–2214. doi: 10.1096/fj.01-0207com. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Botchkareva NV, Huber O, Funa K, Gilchrest BA. Modulation of BMP signaling by noggin is required for induction of the secondary (non-tylotrich) hair follicles. J Invest Dermatol. 2002;118:3–10. doi: 10.1046/j.1523-1747.2002.01645.x. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA. Bone morphogenetic proteins and their antagonists in skin and hair follicle biology. J Invest Dermatol. 2003;120:36–47. doi: 10.1046/j.1523-1747.2003.12002.x. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Sharov AA. BMP signaling in the control of skin development and hair follicle growth. Differentiation. 2004;72:512–26. doi: 10.1111/j.1432-0436.2004.07209005.x. [DOI] [PubMed] [Google Scholar]
- Brugger SM, Merrill AE, Torres-Vazquez J, Wu N, Ting MC, Cho JY, Dobias SL, YI SE, Lyons K, Bell JR, Arora K, Warrior R, Maxson R. A phylogenetically conserved cis-regulatory module in the Msx2 promoter is sufficient for BMP-dependent transcription in murine and Drosophila embryos. Development. 2004;131:5153–51565. doi: 10.1242/dev.01390. [DOI] [PubMed] [Google Scholar]
- Butcher EO. Hair growth on skin transplants in the immature albino rat. Anat Rec. 1936;64:161–171. [Google Scholar]
- Chase H. Growth of the hair. Physiol Rev. 1954:113–126. doi: 10.1152/physrev.1954.34.1.113. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G. Epithelial stem cells: a folliculocentric view. J Invest Dermatol. 2006;126:1459–1468. doi: 10.1038/sj.jid.5700376. [DOI] [PubMed] [Google Scholar]
- Collins HH. Studies of normal moult and of artificially induced regeneration of pelage in Peromyscus. J Exp Zool. 1918;27:73–99. [Google Scholar]
- Craven AJ, Nixon AJ, Ashby MG, Ormandy CJ, Blazek K, Wilkins RJ, Pearson AJ. Prolactin delays hair regrowth in mice. J Endo-crinol. 2006;191:415–425. doi: 10.1677/joe.1.06685. [DOI] [PubMed] [Google Scholar]
- Deutsch A, Dormann S. Cellular Automaton Modeling of Biological Pattern Formation. Basel, Switzerland: A Birkhäuser book; 2005. [Google Scholar]
- Durward A, Rudall KM. Studies on hair growth in the rat. J Anat. 1949;83:325–335. [PMC free article] [PubMed] [Google Scholar]
- Ebling FJ, Johnson E. Systemic influence on activity of hair follicles in skin homografts. J Embryol Exp Morphol. 1961;9:285–93. [PubMed] [Google Scholar]
- Fraser AS, Nay T. Growth of the mouse coat II. Effects of sex and pregnancy. Aust J Biol Sci. 1953;6:645–656. [PubMed] [Google Scholar]
- Fuchs E, Merrill BJ, Jamora C, Dasgupta R. At the roots of a never-ending cycle. Dev Cell. 2001;1:3–25. doi: 10.1016/s1534-5807(01)00022-3. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- Guha U, Mecklenburg L, Cowin P, Kan L, O'guin WM, D'vizio D, Pestell RG, Paus R, Kessler JA. Bone morphogenetic protein signaling regulates postnatal hair follicle differentiation and cycling. Am J Pathol. 2004;165:729–740. doi: 10.1016/S0002-9440(10)63336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang TX, Jung HS, Widelitz RB, Chuong CM. Self-organization of periodic patterns by dissociated feather mesenchymal cells and the regulation of size, number and spacing of primordia. Development. 1999;126:4997–5009. doi: 10.1242/dev.126.22.4997. [DOI] [PubMed] [Google Scholar]
- Johnson E. Quantitative studies of hair growth in the albino rat. III. The role of the adrenal glands. J Endocrinol. 1958a;16:360–368. doi: 10.1677/joe.0.0160360. [DOI] [PubMed] [Google Scholar]
- Johnson E. Quantitative studies of hair growth in the albino rat. II. The effect of sex hormones. J Endocrinol. 1958b;16:351–359. doi: 10.1677/joe.0.0160351. [DOI] [PubMed] [Google Scholar]
- Kobielak K, Pasolli HA, Alonso L, Polak L, Fuchs E. Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA. J Cell Biol. 2003;163:609–623. doi: 10.1083/jcb.200309042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulessa H, Turk G, Hogan BL. Inhibition of Bmp signaling affects growth and differentiation in the anagen hair follicle. EMBO J. 2000;19:6664–66674. doi: 10.1093/emboj/19.24.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Liu J, Wu T, Plikus M, Jiang TX, Bi Q, Liu YH, Muller-Rover S, Peters H, Sundberg JP, Maxson R, Maas RL, Chuong CM. ‘Cyclic alopecia’ in Msx2 mutants: defects in hair cycling and hair shaft differentiation. Development. 2003;130:379–389. doi: 10.1242/dev.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer M, Handjiski B, Paus R. Hair growth modulation by topical immunophilin ligands: induction of anagen, inhibition of massive catagen development, and relative protection from chemotherapy-induced alopecia. Am J Pathol. 1997;150:1433–1441. [PMC free article] [PubMed] [Google Scholar]
- Militzer K. Hair growth pattern in nude mice. Cells Tissues Organs. 2001;168:285–94. doi: 10.1159/000047845. [DOI] [PubMed] [Google Scholar]
- Muller-Rover S, Handjiski B, Van Der Veen C, Eichmuller S, Foitzik K, Mckay IA, Stenn KS, Paus R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- Park YG, Hayasaka S, Takagishi Y, Inouye M, Okumoto M, Oda S. Histological characteristics of the pelage skin of rough fur mice (C3H/HeJ-ruf/ruf) Exp Anim. 2001;50:179–182. doi: 10.1538/expanim.50.179. [DOI] [PubMed] [Google Scholar]
- Paus R. Principles of hair cycle control. J Dermatol. 1998;25:793–802. doi: 10.1111/j.1346-8138.1998.tb02507.x. [DOI] [PubMed] [Google Scholar]
- Paus R, Stenn KS, Link RE. Telogen skin contains an inhibitor of hair growth. Br J Dermatol. 1990;122:777–784. doi: 10.1111/j.1365-2133.1990.tb06266.x. [DOI] [PubMed] [Google Scholar]
- Paus R, Foitzik K. In search of the ≪hair cycle clock≫: a guided tour. Differentiation. 2004;72:489–511. doi: 10.1111/j.1432-0436.2004.07209004.x. [DOI] [PubMed] [Google Scholar]
- Plikus M, Chuong CM. Complex hair cycle domain patterns and regenerative hair waves in living rodents. J Invest Dermatol. 2008;128:1071–1080. doi: 10.1038/sj.jid.5701180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Mayer J, De La Cruz D, Baker RE, Maini PK, Maxson R, Chuong CM. Cyclic dermal BMP signaling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus M, Wang WP, Liu J, Wang X, Jiang TX, Chuong CM. Morpho-regulation of ectodermal organs: integument pathology and phenotypic variations in K14-Noggin engineered mice through modulation of bone morphogenic protein pathway. Am J Pathol. 2004;164:1099–1114. doi: 10.1016/S0002-9440(10)63197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Sundberg JP, Chuong CM. The mouse in biomedical research. Academic Press; 2006. Mouse Skin Ectodermal Organs; pp. 691–694. [Google Scholar]
- Rose J, Garwood T, Jaber B. Prolactin receptor concentrations in the skin of mink during the winter fur growth cycle. J Exp Zool. 1995;271:205–210. doi: 10.1002/jez.1402710307. [DOI] [PubMed] [Google Scholar]
- Stenn KS, Paus R. Control of hair follicle cycling. Physiol Rev. 2001;81:49–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Hirata M, Kondo S. Traveling stripes on the skin of a mutant mouse. Proc Natl Acad Sci USA. 2003;100:9680–9685. doi: 10.1073/pnas.1731184100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyttendaele H, Panteleyev AA, De Berker D, Tobin DT, Christiano AM. Activation of Notch1 in the hair follicle leads to cell-fate switch and Mohawk alopecia. Differentiation. 2004;72:396–409. doi: 10.1111/j.1432-0436.2004.07208006.x. [DOI] [PubMed] [Google Scholar]
- Van Steensel MA, Happle R, Steijlen PM. Molecular genetics of the hair follicle: the state of the art. Proc Soc Exp Biol Med. 2000;223:1–7. doi: 10.1046/j.1525-1373.2000.22301.x. [DOI] [PubMed] [Google Scholar]
- Wolfram S. A new kind of science. Wolfram Media 2002 [Google Scholar]
- Yuhki M, Yamada M, Kawano M, Iwasato T, Itohara S, Yoshida H, Ogawa M, Mishina Y. BMPR1A signaling is necessary for hair follicle cycling and hair shaft differentiation in mice. Development. 2004;131:1825–1833. doi: 10.1242/dev.01079. [DOI] [PubMed] [Google Scholar]
- Zhang J, He XC, Tong WG, Johnson T, Wiedemann LM, Mishina Y, Feng JQ, Li L. BMP signaling inhibits hair follicle anagen induction by restricting epithelial stem/progenitor cell activation and expansion. Stem Cells. 2006;24:2826–2839. doi: 10.1634/stemcells.2005-0544. [DOI] [PubMed] [Google Scholar]


