Abstract
Objective
To study the interactive action of serum amyloid A (SAA), group IIA secretory phospholipase A2 (sPLA2-IIA) and cholesteryl ester transfer protein (CETP) on HDL remodeling and cholesterol efflux during the acute phase (AP) response elicited in humans following cardiac surgery.
Methods and Results
Plasma was collected from patients prior to (pre-AP), 24 hours after (AP- 1d) and 5 days after cardiac surgery (AP- 5d). SAA levels were increased 16-fold in AP- 1d samples. The activity of sPLA2-IIA was increased from 77.7 ± 38.3 U/ml (pre-AP) to 281.4 ± 57.1 U/ml (AP- 1d; p < 0.001). CETP mass and activity reduction was commensurate to the reduction of HDL cholesterol levels. The combined action of SAA, sPLA2-IIA and CETP in vitro markedly remodeled HDL with the generation of lipid-poor apoA-I from both pre-AP and AP- 1d HDL. The net result of this remodeling was a relative preservation of ABCA1 and ABCG1-dependent cholesterol efflux during the acute phase response.
Conclusions
Our results show that the many and complex changes in plasma proteins during the acute phase response markedly remodel HDL with functional implications, particularly the relative retention of cholesterol efflux capacity.
Keywords: SAA, HDL, CETP, apoA-I, inflammation
Inflammation induces major changes in HDL levels and composition. Mediators of inflammation such as TNF-α and IL-6 induce expression of serum amyloid A1 and group IIA secretory phospholipase A2 (sPLA2-IIA)2 which dramatically alter HDL apolipoprotein content and levels respectively. Acute phase SAA in the plasma is associated with HDL, where it can comprise the major apolipoprotein3. The increase in sPLA2-IIA activity results in hydrolysis of HDL surface phospholipids and a decrease in HDL particle size4. The plasma cholesteryl ester transfer protein (CETP) is an integral component of reverse cholesterol transport and regulates HDL cholesterol concentrations. By promoting the transfer of cholesteryl esters (CE) from HDL to apoB-containing lipoprotein particles, HDL-derived CE is taken up via the LDL receptor and cleared by the liver5. An additional result of CETP action is the generation of lipid-poor apoA-I6, a key acceptor in ATP-binding cassette transporter AI (ABCA1)-mediated lipid efflux7. The presence of SAA on HDL holds the potential to impact both the CE transfer and the apoA-I liberating ability of CETP. sPLA2-IIA could also impact the latter action of CETP as apoA-I was shown to dissociate more readily from CETP-remodeled reconstituted HDL after hydrolysis by bee venom phospholipase A28.
Given the centrality of inflammation in atherogenesis, there is a paucity of information regarding CETP function when acute phase HDL is the “substrate”. In the present study, we used plasma from patients undergoing cardiac surgery with cardiopulmonary bypass as a “standardized” insult where the oxygenator membrane activates macrophages to produce cytokines9. We characterized the SAA-containing AP HDL during the acute phase to define the polydisperse HDL “substrate” that CETP would encounter. We further investigated CETP function in the acute phase, particularly as it relates to the presence of SAA and sPLA2 on AP HDL, with respect to its CE transfer and apoA-I liberating functions.
Teleologically, the dramatic changes in HDL composition and metabolism during inflammation must serve a short term purpose to allow the organism to survive a noxious assault. Acute tissue injury results in cell death with large quantities of cell membranes rich in phospholipids and cholesterol generated. Macrophages are mobilized to such sites, ingest these fragments and acquire considerable lipid load 10. We thus examined the influence of the AP response on the ability of serum to promote cholesterol efflux as a removal mechanism to mobilize this cholesterol in an ABCA1 and ABCG1 dependent manner.
Methods
Human subjects
Patients undergoing cardiac surgery donated plasma prior to (pre-acute phase, pre-AP), 24 hr post-operatively (acute phase, AP- 1d), and at discharge, 5 days after surgery (AP- 5d) as outlined in the online data supplement. This study was approved by the University of Kentucky Medical Institutional Review Board (IRB). For the full descriptions of the methods used, please see the supplemental materials.
Statistical analyses
Data are presented as mean ± SEM. Differences between pre-AP and AP parameters were tested by paired t-test (SigmaStat 3.5). Statistical analyses between pre-AP, AP- 1d and AP- 5d were performed using repeated measures one-way ANOVA with the Holm-Sidak multiple comparisons test. Significance was set at p < 0.05. A Wilcoxon signed rank test was used for post-test of CETP mass. The power in all tests was > 0.9.
Results
SAA, sPLA2, CETP and HDL in Acute Phase (AP) plasma
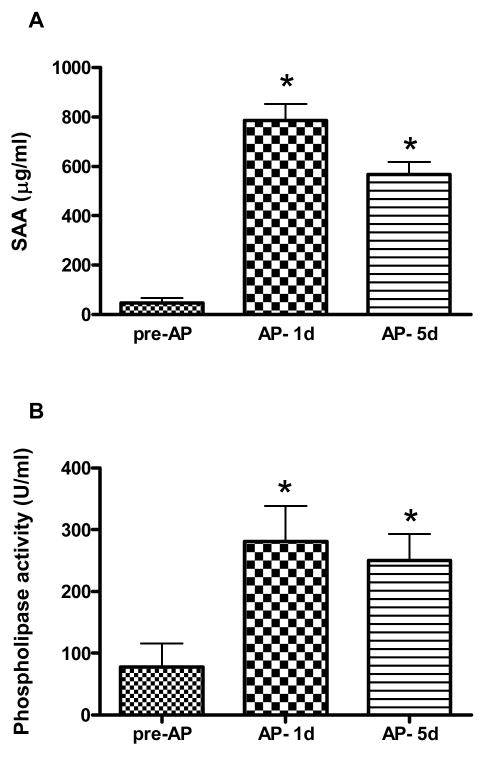
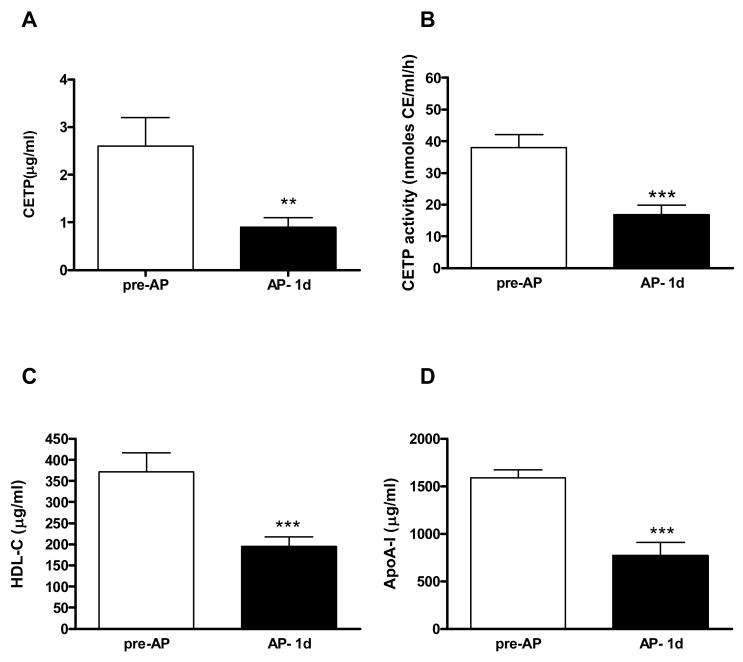
As expected, plasma SAA levels increased from pre-AP levels of 47.2 ± 19.9 μg/ml to 785.6 ± 66.4 μg/ml in AP- 1d samples (p < 0.05) and were still elevated at discharge (AP- 5d; 567.2 ± 50.2 μg/ml; p < 0.05; Fig 1A). The activity of sPLA2-IIA followed a similar pattern: 77.7 ± 38.3 U/ml (pre-AP), 281.4 ± 57.1 U/ml (AP- 1d) and 250.5 ± 43 U/ml (AP- 5d) (p < 0.05; Fig 1B). Quantitative immunoblot analysis showed a 2.9 fold reduction in CETP mass from 2.6 ± 0.6 μg/ml in pre-AP plasma to 0.9 ± 0.2 μg/ml in AP- 1d plasma, (Fig 2A p < 0.01). Consistent with this, CETP activity was 2.2-fold lower in AP compared to pre-AP plasma (16.9 ± 3.0 nmol/ml/h and 37.9 ± 4.1 nmol/ml/h respectively, p < 0.001 (Fig 2B)). HDL-C levels were reduced 1.9 fold, from 372.2 ± 44.6 μg/ml to 195.0 ± 22.2 μg/ml (p < 0.001; Fig 2C) and apoA-I dropped from 1590.0 ± 84.2 μg/ml to 771.4 ± 138.7 μg/ml (p < 0.001; Fig 2D). The decrease in CETP activity was commensurate with the reduction in HDL-C and apoA-I, hence the activity of CETP normalized to HDL-C or apoA-I levels was not different between pre-AP and AP plasma (not shown).
Figure 1.
SAA (A) and sPLA2 (B) concentrations in pre-AP, AP- 1d and AP- 5d plasma. Data is presented as mean ± SEM. n=12 (SAA); n=6 (sPLA2); * p < 0.05 versus pre-AP by one way repeated measures ANOVA.
Figure 2.
CETP, HDL cholesterol and apoA-I are reduced in AP- 1d plasma. (A) Plasma CETP concentrations were quantified by densitometric analysis of Western blots. (B) CETP activity (C) HDL-C concentrations and (D) apoA-I concentrations in pre-AP and AP- 1d plasma. ** p < 0.01, *** p < 0.001 by paired t-test.
Characterization of pre-AP and AP HDL by immunoaffinity chromatography
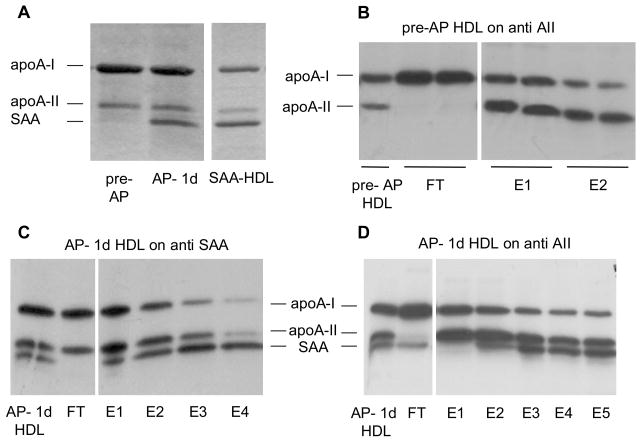
It was reported that the presence of apoA-II on reconstituted HDL particles inhibits the CETP-mediated dissociation of apoA-I11. In order to assess the influence of SAA, we studied pre-AP and AP- 1d HDL as well as HDL2 enriched in vitro with SAA (SAA-HDL). We subjected 125I-HDL to immunoaffinity chromatography to determine the proportion of the LpAI fraction (ie HDL particles lacking both apoA-II and SAA, containing only apoA-I) in AP HDL. Fig 3A is a Coomassie stained SDS-PAGE gel showing the major apolipoproteins present in these HDL. Whereas SAA is virtually undetectable in pre-AP HDL, it is a major component of AP- 1d HDL (27.2% of total protein by mass). In SAA-HDL, SAA comprised a major apolipoprotein. Fig 3B shows the autoradiograph (ARG) of the SDS-PAGE analysis of the fractions when pre-AP HDL was passed through an anti-apoA-II column so that the FT comprises only LpAI particles. ApoA-II containing particles were eluted from the column with sequential chaotropic washes (E1 and E2). In pre-AP HDL, 84% of the total apoA-I counts were in LpAI particles, indicating abundant CETP substrate.
Figure 3.
Apolipoprotein characterization of pre-AP HDL, AP- 1d HDL and SAA-HDL. (A) SDS gel of HDL (5 μg total protein). (B) 125I-pre-AP HDL (5 μg) was passed through an anti-apoA-II immunoaffinity column and fractions were electrophoresed and autoradiographed as outlined in the methods (ARG). (C) 125I-AP HDL (10 μg) was passed through an anti-SAA immunoaffinity column with subsequent electrophoresis and ARG as described in (B). (D) 125I-AP HDL (10 μg) was passed through an anti-apoA-II immunoaffinity column with subsequent electrophoresis and ARG as described in (B). Note: the gels in B–D were loaded on the basis of 2000 cpm per lane and since the majority of counts were present in E1 and E2, E3–E5 quantitatively represent a smaller percentage of total protein mass.
When AP- 1d HDL was passed through an anti-SAA column, 20% of the total apoA-I counts and approximately 5% of the apoA-II counts were in the FT, the remainder associated with SAA-containing particles (Fig 3C). Thus, 80% of apoA-I in AP HDL was present on particles that also contained SAA or both SAA and apoA-II. This indicates that less than 20% of apoA-I in AP HDL is present on LpA-I particles that contain neither apoA-II nor SAA. Specificity of the column was verified with pre-AP HDL passed through the same column. This resulted in 97% of the counts being retrieved in the FT indicating negligible non-specific binding.
In the case of AP HDL passed through an anti-apoA-II column (Fig 3D), 66% of the apoA-I counts and 52% of the SAA counts in AP HDL were in the FT fraction. This indicated that SAA was distributed relatively equally between particles that contain only apoA-I and those that contain both apoA-I and apoA-II. The majority of apoA-I in AP HDL (66%) was present in particles containing no apoA-II, as in the case of pre-AP HDL. However, the majority of such particles contained additional SAA (Fig 3C&D) reducing the LpAI fraction in AP HDL.
Comparative displacement of apoA-I in pre-AP and AP HDL by CETP
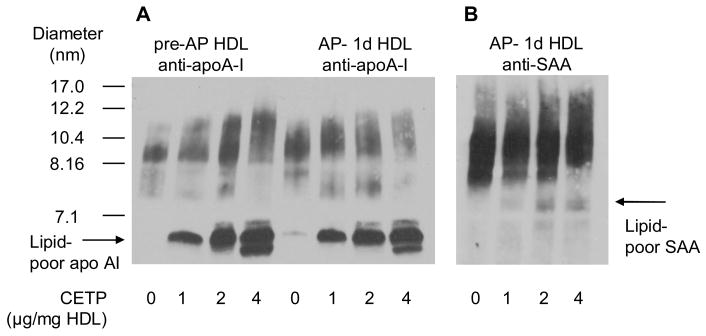
Since ultracentrifugation displaces CETP from HDL,12 recombinant CETP was exogenously added in the remodeling experiments. The CETP activity in the incubations corresponded to the activity of CETP in normal human plasma. When the total pre-AP and AP HDL were incubated with CETP in vitro for 24 hr, a dose-dependent dissociation of apoA-I was observed (Fig 4A). The dissociation of apoA-I from pre-AP and AP HDL was comparable. Although it appears that 50% or more of the apoA-I is in the lipid-poor state, the enhanced immunoreactivity of dissociated apoA-I precludes exact quantification. In addition to generating lipid-poor apoA-I, CETP action also resulted in the formation of larger HDL particles which are likely TG-enriched due to CE/TG exchange. CETP action on AP HDL liberated a very limited amount of “lipid-poor” SAA (Fig 4B). Recombinant SAA exhibited slower mobility than apoA-I on gradient gels, likely due to increased aggregation under the non-denaturing conditions (Fig 4B). SAA is dispersed on a broader spectrum of particle sizes, some likely containing little apoA-I (compare Fig 4A lane 8 and Fig 4B lane 4).
Figure 4.
Dissociation of lipid-poor apoA-I from pre-AP and AP- 1d HDL following remodeling by CETP. HDLs were incubated with CETP in the presence of VLDL for 24 hr at 37°C as outlined in the methods. Reactions were analyzed by Western blot for (A) apoA-I and (B) SAA. The migration of lipid-poor apoA-I and SAA are marked with arrows.
Comparative displacement of apoA-I in pre-AP and AP HDL by concomitant action of CETP and sPLA2-IIA
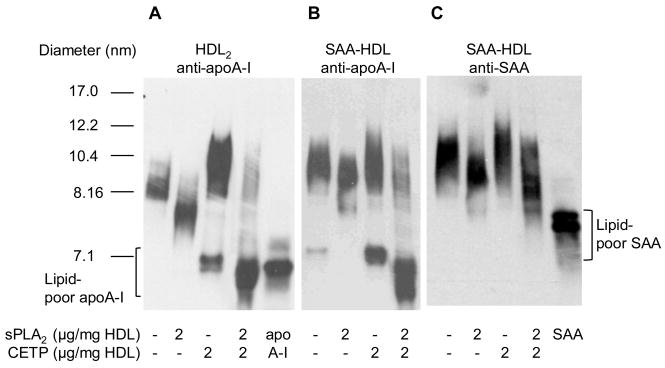
During the acute phase, dissociation of apoA-I from HDL could be influenced by acute phase secretory phospholipase (ie sPLA2-IIA) which is present in plasma and associates with HDL13. We thus used SAA-HDL (Fig. 1A) to study the remodeling of HDL by CETP and sPLA2-IIA. Treatment of SAA-HDL with CETP alone resulted in the generation of comparable amounts of lipid-poor apoA-I (Fig 5B, lane 3) as observed with control HDL2 (Fig 5A, lane 3). CETP treatment did not result in the displacement of significant amounts of lipid-poor SAA from SAA-HDL2 (Fig 5C, lane 3). Surface hydrolysis of HDL phospholipids by sPLA2 action alone resulted in a reduction in HDL particle size not accompanied by the dissociation of apoA-I or SAA (Fig 5A, B, C- lane 2). However the combined action of sPLA2 and CETP converted the majority of the apoA-I from the HDL-bound to the lipid-poor form in both HDL2 and SAA-HDL (Fig 5A & B-lane 4). This combined action may also result in the dissociation of limited amounts of lipid-poor SAA from SAA-HDL(Fig 5C- lane 4). We conclude that CETP action on the core, and sPLA2-IIA hydrolysis on the HDL surface, synergize to liberate lipid-poor apoA-I.
Figure 5.
The combined action of sPLA2-IIA and CETP on HDL2 and SAA-HDL. HDL were incubated with sPLA2-IIA and CETP as set out in the methods. Reactions were analyzed by Western blot for apoA-I in (A) HDL2 and (B) SAA-HDL and (C) SAA blot of SAA-HDL.
ABCA1 and ABCG1-dependent cholesterol efflux
To test the effects of the acute phase response on HDL function, efflux assays were carried out using pre-AP, AP- 1d and AP- 5d serum diluted to 2.5%. When compared to pre-AP serum, ABCA1-dependent efflux was not significantly decreased at AP- 1d despite a highly significant 53% fall in plasma apoA-I concentrations (Table 1). At AP- 5d, ABCA1 efflux was still maintained despite apoA-I remaining 1.5 fold lower than pre-AP levels. Similarly, ABCG1 efflux was modestly reduced at AP- 1d whilst HDL-C decreased by 48.1%. When efflux was normalized to HDL-C concentrations, there was a doubling in the ABCG1-dependent cholesterol efflux efficiency of AP- 1d serum compared to pre-AP serum, and this was maintained at AP-5d. Thus cholesterol efflux appears to be relatively well maintained in the acute phase response despite large reductions in HDL-C and apoA-I.
Table 1.
ABCA1- and ABCG1- dependent cholesterol efflux was determined as outlined in the methods. Efflux experiments were performed at 37 °C by incubating cells with serum (diluted to 2.5%) from patients pre-AP, AP1- d and AP- 5d for 16 h in DMEM containing 0.2% BSA. Values represent the average of triplicate determinations of n=6–8 patients. Results are presented as a percentage of pre-AP serum efflux. ApoA-I was measured using an automated turbidimetric immunoassay (Mayo Medical Laboratories, Rochester, MN). HDL was quantitated using a commercial kit.
| pre-AP | AP- 1d | AP- 5d | |
|---|---|---|---|
| ABCA1-efflux (% of pre-AP) | 100 | 82.5 ± 3.5 | 99.2 ± 2.9 |
| ABCG1- efflux (% of pre-AP) | 100 | 70.0 ± 14.3a | 108.5 ± 16.7 |
| plasma apoA-I (μg/ml) | 1161.7 ± 117.6b | 545.0 ± 62.4 | 785.0 ± 36.6 |
| plasma HDL-C (μg/ml) | 389.7 ± 60.5 | 196.6 ± 39.1c | 238.7 ± 27.9 |
p<0.05 vs pre-AP and AP- 5d;
p<0.001 vs AP- 1d and AP- 5d;
p < 0.5 versus pre-AP.
Discussion
Data presented here indicate the following. (i) The reduction in CETP concentration and activity during the acute phase response is commensurate with the reduction in HDL levels. (ii) Despite the striking alterations in HDL composition during the acute phase, CETP activity was maintained both with respect to its cholesteryl ester transfer function and its capacity to liberate apoA-I. (iii) sPLA2-IIA enhances CETP’s ability to liberate apoA-I and this is not impaired by the presence of SAA on the HDL. (iv) Although SAA has been shown to be an effective acceptor in lipid efflux, CETP action liberates it to a limited extent in a lipid-poor form. (v) The interplay between the numerous acute phase proteins impacting HDL remodeling result in the relative preservation of ABCA1 and ABCG1- dependent cholesterol efflux.
There is an inverse relationship between CETP activity and HDL concentrations in the normal state14. This was the basis for developing CETP inhibitors to increase HDL levels for potential therapeutic benefit. During inflammation this inverse relationship does not hold as both CETP and HDL levels are reduced. Studies have shown that inflammatory cytokines reduce CETP transcription and levels15,16. This could result in increased HDL levels. However our data shows a commensurate decrease in HDL and CETP in the acute phase. This suggests that during inflammation factors operate to reduce HDL despite the normal tendency of CETP to increase HDL. A number of mechanisms could operate to decrease plasma HDL during the acute phase. It was originally assumed that SAA enrichment of HDL was the basis for decreased apoA-I and HDL levels. However, this is unlikely since the decrease in plasma HDL during inflammation occurs rapidly, before SAA accumulation17. An alternative explanation for decreased HDL levels during inflammation could be remodeling by acute phase group II phospholipases, particularly sPLA2-IIA that increase HDL catabolism18, 19. SAA itself can also enhance the activity of sPLA220. The proinflammatory cytokines that induce SAA and sPLA2-IIA, simultaneously decrease apoA-I expression21. Thus the decreased levels of apoA-I and increased levels of SAA on HDL during inflammation are due at least in part to reciprocal coordinated regulation. Finally, the combined remodeling action of sPLA2-IIA and CETP during the acute phase response (Fig. 5) may also result in the increased catabolism of HDL. Given the numerous factors that operate during inflammation it is notable that the ratio of CETP to HDL is maintained. CETP reduction during inflammation could be viewed as a defensive adaptation to prevent “excessive” HDL reduction mediated by the mechanisms discussed.
It was reported that apoA-II abrogates the CETP-mediated liberation of apoA-I from reconstituted HDL containing both apoA-I and apoA-II11. In pre-AP HDL, we demonstrated that approximately 80% of apoA-I is present in LpAI particles (Fig. 3B). Lipid-poor apoA-I dissociating from pre-AP HDL in our study is thus likely derived from these LpAI particles. In contrast, in AP HDL, less than 20% of the apoA-I was present as LpAI, the remainder being associated with SAA and/or apoA-II (Fig. 3C). However, CETP action resulted in the liberation of the bulk of apoA-I from AP HDL. This establishes that SAA, in contrast to apoA-II, does not interfere with the liberation of apoA-I induced by CETP remodeling.
Our data indicate that SAA is present on most AP HDL particles (Fig. 3C). Unlike SAA, CETP is present as a dynamic exchange protein rather than a structural protein and undergoes rapid bidirectional transfer between HDL particles and acceptors. At any given time, only ~1 in 1,000 HDL particles carry a CETP molecule. The fact that the activity of CETP when normalized to HDL-C is unaltered during the acute phase suggests that CETP dynamics are also unchanged during the APR. Our results strongly suggest that any decrease in total cholesteryl ester transfer from HDL during the acute phase is the result of the concomitant reduction of plasma CETP and HDL-C rather than the reduced functionality of the HDL/CETP interaction.
It is notable that CETP action results in an apparent increase in size of the HDL particles (Fig 4 and 5). In addition to liberating apoA-I, CETP-mediated remodeling has been reported to result in particle fusion22. The increased particle size of CETP-remodeled HDL may also relate to the exchange of CE for TG by CETP action. As TG molecules are larger than CE molecules, HDL size would increase as TG content increases23. CETP action on HDL results in core/surface disequilibrium that is alleviated by the dissociation of lipid-poor apoAI24. Notably, in our study, CETP-remodeling of HDL resulted in both an increase in size of the particles, as well as the dissociation of lipid-poor apoA-I. Increased sPLA2-IIA activity during the acute-phase response may further potentiate the generation of lipid-poor apoA-I.
The increased sPLA2 activity in acute phase plasma (Fig 1B) is likely due to an increase in sPLA2-IIA25. sPLA2-IIA was shown to be present in atherosclerotic plaques bound to heparan-sulfate proteoglycans of the subendothelial extracellular matrix2. Proteoglycan binding of sPLA2-IIA serves to “concentrate” the enzyme and it is more active in the bound form26. Thus the effect of sPLA2-IIA on HDL remodeling may be more pronounced in the intima of a vessel. Furthermore, sPLA2 is more active when SAA is present on HDL.20 It may also be relevant that like sPLA2, SAA is also bound by proteoglycans in lesions27. Although efflux of cholesterol from macrophages at this site represents only a small fraction of overall cellular cholesterol efflux, it is critically protective in the context of atherosclerosis7. The interaction of sPLA2 and SAA might constitute a defensive mechanism against lipid accumulation. SAA-HDL is particularly enriched in SAA compared to AP HDL, with SAA present on 80% of particles3. In our study, CETP action on SAA-HDL liberated significant amounts of lipid-poor apoA-I. This confirms that the presence of SAA does not impair the dissociation of apoA-I. In our study, there was no clear evidence that SAA was significantly displaced in a lipid-poor form by either CETP or sPLA2-IIA (Figs. 4 & 5), though one has to recognize the limitations of analyzing SAA on non-denaturing gels as its tendency to self-aggregate and associate with the acrylamide matrix is well established.
Given the extensive remodeling of HDL during the acute phase that affects both the polydisperse particles themselves as well as the equilibrium between bound and free apolipoproteins, we evaluated the integral of all these actions on ABCA1 and ABCG1-dependent cholesterol efflux. We show an overall preservation of cholesterol efflux capacity of serum during the acute phase response. A large body of evidence suggested that HDL is part of the innate immune system28, and that acute phase HDL remodeling could impact the unique cargo of proteins on HDL29 reducing its anti-inflammatory functions. For example, the incorporation of SAA and sPLA2-IIA onto AP HDL particles results in the loss of paraoxonase activity of HDL30. During acute injury when macrophages accumulate lipid, relative preservation of efflux mechanisms could be more beneficial than the impairment of the anti-inflammatory properties of HDL. However during chronic inflammatory conditions, the latter might constitute a much more important risk factor for atherogenesis.
Inhibition of CETP results in elevated HDL levels14, but this could abrogate its two anti-atherogenic functions. The recent cessation of a human clinical trial testing such an inhibitor31 illustrates the need to better understand CETP function, not only on circulating lipoproteins, but also on apoA-I liberation at the level of the atherosclerotic lesion in the vessel wall. One can imagine a scenario where CETP inhibition could increase plasma HDL but also alter the equilibrium between HDL and lipid-poor apoA-I and consequently efflux potential. Our results indicate that AP HDL is not impaired in its ability to liberate apoA-I following CETP remodeling. This combined with the potentiation of apoA-I release by the combined action of CETP and the acute phase sPLA2-IIA, supports our results showing that during the acute phase, cholesterol effluxing capacity may be preserved despite a reduction of plasma HDL. However, prolonged inflammation and continual HDL remodeling may eventually lead to pro-atherogenic conditions by limiting the levels of HDL and apolipoprotein cholesterol acceptors and the pro-inflammatory nature of AP HDL having an impact.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the technical assistance of John Cranfill, Nathan Whitaker, Nick Whitaker, Matt Mains and Connie Dampier.
Funding
This study was supported by an NIH Program Project Grant (PO1HL086670) to D.R.v.d.W and a VA Merit Review Funded by Veterans Affairs VACO to F.C.D.
Footnotes
Disclosures
No disclosures.
References
- 1.Cabana VG, Siegel JN, Sabesin SM. Effects of the acute phase response on the concentration and density distribution of plasma lipids and apolipoproteins. J Lipid Res. 1989;30:39–49. [PubMed] [Google Scholar]
- 2.Menschikowski M, Hagelgans A, Siegert G. Secretory phospholipase A2 of group IIA: is it an offensive or a defensive player during atherosclerosis and other inflammatory diseases? Prostaglandins Other Lipid Mediat. 2006;79:1–33. doi: 10.1016/j.prostaglandins.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Coetzee GA, Strachan AF, van der Westhuyzen DR, Hoppe HC, Jeenah MS, de Beer FC. Serum amyloid A-containing human high density lipoprotein 3. Density, size, and apolipoprotein composition. J Biol Chem. 1986;261:9644–9651. [PubMed] [Google Scholar]
- 4.de Beer FC, de Beer MC, van der Westhuyzen DR, Castellani LW, Lusis AJ, Swanson ME, Grass DS. Secretory non-pancreatic phospholipase A2: influence on lipoprotein metabolism. J Lipid Res. 1997;38:2232–2239. [PubMed] [Google Scholar]
- 5.Barter PJ. Hugh sinclair lecture: the regulation and remodelling of HDL by plasma factors. Atheroscler Suppl. 2002;3:39–47. doi: 10.1016/s1567-5688(02)00041-7. [DOI] [PubMed] [Google Scholar]
- 6.Rye KA, Hime NJ, Barter PJ. The influence of cholesteryl ester transfer protein on the composition, size, and structure of spherical, reconstituted high density lipoproteins. J Biol Chem. 1995;270:189–196. doi: 10.1074/jbc.270.1.189. [DOI] [PubMed] [Google Scholar]
- 7.Lewis GF, Rader DJ. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ Res. 2005;96:1221–1232. doi: 10.1161/01.RES.0000170946.56981.5c. [DOI] [PubMed] [Google Scholar]
- 8.Rye KA, Duong MN. Influence of phospholipid depletion on the size, structure, and remodeling of reconstituted high density lipoproteins. J Lipid Res. 2000;41:1640–1650. [PubMed] [Google Scholar]
- 9.Hacquebard M, Ducart A, Schmartz D, Malaisse WJ, Carpentier YA. Changes in plasma LDL and HDL composition in patients undergoing cardiac surgery. Lipids. 2007;42:1143–1153. doi: 10.1007/s11745-007-3114-9. [DOI] [PubMed] [Google Scholar]
- 10.Tam SP, Ancsin JB, Tan R, Kisilevsky R. Peptides derived from serum amyloid A prevent, and reverse, aortic lipid lesions in apoE−/− mice. J Lipid Res. 2005;46:2091–2101. doi: 10.1194/jlr.M500191-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Rye KA, Wee K, Curtiss LK, Bonnet DJ, Barter PJ. Apolipoprotein A-II inhibits high density lipoprotein remodeling and lipid-poor apolipoprotein A-I formation. J Biol Chem. 2003;278:22530–22536. doi: 10.1074/jbc.M213250200. [DOI] [PubMed] [Google Scholar]
- 12.Tall AR. Plasma cholesteryl ester transfer protein. J Lipid Res. 1993;34:1255–1274. [PubMed] [Google Scholar]
- 13.Gijon MA, Perez C, Mendez E, Sanchez Crespo M. Phospholipase A2 from plasma of patients with septic shock is associated with high-density lipoproteins and C3 anaphylatoxin: some implications for its functional role. Biochem J. 1995;306 (Pt 1):167–175. doi: 10.1042/bj3060167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Grooth GJ, Klerkx AH, Stroes ES, Stalenhoef AF, Kastelein JJ, Kuivenhoven JA. A review of CETP and its relation to atherosclerosis. J Lipid Res. 2004;45:1967–1974. doi: 10.1194/jlr.R400007-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Hardardottir I, Moser AH, Fuller J, Fielding C, Feingold K, Grunfeld C. Endotoxin and cytokines decrease serum levels and extra hepatic protein and mRNA levels of cholesteryl ester transfer protein in syrian hamsters. J Clin Invest. 1996;97:2585–2592. doi: 10.1172/JCI118707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masucci-Magoulas L, Moulin P, Jiang XC, Richardson H, Walsh A, Breslow JL, Tall A. Decreased cholesteryl ester transfer protein (CETP) mRNA and protein and increased high density lipoprotein following lipopolysaccharide administration in human CETP transgenic mice. J Clin Invest. 1995;95:1587–1594. doi: 10.1172/JCI117832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, Grunfeld C. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res. 2004;45:1169–1196. doi: 10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.de Beer FC, Connell PM, Yu J, de Beer MC, Webb NR, van der Westhuyzen DR. HDL modification by secretory phospholipase A(2) promotes scavenger receptor class B type I interaction and accelerates HDL catabolism. J Lipid Res. 2000;41:1849–1857. [PubMed] [Google Scholar]
- 19.Tietge UJ, Maugeais C, Cain W, Grass D, Glick JM, de Beer FC, Rader DJ. Overexpression of secretory phospholipase A(2) causes rapid catabolism and altered tissue uptake of high density lipoprotein cholesteryl ester and apolipoprotein A-I. J Biol Chem. 2000;275:10077–10084. doi: 10.1074/jbc.275.14.10077. [DOI] [PubMed] [Google Scholar]
- 20.Pruzanski W, de Beer FC, de Beer MC, Stefanski E, Vadas P. Serum amyloid A protein enhances the activity of secretory non-pancreatic phospholipase A2. Biochem J. 1995;309 (Pt 2):461–464. doi: 10.1042/bj3090461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han CY, Chiba T, Campbell JS, Fausto N, Chaisson M, Orasanu G, Plutzky J, Chait A. Reciprocal and coordinate regulation of serum amyloid A versus apolipoprotein A-I and paraoxonase-1 by inflammation in murine hepatocytes. Arterioscler Thromb Vasc Biol. 2006;26:1806–1813. doi: 10.1161/01.ATV.0000227472.70734.ad. [DOI] [PubMed] [Google Scholar]
- 22.Rye KA, Hime NJ, Barter PJ. Evidence that cholesteryl ester transfer protein-mediated reductions in reconstituted high density lipoprotein size involve particle fusion. J Biol Chem. 1997;272:3953–3960. doi: 10.1074/jbc.272.7.3953. [DOI] [PubMed] [Google Scholar]
- 23.Borggreve SE, De Vries R, Dullaart RP. Alterations in high-density lipoprotein metabolism and reverse cholesterol transport in insulin resistance and type 2 diabetes mellitus: role of lipolytic enzymes, lecithin: cholesterol acyltransferase and lipid transfer proteins. Eur J Clin Invest. 2003;33:1051–1069. doi: 10.1111/j.1365-2362.2003.01263.x. [DOI] [PubMed] [Google Scholar]
- 24.Rye KA, Barter PJ. Formation and metabolism of prebeta-migrating, lipid-poor apolipoprotein A-I. Arterioscler Thromb Vasc Biol. 2004;24:421–428. doi: 10.1161/01.ATV.0000104029.74961.f5. [DOI] [PubMed] [Google Scholar]
- 25.Nevalainen TJ, Eerola LI, Rintala E, Laine VJ, Lambeau G, Gelb MH. Time-resolved fluoroimmunoassays of the complete set of secreted phospholipases A2 in human serum. Biochim Biophys Acta. 2005;1733:210–223. doi: 10.1016/j.bbalip.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Sartipy P, Johansen B, Camejo G, Rosengren B, Bondjers G, Hurt-Camejo E. Binding of human phospholipase A2 type II to proteoglycans. J Biol Chem. 1996;271:26307–26314. doi: 10.1074/jbc.271.42.26307. [DOI] [PubMed] [Google Scholar]
- 27.O’Brien KD, McDonald TO, Kunjathoor V, Eng K, Knopp EA, Lewis K, Lopez R, Kirk EA, Chait A, Wight TN, deBeer FC, LeBoeuf RC. Serum amyloid A and lipoprotein retention in murine models of atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:785–790. doi: 10.1161/01.ATV.0000158383.65277.2b. [DOI] [PubMed] [Google Scholar]
- 28.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res. 2004;95:764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 29.Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, Byun J, Vuletic S, Kassim S, Singh P, Chea H, Knopp RH, Brunzell J, Geary R, Chait A, Zhao XQ, Elkon K, Marcovina S, Ridker P, Oram JF, Heinecke JW. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest. 2007;117:746–756. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Lenten BJ, Hama SY, de Beer FC, Stafforini DM, McIntyre TM, Prescott SM, La Du BN, Fogelman AM, Navab M. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest. 1995;96:2758–2767. doi: 10.1172/JCI118345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cholesterol: the good, the bad, and the stopped trials. Lancet. 2006;368:2034. doi: 10.1016/S0140-6736(06)69815-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.