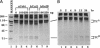Abstract
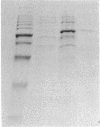
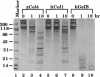
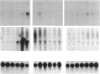
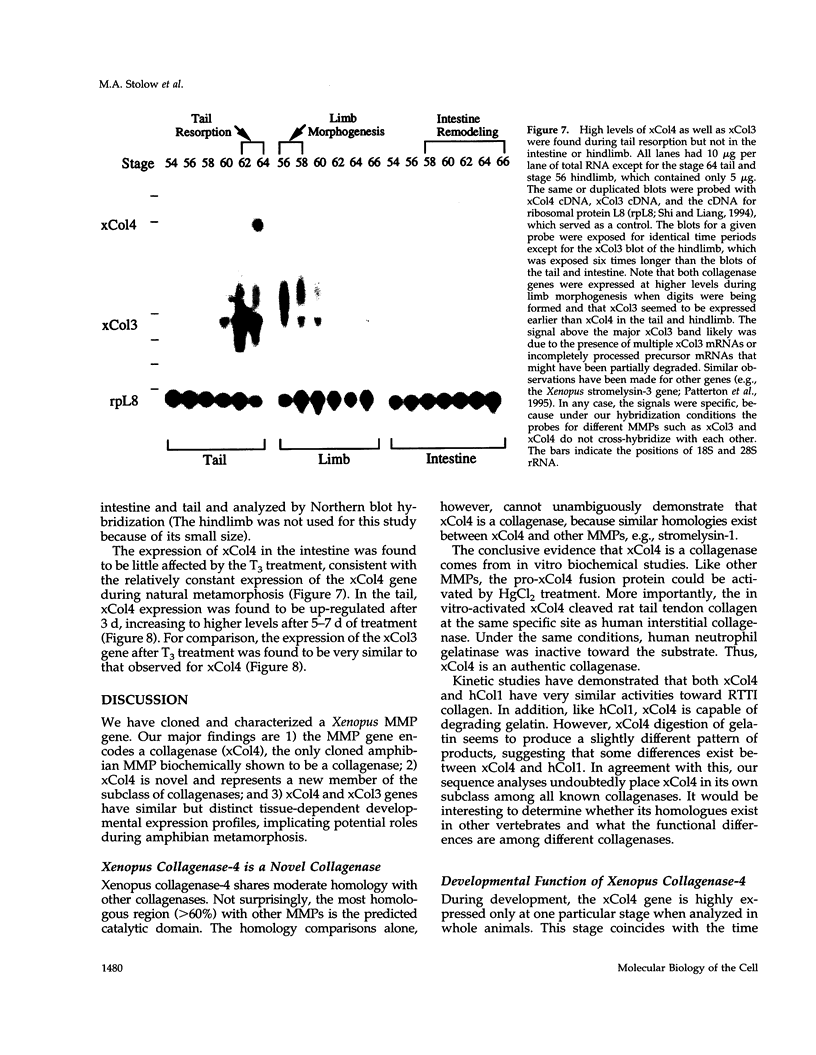
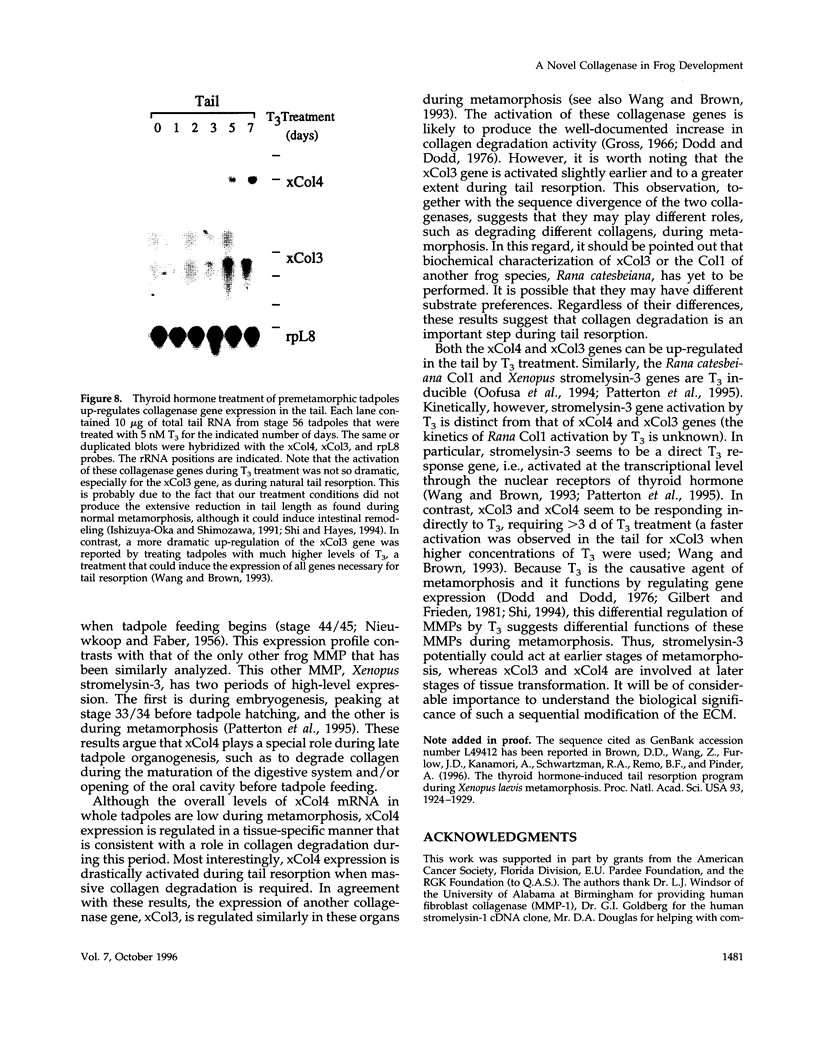
Matrix metalloproteinases (MMPs) participate in extracellular matrix remodeling and degradation and have been implicated in playing important roles during organ development and pathological processes. Although it has been hypothesized for > 30 years that collagenase activities are responsible for collagen degradation during tadpole tail resorption, none of the previously cloned amphibian MMPs have been biochemically demonstrated to be collagenases. Here, we report a novel matrix metalloproteinase gene from metamorphosing Xenopus laevis tadpoles. In vitro biochemical studies demonstrate that this Xenopus enzyme is an interstitial collagenase and has an essentially identical enzymatic activity toward a collagen substrate as the human interstitial collagenase. Sequence comparison of this enzyme to other known MMPs suggests that the Xenopus collagenase is not a homologue of any known collagenases but instead represents a novel collagenase, Xenopus collagenase-4 (xCol4, MMP-18). Interestingly, during development, xCol4 is highly expressed only transiently in whole animals, at approximately the time when tadpole feeding begins, suggesting a role during the maturation of the digestive tract. More importantly, during metamorphosis, xCol4 is regulated in a tissue-dependent manner. High levels of its mRNA are present as the tadpole tail resorbs. Similarly, its expression is elevated during hindlimb morphogenesis and intestinal remodeling. In addition, when premetamorphic tadpoles are treated with thyroid hormone, the causative agent of metamorphosis, xCol4 expression is induced in the tail. These results suggest that xCol4 may facilitate larval tissue degeneration and adult organogenesis during amphibian metamorphosis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aimes R. T., Quigley J. P. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J Biol Chem. 1995 Mar 17;270(11):5872–5876. doi: 10.1074/jbc.270.11.5872. [DOI] [PubMed] [Google Scholar]
- Basset P., Bellocq J. P., Wolf C., Stoll I., Hutin P., Limacher J. M., Podhajcer O. L., Chenard M. P., Rio M. C., Chambon P. A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature. 1990 Dec 20;348(6303):699–704. doi: 10.1038/348699a0. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen B., Moore W. G., Taylor R. E., Bhown A. S., Birkedal-Hansen H. Monoclonal antibodies to human fibroblast procollagenase. Inhibition of enzymatic activity, affinity purification of the enzyme, and evidence for clustering of epitopes in the NH2-terminal end of the activated enzyme. Biochemistry. 1988 Sep 6;27(18):6751–6758. doi: 10.1021/bi00418a016. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H., Moore W. G., Bodden M. K., Windsor L. J., Birkedal-Hansen B., DeCarlo A., Engler J. A. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4(2):197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Wang Z., Furlow J. D., Kanamori A., Schwartzman R. A., Remo B. F., Pinder A. The thyroid hormone-induced tail resorption program during Xenopus laevis metamorphosis. Proc Natl Acad Sci U S A. 1996 Mar 5;93(5):1924–1929. doi: 10.1073/pnas.93.5.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov S., Almouzni G., Dasso M., Wolffe A. P. Chromatin transitions during early Xenopus embryogenesis: changes in histone H4 acetylation and in linker histone type. Dev Biol. 1993 Nov;160(1):214–227. doi: 10.1006/dbio.1993.1299. [DOI] [PubMed] [Google Scholar]
- Freije J. M., Díez-Itza I., Balbín M., Sánchez L. M., Blasco R., Tolivia J., López-Otín C. Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas. J Biol Chem. 1994 Jun 17;269(24):16766–16773. [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanville R. W., Breitkreutz D., Meitinger M., Fietzek P. P. Completion of the amino acid sequence of the alpha 1 chain from type I calf skin collagen. Amino acid sequence of alpha 1(I)B8. Biochem J. 1983 Oct 1;215(1):183–189. doi: 10.1042/bj2150183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg G. I., Wilhelm S. M., Kronberger A., Bauer E. A., Grant G. A., Eisen A. Z. Human fibroblast collagenase. Complete primary structure and homology to an oncogene transformation-induced rat protein. J Biol Chem. 1986 May 15;261(14):6600–6605. [PubMed] [Google Scholar]
- Gross J., Harper E., Harris E. D., McCroskery P. A., Highberger J. H., Corbett C., Kang A. H. Animal collagenases: specificity of action, and structures of the substrate cleavage site. Biochem Biophys Res Commun. 1974 Nov 27;61(2):605–612. doi: 10.1016/0006-291x(74)91000-6. [DOI] [PubMed] [Google Scholar]
- Gross J. How tadpoles lose their tails. J Invest Dermatol. 1966 Oct;47(4):274–277. [PubMed] [Google Scholar]
- Hasty K. A., Pourmotabbed T. F., Goldberg G. I., Thompson J. P., Spinella D. G., Stevens R. M., Mainardi C. L. Human neutrophil collagenase. A distinct gene product with homology to other matrix metalloproteinases. J Biol Chem. 1990 Jul 15;265(20):11421–11424. [PubMed] [Google Scholar]
- Herrin D. L., Schmidt G. W. Rapid, reversible staining of northern blots prior to hybridization. Biotechniques. 1988 Mar;6(3):196-7, 199-200. [PubMed] [Google Scholar]
- Highberger J. H., Corbett C., Dixit S. N., Yu W., Seyer J. M., Kang A. H., Gross J. Amino acid sequence of chick skin collagen alpha 1(I)-CB8 and the complete primary structure of the helical portion of the chick skin collagen alpha 1(I) chain. Biochemistry. 1982 Apr 27;21(9):2048–2055. doi: 10.1021/bi00538a011. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A., Shimozawa A. Development of the connective tissue in the digestive tract of the larval and metamorphosing Xenopus laevis. Anat Anz. 1987;164(2):81–93. [PubMed] [Google Scholar]
- Ishizuya-Oka A., Shimozawa A. Induction of metamorphosis by thyroid hormone in anuran small intestine cultured organotypically in vitro. In Vitro Cell Dev Biol. 1991 Nov;27A(11):853–857. doi: 10.1007/BF02630987. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A., Ueda S., Shi Y. B. Transient expression of stromelysin-3 mRNA in the amphibian small intestine during metamorphosis. Cell Tissue Res. 1996 Feb;283(2):325–329. doi: 10.1007/s004410050542. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lefebvre O., Wolf C., Limacher J. M., Hutin P., Wendling C., LeMeur M., Basset P., Rio M. C. The breast cancer-associated stromelysin-3 gene is expressed during mouse mammary gland apoptosis. J Cell Biol. 1992 Nov;119(4):997–1002. doi: 10.1083/jcb.119.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy B., Cleasby A., Hassell A. M., Longley K., Luther M. A., Weigl D., McGeehan G., McElroy A. B., Drewry D., Lambert M. H. Structure of the catalytic domain of fibroblast collagenase complexed with an inhibitor. Science. 1994 Jan 21;263(5145):375–377. doi: 10.1126/science.8278810. [DOI] [PubMed] [Google Scholar]
- Mallya S. K., Mookhtiar K. A., Gao Y., Brew K., Dioszegi M., Birkedal-Hansen H., Van Wart H. E. Characterization of 58-kilodalton human neutrophil collagenase: comparison with human fibroblast collagenase. Biochemistry. 1990 Nov 27;29(47):10628–10634. doi: 10.1021/bi00499a008. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M. The matrix-degrading metalloproteinases. Bioessays. 1992 Jul;14(7):455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Harris E. D., Jr, Chung E., Finch J. E., Jr, McCroskery P. A., Butler W. T. Cleavage of Type II and III collagens with mammalian collagenase: site of cleavage and primary structure at the NH2-terminal portion of the smaller fragment released from both collagens. Biochemistry. 1976 Feb 24;15(4):787–792. doi: 10.1021/bi00649a009. [DOI] [PubMed] [Google Scholar]
- Mookhtiar K. A., Van Wart H. E. Purification to homogeneity of latent and active 58-kilodalton forms of human neutrophil collagenase. Biochemistry. 1990 Nov 27;29(47):10620–10627. doi: 10.1021/bi00499a007. [DOI] [PubMed] [Google Scholar]
- Moore W. M., Spilburg C. A. Purification of human collagenases with a hydroxamic acid affinity column. Biochemistry. 1986 Sep 9;25(18):5189–5195. doi: 10.1021/bi00366a031. [DOI] [PubMed] [Google Scholar]
- Muller D., Quantin B., Gesnel M. C., Millon-Collard R., Abecassis J., Breathnach R. The collagenase gene family in humans consists of at least four members. Biochem J. 1988 Jul 1;253(1):187–192. doi: 10.1042/bj2530187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oofusa K., Yomori S., Yoshizato K. Regionally and hormonally regulated expression of genes of collagen and collagenase in the anuran larval skin. Int J Dev Biol. 1994 Jun;38(2):345–350. [PubMed] [Google Scholar]
- Patterton D., Hayes W. P., Shi Y. B. Transcriptional activation of the matrix metalloproteinase gene stromelysin-3 coincides with thyroid hormone-induced cell death during frog metamorphosis. Dev Biol. 1995 Jan;167(1):252–262. doi: 10.1006/dbio.1995.1021. [DOI] [PubMed] [Google Scholar]
- Ranjan M., Wong J., Shi Y. B. Transcriptional repression of Xenopus TR beta gene is mediated by a thyroid hormone response element located near the start site. J Biol Chem. 1994 Oct 7;269(40):24699–24705. [PubMed] [Google Scholar]
- Sang Q. A., Douglas D. A. Computational sequence analysis of matrix metalloproteinases. J Protein Chem. 1996 Feb;15(2):137–160. doi: 10.1007/BF01887395. [DOI] [PubMed] [Google Scholar]
- Sang Q. X., Birkedal-Hansen H., Van Wart H. E. Proteolytic and non-proteolytic activation of human neutrophil progelatinase B. Biochim Biophys Acta. 1995 Sep 6;1251(2):99–108. doi: 10.1016/0167-4838(95)00086-a. [DOI] [PubMed] [Google Scholar]
- Saus J., Quinones S., Otani Y., Nagase H., Harris E. D., Jr, Kurkinen M. The complete primary structure of human matrix metalloproteinase-3. Identity with stromelysin. J Biol Chem. 1988 May 15;263(14):6742–6745. [PubMed] [Google Scholar]
- Seltzer J. L., Adams S. A., Grant G. A., Eisen A. Z. Purification and properties of a gelatin-specific neutral protease from human skin. J Biol Chem. 1981 May 10;256(9):4662–4668. [PubMed] [Google Scholar]
- Seltzer J. L., Akers K. T., Weingarten H., Grant G. A., McCourt D. W., Eisen A. Z. Cleavage specificity of human skin type IV collagenase (gelatinase). Identification of cleavage sites in type I gelatin, with confirmation using synthetic peptides. J Biol Chem. 1990 Nov 25;265(33):20409–20413. [PubMed] [Google Scholar]
- Seltzer J. L., Weingarten H., Akers K. T., Eschbach M. L., Grant G. A., Eisen A. Z. Cleavage specificity of type IV collagenase (gelatinase) from human skin. Use of synthetic peptides as model substrates. J Biol Chem. 1989 Nov 25;264(33):19583–19586. [PubMed] [Google Scholar]
- Shi Y. B., Hayes W. P. Thyroid hormone-dependent regulation of the intestinal fatty acid-binding protein gene during amphibian metamorphosis. Dev Biol. 1994 Jan;161(1):48–58. doi: 10.1006/dbio.1994.1006. [DOI] [PubMed] [Google Scholar]
- Shi Y. B., Ishizuya-Oka A. Biphasic intestinal development in amphibians: embryogenesis and remodeling during metamorphosis. Curr Top Dev Biol. 1996;32:205–235. doi: 10.1016/s0070-2153(08)60429-9. [DOI] [PubMed] [Google Scholar]
- Shi Y. B., Liang V. C. Cloning and characterization of the ribosomal protein L8 gene from Xenopus laevis. Biochim Biophys Acta. 1994 Mar 1;1217(2):227–228. doi: 10.1016/0167-4781(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Aznavoorian S., Liotta L. A. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- Talhouk R. S., Bissell M. J., Werb Z. Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution. J Cell Biol. 1992 Sep;118(5):1271–1282. doi: 10.1083/jcb.118.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryggvason K., Höyhtyä M., Salo T. Proteolytic degradation of extracellular matrix in tumor invasion. Biochim Biophys Acta. 1987 Nov 25;907(3):191–217. doi: 10.1016/0304-419x(87)90006-0. [DOI] [PubMed] [Google Scholar]
- Van Wart H. E., Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Brown D. D. Thyroid hormone-induced gene expression program for amphibian tail resorption. J Biol Chem. 1993 Aug 5;268(22):16270–16278. [PubMed] [Google Scholar]
- Welgus H. G., Jeffrey J. J., Eisen A. Z. The collagen substrate specificity of human skin fibroblast collagenase. J Biol Chem. 1981 Sep 25;256(18):9511–9515. [PubMed] [Google Scholar]
- Whitham S. E., Murphy G., Angel P., Rahmsdorf H. J., Smith B. J., Lyons A., Harris T. J., Reynolds J. J., Herrlich P., Docherty A. J. Comparison of human stromelysin and collagenase by cloning and sequence analysis. Biochem J. 1986 Dec 15;240(3):913–916. doi: 10.1042/bj2400913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S. M., Collier I. E., Kronberger A., Eisen A. Z., Marmer B. L., Grant G. A., Bauer E. A., Goldberg G. I. Human skin fibroblast stromelysin: structure, glycosylation, substrate specificity, and differential expression in normal and tumorigenic cells. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6725–6729. doi: 10.1073/pnas.84.19.6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]
- Yoshizato K. Biochemistry and cell biology of amphibian metamorphosis with a special emphasis on the mechanism of removal of larval organs. Int Rev Cytol. 1989;119:97–149. doi: 10.1016/s0074-7696(08)60650-6. [DOI] [PubMed] [Google Scholar]
- Zehr B. D., Savin T. J., Hall R. E. A one-step, low background coomassie staining procedure for polyacrylamide gels. Anal Biochem. 1989 Oct;182(1):157–159. doi: 10.1016/0003-2697(89)90734-3. [DOI] [PubMed] [Google Scholar]