Abstract
Malignant gliomas manifest frequent tumor recurrence after surgical resection and/or other treatment, due to its nature of invasiveness and dissemination. The recognized brain tumor-tracking property of neural progenitor/stem cells (NSCs) opened the possibility of targeting malignant brain tumors using NSCs. We and others have previously demonstrated that fetal NSCs can be utilized to deliver therapeutic molecules to brain tumors. Our recent work has further shown that gene delivery by bone marrow-derived NSCs (BM-NSCs) achieves therapeutic effects in a glioma model. In this study, we isolate and characterize BM-NSCs which also express the chemokine receptor CXCR4. We show that CXCR4 is required for their chemotaxis and extracelluilar matrix invasion against a gradient of glioma soluble factors. Furthermore, LacZ-labeled BM-NSCs implanted in the contralateral side of the brain were shown to track gliomas as early as day 1, and increased through day 3 and day 7. Intracranial glioma tracking by BM-NSCs is significantly inhibited by pre-incubation of BM-NSCs with a blocking anti-CXCR4 antibody, suggesting a CXCR4-dependent tracking mechanism. Glioma tracking BM-NSCs were found to express progenitor/stem cell markers, as well as CXCR4. Although BrdU incorporation assays and proliferating antigen staining indicated that tumor tracking BM-NSCs were mostly non-proliferating, these cells survive in the local tumor environment with little apoptosis. Elucidating the molecular mechanism of brain tumor tracking by adult source stem cells may provide basis for the development of future targeted therapy for malignant brain tumors.
Keywords: glioma, neural stem cells, bone marrow, CXCR4, gene delivery
Introduction
Malignant gliomas are characterized by their invasiveness and dissemination, resulting in frequent tumor recurrence after surgical resection and/or other treatment. As one of the strategies to develop novel therapies for such brain tumors, it had been proposed that neural progenitor/stem cells (NSCs) with tumor-tracking capabilities (or glioma tropism) may be utilized as a vehicle to deliver therapeutic entities and to target the tumor (1-5). We had previously engineered Interleukin-12-producing murine primary NSCs and demonstrated that these NSCs migrated towards intracranial gliomas and induced T-cell anti-tumor immunity (3). Using a similar approach, we reported therapeutically effective delivery of tumor necrosis factor-related apoptosis-inducing ligand to human glioblastoma xenografts (6). We had further identified that the tumor-tropic NSCs comprise largely of chemokine CXC receptor 4 (CXCR4)-expressing progenitors (4).
Increasing evidence suggests that a practical source of NSCs may be from bone marrow-derived stem cells (7-15). Such adult NSCs not only possesses multipotency in differentiation, but also have the tumor-tracking capability (5, 16). For instance, we have shown that bone marrow-derived mouse NSC could migrate and deliver Interleukin-23 to the site of orthotopic glioma, inducing anti-tumor activity (5).
The mechanism underlying the glioma tracking property of NSCs is not well understood. We have previously demonstrated that glioma cells secrete chemokine stroma-derived factor-1 (SDF-1/CXCL-12) and that fetal NSCs migrate toward glioma-conditioned medium in a CXCR4-dependent manner (4) Honeth et al reported that CXCR3 may also be important in glioma tracking using a neural progenitor cell line (17). Whether a similar mechanism underlies tumor tropism of bone marrow stem cells is an open question. Chemokines and their cognate receptors are generally known to be critical in bone marrow stem cell homing and chemotaxis. However, Schichor and colleagues reported that vascular endothelial growth factor A (VEGF-A) played a role in attracting bone marrow stromal cells toward glioma cells (18). On the other hand, Tabatabai et al experimented with hematopoietic stem/progenitor cells and demonstrated that SDF-1/CXCR4 axis is essential for their glioma tracking property (19).
In this study, we isolated neurospheres from bone marrow stem cells and demonstrated that these bone marrow-derived NSCs (BM-NSC) express CXCR4. In migration and invasion assays, we report that glioma tropism of BM-NSCs is CXCR4-dependent. Furthermore, blocking CXCR4 significantly inhibited intracranial glioma tracking by BM-NSCs.
Materials and Methods
Isolation and culture of bone marrow-derived neural stem cells
Isolation of bone marrow-derived stem cells was performed as previously described (5, 9). Briefly, whole bone marrow was harvested from the femurs of adult Fisher F344 rats and mononuclear cells were isolated by gradient density centrifugation. Cells were plated on poly-D-lysine/fibronectin–coated wells and cultured for 10 days in DMEM/F12 medium supplemented with 15% qualified fetal bovine serum (FBS) plus 20 ng/ml of bFGF, 20 ng/ml of EGF (Peprotech, Rocky Hill, NJ) before switching into neural stem cell medium: DMEM/F12 medium supplemented with B27 (Invitrogen, Carlsbad, CA), EGF and FGF. Differentiation medium contains DMEM/F-12, B27/N2, retinoic acid (1 mM; Sigma), dibutyril cyclic AMP (1 mM; Sigma), ciliary neurotrophic factor (CNTF; 20 ng/ml), brain-derived neurotrophic factor (BDNF; 20 ng/ml), and platelet-derived growth factor (PDGF; 20 ng/ml). The differentiation medium was replenished every three days, and cells were differentiated for 15–25 days.
Cell culture of glioma cells
RG2 cells were cultured in DMEM supplemented with 10% fetal bovine serum, 2 mmol/L l-glutamine, and antibiotics (all the reagents from Invitrogen).
Immunocytochemistry, immunohistochemistry and β-gal staining
Immunocytochemistry analysis of neural stem cells and differentiated cells was described before(5, 20). Brains harvested from NSC-LacZ inoculated tumor-bearing animals were frozen on dry ice, sectioned using a cryostat, and mounted on slides. Paraffin embedded tissue sections were dried for 2h at 60 °C, dewaxed in xylene and rehydrated. Incubation with antibodies was performed overnight at 4 °C. The following antibodies were used: nestin (1:200), CD133 (1:20), GFAP (1:1,000), βIII-tubulin (1:200), tuj (1:1,000), A2B5 (5 mg/ml), MBP (1:150), CNPase (1:200), and CXCR4 (1:200). Immunodetection was performed using the Elite Vector Stain ABC System (Vector Laboratories). For histological visualization of LacZ-expressing NSCs, sections were stained with X-gal as per routine protocol and then counterstained with H/E where indicated. Adjacent tissue sections were fixed in acetone. Staining was performed as per standard immunohistochemistry protocols using primary antibodies against β-galactosidase. For photography and quantification of X-gal-stained cells, a Carl Zeiss AxioCam HR camera and the software AxioVision is used to measure the areas of NSCs (Carl Zeiss). At lease sections from three animals were used for quantification.
Cell migration and invasion assays
All migration assays were performed using a fluorometric chemotaxis chamber system (Chemicon, MA, 8μm pore) following manufacturer manual. Briefly, 250 μl cell suspension containing 1×106 cells/mL BM-NSCs were added into the upper chamber, with or without blocking anti-CXCR4 antibody (Torrey Pines Biolabs, NJ, 20μg/ml) or control IgG isotype. Cells were preincubated with antibody or control for 30 minutes at room temperature prior to the assay. Lower chambers were filled with 400 μl basal medium, with or without 10% FBS, 10% RG2 cells-conditioned media or control medium. After incubation at 37C for 4 hours, migrated cells in the lower chamber were collected and quantitatively analyzed using a fluorometer (Molecular Devices, CA). Invasion assays were performed using BD Biocoat® Matrigel Invasion Chamber, following product manual. Briefly, BM-NSCs were incubated with blocking antibody or control IgG, and placed in top chambers, while glioma cells-conditioned medium or controls were added to lower chambers. After 22-24h incubation at 37 C, non-invading cells were removed and invading cells were stained with 1% Toluidine blue in 1% borax. Invading cells were photographed, counted, and background-subtracted. At least four random fields were counted for each well. All experiments were performed in triplicate.
Intracranial cell transplantation
Fisher rat F344 (6–8 weeks old; Charles River Laboratories, Wilmington, MA, USA) were anesthetized with i.p. ketamine and medetomidine, and stereotactically implanted with RG2 glioma cells (50,000 per rat) or saline in 3 μl of 1.2% methylcellulose/MEM in the right striatum. At day 7 post-implantation, animals received intratumoral inoculations of 2×105 bone marrow-derived neural stem cells expressing β-galactosidase (NSC-LacZ) in 5 ml of serum and virus-free media, injected directly into established tumor using the same burr hole and stereotactic coordinates, or in the contra-lateral side. The implanted rats were euthanized at day1, 3, 7, and 14, by intracardic perfusion–fixation with 4% paraformaldehyde. Five animals for each group for each time point were included. The experiment was repeated once under identical conditions. Brain tissues were retrieved for frozen section and analysis. All animals used were experimented in strict accordance with the Institutional Animal Care and Committee Guidelines at Cedars-Sinai Medical Center.
Statistical Methods
Data are from three independent experiments and are expressed as mean ± SE. To test whether variables differed across two groups, we used student t-test (unpaired, two-tailed).
Results
While fetal neural stem cells have been instrumental in studying brain tumor tracking and gene delivery, access of NSCs from adult sources may be critical for clinical applications. We and others had demonstrated that subpopulations of progenitor/stem cells isolated from bone marrow had brain tumor-tracking capability (5, 18, 19). In order to isolate relatively pure BM-NSCs for glioma tracking, we followed a procedure that we had previously used to isolate human BM-NSCs (9). After expansion and switching to neural stem cell medium, a subpopulation of bone marrow-derived cells forms neurospheres that are continuously propagated (Figure 1A). These neurosphere-forming cells maintain high expression of neural stem cell marker Nestin, as well as moderate expression of CD133 and A2B5 (Figure 1B). Furthermore, when cultured under differentiation conditions, these cells can differentiate into astrocytes, neurons, and oligodendrocytes (Supplementary Figure S1). Under our NSC culture conditions, these BM-NSCs can be propagated for at least 20 passages without losing self-renewal and differentiation potential, thus providing a renewable source for further glioma tracking study.
Figure 1.
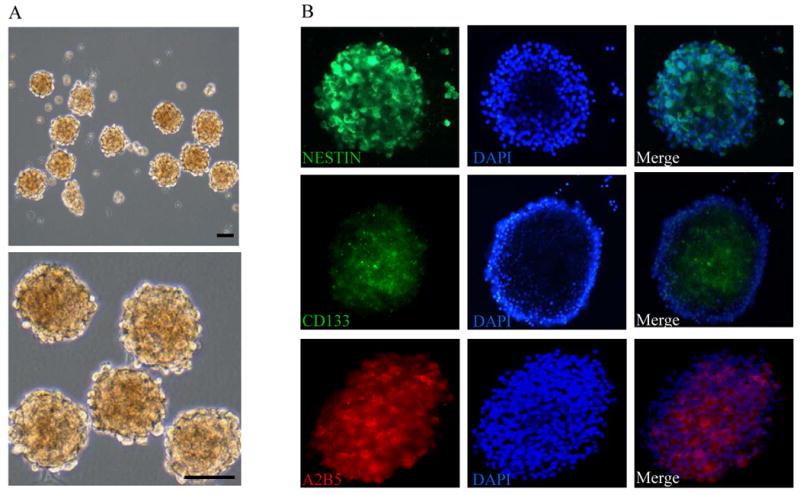
Isolation and characterization of bone marrow-derived neural stem cells. A, Bone marrow-derived NSCs form neural spheres and maintain self-renewal capacity in neural stem cell medium. scale bar, 100 μm. B, BM-NSCs express typical neural /stem cell/progenitor markers, nestin, CD133, and A2B5. Nestin is highly expressed in BM-NSCs, while CD133 and A2B5 is expressed in only a fraction of cells.
We had previously reported that fetal NSCs express chemokine receptor CXCR4 and that it mediates NSC chemotaxis toward gliomas(4). Here we show that BM-derived neurospheres also express CXCR4, as demonstrated by immunostaining and flow cytometry analysis (Figure 2A, B). The RG2 cell conditioned medium contains SDF-1 protein with a concentration of 0.03 ng/mL as determined by ELISA assays. To test if CXCR4 plays a role in BM-NSC migration, we measured cell migration under several conditions using a two-chamber system. As expected, 10% FBS induced robust cell migration, compared to the background level in the control group (Figure 2C). In support of the glioma tropism of BM-NSCs, glioma-conditioned medium (1:10 diluted in neural stem cell medium) induced high level cell migration towards the gradient. Importantly, incubation of BM-NSCs with a blocking anti-CXCR4 antibody significantly inhibited cell migration by 21% (Figure 2C). To further test if glioma-conditioned medium could induce BM-NSC invasion through extracellular matrix and if CXCR4 is also important in BM-NSC invasion, we performed cell invasion assays using chambers separated by matrix-coated porous membrane. At 24 h, invading cells were processed and photographed. As shown in Figure 2D, there is little cell invasion in the absence of chemoattractants, whereas both 10% FBS and 1:10 diluted glioma-conditioned medium induced strong cell invasion (Figure 2D,a-c). Pre-incubation of BM-NSCs with a blocking CXCR4 antibody reduced the cell invasion to near the background level (Figure 2D,d). Quantification of cell invasion indicated that blocking CXCR4 inhibited cell invasion by 86%, suggesting that blocking CXCR4 inhibited both chemotaxis and cell penetration of extracellular matrix. Together, these observations clearly suggest that CXCR4 is required for glioma factors-induced BM-NSC migration and invasion.
Figure 2.
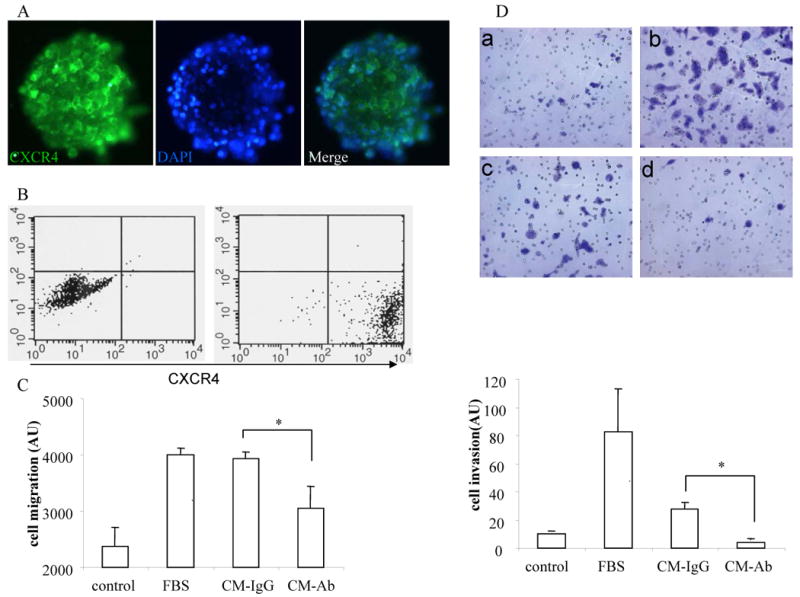
CXCR4 is required for chemotactic migration and invasion of BM-NSCs towards glioma secreted factors. A, Immunostaining showing expression of CXCR4 by BM-NSCs. B, FACS analysis indicating CXCR4 expression in cultured BM-NSCs. (left) isotype control. (right) anti-CXCR4 antibody. C, BM-NSCs migration towards 1:10 diluted glioma-conditioned medium is inhibited in the presence of a blocking anti-CXCR4 antibody. Stem cell growth medium and the medium containing 10% FBS were used as controls, respectively. D, (top) Representative pictures showing BM-NSCs invasion towards medium (a), medium with 10% FBS (b), medium with 1:10 glioma-conditioned medium in the absence (c) and presence (d) of a blocking anti-CXCR4 antibody, respectively. (bottom) Quantification of cell invasion indicated that cell invasion towards glioma-conditioned medium is inhibited when CXCR4 is blocked. * p<0.05.
To study in vivo glioma tracking property of BM-NSCs and its mechanism, we implanted rat glioma cells into the rat brain seven days before placing congenic LacZ-expressing BM-NSCs into the contralateral side. At seven days, LacZ-labeled BM-NSCs were detected at the tumor site, as revealed by β-gal colormetric staining (Figure 3A, Supplementary Figure S2). To test the dynamics of glioma tracking process by BM-NSCs, brain sections were prepared and examined at day 1, day 3, day 7 and day 14 post-implantation of LacZ-BM-NSCs. As shown in Figure 3B, glioma tropic LacZ-BM-NSCs were seen at tumor site as early as at day 1, but they increased at day 3 and further increased at day 7, leveling off at day 14. To investigate if cell surface receptor CXCR4 is required for glioma tracking by BM-NSCs, we pre-incubated LacZ-BM-NSCs with blocking anti-CXCR4 antibody or isotype IgG as control for 4 h before implantation. At day 7, there were more LacZ-BM-NSCs around needle track in the contralateral side for the antibody-treated group than for the isotype IgG-treated control group, whereas much less LacZ-BM-NSCs were detected at the tumor site for the antibody-treated group (Figure 3). Quantification of samples from multiple animals indicated significant reduction of glioma-tracking BM-NSCs after blocking receptor CXCR4 (Figure 3). Thus, glioma-tracking capability by BM-NSCs is, at least in part, CXCR4-dependent.
Figure 3.
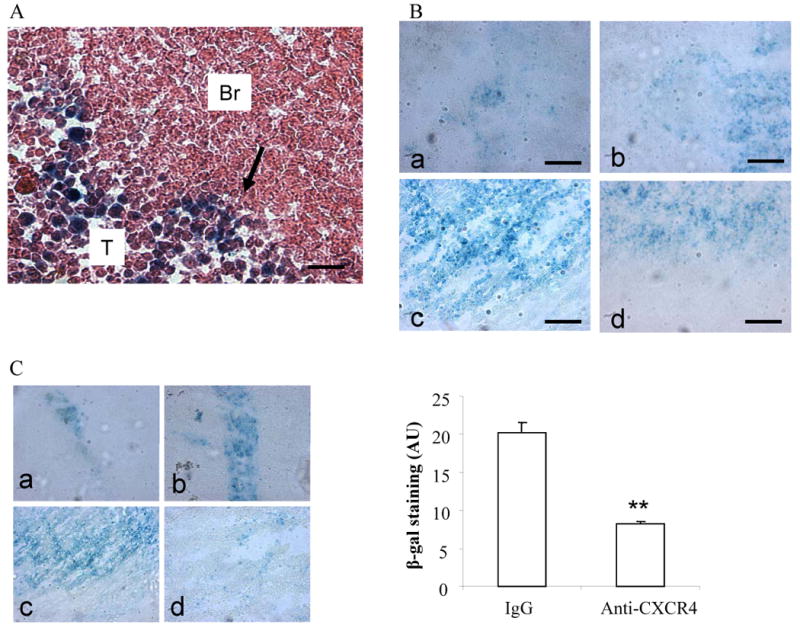
Glioma tracking capability of BM-NSCs is CXCR4-dependent. A, Glioma tropism of LacZ-BM-NSCs. At 7 day post-implantation of RG2 glioma cells or saline as control, LacZ-BM-NSCs were implanted on the contralateral side or inside the tumor (positive control). Brain sections were harvest after seven days and processed for β-gal staining and H&E counterstaining. Graph shows LacZ-BM-NSCs migrating into the glioma on contra-lateral side. Arrow indicates the front of tracking NSCs. Br, brain. T, tumor. B, Time-course of glioma tracking by LacZ-BM-NSCs. β-gal staining of brain sections at day 1(a), day 3 (b), day 7 (c), and day 14 (d) post-implantation of LacZ-BM-NSCs on the contralateral side. C, (left) Effect of blocking CXCR4 on glioma tracking by LacZ-BM-NSCs. β-gal staining of brain sections at day 7 post-implantation of LacZ-BM-NSCs showing injection sides (a and b) and tumor-containing contralateral sides (c and d), in the presence of 20 μg/ml of isotype IgG (a and c) or anti-CXCR4 antibody (b and d). (right) Quantification of β-gal staining of contralateral sides showing significant reduction of LacZ-BM-NSCs migrated to the tumor sites. ** p<0.01.
Our previous study of glioma tracking by fetal neural stem cells indicated that glioma tropic cells consist of mainly A2B5+ precursor cells(4). To test if glioma-tropic BM-NSCs share similar marker expression and undifferentiated status, we double-stained day 7 brain sections on both tumor and contralateral sides to examine A2B5 and β-gal expression. The largely overlapping patterns of both marker stainings suggest that glioma-tracking BM-NSCs maintain undifferentiated progenitor phenotypes (Figure 4).
Figure 4.
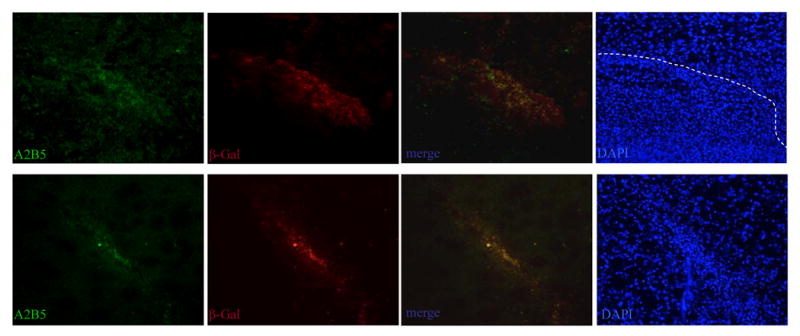
Glioma tracking cells are mainly A2B5+ progenitor cells. Immunostaining of A2B5 (green) and β-gal (red) with brain sections at day 7 post-implantation of LacZ-BM-NSCs showing the contralateral side (top) and the injection side (bottom). The merged pictures show that brain tumors tropic BM cells, as well as residual BM cells along the needle tracts, express progenitor marker A2B5. DAPI staining of nuclei reveals tumor boundary (dashed line) and injection site needle tracts (bottom).
Next, we asked if the complex local environment inside the tumor might damage BM-NSCs and cause their apoptosis. TUNEL assays were performed on brain sections at day 3 and day 7 to detect apoptotic cells. Figure 5 shows that both at day 3 and day 7, while sporadic apoptosis was detected in tumor cells, there was no apparent apoptosis in β-gal+ BM-NSCs detected. This result suggests that BM-NSCs can survive at the tumor sites at least for the period of we examined. Furthermore, with BrdU incorporation assays, we were unable to detect BrdU-labeled BM-NSCs, although some neighboring tumor cells were BrdU-positive, indicating that at least the majority of tumor tropic BM-NSCs were non-dividing cells(Supplementary Figure S3). Unlike the glioma cells, these tumor tracking cells did not express proliferation antigen Ki67 under our experimental conditions (Supplementary Figure S2).
Figure 5.
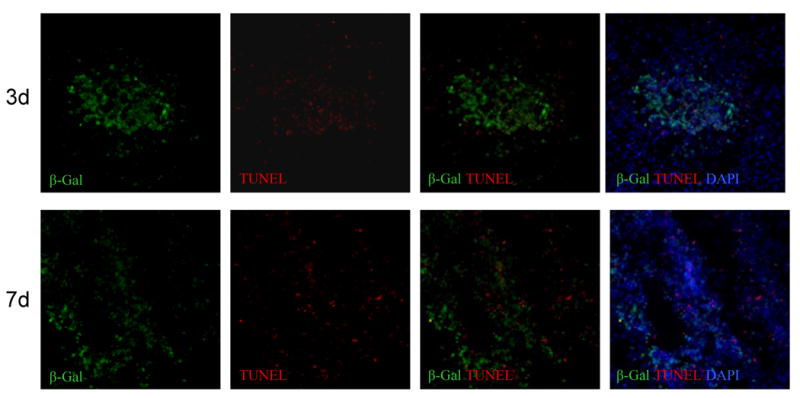
Sustained survival of bone marrow-derived cells in the brain tumor. TUNEL (red) and β-gal (green) double-staining of brain sections at day 3 (top) and day 7 (bottom) post-implantation of LacZ-BM-NSCs. The merged pictures show that apoptotic cells are detected in tumor cells, but not in BM cells.
Finally, co-immunostaining of β-gal and CXCR4 with day 7 brain sections suggested that BM-NSCs express CXCR4 both on the contralateral side and inside the tumor, further supporting the CXCR4 plays a critical role in glioma tracking by BM-NSCs (Supplementary Figure S4).
Discussion
The brain tumor-tracking property of NSCs had made it possible to deliver imaging or therapeutic molecules to intracranial tumor sites(5, 21, 22) It has also been shown that subpopulations of progenitor/stem cells isolated from adult bone marrow shared this brain tumor-tracking capability, making bone marrow-derived cells a practical source of potential autologous cell-based cancer therapy(5, 18, 19). In this study, we isolated and characterized bone marrow-derived NSCs that can be used for brain tumor tracking. We show that BM-NSCs migrate against a gradient of glioma factors and invade extracellular matrix in a CXCR4-dependent manner. Moreover, blocking CXCR4 significantly inhibited intracranial glioma tracking capability by BM-NSCs.
The phenomenon of brain tumor tracking by neural stem cells appeared to be a natural response to brain injury. It has been reported that endogenous neural progenitor/stem cells from ipsilateral dorsal tip of the subventricular zone (SVZ) migrated along the ventral margin of the corpus callosum and infiltrated gliomas(23). Furthermore, exogenous adult NSCs and bone marrow stromal cells can cross the blood-brain barrier and track intracranial brain tumor, as demonstrated using magnetic resonance imaging (MRI)(7). Based on their glioma-tracking property, we had previously successfully delivered immune-modulating and antitumor molecules into the brain tumors to achieve therapeutic effects, using both fetal neural stem cells and bone marrow-derived stem-like cells (3, 5, 6). Although other groups had also reported brain tumor tracking by bone marrow-derived cells (16, 18, 19, 23), we had isolated phenotypically and functionally defined BM-NSCs and demonstrated their brain tumor-tracking property (5, 9). In this study, we further define the molecular mechanism underlying brain tumor-tracking by BM-NSCs.
CXCR4 is known to mediate bone marrow-derived cell trafficking under normal and pathological conditions(24-27). We have previously shown that CXCR4 is important for glioma tropism of fetal NSCs (4). Furthermore, Tabatabai et al demonstrated that SDF-1/CXCR4 axis is also essential for glioma tracking by hematopoietic stem/progenitor cells (19). However, Schichor and colleagues reported that vascular endothelial growth factor A (VEGF-A) played a role in attracting bone marrow stromal cells toward glioma cells (18). One reason of this discrepancy could be that they used different subpopulations of bone marrow-derived cells. While Tabatabai et al used CD34+ stem/progenitor cells, Schichor et al isolated adherent mononuclear bone marrow cells (18, 19). In our study, we isolated neurosphere-forming BM-NSCs and show that these cells express CXCR4. Furthermore, we demonstrated that both in vitro and in vivo BM-NSC tracking of gliomas is CXCR4-dependent. We can not rule out that additional signaling mechanisms may also be involved, or whether possible crosstalks between signaling pathways may regulate the process. In fact, several studies indicated that SDF-1/CXCR4 is regulated by diverse signaling molecules (28-31).
The utility of BM-NSCs as a vehicle for targeted gene delivery to the brain requires that these cells survive the complex local tumor environment. Our study suggested that BM-NSCs migrated into the tumor express undifferentiated progenitor markers. Although these tumor-infiltrating BM-NSCs appeared to be non-proliferating based on BrdU incorporation assays and Ki67 staining, there was no apparent apoptosis in these cells. However, we found that there is a decrease of tumor-infiltrating BM-NSCs at day 14, suggesting that they are not self-renewable and may not sustain for longer term. The non-proliferating feature of tumor-tracking of BM-NSCs is in contrast to the results from tumor-tracking endogenous NSCs, which were shown to be proliferating (22). This difference may reflect the intrinsic difference in response to local environment between brain-derived NSCs and BM-NSCs. Alternatively, different experimental system may account for the different results. Nevertheless, these BM-NSCs may provide a valuable means for brain tumor targeting and gene delivery for a short term and could achieve therapeutic effects, as demonstrated by our previous studies (5, 32).
In summary, we demonstrated that BM-NSCs express chemokine receptor CXCR4 and that CXCR4 is required for their chemotaxis and invasion against a gradient of glioma soluble factors. Intracranial glioma tracking by BM-NSCs is CXCR4-dependent. Elucidating the molecular mechanism of brain tumor tracking by adult source stem cells may provide a basis for the development of future targeted therapy for malignant brain tumors.
Supplementary Material
Acknowledgments
We thank Iman Abdulkadir for technical help in tissue culture and Akop Seksenyan for critically reading this manuscript.
This work was supported in part by R01 NS048959. and grant NS048879 to J.S.Y..
Abbreviation list
- CXCR4
chemokine CXC receptor 4
- NSC
neural progenitor/stem cells
- BM-NSCs
bone marrow-derived NSCs
- SDF-1/CXCL-12
stroma-derived factor-1/CXC chemokine ligand-12
- EGF
epidermal growth factor
- bFGF
basic fibroblast growth factor
- VEGF
vascular endothelial growth factor
- SVZ
subventricular zone
- TUNEL
Terminal Deoxynucleotidyl Transferase Mediated dUTP Nick End Labeling
Footnotes
The authors declare no conflict of interest.
References
- 1.Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000;97:12846–51. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benedetti S, Pirola B, Pollo B, et al. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med. 2000;6:447–50. doi: 10.1038/74710. [DOI] [PubMed] [Google Scholar]
- 3.Ehtesham M, Kabos P, Kabosova A, Neuman T, Black KL, Yu JS. The use of interleukin 12-secreting neural stem cells for the treatment of intracranial glioma. Cancer Res. 2002;62:5657–63. [PubMed] [Google Scholar]
- 4.Ehtesham M, Yuan X, Kabos P, et al. Glioma tropic neural stem cells consist of astrocytic precursors and their migratory capacity is mediated by CXCR4. Neoplasia. 2004;6:287–93. doi: 10.1593/neo.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan X, Hu J, Belladonna ML, Black KL, Yu JS. Interleukin-23-expressing bone marrow-derived neural stem-like cells exhibit antitumor activity against intracranial glioma. Cancer Res. 2006;66:2630–8. doi: 10.1158/0008-5472.CAN-05-1682. [DOI] [PubMed] [Google Scholar]
- 6.Ehtesham M, Kabos P, Gutierrez MA, et al. Induction of glioblastoma apoptosis using neural stem cell-mediated delivery of tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res. 2002;62:7170–4. [PubMed] [Google Scholar]
- 7.Zhang Z, Jiang Q, Jiang F, et al. In vivo magnetic resonance imaging tracks adult neural progenitor cell targeting of brain tumor. Neuroimage. 2004;23:281–7. doi: 10.1016/j.neuroimage.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 8.Zurita M, Vaquero J, Oya S, Bonilla C, Aguayo C. Neurotrophic Schwann-cell factors induce neural differentiation of bone marrow stromal cells. Neuroreport. 2007;18:1713–7. doi: 10.1097/WNR.0b013e3282f0d3b0. [DOI] [PubMed] [Google Scholar]
- 9.Zeng Z, Yuan X, Liu G, et al. Manipulation of proliferation and differentiation of human bone marrow-derived neural stem cells in vitro and in vivo. J Neurosci Res. 2007;85:310–20. doi: 10.1002/jnr.21131. [DOI] [PubMed] [Google Scholar]
- 10.Shiota M, Heike T, Haruyama M, et al. Isolation and characterization of bone marrow-derived mesenchymal progenitor cells with myogenic and neuronal properties. Exp Cell Res. 2007;313:1008–23. doi: 10.1016/j.yexcr.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 11.Berger F, Gay E, Pelletier L, Tropel P, Wion D. Development of gliomas: potential role of asymmetrical cell division of neural stem cells. Lancet Oncol. 2004;5:511–4. doi: 10.1016/S1470-2045(04)01531-1. [DOI] [PubMed] [Google Scholar]
- 12.Kim S, Honmou O, Kato K, et al. Neural differentiation potential of peripheral blood- and bone-marrow-derived precursor cells. Brain Res. 2006;1123:27–33. doi: 10.1016/j.brainres.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonilla S, Silva A, Valdes L, Geijo E, Garcia-Verdugo JM, Martinez S. Functional neural stem cells derived from adult bone marrow. Neuroscience. 2005;133:85–95. doi: 10.1016/j.neuroscience.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 14.Magaki T, Kurisu K, Okazaki T. Generation of bone marrow-derived neural cells in serum-free monolayer culture. Neurosci Lett. 2005;384:282–7. doi: 10.1016/j.neulet.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki T, Izumoto S, Wada K, et al. Inhibition of glioma cell proliferation by neural stem cell factor. J Neurooncol. 2005;74:233–9. doi: 10.1007/s11060-004-7118-5. [DOI] [PubMed] [Google Scholar]
- 16.Lee J, Elkahloun AG, Messina SA, et al. Cellular and genetic characterization of human adult bone marrow-derived neural stem-like cells: a potential antiglioma cellular vector. Cancer Res. 2003;63:8877–89. [PubMed] [Google Scholar]
- 17.Honeth G, Staflin K, Kalliomaki S, Lindvall M, Kjellman C. Chemokine-directed migration of tumor-inhibitory neural progenitor cells towards an intracranially growing glioma. Exp Cell Res. 2006;312:1265–76. doi: 10.1016/j.yexcr.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 18.Schichor C, Birnbaum T, Etminan N, et al. Vascular endothelial growth factor A contributes to glioma-induced migration of human marrow stromal cells (hMSC) Exp Neurol. 2006;199:301–10. doi: 10.1016/j.expneurol.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 19.Tabatabai G, Bahr O, Mohle R, et al. Lessons from the bone marrow: how malignant glioma cells attract adult haematopoietic progenitor cells. Brain. 2005;128:2200–11. doi: 10.1093/brain/awh563. [DOI] [PubMed] [Google Scholar]
- 20.Xu Q, Yuan X, Liu G, Black KL, Yu JS. Hedgehog signaling regulates brain tumor-initiating cell proliferation and portends shorter survival for patients with PTEN-coexpressing glioblastomas. Stem Cells. 2008;26:3018–26. doi: 10.1634/stemcells.2008-0459. [DOI] [PubMed] [Google Scholar]
- 21.Shah K, Bureau E, Kim DE, et al. Glioma therapy and real-time imaging of neural precursor cell migration and tumor regression. Ann Neurol. 2005;57:34–41. doi: 10.1002/ana.20306. [DOI] [PubMed] [Google Scholar]
- 22.Tang Y, Shah K, Messerli SM, Snyder E, Breakefield X, Weissleder R. In vivo tracking of neural progenitor cell migration to glioblastomas. Hum Gene Ther. 2003;14:1247–54. doi: 10.1089/104303403767740786. [DOI] [PubMed] [Google Scholar]
- 23.Duntsch C, Zhou Q, Weimar JD, Frankel B, Robertson JH, Pourmotabbed T. Up-regulation of neuropoiesis generating glial progenitors that infiltrate rat intracranial glioma. J Neurooncol. 2005;71:245–55. doi: 10.1007/s11060-004-2156-6. [DOI] [PubMed] [Google Scholar]
- 24.Kucia M, Reca R, Miekus K, et al. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23:879–94. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- 25.Otsuru S, Tamai K, Yamazaki T, Yoshikawa H, Kaneda Y. Circulating bone marrow-derived osteoblast progenitor cells are recruited to the bone-forming site by the CXCR4/stromal cell-derived factor-1 pathway. Stem Cells. 2008;26:223–34. doi: 10.1634/stemcells.2007-0515. [DOI] [PubMed] [Google Scholar]
- 26.Son BR, Marquez-Curtis LA, Kucia M, et al. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24:1254–64. doi: 10.1634/stemcells.2005-0271. [DOI] [PubMed] [Google Scholar]
- 27.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–88. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 28.Broxmeyer HE, Hangoc G, Cooper S, Campbell T, Ito S, Mantel C. AMD3100 and CD26 modulate mobilization, engraftment, and survival of hematopoietic stem and progenitor cells mediated by the SDF-1/CXCL12-CXCR4 axis. Ann N Y Acad Sci. 2007;1106:1–19. doi: 10.1196/annals.1392.013. [DOI] [PubMed] [Google Scholar]
- 29.Kim HK, De La Luz Sierra M, Williams CK, Gulino AV, Tosato G. G-CSF down-regulation of CXCR4 expression identified as a mechanism for mobilization of myeloid cells. Blood. 2006;108:812–20. doi: 10.1182/blood-2005-10-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pello OM, Moreno-Ortiz Mdel C, Rodriguez-Frade JM, et al. SOCS up-regulation mobilizes autologous stem cells through CXCR4 blockade. Blood. 2006;108:3928–37. doi: 10.1182/blood-2006-02-006353. [DOI] [PubMed] [Google Scholar]
- 31.Petit I, Szyper-Kravitz M, Nagler A, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–94. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 32.Hu J, Yuan X, Belladonna ML, et al. Induction of potent antitumor immunity by intratumoral injection of interleukin 23-transduced dendritic cells. Cancer Res. 2006;66:8887–96. doi: 10.1158/0008-5472.CAN-05-3448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


