Abstract
Clonally expanded mitochondrial DNA (mtDNA) deletions accumulate with age in human substantia nigra (SN) and high levels cause respiratory chain deficiency. In other human tissues, mtDNA point mutations clonally expand with age. Here, the abundance of mtDNA point mutations within single SN neurons from aged controls was investigated. From 31 single cytochrome c oxidase normal SN neurons, only one clonally expanded mtDNA point mutation was identified, suggesting in these neurons mtDNA point mutations occur rarely, whereas mtDNA deletions are frequently observed. This contrasts observations in mitotic tissues and suggests that different forms of mtDNA maintenance may exist in these two cell types.
Keywords: clonal expansion, mtDNA, neurons, point mutations
The mitochondrial genome is present in multiple copies, the number varying depending on the energy requirement of the cell. Mitochondrial DNA encodes key respiratory chain proteins, as well as RNA machinery required for intra-mitochondrial protein synthesis (Anderson et al., 1981). Mitochondrial DNA defects are an important cause of mitochondrial disease with an incidence of ∼1 in 10 000 adults clinically affected in the UK (Schaefer et al., 2008). Although patient symptoms are variable, severe and progressive neurological problems are a common feature (Taylor & Turnbull, 2005).
Mitochondrial DNA mutations accumulate with age in many tissues from human subjects (Liu et al., 1998; Fayet et al., 2002; Nekhaeva et al., 2002; Taylor et al., 2003), although their contribution to the human aging process remains uncertain. Both mtDNA point mutations and large-scale deletions clonally expand to high levels (> 60%) leading to respiratory chain deficiency. Support for a direct role for mtDNA mutations in aging resulted from the generation of mouse models with a proof-reading deficient version of PolgA; these mice have high levels of mtDNA mutations and develop a premature aging phenotype (Trifunovic et al., 2004; Kujoth et al., 2005; Vermulst et al., 2008).
Although both mtDNA deletions and point mutations are observed in human cells, few studies have examined the presence of both in the same tissue. This may give insight into the mechanisms by which they arise and clonally expand. We previously detected high levels of mtDNA deletions in substantia nigra (SN) neurons from aged humans (Bender et al., 2006; Krishnan et al., 2008; Reeve et al., 2008). In this study, we wished to determine if these cells also contained clonally expanded mtDNA point mutations.
Frozen midbrain sections were cut at 20 μm from five elderly controls (Table 1). Mitochondrial dysfunction was determined using sequential histochemical staining for the activities of cytochrome c oxidase (COX) and succinate dehydrogenase (Brierley et al., 1998). This revealed the presence of both COX normal and deficient SN neurons. Single COX normal cells were chosen for analysis to allow comparison with mtDNA deletion levels from our previous study (Bender et al., 2006).
Table 1.
Table showing the details of the subjects used in this study
| Subject number | Age | pm delay (h) | Sex | mtDNA haplogroup | Haplogroup markers | Percentage COX-deficient neurons in the SN (Bender et al., 2006) | Percentage deletion from 25 COX normal neurons (Bender et al., 2006) |
|---|---|---|---|---|---|---|---|
| 1 | 75 | 30 | M | K | m.3480A>G, m.9698T>C, m.10550A>G, m.11299T>C | 0.61 | 40 |
| 2 | 72 | 28 | F | J | m.11251A>G, m.12612A>G, m.13708G>A, m.15452C>A | 0.35 | 45 |
| 3 | 75 | 9 | F | H2 | m.1438A>G, m.4769A>G, m.8860A>G, m.15326A>G | 0.26 | 42 |
| 4 | 81 | 29 | F | H2 | m.1438A>G, m.4769A>G, m.8860A>G, m.15326A>G | 0.12 | 33 |
| 5 | 89 | 27 | M | X | m.6221T>C, m.6371C>T, m.13996A>G, m.14470T>C | 0.42 | 56 |
pm, post mortem; COX, cytochrome c oxidase; SN, substantia nigra.
The entire mtDNA was sequenced from 31 single SN neurons as previously described (Taylor et al., 2001). Analysis of single cell mtDNA sequences is problematic as highlighted previously (Bandelt et al., 2008). Low mtDNA copy number in single cells can result in contamination from nuclear pseudogenes or result in PCR-induced errors. To ensure detection of only true clonally expanded mutations, we re-sequenced all putative mutations with DNA from the initial cell lysate; repeating the second-round PCR, to confirm the presence of a mutation by at least two, separate PCR reactions. Following re-sequencing, we excluded 10 mutations detected only on the initial PCR reaction. None were recognized polymorphisms, suggesting the mutations arose during PCR amplification.
In total, we analysed 31 cells (513 639 bp). Germline polymorphisms were found in all cells from each subject. All changes from the revised Cambridge Reference Sequence (Andrews et al., 1999) (n = 730) were recognized polymorphic variants (MITOMAP, 2009, Ingman & Gyllensten, 2006), permitting the determination of mitochondrial haplogroups, for all subjects (Table 1).
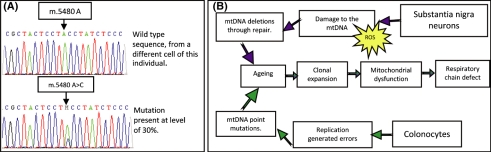
True clonally expanded point mutations were those present only once in a single cell from an individual. Using these criteria, we detected only one clonally expanded point mutation (Table 1, Fig. 1A). This m.5480A>C transversion found within the MTND2 gene, NADH ubiquinone oxidoreductase (complex I) subunit 2, was synonymous and was heteroplasmic (∼30%) based on the relative abundance of peaks in the sequence chromatogram. This shows that clonal expansion of mtDNA point mutations is a rare event in single SN neurons, consistent with previous data from a small number of COX-deficient cells (Bender et al., 2006). The single clonally expanded point mutation detected does not confer an amino acid change and is therefore unlikely to be pathogenic. Based on these data, only 3% (1/31) of SN neurons from the elderly subjects harbour a clonally expanded point mutation, a very different scenario to mtDNA deletions, where high levels of deletions were detected in COX normal neurons from the same subjects (Table 1) and other elderly subjects (Bender et al., 2006; Kraytsberg et al., 2006).
Fig. 1.
(A) Sequence electropherogram of the clonally expanded point mutation detected within the SN neurons. (B) Flow diagram to illustrate how different mtDNA mutations may be preferentially formed in mitotic and postmitotic tissues. Both mutation types lead to mitochondrial dysfunction with age. ROS = Reactive oxygen species.
Both mitotic and postmitotic cells show clonal expansion of mtDNA mutations with age, but it appears that there are marked differences in the type of mutations detected. In buccal epithelium cells and cardiomyocytes, clonally expanded mtDNA point mutations have been documented; however, mtDNA deletions were only detected in the cardiomyocytes (Bodyak et al., 2001; Nekhaeva et al., 2002). In a separate study, in three different tissues (skeletal muscle, heart and kidney), the 4977 bp mtDNA deletion and an m.3243 A>G point mutation accumulated with age. However, deletion levels were much higher in skeletal muscle and point mutation accumulation occurred earlier in the kidney and heart reaching higher levels than in skeletal muscle (Liu et al., 1998). These studies imply that there is preferential formation and/or accumulation of mtDNA point mutations in mitotic tissues, whereas, mtDNA deletions accumulate in postmitotic tissues (Fig. 1B).
Mitochondrial DNA replication occurs in postmitotic cells, even though the cell cycle is suspended. The rate of mtDNA turnover remains unknown, but it is thought to be much slower in neurons than in dividing cells (Wang et al., 1997). Substantia nigra neurons are particularly vulnerable to reactive oxygen species damage, due in part to their high iron content and through dopamine metabolism (Halliwell, 1992). Thus the high levels of mtDNA deletions are most likely to be caused during repair of the reactive oxygen species damage (Krishnan et al., 2008). In mitotic tissues where cell turnover occurs more frequently, mtDNA mutations are more likely to be generated by replication errors (Fig. 1B).
In conclusion, we have described low levels of clonally expanded point mutations in SN neurons, which contrast the high levels of mtDNA deletions previously reported. This further supports the difference in mtDNA mutation types detected between mitotic and postmitotic cells, and implies that different mechanisms of mtDNA maintenance exist within different cell types.
Acknowledgments
This work was supported by the Alzheimer’s Research Trust, the Wellcome Trust and the Newcastle University Centre for Brain Ageing and Vitality, part of the cross council Lifelong Health and Wellbeing Initiative with funding from the BBSRC, EPSRC, ESRC and MRC.
References
- Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJ, Staden R, Young IG. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Andrews RM, Kubacka I, Chinnery PF, Lightowlers RN, Turnbull DM, Howell N. Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat. Genet. 1999;23:147. doi: 10.1038/13779. [DOI] [PubMed] [Google Scholar]
- Bandelt HJ, Salas A, Taylor RW, Yao YG. Exaggerated status of “novel” and “pathogenic” mtDNA sequence variants due to inadequate database searches. Hum. Mutat. 2008;30:191–196. doi: 10.1002/humu.20846. [DOI] [PubMed] [Google Scholar]
- Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, Taylor RW, Turnbull DM. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat. Genet. 2006;38:515–517. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- Bodyak ND, Nekhaeva E, Wei JY, Khrapko K. Quantification and sequencing of somatic deleted mtDNA in single cells: evidence for partially duplicated mtDNA in aged human tissues. Hum. Mol. Genet. 2001;10:17–24. doi: 10.1093/hmg/10.1.17. [DOI] [PubMed] [Google Scholar]
- Brierley EJ, Johnson MA, Lightowlers RN, James OF, Turnbull DM. Role of mitochondrial DNA mutations in human aging: implications for the central nervous system and muscle. Ann. Neurol. 1998;43:217–223. doi: 10.1002/ana.410430212. [DOI] [PubMed] [Google Scholar]
- Fayet G, Jansson M, Sternberg D, Moslemi AR, Blondy P, Lombes A, Fardeau M, Oldfors A. Ageing muscle: clonal expansions of mitochondrial DNA point mutations and deletions cause focal impairment of mitochondrial function. Neuromuscul. Disord. 2002;12:484–493. doi: 10.1016/s0960-8966(01)00332-7. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Reactive oxygen species and the central nervous system. J. Neurochem. 1992;59:1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- Ingman M, Gyllensten U. mtDB: Human Mitochondrial Genome Database, a resource for population genetics and medical sciences. Nucleic Acids Res. 2006;34:D749–D751. doi: 10.1093/nar/gkj010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, Kowall NW, Khrapko K. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat. Genet. 2006;38:518–520. doi: 10.1038/ng1778. [DOI] [PubMed] [Google Scholar]
- Krishnan KJ, Reeve AK, Samuels DC, Chinnery PF, Blackwood JK, Taylor RW, Wanrooij S, Spelbrink JN, Lightowlers RN, Turnbull DM. What causes mitochondrial DNA deletions in human cells? Nat. Genet. 2008;40:275–279. doi: 10.1038/ng.f.94. [DOI] [PubMed] [Google Scholar]
- Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- Liu VW, Zhang C, Nagley P. Mutations in mitochondrial DNA accumulate differentially in three different human tissues during ageing. Nucleic Acids Res. 1998;26:1268–1275. doi: 10.1093/nar/26.5.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITOMAP MITOMAP: A Human Mitochondrial Genome Database. 2009. Available at: http://www.mitomap.org (Accessed: 8 June 2009)
- Nekhaeva E, Bodyak ND, Kraytsberg Y, McGrath SB, Van Orsouw NJ, Pluzhnikov A, Wei JY, Vijg J, Khrapko K. Clonally expanded mtDNA point mutations are abundant in individual cells of human tissues. Proc. Natl. Acad. Sci. USA. 2002;99:5521–5526. doi: 10.1073/pnas.072670199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve AK, Krishnan KJ, Elson JL, Morris CM, Bender A, Lightowlers RN, Turnbull DM. Nature of mitochondrial DNA deletions in Substantia Nigra neurons. Am. J. Hum. Genet. 2008;82:228–235. doi: 10.1016/j.ajhg.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer AM, McFarland R, Blakely EL, He L, Whittaker RG, Taylor RW, Chinnery PF, Turnbull DM. Prevalence of mitochondrial DNA disease in adults. Ann. Neurol. 2008;63:35–39. doi: 10.1002/ana.21217. [DOI] [PubMed] [Google Scholar]
- Taylor RW, Turnbull DM. Mitochondrial DNA mutations in human disease. Nat. Rev. Gen. 2005;6:389–402. doi: 10.1038/nrg1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RW, Taylor GA, Durham SE, Turnbull DM. The determination of complete human mitochondrial DNA sequences in single cells: implications for the study of somatic mitochondrial DNA point mutations. Nucleic Acids Res. 2001;29:E74. doi: 10.1093/nar/29.15.e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RW, Barron MJ, Borthwick GM, Gospel A, Chinnery PF, Samuels DC, Taylor GA, Plusa SM, Meddham SJ, Greaves LC, Kirkwood BL, Turnbull DM. Mitochondrial mutations in human colonic crypt stem cells. J. Clin. Invest. 2003;112:1351–1360. doi: 10.1172/JCI19435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly YM, Gidlof S, Oldfors A, Wibom R, Tornell J, Jacobs HT, Larsson NG. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- Vermulst M, Wanagat J, Kujoth GC, Bielas JH, Rabinovitch PS, Prolla TA, Loeb LA. DNA deletions and clonal mutations drive premature aging in mitochondrial mutator mice. Nat. Genet. 2008;40:392–394. doi: 10.1038/ng.95. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Nutter LM, Thayer SA. Insensitivity of cultured rat cortical neurons to mitochondrial DNA synthesis inhibitors: evidence for a slow turnover of mitochondrial DNA. Biochem. Pharmacol. 1997;54:181–187. doi: 10.1016/s0006-2952(97)00158-5. [DOI] [PubMed] [Google Scholar]