Abstract
WOOLEY, C.M., S. XING, R.W. BURGESS, G.A. COX, AND K.L. SEBURN. Age, experience and genetic background influence treadmill walking in mice. PHYSIOL. BEHAV. XX(X), XXX-XXX, 2008 – The use of a treadmill to gather data for gait analysis in mice is a convenient, sensitive method to evaluate motor performance. However, evidence from several species, including mice, shows that treadmill locomotion is a novel task that is not equivalent to over ground locomotion and that may be particularly sensitive to the test environment and protocol. We investigated the effects of age, genetic background and repeated trials on treadmill walking in mice and show that these factors are important considerations in the interpretation of gait data. Specifically we report that as C57BL/6J (B6) mice age, the animals use progressively longer, less frequent strides to maintain the same walking speed. The increase is most rapid between 1 and 6 months of age and is explained, in part, by changes in size and weight. We also extended previous findings showing that repeat trials cause mice to modify their treadmill gait pattern. In general, B6 mice tend to take shorter, more frequent steps and adopt a wider dynamic stance with repeated walking trials. The nature and extent of the response changes with both the number and timing of the trials and was observed with inter-trial intervals as long as 3 months. Finally, we compared the gait pattern of an additional seven inbred strains of mice and found significant variation in the length and frequency of strides used to maintain the same walking speed. The combined results offer the bases for further mechanistic studies and can be used to guide optimal experimental design.
Keywords: quadruped gait, strain differences, motor behavior
Introduction
Gait measures have been successfully used to quantify motor function in the mouse for many years 8,11,30. The use of gait analysis in mice has increased as the number of murine models of motor dysfunction and new technologies for data collection and analysis has grown 1,10,24,28,31,43,50,51. We and others have used a simplified approach to quantify murine gait by analysis of digital video recordings made during treadmill locomotion 2,20,29,45,50. The use of a treadmill offers significant advantages, most notably the ability to standardize the speed of locomotion. However, it is also well-documented that the mechanics of treadmill locomotion differ significantly from normal over-ground locomotion, and that gait measurements taken under the two conditions are not equivalent for humans, cats, rats or mice 9,25,38,49. In the present study we investigated three factors that are important considerations for interpretation of murine gait data collected on the treadmill: 1) testing protocols, in particular the influence of repeated trials, 2) age and, 3) genetic background.
Previous studies and our own findings 50 emphasize that for mice treadmill locomotion is a novel behavior that may be particularly sensitive to the testing protocol and experimental design employed. In our previous study of early gait defects in a model of motoneuron disease, we also discovered that wild-type mice modify their walking strategy with repeat trials on the treadmill 50. Unfortunately the design of that study did not allow a clear understanding of these effects. Here we report results from a series of studies that were undertaken to clarify the effects of repeat trials and provide data that could be used to guide development of experimental protocols.
Other observations from ongoing studies in our laboratories suggested that treadmill gait might also be sensitive to age-related changes. Gait patterns are known to change with age in several species 7,40,41,44,48, but a previous report on the gait of freely walking mice found no significant age-related changes that were not due to changes in velocity 12. This is a somewhat surprising finding given the significant changes in the size and weight of the mouse and it is currently not known if such changes affect treadmill gait patterns. To address this question we measured gait parameters from C57BL/6J mice during the first year of life.
A third parameter relevant to mouse studies, in addition to the testing paradigm and age, is genetic background. A large number of inbred mouse strains have been developed for use in laboratory study. Strains have different origins 3 and the resulting genetic variation is extensive 39 and produces a range of differences in most phenotypes 26,36 (http://www.jax.org/phenome). The effect of this genetic variation on treadmill gait in mice has received little attention. Variation in size, weight and skeletal morphometry (e.g. 22) are obvious examples of differences that could contribute to the variation in standard gait measurements. Less obviously, strain differences in anxiety have recently been shown to contribute to differences in gait patterns 33, demonstrating that behavioral traits can affect the novel task of walking on a treadmill. To begin to address the contribution of genetic background we have examined the treadmill gait measurements of seven different inbred strains of mice. These strains comprise a subset of a panel of 32 inbred mouse strains included in an ongoing comprehensive aging study. The project aims to compare a variety of phenotypic measures of the different inbred strains across their lifespan (http://www.agingmice.org/).
Materials and Methods
Mice
Eight different inbred strains of mice were used. All mice were obtained from The Jackson Laboratory (Bar Harbor, USA). Detailed studies on aging and the effects of repeated trials used C57BL6/J mice (abbrev. B6). The strain comparison study used the following inbred strains: 129S1/SvImJ; BALB/cByJ; C3H/HeJ; C57BL/10J; DBA/2J; NON/ShiLtJ; SM/J. Strain abbreviations are respectively: 129S1; BALB; C3H; B10; DBA; NON and SM. Mice were maintained in humidity and temperature controlled rooms with 12:12 dark:light cycle. Mice were provided NIH-mouse/rat diet with 4% fat (#5K54, PMI Feeds Inc., St. Louise MO) ad libitum with free access to water (HCL acidified, pH 2.8-3.2). All procedures performed on the animals were reviewed and approved by the Institutional Animal Care and Use Committee of The Jackson Laboratory.
Gait analysis system
The system is configured to allow video capture from below the mice during treadmill locomotion (Clever Sys Inc, Reston VA). Hardware consists of a treadmill with a clear belt that has a mirror mounted directly below and set at an angle of 45 degrees. A digital video camera (Basler A301fc) is mounted in a translucent white plexiglas box beside the treadmill at the level of the mirror chamber and focused on the reflected image of the underside of the mouse. A rectangular, opaque plexigas testing chamber (20 × 4 × 16.5 cm) is mounted over the tread and serves to keep the mouse within view of the camera. Indirect illumination of the ventral side of the mouse is achieved via the mirror and lighting is adjusted to obtain optimal contrast between the paws and body of the mouse. The digital camera operates at 100 fps with a resolution of 658 × 494 pixels and the camera output is fed directly (Firewire IEEE-1394) to a 2.4GHz, 1GB RAM computer. The system described above was used for all data collection with the exception of the strain data which were collected using an identical camera and video capture software but used the prototype hardware described previously (50.
Digital video recording software is used to capture the raw video images and convert them to MPEG format. The MPEG file is then exported to custom analysis software (Treadscan™ 1.0, Clever Sys, Inc., Reston, VA, USA) that precisely tracks the body and paws position of the mice during locomotion and determines when each paw is in contact with the tread. Analysis is interactive and provides frame-by-frame feedback that allows the user to confirm that the mouse is walking with a consistent pattern and that the paws are tracked accurately. Measurements of improper strides (e.g. paw not being tracked, mouse moving forward or back on tread) can be flagged and excluded from the data output.
Standard video capture protocol
Camera settings, lighting and treadmill speed (0.20 m/s for all studies) were preset and background images including distance calibration were captured, prior to introduction of the mouse. Each animal was placed into the testing chamber where it could be seen live on the computer screen and within 15 seconds the treadmill was turned on and operating at the set speed. The animal was recorded for a preset fixed number of frames (796 frames) after which the treadmill was stopped and the mouse removed from the testing chamber. Each mouse was in the testing chamber for a maximum total of 45-60 seconds. The tread was cleaned between trials.
Gait parameters
The Treadscan™ software determines when individual paws are in contact with the treadmill and then uses this determination to derive more than 20 different measures including standard time-domain gait parameters (stride, stance and swing time). A step cycle or stride consists of two consecutive contacts of a given foot. We have established that at least five strides for each foot be included for a valid data set 50. Only strides recorded when the mice are walking in a fixed position relative (i.e. at the preset speed) to the camera are included. Whenever possible consecutive strides are used but because intervening aberrant strides do occur (e.g. jumps or partial steps) as do periods of “stop and go” walking, data from most animals includes 2 or 3 sets of consecutive strides for a given trial. For these studies between 5 and 20 strides were collected for every animal (mean=11±3.6). Values for each parameter were calculated using the average of all valid strides for each of the four paws. For comparisons of front and rear paws the right and left paws were averaged for each animal.
Definition of gait measures
Time domain measures are calculated by multiplying the number of elapsed frames by the frame rate/1000. A known distance is measured from a ruler captured in the calibration video and stored for calculation of distances and speed. 1) Stride time is the time between two consecutive foot contacts. 2) Stance time (phase) is the amount of time the foot is in contact with the tread during each stride. 3) Swing time (phase) is the amount of time the foot is not in contact with the tread during each stride. 4) Stride length (mm) is calculated for each foot as the product of the overall average running speed and the stride time. Corrections are made for any relative foot placement differences. For example if the foot consistently contacts the tread in the same location (i.e. pixel coordinates) then no adjustment is made. 5) Dynamic stance width (front and rear) is the perpendicular distance between the left and right paws measured at the midpoint (pixel coordinate) of the stance phase.
Experimental design and data analysis
All mice were moved from the large colony room to racks in a smaller testing room at least 24 hours prior to testing. Reducing the amount of noise and activity in their surroundings and otherwise ensuring the mice have not been disturbed (e.g. cage changes) prior to testing improves the compliance of the mice. Nonetheless, in our laboratory ~10% of B6 mice will not walk sufficiently well for useful data to be collected. The compliance among the other inbred strains with treadmill walking is variable with some strains being almost totally non-compliant (e.g. A/J, BUB/BnJ) and among compliant strains compliance declines with age (unpublished observations). All experimental groups included equal numbers of males and females to balance for possible sex differences that become evident with large samples 50.
Repeated trials study
Three experimental groups comprised of twenty 8-week-old mice (10 males, 10 females) were randomly selected for three separate experiments. In most experiments some mice were excluded for non-compliance as indicated. The standard video capture protocol was used for all groups (see above). In the first experiment mice were tested twice with a 1-week inter-trial interval (2 females excluded, final n=18) In the second experiment mice were tested three times with a 3-minute inter-trial interval between the first two trials and an inter-trial interval of 1 week between the second and third trials (2 males excluded, final n=18). In the final experiment mice were tested six times with inter-trial interval of 3-minutes between the first five and a 1 week inter-trial interval between the 5 and 6 trials (see results for numbers excluded).
Aging study
Fifty B6 mice were selected and divided into 5 groups of 10 mice each (5 males, 5 females) and each group was assigned a test age of 1, 2, 6, 9 or 12 months. Mice in each naïve group were given a single trial at the designated test age using the standard video capture protocol. Concurrent with the naïve groups of different aged mice we also tested three additional groups of mice that were repeat-tested across the age span. Mice were assigned to groups that were tested using the standard capture protocol at 3 month intervals beginning at 3, 6 or 9 months of age. This design allowed us to evaluate repeat testing effects in 12 month-old mice subjected to one, two or three previous trials with a 3 month inter-trial interval.
Strain comparison study
These data are a subset of data collected as part of a large, ongoing, integrated aging project (http://www.agingmice.org). The purpose of the project is to establish a public database containing a large variety of phenotypic measures for 32 different inbred strains at ages between 6 months and death. These data are meant to provide baseline data for further studies into the biology of aging. The gait data included here and all other data sets associated with the project are available to the public (http://phenome.jax.org/). The project design required that ten naïve mice of each strain (5 males, 5 females) be tested at 6 month intervals across their lifespan beginning at 6 months of age. The standard video capture protocol was used. Unfortunately the compliance of the mice with the testing protocol was poor (see Results) and resulted in incomplete data sets for all strains attempted.
Statistics
Unless otherwise noted a 2-way analysis of variance (ANOVA) was employed. Repeat trial experiments used paw (front and rear) and trial as factors. B6 naïve groups were compared using paw and sex as factors. Strain studies used and a Tukey honest significant difference (HSD) test was used to compare individual means when appropriate. A probability value of ≤0.05 was used as a limit for declaring statistical significance for all studies. Data from experiments where animals were tested more than once were compared with a repeat measures design. Multiple regression analysis was used to evaluate the contribution of morphometric measures to age-related changes in treadmill gait parameters.
Results
Effects of repeated testing
We previously reported that repeated trials cause gait modifications in wild-type mice 50. We undertook a series of experiments to better understand this phenomenon and help develop experimental strategies that might avoid confounding effects.
Single retest – 1 week inter-trial interval
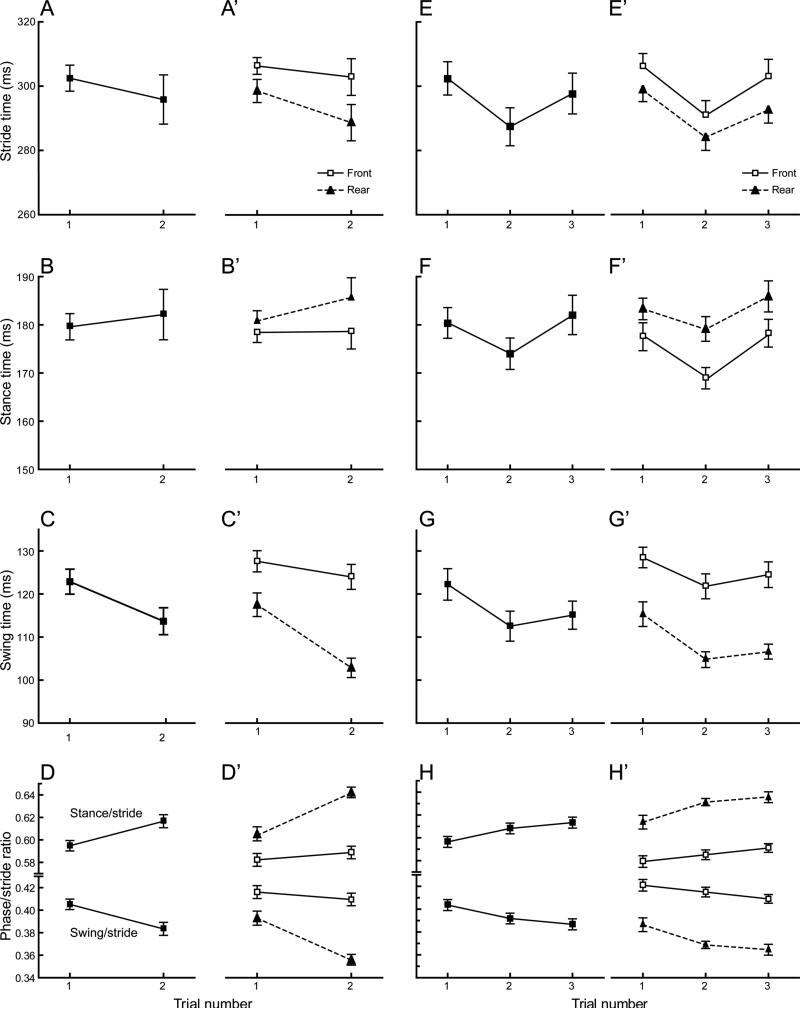
An initial experiment tested the effects of a single treadmill trial on gait parameters recorded during a second trial one week later. Of the basic gait parameters, only swing time was significantly different in the second trial (p=0.001) (Fig. 1c). A reduced swing phase was evident for both front and rear paws but the effect was larger for the rear (p=0.0002) than for the front paws (p=0.05) (Fig 1c’). In contrast there was no trial effect for stance (p=0.33) due to a marked increase in variability (Fig. 1b) that was caused by an inconsistent response among individual mice. Approximately half of the mice showed increases (10 and 12 mice, front and rear paws respectively) while the remainder decreased or showed no change. The lack of change in the stance phase, and the relatively small reduction in the forelimb swing phase, both contributed to the absence of a significant change in overall stride time (p=0.15)(Fig. 1a). However, the discrepant changes in swing and stance phases did cause a decrease in the overall swing/stride ratio and an accompanying increase in the stance/stride ratio (p<0.001, Fig. 1d) again due to adjustments in the rear paws only (p<0.001, Fig. 1d’). Finally. we compared the dynamic stance width between trials for the front and rear paws and found no change for the front paws (p=0.99) but a significantly wider rear stance during the second trial (23.9±0.5 vs. 24.6±0.5; p<0.001).
Figure 1.
The effects of repeated trials of treadmill walking were tested in two separate groups of mice. (a-d) The first group performed two trials separated by one-week. In the second trial there is significant decrease in overall swing phase that is driven primarily by changes in the rear paws but overall stride and stance time are unchanged. (e-h) The second group performed three trials, with an inter-trial interval of 3 minutes (trials 1-2) and 1 week (trials 2-3). During the second trial mice walked with a shortened stride and changes were evident for both stance and swing phases and in both front and rear paws. The third trial, one week later, showed changes that were remarkably similar to those observed in the first experiment when only two-trials were performed with the same inter-trial interval (compare trials 1 and 2 in a-c with trials 1 and 3 in e-g). Values in a-h are calculated for each animal as the average of all four paws, in a’-h’ the two front and two rear paws were averaged for each animal. All plots show the mean +/− s.e. for each group.
Together these data confirm that a single brief walking trial on the treadmill causes mice to walk differently during a second session one week later. The observed response would result in greater stability through an increase in stance phase, and decrease in swing phase duration relative to the overall stride cycle.
Two retests – 3 minute and 1-week inter-trial interval
We next conducted an experiment to test the effects of performing two closely spaced trials within the same session. Mice were given two initial trials with a brief 3 minute inter-trial interval followed by a third trial one week later.
Before analyzing data for this experiment we first confirmed that separate naive groups of B6 mice gave similar results in the initial trial. Comparison of data for the B6 mice used in this experiment and the preceding experiment showed no differences for stride, stance and swing measurements (F<0.3 (1,70), p≥0.6; compare Trial 1 in Fig. 1 a-d with Trial 1 in e-h). This further establishes reliability of the device and the standard video collection protocol.
When we analyzed the data for trial effects we found significant overall effects for stride, stance and swing time (F ≥ 8.7 (2,34), p≤ 0.001) that were similar for both paws (F≤2.0 (2,34), p≥0.15). We then focused on the effects of the 3-minute inter-trial interval (Trial 1 vs. 2) and confirmed significant differences between trial 1 and 2 for stride time and both stance and swing phase times (p≤0.003) (Fig. 1e-g). However, swing phase times again changed to a greater extent than stance phase (compare Fig. 1f, g) and led to significant reduction in the swing/stride ratio and a corresponding increase in the stance/stride ratio (p<0.01) (Fig 1h). Examination of dynamic stance width found no changes with the brief inter-trial interval (Trial 1 vs 2; p≥0.21).
Next we examined the effect of the two initial trials on the third trial measures taken one week later. The overall response of the mice to a 1-week inter-trial interval in this study was similar to that observed in the previous experiment with the same interval. The swing phase recorded in the third trial remained significantly lower than initial values (p<0.02) and was not different than average swing time recorded in the second trial (p=0.55) (Fig. 1g). Third trial stride and stance times were not different than those recorded during the initial trial (p>0.3) and were significantly increased (p≤0.008) relative to second trial measures (Fig. 1e,f). As in the previous experiment the combined outcome of these changes was a significantly shorter swing/stride ratio and longer stance/stride ratio for the rear paws in the third trial (p<0.001). We also observed changes in dynamic stance width that were similar to those previously observed with the 1-week inter-trial interval. Front paws stance width did not change (p≥0.65) but mice adopted a wider stance with the rear paws in the third trial (24.5±0.3) compared to both the first (23.8±0.4) and second trials (23.2±0.4) (p≤0.044).
These data show that a 3-minute inter-trial interval has a different, more robust effect on gait parameters than a 1-week inter-trial interval. Specifically, with the shorter interval, mice reduce their stride time by decreasing both swing and stance phase times such that in the second trial mice are using shorter more frequent steps to achieve the same speed.
Multiple retest effect – 3 minute and 1-week inter-trial intervals
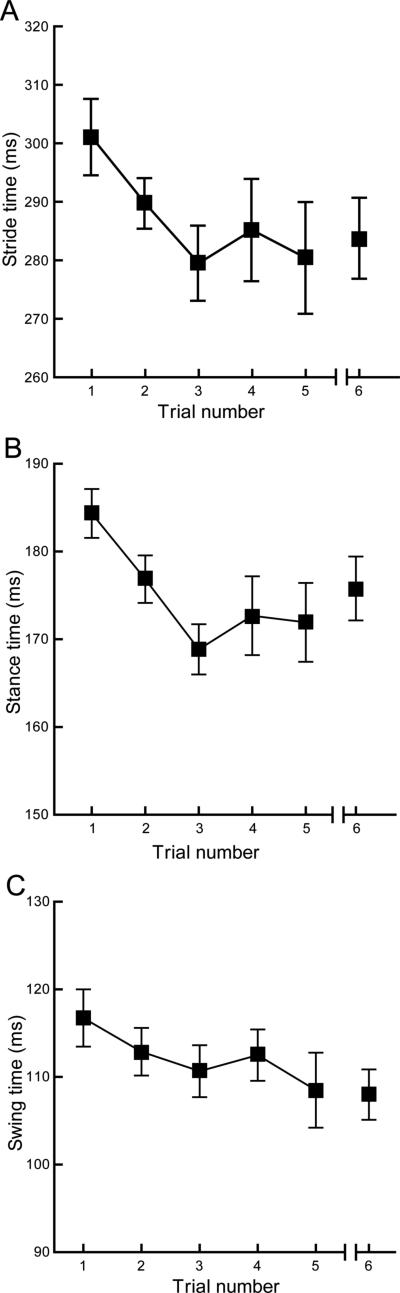
Given the effect observed with two rapid trials we hypothesized that additional initial trials might stabilize treadmill gait parameters. Thus we designed a third experiment to evaluate a rapid “training” protocol that might allow efficient repeat testing. The training consisted of five sequential trials with 3-minute inter-trial intervals. Data was then recorded in a sixth trial one week later to evaluate stability.
Although the training strategy was generally successful (see below) it was also plagued by poor compliance of the mice. In the current experiment only 12 of the 20 mice walked sufficiently well in the sixth trial, the following week, to allow us to acquire valid data. Compliance was also variable for a given mouse in that some would behave well for one, two or several trials and then not the next. For example although we obtained data from at least 16 mice for each training trial only 12 mice walked well in all 5 trials (3 minute inter-trial) and only 7 of these 12 mice completed the entire protocol.
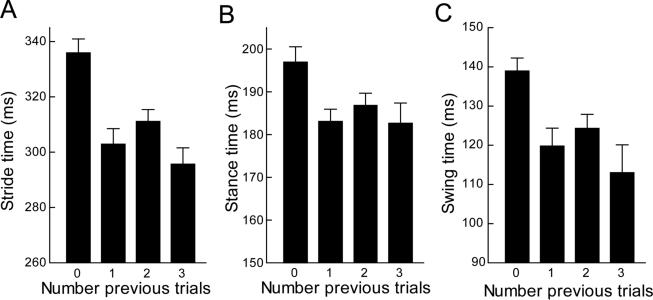
Due to the missing data for nearly half the mice in the final trial, we did not perform the planned repeated ANOVA and considered all data for each trial. Stride, stance and swing times decreased progressively through the 1st three trials and then stabilized for trials 4 and 5 (Fig. 2). Five training trials also were sufficient for the modifications to persist when mice performed a sixth trial one week later. Comparison of mean values for the 3rd and 6th trials found no difference in any of the three basic measures, either of the phase/stride ratios or for dynamic stance width (Student's t, p>0.09, all comparisons).
Figure 2.
Mice in this study performed five initial trials separated by 3 minutes and then a sixth trial 1 week later. (a-c) Stride, stance and swing times decreased progressively from trial 1 to 3 and then stabilized for the remaining two trials. Comparison of values from the 3rd and 6th trials showed no differences for these measures. Compliance was poor and variable (trials 1-5, n=16 – 19; trial 6, n=12). Values are calculated for each animal as the average of all four paws. All plots show the mean +/− s.e. for each trial.
These data demonstrate that as few as five training trials “reset” and stabilize basic treadmill gait parameters for seven days. These data provide a basis for developing repeat testing strategies which are discussed below.
Aging
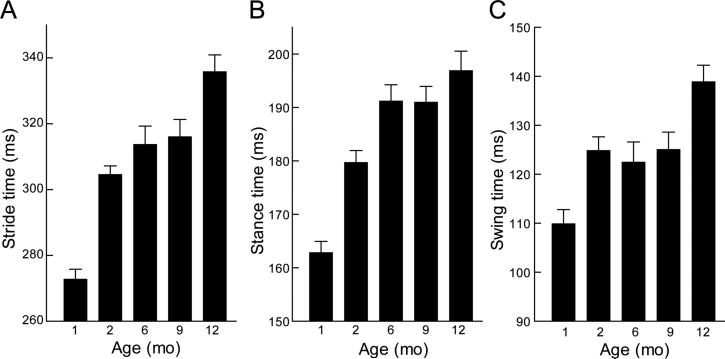
To determine if treadmill gait is stable as mice age and grow we compared the gait of five naïve groups of B6 mice that were tested at 1, 2, 6, 9 or 12 months of age. An age-related increase in stride, stance and swing times (main effect of age; F>11(4,90), p<1 × 10−4) was evident across the entire age span but the increases were greatest between 1 and 2 months of age (Fig 3). Post hoc analysis of individual means showed that the youngest mice had significantly shorter stride, stance and swing times than mice at all older ages (1<2,6,9,12 months, p≤ 0.03). In agreement with changes in the time domain measures, stride length was increased concomitant with a decrease in stride frequency (F>22(4,90), p< 1× 10-4) for both paws (Table 1) (no interaction) confirming that older B6 mice take longer, less frequent steps than young mice to achieve the same walking speed on the treadmill.
Figure 3.
Primary gait parameters for five naive groups of C57BL/6J mice (n=10 per group) tested at different ages. A significant age-related increase was found for overall stride time and both stance and swing phases. Values of each animal are the average of all four paws and data are shown as the mean +/− S.E. for each group.
Table 1.
C57BL/6J mice take longer less frequent steps as they age. Most of the increase takes place between 1 and 2 months of age during the period of rapid growth but as for time domain measures smaller increases are evident between other age points.
| Stride length (mm) | Stride frequency (steps/s) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Front | Rear | Front | Rear | |||||||||
| Age | Mean ± s.e. | −95% | +95% | Mean ± s.e. | −95% | +95% | Mean ± s.e. | −95% | +95% | Mean ± s.e. | −95% | +95% |
| 1 | 48.8 ± 1.0 | 46.4 | 51.2 | 47.9 ± 0.9 | 46 | 49.9 | 3.7 ± 0.06 | 3.5 | 3.8 | 3.7 ± 0.06 | 3.6 | 3.8 |
| 2 | 57.4 ± 0.6 | 56.2 | 58.7 | 55.6 ± 1.0 | 53.4 | 57.9 | 3.2 ± 0.02 | 3.2 | 3.3 | 3.4 ± 0.05 | 3.3 | 3.5 |
| 6 | 60.7 ± 1.8 | 56.7 | 64.7 | 58.3 ± 1.6 | 54.6 | 62.1 | 3.1 ± 0.08 | 3.0 | 3.3 | 3.3 ± 0.10 | 3.1 | 3.6 |
| 9 | 58.6 ± 1.3 | 55.8 | 61.5 | 56.5 ± 1.0 | 54.1 | 58.8 | 3.2 ± 0.07 | 3 | 3.3 | 3.4 ± 0.08 | 3.2 | 3.6 |
| 12 | 62.5 ± 1.3 | 59.6 | 65.5 | 57.9 ± 1.1 | 55.6 | 60.4 | 2.9 ± 0.05 | 2.8 | 3 | 3.2 ± 0.08 | 3.0 | 3.3 |
Closer examination of the age-related changes in stance and swing phases showed that between 1 and 2 months of age increased stride time was achieved by adjustments during both phases. However, at later ages, stance and swing phases were not adjusted in parallel. Stance phase continued to increase up to 6 months (2<6, p=0.01) and then remained stable to 12 months of age (6 = 9 = 12, p>0.5) (Fig. 3b). In contrast, swing phase did not change between 2 and 9 months (2 = 6 = 9, p>0.5) but then increased further between 9 and 12 months of age (p=0.01) and swing phase was significantly longer at 12 months than at all younger ages (p≤0.01) (Fig 3c).
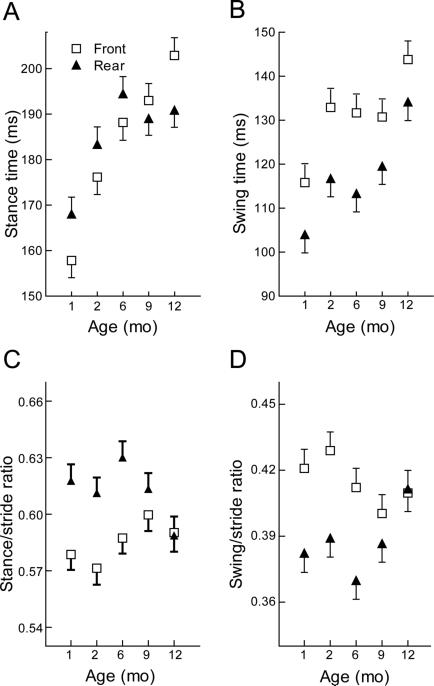
Although changes in overall stride time and swing phase were similar for both paws (no interaction effect, p≥0.55) there was a significant paw by age interaction for the stance phase (F=2.9(4,90), p=0.02) (Fig 4a, b). We previously observed that normal 2 month old mice have rear paw stance times that are slightly longer, and swing times that are commensurately shorter, than front paw stance times 50. A similar rear/front relationship is evident in the current data at 1, 2 and 6 months and though the relationship persists for swing time, it is lost for stance time (Fig. 4a,b). The overall effect of these discrepant changes is most easily seen by expressing stance and swing phases as a proportion of stride time. The ultimate result, by 12 months of age, is a convergence of the front and rear paw phase ratios (Fig 4c,d). The typical difference between front and rear paw phase ratios persists to six months of age (p≤ 0.04) but are no longer different at 9 months (p≥0.9) (Fig. 4c,d) The age-related change in phase ratios is evident for both front and rear paws but changes in the rear paws are greater (age by paw interaction effect (F=2.7(4,90), p=0.03). Post hoc analysis showed significant phase ratio changes between 6 and 12 months for the rear paws (p≤0.03) but not the front paws (p<0.9).
Figure 4.
a) Age-related changes in stance phase duration are similar for front and rear paws until 6 months of age when rear paws stabilize. b) Age-related changes in swing phase duration for front and rear paws remain parallel through the entire 12 months studied. c,d) Overall effect of discrepant changes in stance and swing is a loss of a front/rear differences in phase ratios that is driven by changes in rear limbs. For this plot values of each animal (n=10 per group) are calculated as averages of the two front and two rear paws. Data are shown as the mean +/− S.E. for each group.
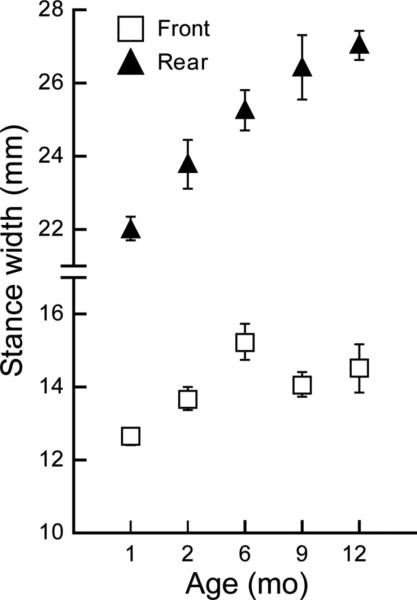
We also examined the dynamic stance width of the front and rear paws during treadmill walking and found the distance between the rear paws increased steadily with age (Fig. 5) (F=11.6 (4, 45), p<1 × 10−4). The rear stance width of 1 month old mice was significantly narrower than that of 6,9 and 12 month old animals (p<2 × 10-4) and 2 month old stance width was significantly less than both 9 and 12 month measures (p< 0.04). The overall age-related increase in front stance width (F=4.9 (4, 45), p=0.002) was due to changes between 1 and 6 months of age (Fig. 5; 1>6, p=0.001) but then remained stable up to 12 months.
Figure 5.
A significant age-related change in dynamic stance width was found for both the front (1-month<6; 2month=6,9,12) and rear paws (1month<6,9,12; 2 month<9,12). Values of each animal are calculated as average of the two rear paws. Data are shown as the mean +/− S.E. for each group. Note Y-axis scale is broken to accommodate differences in front and rear paw distances.
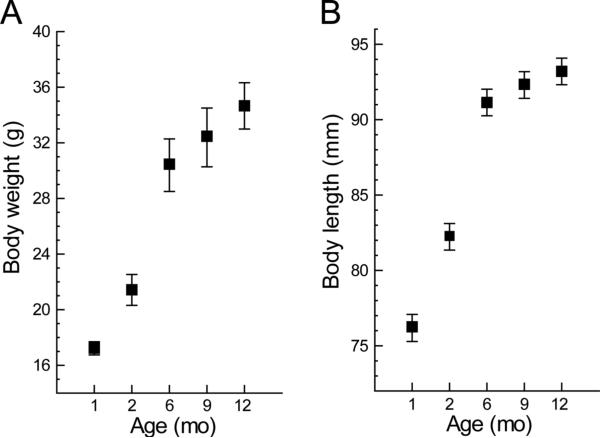
An obvious explanation for the observed age-related changes in treadmill gait parameters is the accompanying increase in the size and weight of mice during the first year. The growth curves for the mice studied (Fig. 6) have a pattern similar to that for age-related changes in overall stride time (Fig. 3a); changes in size and weight are more rapid at younger ages. For example, between 1 and 2 months of age average body weight increased (1.0 g/wk) at double the rate recorded between 2 and 6 months (0.5 g/wk) and then slowed further between 6 and 12 months (< 0.2 g/wk) (Fig. 6a). Similarly body length increased rapidly between 1 and 2 months (1.4 mm/wk), more modestly between 2 and 6 months (0.5 mm/wk) and then very little between 6 and 12 months (0.25 mm/wk) (Fig. 6b).
Figure 6.
Growth curves of C57BL/6J mice show typical rapid increases in both body weight and length (nose to anus) at younger ages. This pattern of change is similar to that seen for treadmill gait in Figure 1 above. Regression analysis (see text) confirmed that both measures contribute significantly to age-related changes in gait parameters. Values are mean ± s.e. of each group.
To determine the extent that these growth measures predict changes in gait we performed a multiple regression analysis of rear stride time and stance width using body weight and length as independent variables. Significant correlations were found for both dependent variables. Together body weight and length accounted for 36 and 60% of the age-related variability in rear stride time and stance width respectively. Examination of beta coefficients revealed that while neither body length nor body weight contributes significantly on its own to predicting rear stride time, body length is a relatively better predictor. The same analysis for rear stance width, showed both length and weight contributed significantly and equally to its prediction (Table 2)
Table 2.
Age-related changes in body weight and length together explain ~40% of the variation in rear stride time and ~60% of rear stance width. Beta coefficients allow evaluation of the relative contribution of each independent variable to the prediction of the dependent variable. Rear stance width is predicted equally well by length and weight separately while rear stride time is better predicted by length than by weight.
| Beta coefficient | p-level | ||||||
|---|---|---|---|---|---|---|---|
| Multiple R | R2 | p | Body weight (g) | Body length (mm) | Body weight (g) | Body length (mm) | |
| Rear stride time (ms) | 0.60 | 0.36 | <0.0001 | 0.23±0.23 | 0.39±0.23 | 0.32 | 0.10 |
| Rear stance width (mm) | 0.78 | 0.61 | <0.0001 | 0.41±0.18 | 0.40±0.18 | 0.03 | 0.03 |
Together these data show that as B6 mice age they take longer, less frequent strides and widen the rear paw stance while walking on a treadmill. Regression analysis suggests these changes are partly due to the growth of the mice, particularly at younger ages (<6 months).
Effect of repeat testing in older mice
We were also interested in determining if repeat testing effects could be produced in older animals and with a much longer inter-trial interval of 3 months. To do this we compared data collected from a cohort of naïve 12 month-old animals to three independent groups of age-matched mice that were tested previously one, two or three times. To our surprise, despite a 3-month inter-trial interval, each of the three independent groups that were repeat-tested had significantly shorter stride times than their respective age-matched naïve group (p≤0.003) (Fig. 7). This was due to significant reductions in both stance and swing phase for two of the repeat groups (naive<1 and 3 previous trials; p≤0.02) while the remaining repeat group (2 previous trials) showed the same trend but did not reach statistical significance (p=0.10, 0.07 stance and swing respectively). There were no differences between the three repeat groups for any of the measures (p>0.6) and both paws were affected similarly (no interaction effects). We also noted a somewhat greater decrease in the swing phase than in the stance phase with the 3-month inter-trial interval (Fig.7). Similar to results with a 1-week retest this discrepancy tended to reduce the swing/stride ratio and increase stance/stride ratio, however with the longer interval this effect did not reach statistical significance for either measure (p=0.10). Finally, we were interested to see if mice adopted a wider stance width with the rear paws (see above) as observed with the 1-week inter-trial interval. No significant differences were found, for either the front or rear paws, between the naïve group and any of the retest groups (p≥0.3).
Figure 7.
Data shown were collected from four separate groups of B6 mice tested at 12 months of age (n=10). Three of the groups had also been tested previously; one at 9 months (1 trial), one at 6 and 9 months (2 trials), one at 3, 6 and 9 months (3 trials). The stride time of the naïve group (0 retests) is significantly longer than each of the 3 groups that were tested previously. These data demonstrate a significant, non-additive effect of repeat testing with a 3-month inter-trial interval. Values in are calculated for each animal as the average of all four paws and plots show the mean +/− s.e. for each group.
These results confirm an effect of repeat testing with a 3 month inter-trial interval in three separate groups of 12-month-old mice and further show that, at this interval, trial effects are not additive.
Inbred strains
We chose B6 mice for the preceding detailed studies on aging and trial effects because most of the disease models studied in our own laboratory are maintained on this background and it is extensively used in many laboratories. As part of larger integrated aging project, (www.agingmice.org) we compared the treadmill gait of different strains of inbred mice as they aged.
Strains differ in compliance
We were unable to obtain a complete data set for treadmill gait for any of the strains because of poor compliance with the testing protocol. Upon initial testing, at six months of age, several of the strains either would not walk at all or did not walk consistently enough to obtain valid data. In other strains, the mice complied at 6 months but then were either unwilling or unable to walk at the prescribed speed at later ages. Up to 18 months the mice generally still moved well with no overt impairments while by 24 months some overt motor deficits could be observed. In these older animals reducing the tread speed to a slower walking pace would sometimes improve their performance. These anecdotal results point to complications for the use of treadmill gait as a longitudinal assay for aging studies. Strategies are discussed below for possibly circumventing these problems. Nonetheless, useful data sets were obtained at 6 months of age from naïve cohorts of seven inbred strains. Three of these strains also completed walking trials at 18 months of age.
Strains differ in treadmill walking strategy
We compared the relationship between mean stride length and stride frequency of the rear limbs for each strain at 6-months of age to look for differences in the treadmill gait pattern among the seven strains.
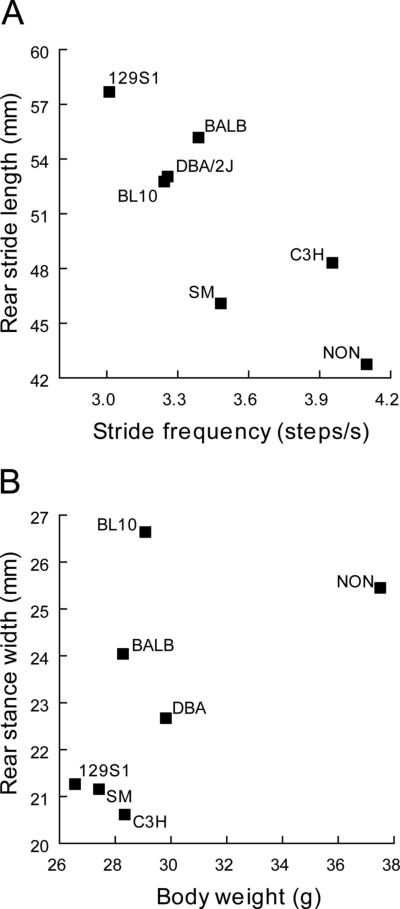
At the same walking speed the seven strains showed significant variation with 129S1 mice using the longest steps with a rate of ~ 3 per second compared to NON mice that took, on average, much shorter steps at a rate of more than 4 per second (Fig. 8a). Significant strain differences were present for both measures (F≥18.9 (6,1), p<1 × 10−4) but overall males and females were not different (F≤0.02 (6,1), p≥0.8). The NON and SM strain were the most distinct with significantly shorter strides than, respectively, four and five of the other six strains (Table 3) Similar results were found for an analysis of rear stance width (Fig. 8b); a significant strain effect (F≥21.3 (6,1), p<1 × 10−6) and no sex difference (F≥3.6 (6,1), p=0.06). However, strain differences were more distributed with each strain showing significant differences with 2 or more of the other strains (Table 4).
Figure 8.
Data shown were recorded each strain at 6 months of age. (a) Strains employ different strategies for treadmill walking at the same speed with significant differences in stride length and frequency. (b) Differences in stance width of rear paws are due in part to differences in body weight between strains (see text) but other factors also contribute. This is emphasized by the NON mice which are markedly heavier than all other strains but have the shortest stride and do not have the largest stance width. Values are mean ± s.e. of each group.
Table 3.
Results of post-hoc analysis (Tukey HSD) of rear stride length comparisons between strains. The large NON mice have shorter strides than all strains except for SM which is among the smallest of strains.
| Strain | (1) 57.7 | (2) 55.2 | (3) 48.3 | (4) 52.8 | (5) 53.1 | (6) 42.8 | (7) 46.1 | |
|---|---|---|---|---|---|---|---|---|
| 129S1/SvImJ | (1) | 0.80 | 0.0001 | 0.11 | 0.15 | 0.0001 | 0.0001 | |
| BALB/cByJ | (2) | 0.80 | 0.0007 | 0.72 | 0.86 | 0.0001 | 0.0002 | |
| C3H/HeJ | (3) | 0.0001 | 0.0007 | 0.07 | 0.08 | 0.0103 | 0.87 | |
| C57BL/10J | (4) | 0.11 | 0.724 | 0.07 | 1.0 | 0.0001 | 0.0070 | |
| DBA/2J | (5) | 0.15 | 0.86 | 0.08 | 1.0 | 0.0001 | 0.0040 | |
| NON/ShiLtJ | (6) | 0.0001 | 0.0001 | 0.01 | 0.0001 | 0.0001 | 0.55 | |
| SM/J | (7) | 0.0001 | 0.0002 | 0.87 | 0.0066 | 0.0041 | 0.55 |
Table 4.
Results of post-hoc analysis of rear stance width comparisons between strains. With the exception of NON mice body weights are similar yet each of these six strains are significantly different than 2 or more other strains.
| Strain | (1) 21.267 | (2) 24.024 | (3) 20.609 | (4) 26.646 | (5) 22.668 | (6) 25.448 | (7)21.118 | |
|---|---|---|---|---|---|---|---|---|
| 129S1/SvImJ | (1) | 0.01 | 0.98 | 0.0001 | 0.57 | 0.0001 | 1.0 | |
| BALB/cByJ | (2) | 0.01 | 0.0002 | 0.004 | 0.51 | 0.36 | 0.003 | |
| C3H/HeJ | (3) | 0.98 | 0.0002 | 0.0001 | 0.08 | 0.0001 | 0.99 | |
| C57BL/10J | (4) | 0.0001 | 0.004 | 0.0001 | 0.0 | 0.58 | 0.000 | |
| DBA/2J | (5) | 0.57 | 0.51 | 0.08 | 0.0001 | 0.0048 | 0.35 | |
| NON/ShiLtJ | (6) | 0.0001 | 0.36 | 0.0001 | 0.58 | 0.005 | 0.000 | |
| SM/J | (7) | 1.0 | 0.003 | 0.99 | 0.0001 | 0.35 | 0.0001 |
We were also interested in examining the relationship of the front and rear paw stance phases for these strains. The stance phase of the rear limbs is typically slightly longer than for the forelimbs and is reflected in a greater stance/stride ratio (Fig. 4c above). Stance/stride ratios showed significant variation across strains and only two strains (BALB, B10) showed larger stance/stride ratios for the rear paws (p≤ 0.002) at 6 months. We took this analysis one step further to confirm that the front:rear relationship was similar for males and females of a given strain. This was confirmed with the exception of 129 and SM strains which each showed the typical relationship for females but not for males (Table 5).
Table 5.
Relationship between the stance phase ratio of front and rear paws for seven inbred strains at 6 months of age. Only BALB and B10 mice have the expected FL:HL relationship for stance phase ratios (HL>FL). SM and 129S1 mice show the typical relationship for females but not for males.
| Stance/Stride | ||||
|---|---|---|---|---|
| Strain | Sex | N | Front | Rear |
| C56BL/10J | M | 8 | 0.60 +/− 0.008 | 0.68 +/− 0.007 |
| F | 7 | 0.60 +/− 0.007 | 0.66 +/− 0.005 | |
| DBA/2J | M | 6 | 0.65 +/− 0.006 | 0.64 +/− 0.012 |
| F | 7 | 0.64 +/− 0.012 | 0.64 +/− 0.010 | |
| NON/ShiLtJ | M | 8 | 0.69 +/− 0.008 | 0.70 +/− 0.015 |
| F | 9 | 0.68 +/− 0.006 | 0.70 +/− 0.006 | |
| 129S1/SvImJ | M | 4 | 0.62 +/− 0.007 | 0.58 +/− 0.006 |
| F | 7 | 0.56 +/− 0.025 | 0.64 +/− 0.018 | |
| BALB/cByJ | M | 8 | 0.61 +/− 0.014 | 0.68 +/− 0.009 |
| F | 7 | 0.64 +/− 0.011 | 0.67 +/− 0.012 | |
| C3H/HeJ | M | 7 | 0.61 +/− 0.011 | 0.60 +/− 0.017 |
| F | 8 | 0.62 +/− 0.013 | 0.60 +/− 0.015 | |
| SM/J | M | 6 | 0.65 +/− 0.014 | 0.66 +/− 0.012 |
| F | 5 | 0.60 +/− 0.027 | 0.65 +/− 0.013 | |
In the analysis of B6 mice above we show that age-related changes in body weight contribute to the variability in rear stride time and, to a somewhat greater extent, rear stance width. When we examined these relationships across the seven strains including all animals (n=96) we also found weak significant correlations between body weight and rear stance width (r=0.29, p=0.004, r2=0.094) or rear stride length (r=−0.32, p=0.002, r2=0.10). Thus, at 6 months of age only ~10% of the variation in these two gait parameters is due to the differences in body weight across these seven strains. Close examination of the data emphasizes that variation in gait parameters is due to factors other than weight (size). Mean rear stance widths were clearly different (Tukey, p≤0.013) even though six of the seven strains had very similar body weights (Fig. 8b; range ~26-30 g; Tukey, p≥ 0.66). Moreover, the lightest (129) and heaviest strains (NON) had, respectively the longest and shortest strides and the NON mice had an average rear stance width in the range of mice that were on average 12-15 grams lighter (Fig. 8).
Age-related change in treadmill gait in three strains
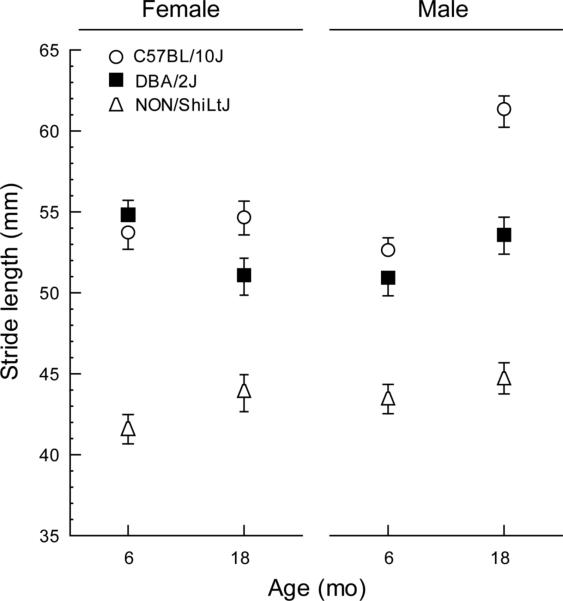
When we compared the 6 and 18 month data collected for B10, DBA and NON mice our first observation was an overall decrease in body weight between 6 and 18 months (Table 6) (age effect (F=13, (1, 67), p<0.001) that was consistent across strains for both sexes (no interactions (F≤1.7 (2,67), p>0.2). However, the weight losses were not significant for separate comparisons of males and females within each strain. Changes in body weight would predict an accompanying reduction in stride length between six and eighteen months. Instead stride length showed an overall increase (age effect F=12, (1, 134), p<0.001) for both paws (no interaction) but this effect was modest and varied both between strains and sexes (Fig. 9) (age by strain by sex interaction (F=6, (2,134), p=0.005). In post hoc comparisons only B10 mice had a significantly greater stride length at 18 months and this effect was due to an increase for male mice (p<1×10−4) while B10 females were unchanged (p=0.99).
Table 6.
Weight and stance phase ratio changes between 6 and 18 month for three inbred strains. Males and females for each strain showed a mild weight loss between 6 and 18 months. Stance phase ratios of front and rear paws were stable for NON and DBA but not for B10 mice. B10 mice showed the typical front:rear relationship at 6 months but, as shown above for closely related B6 mice (Fig 4c,d) the front:rear stance phase ratios converged at 18 months.
| Stance/Stride | ||||||
|---|---|---|---|---|---|---|
| Strain | Sex | N | Age | Weight | Front | Rear |
| C56BL/10J |
M | 8 | 6 | 31.9 +/− 0.88 | 0.60 +/− 0.008 | 0.68 +/− 0.007 |
| M | 7 | 18 | 27.4 +/− 1.6 | 0.57 +/− 0.013 | 0.61 +/− 0.013 | |
| F | 7 | 6 | 25.8 +/− 0.60 | 0.60 +/− 0.007 | 0.66 +/− 0.005 | |
| F |
6 |
18 |
23.0 +/− 1.2 |
0.60 +/− 0.008 |
0.64 +/− 0.011 |
|
| DBA/2J |
M | 6 | 6 | 33.3 +/− 1.4 | 0.65 +/− 0.006 | 0.64 +/− 0.012 |
| M | 5 | 18 | 26.0 +/− 1.8 | 0.66 +/− 0.012 | 0.64 +/− 0.009 | |
| F | 7 | 6 | 26.9 +/− 1.7 | 0.64 +/− 0.012 | 0.64 +/− 0.010 | |
| F |
5 |
18 |
23.7 +/− 0.96 |
0.62 +/− 0.020 |
0.62 +/− 0.013 |
|
| NON/ShiLtJ | M | 8 | 6 | 43.5 +/− 2.1 | 0.69 +/− 0.008 | 0.70 +/− 0.015 |
| M | 7 | 18 | 37.1 +/− 3.6 | 0.67 +/− 0.009 | 0.67 +/− 0.011 | |
| F | 8 | 6 | 31.5 +/− 2.2 | 0.68 +/− 0.006 | 0.70 +/− 0.006 | |
| F | 8 | 18 | 30.8 +/− 2.1 | 0.67 +/− 0.01 | 0.71 +/− 0.007 | |
Figure 9.
Naïve cohorts of mice were tested at 6 and 18 months of age for each strain. There was a significant overall increase in stride length when data were collapsed across sex and strain. However, when males and females were considered separately within each strain, only male B10 mice had a significantly longer stride. Overall these suggest that stride length remains stable up to 18 months for these three strains. Note the variation among males and females for B10 and DBA mice. Values are calculated for each animal as the average of all four paws and plotted as the mean ± s.e. of each group.
Finally we compared the stance/stride ratio of B10 mice at 6 and 18 months of age to determine if the age-related change in the FL: HL relationship we observed for B6 (Fig 4c above) was also evident in B10 mice which are closely related to B6. Consistent with our B6 results the significant difference in stance/stride ratio between the front and rear paws that was present at 6 months, was no longer significant at 18 months for either males or females (Table 6). Note that we did not examine the other two strains for this change because they did not show the FL:HL initially (Table 5).
Discussion
The combined results of these studies confirm that treadmill gait is a sensitive motor assay for mice, but also demonstrate that it should be considered a novel behavior that can be affected by age and experience. These findings are consistent with results of studies in other quadrapeds 4,7 and humans 9,14,16,46,47. We also show an influence of genetic background on treadmill gait that contrasts with the absence of comparable differences among strains during over ground locomotion when mice select their walking velocity 5.
These findings point to some general considerations for the use of treadmill gait as an assay of neuromuscular/neurological function in mice. First, interpretation of results from longitudinal studies is complicated by the effects of repeated exposures (shorter strides) and, depending on the age span studied and duration of the study, an opposing age-related effect (longer strides) due, in part, to changes in size and weight. In studies where standard inbred mice are used these complications can be easily avoided by using age-matched groups of naïve mice and strict adherence to a standardized trial protocol that includes exclusion of non-compliant mice.
Second, studies comparing different inbred strains or studies of strains with engineered or spontaneous mutations may require additional controls. Engineering knockout or transgenic mice often results in a mixed genetic background in early generations that can increase phenotypic variability or, as the genetic background is fixed in subsequent generations, lead to a modified phenotype 23. If strains are to be maintained on a mixed background litter effects need to be considered 42 and otherwise the phenotype should be monitored carefully for several backcross generations until the background is fully fixed. A related issue is that mutations may cause affected mice to be smaller and lighter or, in the case of degenerative conditions, cause a progressive age-related weight loss. In such cases possible effects due to differences in size might be avoided by testing at earlier ages before differences in size are evident or by using weight-matched controls.
Third, while the preceding considerations can guide experimental design the pertinence to any given model or treatment may vary. Our previous findings provide an example. The disease process in SOD1G93A transgenic ALS model 19 causes an increase in stride length prior to the appearance of overt effects 50. A change in this direction might be obscured by the shortening effect of repeat testing and, at later disease stages, confounded by progressive weight loss caused by disuse atrophy. However, in that study only control mice responded significantly to repeat testing and gait differences were detected prior to significant weight differences 50 and, at later stages when the transgenic mice are overtly affected, stride lengthens further despite significant weight loss (unpublished observations). On the one hand, this example demonstrates that a longitudinal design may be used and that repeated treadmill trials may even provide an additional variable. On the other hand, given the current findings, it may be advisable for longitudinal studies to either include concurrent naïve groups or to establish the nature of the response to treatment/disease at a single time point in advance. Whether such requirements negate the efficiency gained by a longitudinal study will depend on the specifics of each experiment. It should also be considered that gait analysis during over ground locomotion may be preferable to the treadmill for certain experiments. This approach allows walking velocity to vary but offers a sensitive, reliable index of motor performance that is unaffected by repeat trials 15 and age 12.
Repeated trial studies
The purpose of these studies was to better understand how multiple trials affected treadmill gait parameters with the practical goal of improving strategies for longitudinal studies. The results confirm and extend previous results 50 by showing: 1) that the extent and nature of the modifications depend on the number and timing of repeated trials and, 2) that effects are seen even with inter-trial intervals as long as three months.
These studies provide a starting point for study of the mechanism(s) underlying the observed trial effects. A consistent pattern is evident in the present data which shows that at the longer intervals (≥1 week) changes are characterized by a reduction in the swing phase that is more pronounced for the rear limbs. In contrast, for the rapid retests, the shortened strides are caused by reductions in stance and swing phases and are evident for both front and rear paws.
These studies do not establish the mechanism(s) for the repeated trial effect but two general possibilities can be considered. One possibility is that the effect is a reflex habituation response to the distinct dynamics and altered sensory feedback associated with treadmill walking 17,25. Such a response has been documented in humans and is characterized by a rapid habituation (≤10s) that takes more than 10 minutes to become consistent 9,46. Also, the rapid habituation response is repeated over multiple session but subjects take less time to develop a consistent pattern 47. Our data in mice are generally consistent with this scenario. The effect is rapid as it can be observed with a total walking time of less than 60 seconds (2 trial total) but takes an additional time (≥3 trials) for the pattern to stabilize. One requirement for evaluating this mechanism is the quantification of modifications within a single trial and attempts at these experiments were unsuccessful. Our standard trial is short and mice do not necessarily walk consistently for the entire trial and thus do not provide enough data for valid intra-trial comparisons (e.g. beginning vs. end). At this point computer/software limitations do not allow us to capture video clips of significantly longer trials (3-5 minutes) that might make such an analysis feasible.
A second related possibility is that mice learn with experience and some of our findings suggest a learning/memory component. First, we saw gait modifications in a second trial with a 1-week, and even a 3-month inter-trial interval. Second when mice were given five training trials (3 min. inter-trial) the modified gait pattern stabilized and persisted for 1 week, but only two initial trials (3 min. inter-trial) did not stabilize the gait pattern seen a week later. Our finding that 1, 2 or 3 retests with the 3-month inter-trial interval were similar shows effects are not additive with this long of an interval.
Our current data do not allow us to determine if these effects are due to more rapid within session habituation, as for humans 47, a persistent learned strategy, or some behavioral response, such as anxiety, related to memory of the testing chamber/treadmill. The latter possibility is supported by our observations of a more marked effect on the swing phase and an increase in rear stance width with a 1-week inter-trial interval. B6 mice were shown to have a reduced swing phase, a slightly longer stance phase and a wider stance during over ground locomotion when the environment was manipulated to increase anxiety 33.
Walking is a rhythmic, repetitive motor behavior that is controlled by interactions between central pattern generators and a variety of sensory feedback 13,18. However, some evidence suggests that higher central inputs contribute to swing phase adjustments 35. This view is supported, in mice, by the association of swing phase adjustments (decreases) with increasingly anxiogenic environments 33, changes that would require processing by higher centers to be assessed. The lighted treadmill enclosure likely is a stressful environment and our results suggest that the memory of the enclosure may heighten anxiety and contribute to gait modifications on the treadmill also. Our results further suggest, however, that training, the length of the inter-trial interval and age can affect this response. Five training trials stabilized the swing phase and eliminated the effect on stance width when tested a week later and with a 3-month inter-trial interval changes in stance and swing phases were more comparable and stance width was unaffected.
A practical goal of these studies was to develop an efficient strategy for training mice to establish a new stable baseline. We conclude that a series of five initial training trials can achieve this for at least a week. However, the training had the serious drawback that a week later nearly half the mice refused to walk well enough for valid data to be collected. Given these findings and the resulting need to test additional animals as well as the possible bias introduced by eliminating non-compliant animals, we further conclude that using naïve groups of mice and our standard protocol for data collection, which produces highly reliable results for naïve B6 mice, is a preferable strategy when feasible.
Nonetheless, there are many other potential training strategies, the most obvious of which is to train the mice for longer periods when allowed by the model under study. This would allow mice more time to acclimate, establish a baseline and possibly improve compliance.
Aging
The goal of our aging study was to evaluate the stability of treadmill gait parameters during the first year of life. Our results show that as B6 mice age the same walking speed is achieved by longer, less frequent steps that are also accompanied by an increase in dynamic stance width. We also show that between 40 and 60% of the age-related changes in the gait parameters examined can be attributed to changes in body weight and length, particularly between 1 and 6 months of age. The source of the remaining variability in age-related gait changes is not known and would require additional study. We chose weight and length as simple general measures of growth but it is possible that other morphometric measurements would account more fully for variation in gait measurements. For instance, the length of HL and FL bones increase by 2-3 millmeters between 1 and 5 months of age 2,37 and corresponds with the period when 2we observed relatively rapid changes in treadmill gait parameters.
For freely walking mice a transition from immature to mature gait was described that occurs at around postnatal day 24 12. The transition is characterized by the establishment of stance times that are longer and swing times that are shorter in HL compared to FL. We did not attempt to discern a developmental transition on the treadmill but the mature relationship is also evident for treadmill gait between 30-180 days even as measures show age-related increases. However, the HL to FL relationship for treadmill stance time is lost between 6 and 9 months and is reversed by 12 months. The functional outcome of these changes is to confer somewhat greater stability as the mice come to rely more equally on the front and rear paws for treadmill locomotion.
The age-related changes in the basic gait parameters and the loss of the typical HL:FL relationship that we observed demonstrate that treadmill gait is sensitive to factors that apparently do not affect freely walking mice. A study of two cohorts of freely walking mice over a greater age-span (10 days to 18 months) did not find any significant changes that were not due to changes in velocity. As such, our results add to other data, from mice 25 other quadrapeds 4,7 and humans 14,16 which demonstrated that treadmill and over ground locomotion are not equivalent. Additional study is required to determine why mice walking on the treadmill show age/growth-related changes that are not evident during over ground walking and may necessitate more sophisticated methods (e.g. ground reaction forces, joint kinematics). For instance, freely walking mice might be better able to vary the amount of curvature in their spine to effectively lengthen or shorten overall body length and in turn modify gait parameters to compensate for changes in size and weight. Such adjustments may be constrained on the treadmill by the fixed velocity or by behavioral factors such as anxiety 33 that change with age 27.
Strain differences
Our comparison of gait parameters among the seven inbred strains that completed testing at 6-months of age confirms an influence of genetic background. These data offer a potential starting point for identifying genetic loci associated with differences in treadmill gait. Crosses between closely related strains that are similar in size (weight) but that showed gait differences (e.g.BALB and C3H or DBA and SM) would be most likely to be informative. However, a recent report showing that differences in anxiety can affect gait measures 33 also allow for the possibility that loci associated with traits other than those directly related to motor output during treadmill walking might be identified.
The other major finding of the strain survey is that a proportion of mice for most strains do not walk on the treadmill consistently enough for useful data to be collected even at 6-months of age. Also, naïve groups of mice from strains that did walk well initially often became less compliant with age. As discussed above, it might be possible to improve compliance by allowing more time for acclimation. Alternative strategies, in combination with acclimation, might improve acquisition of useful data from non-compliant strains. For example, although we chose to use a fixed walking speed, it is possible to evaluate gait measures as treadmill speed is progressively increased or at slower speeds. In our hands, it is difficult to obtain valid data below ~15cm/s because mice walk inconsistently with a “stop and go” strategy 50 but others have successfully used speeds as low as 10 cm/sec 25,32.
Aging NON, DBA and B10 mice from 6-18 months has little overall effect on gait parameters even though each of the strains were beginning to show minor weight losses that accompany aging 21. Eighteen-month-old mice are not considered aged, with reported degenerative changes (e.g. sarcopenia) not appearing until ≥ 2 years of age 6,21,34. Of the three strains studied, survival rates at 18 months for B10 and NON mice of both sexes exceed 90% while the survival of DBA mice at the same age is ~70% for males and ~55% for females and (www.jax.org/phenome). The reduced survival rate of female DBA mice is interesting in that these were the only mice to show a decreased stride length at 18 months of age. Whether this is indicative of more rapid aging will require additional study.
In summary these findings indicate that treadmill gait measures are sensitive indicators of gross motor performance in mice and are therefore very useful in evaluating mechanisms and disease models that affect motor function. However, the caveats that apply to other behavioral tests, including repeat testing effects, age, genetic background and compliance, must be carefully considered in the design of gait experiments. As well, we have suggested possible solutions and appropriate controls to overcome some of these factors, but these will vary depending on each animal model and experimental protocol.
Acknowledgments
Work supported by NS05471 (KLS), ALS Association (KLS, GAC), Nathan Shock Center Grant AG 25707 and Ellison Medical Foundation grant to The Jackson Laboratory AG-1A-0204-05
Footnotes
Abbreviations.
References
- 1.Akay T, Acharya HJ, Fouad K, Pearson KG. Behavioral and electromyographic characterization of mice lacking EphA4 receptors. J Neurophysiol. 2006;96(2):642–51. doi: 10.1152/jn.00174.2006. [DOI] [PubMed] [Google Scholar]
- 2.Amende I, Kale A, McCue S, Glazier S, Morgan JP, Hampton TG. Gait dynamics in mouse models of Parkinson's disease and Huntington's disease. J Neuroeng Rehabil. 2005;2:20. doi: 10.1186/1743-0003-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck JA, Lloyd S, Hafezparast M, Lennon-Pierce M, Eppig JT, Festing MF, Fisher EM. Genealogies of mouse inbred strains. Nat Genet. 2000;24(1):23–5. doi: 10.1038/71641. [DOI] [PubMed] [Google Scholar]
- 4.Blaszczyk J, Loeb GE. Why cats pace on the treadmill. Physiol Behav. 1993;53(3):501–7. doi: 10.1016/0031-9384(93)90144-5. [DOI] [PubMed] [Google Scholar]
- 5.Brooks SP, Pask T, Jones L, Dunnett SB. Behavioural profiles of inbred mouse strains used as transgenic backgrounds. I: motor tests. Genes Brain Behav. 2004;3(4):206–15. doi: 10.1111/j.1601-183X.2004.00072.x. [DOI] [PubMed] [Google Scholar]
- 6.Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol. 1988;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchner DM, Cress ME, Esselman PC, Margherita AJ, de Lateur BJ, Campbell AJ, Wagner EH. Factors associated with changes in gait speed in older adults. J Gerontol A Biol Sci Med Sci. 1996;51(6):M297–302. doi: 10.1093/gerona/51a.6.m297. [DOI] [PubMed] [Google Scholar]
- 8.Carter RJ, Lione LA, Humby T, Mangiarini L, Mahal A, Bates GP, Dunnett SB, Morton AJ. Characterization of progressive motor deficits in mice transgenic for the human Huntington's disease mutation. J Neurosci. 1999;19(8):3248–57. doi: 10.1523/JNEUROSCI.19-08-03248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charteris J, Taves C. The process of habituation to treadmill walking: a kinematic analysis. Percept Mot Skills. 1978;47(2):659–66. doi: 10.2466/pms.1978.47.2.659. [DOI] [PubMed] [Google Scholar]
- 10.Clarke KA, Smart L, Still J. Ground reaction force and spatiotemporal measurements of the gait of the mouse. Behav Res Methods Instrum Comput. 2001;33(3):422–6. doi: 10.3758/bf03195396. [DOI] [PubMed] [Google Scholar]
- 11.Clarke KA, Still J. Gait analysis in the mouse. Physiol Behav. 1999;66(5):723–9. doi: 10.1016/s0031-9384(98)00343-6. [DOI] [PubMed] [Google Scholar]
- 12.Clarke KA, Still J. Development and consistency of gait in the mouse. Physiol Behav. 2001;73(12):159–64. doi: 10.1016/s0031-9384(01)00444-9. [DOI] [PubMed] [Google Scholar]
- 13.Delcomyn F. Neural basis of rhythmic behavior in animals. Science. 1980;210(4469):492–8. doi: 10.1126/science.7423199. [DOI] [PubMed] [Google Scholar]
- 14.Elliott BC, Blanksby BA. A cinematographic analysis of overground and treadmill running by males and females. Med Sci Sports. 1976;8(2):84–7. [PubMed] [Google Scholar]
- 15.Fernagut PO, Diguet E, Labattu B, Tison F. A simple method to measure stride length as an index of nigrostriatal dysfunction in mice. J Neurosci Methods. 2002;113(2):123–30. doi: 10.1016/s0165-0270(01)00485-x. [DOI] [PubMed] [Google Scholar]
- 16.Frishberg BA. An analysis of overground and treadmill sprinting. Med Sci Sports Exerc. 1983;15(6):478–85. [PubMed] [Google Scholar]
- 17.Grillner S. Control of locomotion in bipeds, tetrapods, and fish. In: Brooks VB, editor. The nervous system, section I. American Physiological Society; Bethesda, Maryland: 1981. pp. 1179–1236. [Google Scholar]
- 18.Grillner S, Cangiano L, Hu G, Thompson R, Hill R, Wallen P. The intrinsic function of a motor system--from ion channels to networks and behavior. Brain Res. 2000;886(12):224–236. doi: 10.1016/s0006-8993(00)03088-2. [DOI] [PubMed] [Google Scholar]
- 19.Gurney ME. Transgenic-mouse model of amyotrophic lateral sclerosis. N Engl J Med. 1994;331(25):1721–2. doi: 10.1056/NEJM199412223312516. [DOI] [PubMed] [Google Scholar]
- 20.Hampton TG, Stasko MR, Kale A, Amende I, Costa AC. Gait dynamics in trisomic mice: quantitative neurological traits of Down syndrome. Physiol Behav. 2004;82(23):381–9. doi: 10.1016/j.physbeh.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Hamrick MW, Ding KH, Pennington C, Chao YJ, Wu YD, Howard B, Immel D, Borlongan C, McNeil PL, Bollag WB. Age-related loss of muscle mass and bone strength in mice is associated with a decline in physical activity and serum leptin. Bone. 2006;39(4):845–53. doi: 10.1016/j.bone.2006.04.011. others. [DOI] [PubMed] [Google Scholar]
- 22.He J, Rosen CJ, Adams DJ, Kream BE. Postnatal growth and bone mass in mice with IGF-I haploinsufficiency. Bone. 2006;38(6):826–35. doi: 10.1016/j.bone.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 23.Heiman-Patterson TD, Deitch JS, Blankenhorn EP, Erwin KL, Perreault MJ, Alexander BK, Byers N, Toman I, Alexander GM. Background and gender effects on survival in the TgN(SOD1-G93A)1Gur mouse model of ALS. J Neurol Sci. 2005;236(12):1–7. doi: 10.1016/j.jns.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Herbin M, Gasc JP, Renous S. Symmetrical and asymmetrical gaits in the mouse: patterns to increase velocity. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2004;190(11):895–906. doi: 10.1007/s00359-004-0545-0. [DOI] [PubMed] [Google Scholar]
- 25.Herbin M, Hackert R, Gasc JP, Renous S. Gait parameters of treadmill versus overground locomotion in mouse. Behav Brain Res. 2007;181(2):173–9. doi: 10.1016/j.bbr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Holmes A, Wrenn CC, Harris AP, Thayer KE, Crawley JN. Behavioral profiles of inbred strains on novel olfactory, spatial and emotional tests for reference memory in mice. Genes Brain Behav. 2002;1(1):55–69. doi: 10.1046/j.1601-1848.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- 27.Imhof JT, Coelho ZM, Schmitt ML, Morato GS, Carobrez AP. Influence of gender and age on performance of rats in the elevated plus maze apparatus. Behav Brain Res. 1993;56(2):177–80. doi: 10.1016/0166-4328(93)90036-p. [DOI] [PubMed] [Google Scholar]
- 28.Jakeman LB, Chen Y, Lucin KM, McTigue DM. Mice lacking L1 cell adhesion molecule have deficits in locomotion and exhibit enhanced corticospinal tract sprouting following mild contusion injury to the spinal cord. Eur J Neurosci. 2006;23(8):1997–2011. doi: 10.1111/j.1460-9568.2006.04721.x. [DOI] [PubMed] [Google Scholar]
- 29.Kale A, Amende I, Meyer GP, Crabbe JC, Hampton TG. Ethanol's effects on gait dynamics in mice investigated by ventral plane videography. Alcohol Clin Exp Res. 2004;28(12):1839–48. doi: 10.1097/01.alc.0000148103.09378.81. [DOI] [PubMed] [Google Scholar]
- 30.Klapdor K, Dulfer BG, Hammann A, Van der Staay FJ. A low-cost method to analyse footprint patterns. J Neurosci Methods. 1997;75(1):49–54. doi: 10.1016/s0165-0270(97)00042-3. [DOI] [PubMed] [Google Scholar]
- 31.Kurz MJ, Pothakos K, Jamaluddin S, Scott-Pandorf M, Arellano C, Lau YS. A chronic mouse model of Parkinson's disease has a reduced gait pattern certainty. Neurosci Lett. 2007;429(1):39–42. doi: 10.1016/j.neulet.2007.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leblond H, L'Esperance M, Orsal D, Rossignol S. Treadmill locomotion in the intact and spinal mouse. J Neurosci. 2003;23(36):11411–9. doi: 10.1523/JNEUROSCI.23-36-11411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lepicard EM, Venault P, Abourachid A, Pelle E, Chapouthier G, Gasc JP. Spatiotemporal analysis of locomotion in BALB/cByJ and C57BL/6J mice in different environmental conditions. Behav Brain Res. 2006;167(2):365–72. doi: 10.1016/j.bbr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Luff AR. Age-associated changes in the innervation of muscle fibers and changes in the mechanical properties of motor units. Ann N Y Acad Sci. 1998;854:92–101. doi: 10.1111/j.1749-6632.1998.tb09895.x. [DOI] [PubMed] [Google Scholar]
- 35.Miller S, Schomburg ED. Locomotor coordination in the cat. In: Bush BMH, Clarac FS, editors. Coordination of motor behavior. Vol. 24. Cambridge University Press; Cambridge: 1985. pp. 201–220. [Google Scholar]
- 36.Nadler JJ, Zou F, Huang H, Moy SS, Lauder J, Crawley JN, Threadgill DW, Wright FA, Magnuson TR. Large-scale gene expression differences across brain regions and inbred strains correlate with a behavioral phenotype. Genetics. 2006;174(3):1229–36. doi: 10.1534/genetics.106.061481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parker LT, Leamy L. Fluctuating asymmetry of morphometric characters in house mice: the effects of age, sex, and phenotypical extremeness in a randombred population. J Hered. 1991;82(2):145–50. doi: 10.1093/oxfordjournals.jhered.a111049. [DOI] [PubMed] [Google Scholar]
- 38.Pereira JE, Cabrita AM, Filipe VM, Bulas-Cruz J, Couto PA, Melo-Pinto P, Costa LM, Geuna S, Mauricio AC, Varejao AS. A comparison analysis of hindlimb kinematics during overground and treadmill locomotion in rats. Behav Brain Res. 2006;172(2):212–8. doi: 10.1016/j.bbr.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 39.Petkov PM, Ding Y, Cassell MA, Zhang W, Wagner G, Sargent EE, Asquith S, Crew V, Johnson KA, Robinson P. An efficient SNP system for mouse genome scanning and elucidating strain relationships. Genome Res. 2004;14(9):1806–11. doi: 10.1101/gr.2825804. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ridgel AL, Ritzmann RE. Insights into age-related locomotor declines from studies of insects. Ageing Res Rev. 2005;4(1):23–39. doi: 10.1016/j.arr.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Scherder E, Eggermont L, Swaab D, van Heuvelen M, Kamsma Y, de Greef M, van Wijck R, Mulder T. Gait in ageing and associated dementias; its relationship with cognition. Neurosci Biobehav Rev. 2007;31(4):485–97. doi: 10.1016/j.neubiorev.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 42.Scott S, Kranz JE, Cole J, Lincecum JM, Thompson K, Kelly N, Bostrom A, Theodoss J, Al-Nakhala BM, Vieira FG. Design, power, and interpretation of studies in the standard murine model of ALS. Amyotroph Lateral Scler. 2008;9(1):4–15. doi: 10.1080/17482960701856300. others. [DOI] [PubMed] [Google Scholar]
- 43.Starkey ML, Barritt AW, Yip PK, Davies M, Hamers FP, McMahon SB, Bradbury EJ. Assessing behavioural function following a pyramidotomy lesion of the corticospinal tract in adult mice. Exp Neurol. 2005;195(2):524–39. doi: 10.1016/j.expneurol.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 44.Stoll S, Dorner H, Blosch M, Platt D. Age-dependent differences in the gait of rats. Arch Gerontol Geriatr. 1990;10(3):261–8. doi: 10.1016/0167-4943(90)90027-4. [DOI] [PubMed] [Google Scholar]
- 45.Vincelette J, Xu Y, Zhang LN, Schaefer CJ, Vergona R, Sullivan ME, Hampton TG, Wang YX. Gait analysis in a murine model of collagen-induced arthritis. Arthritis Res Ther. 2007;9(6):R123. doi: 10.1186/ar2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wall JC, Charteris J. The process of habituation to treadmill walking at different velocities. Ergonomics. 1980;23(5):425–35. doi: 10.1080/00140138008924758. [DOI] [PubMed] [Google Scholar]
- 47.Wall JC, Charteris J. A kinematic study of long-term habituation to treadmill walking. Ergonomics. 1981;24(7):531–42. doi: 10.1080/00140138108924874. [DOI] [PubMed] [Google Scholar]
- 48.Walton A, Branham A, Gash DM, Grondin R. Automated video analysis of age-related motor deficits in monkeys using EthoVision. Neurobiol Aging. 2006;27(10):1477–83. doi: 10.1016/j.neurobiolaging.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 49.Wetzel MC, Atwater AE, Wait JV, Stuart DC. Neural implications of different profiles between treadmill and overground locomotion timings in cats. J Neurophysiol. 1975;38(3):492–501. doi: 10.1152/jn.1975.38.3.492. [DOI] [PubMed] [Google Scholar]
- 50.Wooley CM, Sher RB, Kale A, Frankel WN, Cox GA, Seburn KL. Gait analysis detects early changes in transgenic SOD1(G93A) mice. Muscle Nerve. 2005;32(1):43–50. doi: 10.1002/mus.20228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zumwalt AC, Hamrick M, Schmitt D. Force plate for measuring the ground reaction forces in small animal locomotion. J Biomech. 2006;39(15):2877–81. doi: 10.1016/j.jbiomech.2005.10.006. [DOI] [PubMed] [Google Scholar]