Abstract
Breast cancer survivors experience cognitive difficulties following chemotherapy, yet the effects of these deficits on functional outcomes have not been systematically evaluated. This study assessed the relationships between post-chemotherapy cognitive difficulties and functional outcomes. Forty-six women with breast cancer were seen at 1-month post-chemotherapy; data were collected on cognitive functioning, psychological variables, and physical symptoms. Wilcoxon Signed Rank analyses revealed cognitive deficits in executive functioning and verbal fluency. Subsequent regression analyses demonstrated that poorer executive functioning was associated with decreased productivity, community involvement, and social role functioning. Poorer quality of life was predicted by depression and reluctance to seek social support, but not cognitive functioning. These findings indicate that executive functioning deficits are associated with important functional outcomes among breast cancer survivors 1-month post-chemotherapy. Thus, treatment efforts should focus on addressing cognitive, as well as psychological and physical, issues among cancer survivors.
Keywords: Breast cancer, Cognitive, Quality of life, Social support, Community integration
INTRODUCTION
Breast cancer is currently the most prevalent malignancy affecting women. Treatments have improved over time, such that the current survival rate across stages is approximately 88% (American Cancer Society, 2006). Coinciding with these improvements in mortality rates, medical and behavioral health care providers alike are increasingly attuned to the experiences women have following treatment completion. Research on quality of life after treatment for cancer abounds (e.g., Carver, Smith, Petronis & Antoni, 2006; Helgeson & Tomich, 2005; Mols, Vingerhoets, Coebergh & van de Poll-Franse, 2005), with efforts underway to identify factors that improve the quality of life experienced by cancer survivors, so that treatments to enhance these factors can be developed.
Cognitive Deficits Following Cancer Treatment
Within the field of cancer survivorship research, one issue receiving increased attention in the past 10 years is the impact of chemotherapy on cognitive functioning. A growing literature suggests that chemotherapy is associated with a decline in cognitive functioning among a subset of women treated for breast cancer (for reviews, see Rugo & Ahles, 2003; Tannock, Ahles, Ganz, & Van Dam, 2004), with prevalence estimates ranging from 17-35% (Ahles & Saykin, 2001). Deficits have specifically been noted in memory (e.g., Ahles et al., 2002; Brezden, Phillips, Abdolell, Bunston, & Tannock, 2000; Meyers, Byrne, & Komaki, 1995; Wienke & Dienst, 1995), attention/concentration (Schagen et al., 1999; van Dam et al., 1998; Van Oosterhout et al., 1996; Wienke & Dienst, 1995), psychomotor speed (Ahles et al., 2002; Meyers et al., 1995; van Dam et al., 1998), and cognitive processing speed (Silberfarb, Philibert, & Levine, 1980; van Dam et al., 1998; Van Oosterhout et al., 1996). Several meta-analyses have revealed small to medium effect sizes across each of these cognitive domains (Falleti, Sanfilippo, Maruff, Weih, & Phillips, 2005; Stewart, Bielajew, Collins, Parkinson, & Tomiak, 2006), with the largest effects for executive functioning and verbal memory (Anderson-Hanley, Sherman, Riggs, Agocha, & Compas, 2003). The etiology of these deficits remains unclear, with several possibilities currently under consideration including: fatigue, hormonal changes, direct neurotoxic effects, and genetic predispositions (for reviews, see Reid-Arndt, 2006; Saykin, Ahles & McDonald, 2003).
While research documents cognitive changes post-chemotherapy, the duration of these observed changes has not been clearly delineated. Some studies suggest these deficits may be long-term, as continued reports of a decline in cognitive functioning (e.g., forgetfulness, increased distractibility, problems concentrating) have been noted among breast cancer survivors at 5-10 years after initial diagnosis (Ahles et al., 2002; Ganz et al., 2002). However, other results from longitudinal and cross-sectional studies reveal that, for some women, cognitive impairments may diminish over time. For example, one series of studies indicated that initial cognitive impairments noted at 2 years post-treatment (Schagen et al., 1999; Van Dam et al., 1998) were no longer present at 4 years post-treatment (Schagen et al., 2002). Another study followed 18 women treated for breast cancer, collecting data pre-chemotherapy, at 6-months post-chemotherapy, and at one year post-chemotherapy (Wefel, Lenzi, Theriault, Davis, & Meyers, 2004). Deficits were documented in attention, processing speed and verbal memory, particularly at 6-months post-chemotherapy; approximately 45% evidenced improvements over time. Finally, one cross-sectional study compared individuals currently undergoing chemotherapy with a group who had completed chemotherapy a median of 2 years prior and a group of healthy controls (Brezden et al., 2000). A general cognitive screen revealed deficits among all women with a history of chemotherapy; importantly, women with a prior history of chemotherapy performed better than those currently undergoing chemotherapy, while the healthy controls performed better than both chemotherapy groups. Together, these data suggest that the decline in cognitive functioning post-chemotherapy may resolve over time among a portion of affected individuals, while some will continue to demonstrate cognitive deficits.
Factors Influencing Functional Outcomes among Breast Cancer Survivors
Although progress has been made in identifying and understanding cognitive deficits following cancer treatment, what has yet to be considered is the functional significance of these deficits. Specifically, while studies provide evidence of statistically significant cognitive weaknesses, there is limited information regarding potential clinical significance (Reid-Arndt, 2006). For example, although relative intra-individual cognitive weaknesses have been found, in general women are performing in the normal range compared to age and education matched peers. Additionally, to these authors' knowledge, there is a lack of research documenting the relationship between cognitive functioning and measures of functional outcomes, despite calls for this type of analysis (e.g., Ahles & Saykin, 2001).
While there is a dearth of information regarding the functional impact of cognitive deficits among BCS, there exists a relatively extensive literature documenting the effects of other variables on functional outcomes such as quality of life, vocational functioning, and social functioning. For example, prior research has identified several psychological and interpersonal factors that are associated with better quality of life, including greater degree of optimism (Carver et al., 2005), strong social support (Friedman et al., 2006), and use of stress management interventions (Antoni et al., 2006). Age also plays a part in predicting quality of life after treatment, with younger survivors reporting poorer mental health outcome than older patients (Wenzel et al., 1999).
Returning to work is both an indicator of recovery and an important contributor to quality of life. In a qualitative study of cancer survivors, many individuals reported that “work brought an added sense of meaning, challenge and accomplishment at a time when their cancer made such meaning particularly important” (Main, Nowels, Cavender, Etschmaier, & Steiner, 2005, p. 1002). Unfortunately, some women experience changes in vocational functioning associated with their diagnosis and treatment of cancer. For example, one study found that approximately one-third of the cancer survivors who were employed prior to diagnosis were no longer working 6 months after their diagnosis, and those who returned worked fewer hours per week on average compared to before their diagnosis (Bradley, Neumark, Bednarek, & Schenk, 2005). Moreover, individuals needed to accommodate treatment plans into their vocational obligations in a variety of ways, including quitting their job or changing employers.
Social functioning is an important part of the multidimensional conceptualization of quality of life, and women undergoing treatment for breast cancer express concerns about the impact of their diagnosis and treatment on social and role functioning (Osoba et al., 2006). In a longitudinal study, breast cancer survivors consistently reported lower social and role functioning compared to a control group of healthy individuals both at the time of diagnosis and up to one year after surgery (Schou, Ekeberg, Sandvik, Hjermstad, & Ruland, 2005). While other domains of quality of life showed signs of improvement over time, social and cognitive functioning were the slowest to recover. Several individual and situation factors have been associated with poorer social role functioning outcomes, such as pessimism (Carver et al., 2005), passive approach to decision-making about treatment plans (Hack, Degner, Watson, & Sinha, 2006), and failure to disclose concerns (Figueiredo, Fries, & Ingram, 2004).
Together, these findings demonstrate the evident impact of cancer treatment on quality of life, vocational functioning, and social functioning, as well as how individual factors may moderator this effect. Interestingly, although there is evidence from other populations (e.g., TBI, multiple sclerosis) that cognitive functioning is another individual variable that can impact functioning in each of these domains (e.g., Girard et al., 1996; Hanks, Rapport, Millis, & Deshpande, 1999; Rao et al., 1991), the link between functional outcomes and cognitive deficits among cancer survivors has yet to be clearly explicated.
The present study represents an initial effort to fill this knowledge gap. Women with Stages 1-3 breast cancer who completed adjuvant chemotherapy were seen at 1-month following chemotherapy, at which time data were collected regarding cognitive, emotional, social, and vocational functioning. The goal of this project was to demonstrate what effects changes in cognitive functioning following chemotherapy for breast cancer may be having on quality of life and functional outcomes in the early stages of survivorship. We predicted that cognitive weaknesses, and particularly executive functioning deficits, would be associated with poorer quality of life and social role functioning among women breast cancer patients one month following completion of chemotherapy.
METHODS
Participants
Forty-six women receiving treatment for Stages I - III primary breast cancer participated in this study. Demographic information for study participants is provided in Table 1. Almost one-half of participants were diagnosed with Stage II breast cancer (47.4%), while 23.7% were diagnosed with Stage I and 28.9% were diagnosed with Stage III. Participants underwent mastectomy or lumpectomy and received adriamycincyclophosphamide with or without paclitaxel or taxotere or a variant of that program; the average number of chemotherapy cycles across all stages was 5.77 (SD = 1.93). Exclusion criteria were identified through a study-specific intake screening questionnaire completed at the first appointment and included: prior history of adjuvant chemotherapy for cancer, past or current neurological illness, and significant psychopathology (e.g., psychosis).
Table 1.
Demographic Information
| Mean Age (years) | 53.38 (9.61) |
| Marital Status | |
| Married | 64% |
| Single | 36% |
| Ethnicity/Race | |
| Caucasian | 100% |
| Mean Education (years) | 14.87 (2.56) |
| Employment Status | |
| 30+ hours/week | 70.7% |
| 10-30 hours/week | 10.4% |
| Not employed | 18.9% |
n = 46
Measures
Demographic data were obtained via a questionnaire created specifically for this study. Information regarding medical variables (e.g., cancer stage, number of chemotherapy cycles, and exposure to other adjuvant therapies including radiation and Tamoxifen) was obtained from the participants' medical records, with their informed consent.
Neuropsychological measures were used to evaluate skills in five cognitive domains: Immediate Memory, Delayed Memory, Attention, Executive Functioning, and Verbal Fluency. Specific tests comprising each of 5 cognitive domains are listed in Table 2. Of note, while verbal fluency is often considered a component of executive functioning because it can be associated with frontal lobe lesions (e.g., Miceli, Caltagirone, Gainotti, Masullo, & Silveri, 1981; Stuss et al., 1998), an a priori decision was made to separate this from other executive functioning measures based on clinical observations that word-finding deficits are one of the most common complaints reported in the cancer treatment centers where this study occurred. Finally, in line with neuropsychological theory and research (e.g., Griffin, Mindt, Rankin, Ritchie, & Scott, 2002; Kareken, Gur, & Saykin, 1995; Lezak, 1995), the Wide Range Achievement Test-3 (WRAT-3) Reading subtest (Wilkinson, 1993) was included as an estimate of overall premorbid functioning. In general, this battery was designed to evaluate a broad range of cognitive domains, and the specific selection of tests was based on neuropsychological batteries used in prior research with cancer patients (e.g., Ahles et al., 2002; Meyers et al., 1995; van Dam et al., 1998;). It included those tests determined to be most sensitive to changes secondary to chemotherapy treatment for cancer.
Table 2.
Neuropsychological Measures
| Cognitive Domain | Measures |
|---|---|
| Immediate Memory | WMS-III Logical Memory Ia |
| WMS-III Visual Reproduction Ia | |
| Delayed Memory | WMS-III Logical Memory IIa |
| WMS-III Visual Reproduction IIa | |
| Rey AVLT Delayed Recallb | |
| Attention | Trail Making Test Ac |
| WAIS-III Digit Spand | |
| Executive Functioning | Trail Making Test Bc |
| Stroop Teste | |
| Verbal Fluency | COWATf |
| Category Fluencyg |
Psychological variables were measured with the Hesitation Scale and the Profile of Mood States - Short Form (POMS-SF). The Hesitation Scale is a 20-item measure of willingness to seek social support. Previous research using this scale (Farmer, Clark & Sherman, 2003) demonstrated that reluctance to seek social support was significantly related to perceptions of lesser social support and lower quality of life. The POMS-SF, which is a 37-item version of the original 65-item POMS, was developed by Shacham (1983) for use with cancer patients. There are six subscales -- Depression, Vigor, Confusion, Tension, Anger, and Fatigue -- with internal consistency reliabilities ranging from 0.78 to 0.91 (Baker, Denniston, Zabora, Polland, & Dudley, 2002).
Social role functioning was measured with a questionnaire designed to assess individuals' perceptions of their competency in fulfilling important social roles (Bettencourt & Sheldon, 2001; Sheldon & Bettencourt, 2002). This measure was developed as part of a body of research examining the theory that successful social role involvement may promote authentic self-expression while still enhancing group connectedness (Bettencourt & Sheldon, 2001; Sheldon & Bettencourt, 2002). This measure has been found to predict subjective well-being (Bettencourt & Sheldon, 2001) and depression among cancer survivors (Talley, Molix, Schlegel & Bettencourt, in press). Subscales from this measure evaluate perceived competency in social roles (internal reliabilities = .78-.80), providing an index of perceived functioning in four possible roles: as a spouse/partner, a parent, an employee, and any other role identified by each participant (e.g., volunteer, caregiver). Participants are asked to indicate their level of agreement using a 6 point Likert scale in response to items such as: “I feel as if I am fully functioning in my role as a spouse/partner.”
Community Integration Questionnaire (CIQ) was administered to measure general community involvement as well as engagement in home activities, social activities, and work activities (Willer, Rosenthal, Kreutzer, Gordon & Rempel, 1993). Research with other groups with chronic medical conditions (e.g., TBI) has reported internal consistencies ranging from .79 to .90 (Corrigan & Deming, 1995).
Functional Assessment of Cancer Therapy-Breast (FACT-B) is a commonly used measure of quality of life in breast cancer survivors. The FACT-B includes items designed to assess quality of life (QOL) in terms of patients' relationships with their physician(s) as well as physical, emotional, social, functional well-being. This measure includes items from the FACT-General as well as 9 additional items specifically selected to evaluate QOL issues in breast cancer survivors. Per a validation study (Brady et al., 1997), the total score from this measure has acceptable internal consistency (Chronbach's alpha=.90) and test-retest reliability (.85). Construct validity was suggested by high correlations with other measures of QOL, such as the Functional Living Index - Cancer (r=.87).
Procedure
Women who were receiving adjuvant chemotherapy treatment for breast cancer were recruited at 3 sites in a Midwestern city (an academic hospital, a private hospital, and a private oncology practice). They were informed of the study by a health care provider working with them (typically their treating physician or nurse) prior to the completion of their last cycle of chemotherapy. Those who expressed an interest in the study were contacted by a member of the research team, either in person or via telephone, so that a description of the study could be provided and any questions answered. All participants provided informed written consent in accordance with the Institutional Review Board informed consent guidelines at each site. Participants then completed a data collection session at 1-month following the completion of their chemotherapy, which was an average of 5 months following the diagnosis of cancer for study participants. Efforts were made to ensure that these appointments coincided with the participants' follow-up appointments with health care providers at each site.
RESULTS
Wilcoxon Signed Rank tests were computed to assess for relative deficits for each cognitive domain. Next, backwards selection regression analyses were conducted to determine what variables predict the outcome measures of interest. For each regression model, possible predictor variables included medical variables (i.e., number of chemotherapy treatments, POMS-SF Fatigue subscale), psychological variables (i.e., POMS-SF Depression subscale, Hesitation Scale), and cognitive variables (i.e., composite indices of Executive Functioning and Verbal Fluency). Three composite indices (Immediate Memory, Delayed Memory, Attention) were excluded from regression analyses based on findings from initial Wilcoxon Signed Rank tests that revealed no evidence of deficits in these domains.
Cognitive functioning
First, composite variables were created for each cognitive domain of interest by converting each individual's raw test scores to z-scores utilizing age, education, and gender based normative data. Thereafter, an average of each person's z-scores for tests comprising each domain was computed.
For the initial analyses, we were interested in determining whether participants may be experiencing a decline in cognitive functioning compared to estimated premorbid abilities. As noted above, in the absence of pre-treatment data, the WRAT-3 Reading test was used as a proxy of premorbid functioning, a procedure that has support in both clinical literature (e.g., Lezak, 1995) and research literature (Johnstone, Hexum, & Ashkanazi, 1995). Thus, for these analyses, a new variable was created reflecting the difference between each individual's estimate of premorbid abilities (WRAT-3 Reading) and her composite score for each domain.
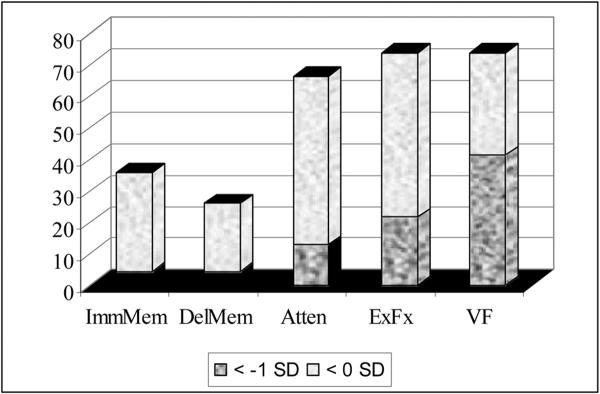
Wilcoxon Signed Rank tests were computed to determine if the mean change in composite test scores was significantly different from zero. Results indicated that at 1-month post-chemotherapy, participants were demonstrating significantly lower scores in Executive Functioning (t = -2.71, p = .01) and Verbal Fluency (t = -4.54, p < .001) than would have been expected based on premorbid estimates. Conversely, the mean Immediate Memory and Delayed Memory scores were significantly higher than expected (Immediate Memory: t = 3.06, p < .01; Delayed Memory: t = 4.08, p < .001). See Table 3 for mean scores. A review of the distribution of difference scores revealed that 21.7% were performing >1 SD below premorbid estimates in Executive Functioning, while 41.3% were performing >1 SD below premorbid estimates in Verbal Fluency (see Figure 1).
Table 3.
Group Mean z (difference) scores* for each Cognitive Domain
| Cognitive Domain | Mean z (difference) score |
|---|---|
| Immediate Memory | .29 (.80)a |
| Delayed Memory | .49 (.81)b |
| Attention | -.04 (.84) |
| Executive Functioning | -.44 (1.10)a |
| Verbal Fluency | -.65 (.97)b |
z(difference) score = (Domain composite z-score) - (WRAT-3 Reading z-score)
Wilcoxon Signed Rank test, p <. 05
Wilcoxon Signed Rank test, p < .001
Figure 1.
Percentage of Participants with Relative Cognitive Weaknesses
Correlations between Outcome Variables
Pearson correlation analyses were performed to better understand the relationships between the multiple measures of functional outcomes, which included FACT-B (Brady et al., 1997), Community Integration Questionnaire (Willer et al., 1993) and the Social Roles Measure (Bettencourt & Sheldon, 2001). As evidenced in Table 4, measures of social functioning (FACT-B Social Well-Being, CIQ Social Integration, Social Roles) were significantly correlated (all r's > .34, all p's < .05). Significant correlations were also observed among measures of productivity (FACT-B Functional Well-Being, CIQ Productivity, CIQ Total: all r's > .35, all p's < .05). Finally, consistent with prior research (Bettencourt & Sheldon, 2001; Talley et al., in press), social role functioning was also correlated with emotional well being (FACT-B Emotional Well-Being, r = .589, p < .01).
Table 4.
Correlations between Outcome Measures
| FACT/SWB Subscale | FACT/RWD Subscale | FACT/EWB Subscale | FACT/FWB Subscale | CIQ/Home Integration Score | CIQ/Social Integration Score | CIQ/Productivity Score | CIQ/Total Score | |
|---|---|---|---|---|---|---|---|---|
| FACT/RWD Subscale | .316* | |||||||
| FACT/EWB Subscale | .571** | .121 | ||||||
| FACT/FWB Subscale | .713** | .363* | .674** | |||||
| CIQ/Home Integration Score | .024 | -.085 | .027 | -.042 | ||||
| CIQ/Social Integration Score | .506** | .021 | .413** | .345* | .390** | |||
| CIQ/Productivity Score | .224 | .157 | .174 | .358* | -.104 | .041 | ||
| CIQ/Total Score | .368* | .079 | .335* | .306* | .677** | .699** | .471** | |
| Social Roles Average | .573** | .128 | .589** | .451** | -.248 | .341* | .026 | .066 |
Correlation is significant at the 0.05 level (2-tailed).
Correlation is significant at the 0.01 level (2-tailed).
Predicting Functional Outcomes
Backwards selection regression analyses were conducted to evaluate the relationships between functional outcomes of interest and several predictor variables. Based on a priori hypotheses that they may impact participants' outcomes, predictors entered into the equation for each model included medical variables (number of chemotherapy treatments, POMS Fatigue subscale) and psychological variables (POMS Depression subscale, Hesitation scale). Additionally, to evaluate the potential effects of cognitive decline on functional outcomes, composite scores from domains where a relative decline in cognitive functioning was evidenced in initial analyses -- Executive Functioning and Verbal Fluency -- were included.
Quality of life
Regression analyses were conducted to determine whether these predictor variables were associated with reports of quality of life. As noted above, the FACT-B index of quality of life is comprised of multiple subscales, each of which was evaluated separately. The model for FACT-B Social Well-Being was significant, F(2, 38) = 8.93, p = .001, accounting for 32% of the variance. Examination of the individual betas showed that higher levels of depression and greater hesitation to seek support were associated with poorer social well-being: POMS Depression was marginally significant at p = .079, while Hesitation was significant at p = .017. Similarly, the model for FACT-B Emotional Well-Being was significant, F(2, 38) = 21.29, accounting for 53% of the variance. A review of the individual betas revealed that POMS Depression and Hesitation were again significant predictors in this model (p = .017 and p < .001, respectively), revealing that higher levels of depression and a greater reluctance to seek social support were also associated with poorer emotional well-being.
The model for FACT-B Functional Well-Being was significant, F(3, 37) = 12.11, p < .001, accounting for 50% of the variance. A review of the individual beta weights revealed that poorer functional well-being, an index of the ability to participate in important daily activities (including work and leisure activities), was associated with greater reluctance to seek social support (Hesitation: p = .001), more chemotherapy treatments (p = .061), and higher levels of fatigue (POMS Fatigue: p = .001). The FACT-B includes a subscale seeking ratings on one's Relationship with his/her Doctor, and this model was significant, F(1, 39) = 4.00, p = .052, accounting for a modest 9% of the variance. A review of betas revealed that greater reluctance to seek social support, indexed by the Hesitation scale, was associated with reports of less satisfying relationships with doctors (p = .052). Finally, the model for the FACT-B Physical Well-Being subscale was also significant, F(1, 39) = 5.162, p < .05, accounting for 12% of variance. POMS Fatigue was the single variable that remained significant in this backwards selection model (p = .029), indicating that increased fatigue was associated poorer physical well-being.
Community involvement and productivity
Regression analyses were conducted to determine what variables were associated with five measures of community involvement and productivity included in this study. As noted above, the Community Integration Questionnaire (CIQ) provides three subscale scores as well as a total score; regression analyses were computed using each as an outcome measure. The model for CIQ Social Integration was significant, F(1, 39) = 5.490, p < .05, with predictors accounting for 12% of the variance. A review of the betas revealed that better executive functioning was associated with enhanced social integration (Executive Functioning: p < .024). Executive functioning (p = .05) as well as depression (POMS Depression: p = .05) were significant predictors accounting for 22% of the variance in a model for CIQ Productivity (F(2, 38) = 5.26, p = .01), indicating that productivity was lowered by poorer executive functioning and increased symptoms of depression. The model for CIQ Home Integration did not reach significance. Finally, the model for the CIQ Total Score was significant, F(2, 38) = 5.73, p < .01, accounting for 23% of the variance. A review of the betas revealed that better executive functioning and less fatigue were associated with enhanced overall community involvement (Executive Functioning: p = .10; POMS Fatigue: p < .05).
Finally, the Social Roles questionnaire was also utilized as an index of participants' perceived effectiveness in important social roles. The model for social roles functioning was significant, F(2, 37) = 9.35, p < .001, accounting for 34% of the variance. A review of the betas revealed that better executive functioning and less reluctance to seek social support were associated with enhanced social role functioning (Executive Functioning: p = .01; Hesitation: p < .01).
DISCUSSION
The present study was undertaken to ultimately provide direction for improving the lives of women breast cancer survivors by offering initial insight into how cognitive deficits relate to functional outcomes in the early stages of recovery following chemotherapy. Although the etiology remains unknown, research to date has suggested that a portion of women will experience modest cognitive decline following chemotherapy (Ahles & Saykin, 2001), and the present study supported this observation. Specifically, there was a significant decline in executive functioning and verbal fluency skills compared to estimated premorbid abilities among participants at 1-month following the completion of chemotherapy. These findings are consistent with reports from other research, which suggest that these complex cognitive skills may be most susceptible to adverse effects of chemotherapy (e.g., Ahles et al., 1998; Freeman & Broshek, 2002; Schagen et al., 1999; van Dam et al., 1998; Wienke & Dienst, 1995;).
Subsequent analyses were conducted to clarify whether these cognitive deficits may be impacting quality of life and daily functioning; findings provided mixed support for this possibility. First, data did not support the hypothesis that the observed cognitive deficits were having a significant impact on quality of life as measured by the FACT-B. Rather, consistent with prior research on quality of life (Visser & Smets, 1998), symptoms of depression were associated with social and emotional well-being among this group of cancer survivors. Also consistent with a growing body of literature (e.g., Tchen et al., 2003) was the finding that increased fatigue is associated with poorer quality of life among women following treatment for breast cancer.
The present research revealed some novel findings regarding quality of life among cancer survivors as well. Specifically, individuals who were more reluctant to seek social support, as measured by the Hesitation Scale, were also more likely to report relatively poorer social and emotional well-being, as well as poorer doctor-patient relationships. These findings are in line with some of the literature on coping, which has suggested that social support seeking as a coping style may have a significant impact on emotional well-being (Holland & Hollahan, 2003; Stanton & Snider, 1993).
While cognitive decline following treatment for breast cancer did not predict quality of life in this study, there was evidence that deficits in executive functioning were adversely impacting other functional outcomes. Specifically, women who were experiencing greater deficits in executive functioning were less engaged in social and community activities, and they were reporting greater difficulties functioning effectively in important social roles (e.g., spouse, parent, employee, etc.). These findings validate breast cancer survivors' anecdotal reports that difficulties with multi-tasking, particularly during treatment and in the early stages following treatment completion, hinder their functioning at work and at home. They are also significant because there may be long-term consequences of an initial decline in cognitive functioning and functional abilities (e.g., women may be more inclined to quit a job or disengage from other important responsibilities during this period). Moreover, while some of these women may experience a recovery in cognitive functioning over time, prior research has indicated that these deficits persist for a portion of those affected. Thus, this study points to the potential need for interventions in the early stages of recovery following treatment for breast cancer.
Several limitations of the study are worth noting. First, although this procedure is consistent with a majority of studies in this area (see Minisini et al., 2004), the absence of pre-chemotherapy data restricts our ability to draw conclusions regarding the extent of cognitive decline following treatment. However, it is also true that cognitive testing immediately prior to starting chemotherapy may not provide the best indication of baseline functioning. Specifically, it is well known that the time from diagnosis to the initiation of treatment is a cognitively taxing and emotionally stressful period. Given the adverse impact that emotional distress can have on cognitive functioning, it could be argued that testing during this period may not provide a true index of premorbid abilities.
Second, multiple analyses were conducted on a dataset having a relatively large number of variables and a modest sample size. Efforts were made to address this limitation in several ways. For example, the number of variables in each model was minimized by creating composites for the neuropsychological test scores, and the number of medical and psychological variables was limited in each model. Moreover, all models were developed based on a priori hypotheses, and efforts were made to uncover converging evidence to support conclusions about relationships between the predictor and outcome variables. Despite this, future projects can improve upon this work by relying on a larger sample size.
While deficits in executive functioning have been among the most consistently documented following treatment for breast cancer (Anderson-Hanley et al., 2003), it is worth noting that conceptually this domain of functioning encompasses a wide range of cognitive abilities. In this study's test battery, two measures were utilized that are often described as indices of set shifting (Trails B) and response inhibition (Stroop test). Research abounds indicating that executive functioning is not a unitary construct, and that in fact different measures of executive functioning rely on intact processing in a variety of neural regions (Posner & Peterson, 1990). Thus it would be informative to administer a broader range of executive functioning measures to women following treatment for cancer. This could help to determine whether it is appropriate to conclude that there are impairments in the broad range of skills typically associated with executive functioning or whether the deficits are relatively more circumscribed.
With regards to understanding the cognitive changes experience with cancer treatment, it is interesting to note that study participants performed better on immediate and delayed memory tasks than would have been predicted based on WRAT-3 Reading test scores. This raises some questions regarding the utility of this measure as an estimate of pre-morbid functioning with this sample. While this test is commonly used in research and clinical work to estimate pre-morbid abilities, research comparing IQ test scores with various estimates of pre-morbid abilities has suggested that the WRAT-3 Reading test may underestimate abilities among individuals with higher intellectual functioning (Johnstone, Callahan, Kapila, & Bouman, 1996; Weins, Bryan, & Crossen, 1993). As a group our participants had a high level of education (33% had completed some college, and an additional 33% had college degrees or higher), which is known to be correlated with higher intellectual abilities (Neisser et al., 1996). If their intellectual functioning was in fact also above average, WRAT-3 Reading scores may have underestimated their pre-morbid functioning, which could have implications for analyses attempting to determine whether cognitive decline had occurred. However, it is noted that the primary goal of this research was to examine relationships between cognitive deficits and functional outcomes, and that regression analyses addressing this aim utilized cognitive composite scores and did not rely on comparisons with estimated pre-morbid abilities.
IMPLICATIONS FOR CLINICAL PRACTICE
Literature on the experiences of cancer survivors has provided ample evidence of a decline in cognitive functioning among a portion of women who have undergone treatment for breast cancer. Data from the present study document this occurrence and further suggest that cognitive difficulties after treatment can have significant implications for real world functioning. Specifically, it appears that those women experiencing cognitive decline following treatment may experience greater difficulties engaging in productive activities, such as being involved in their communities and being effective in important social roles.
These observations provide support for the ongoing development of interventions for cognitive deficits following chemotherapy, which has already received some attention to date. Potentially useful treatments may include medications such as methylphenidate, which has demonstrated positive effects (National Cancer Institute, 2005). Additionally, psychological interventions focused on providing emotional support and training in metacognitive strategies, similar to those being offered for other populations with mild cognitive decline (e.g., mild traumatic brain injury) have promise and are currently being developed (Ferguson et al., 2007; Tannock et al., 2003).
Given the improving survival rates, cancer is increasingly being viewed as a chronic illness, with affected individuals benefiting from long-term follow-up and assistance in dealing with physical and psychological sequelae. Findings from this research suggest that cognitive issues may warrant similar attention in our efforts to understand and address factors that may affect daily functioning and long-term outcomes among cancer survivors.
Acknowledgments
This research was supported by Grant R03 CA108340 from the National Cancer Institute.
References
- Ahles TA, Saykin A. Cognitive Effects of Standard-Dose Chemotherapy in Patients with Cancer. Cancer Investigation. 2001;19:812–820. doi: 10.1081/cnv-100107743. [DOI] [PubMed] [Google Scholar]
- Ahles TA, Saykin A, Furstenberg CT, Cole B, Mott LA, Skalla K, Whedon MB, Bivens S, Mitchell T, Greenberg ER, Silberfarb PM. Neuropsychologic Impact of Standard-Dose Systemic Chemotherapy in Long-Term Survivors of Breast Cancer and Lymphoma. Journal of Clinical Oncology. 2002;20:485–493. doi: 10.1200/JCO.2002.20.2.485. [DOI] [PubMed] [Google Scholar]
- Ahles TA, Silberfarb PM, Herndon J, Maurer H, Kornblith AB, Aisner J, Perry MC, Eaton WL, Zacharski LL, Green MR, Holland JC. Psychologic and Neuropsychologic Functioning of Patients with Limited Small-Cell Lung Cancer Treated With Chemotherapy and Radiation Therapy With or Without Warfarin: A Study by the Cancer and Leukemia Group B. Journal of Clinical Oncology. 1998;16:1954–1960. doi: 10.1200/JCO.1998.16.5.1954. [DOI] [PubMed] [Google Scholar]
- American Cancer Society Facts and Figures 2006 [On-line] 2006 Available: http://www.cancer.org/downloads/STT/CAFF2006PWSecured.pdf.
- Anderson-Hanley C, Sherman ML, Riggs R, Agocha VB, Compas BE. Neuropsychological effects of treatments for adults with cancer: a meta-analysis and review of the literature. Journal of the International Neuropsychological Society. 2003;9(7):967–982. doi: 10.1017/S1355617703970019. [DOI] [PubMed] [Google Scholar]
- Antoni MH, Lechner SC, Kazi A, Wimberly SR, Sifre T, Urcuyo KR, Phillips K, Gluck S, Carver CS. How stress management improves quality of life after treatment for breast cancer. Journal of Consulting and Clinical Psychology. 2006;74(6):1143–1152. doi: 10.1037/0022-006X.74.6.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker F, Denniston M, Zabora J, Polland A, Dudley WN. A POMS short form for cancer patients: psychometric and structural evaluation. Psycho-Oncology. 2002;11(4):273–281. doi: 10.1002/pon.564. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher K.deS. Multilingual Aphasia Examination. AJA Associates; Iowa City, Iowa: 1989. [Google Scholar]
- Bettencourt BA, Sheldon K. Social roles as mechanism for psychological need satisfaction within social groups. Journal of Personality and Social Psychology. 2001;81(6):1131–1143. [PubMed] [Google Scholar]
- Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, Deasy S, Cobleigh M, Shiomoto G. Reliability and validity of the Functional Assessment of Cancer Therapy - Breast quality of life instrument. Journal of Clinical Oncology. 1997;15(3):974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- Bradley CJ, Neumark D, Bednarek HL, Schenk M. Short-term effects of breast cancer on labor market attachment: Results from a longitudinal study. Journal of Health Economics. 2005;24:137–160. doi: 10.1016/j.jhealeco.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Brezden CB, Phillips K, Abdolell M, Bunston T, Tannock IF. Cognitive Function in Breast Cancer Patients Receiving Adjuvant Chemotherapy. Journal of Clinical Oncology. 2000;18:2695–2701. doi: 10.1200/JCO.2000.18.14.2695. [DOI] [PubMed] [Google Scholar]
- Carver CS, Smith R, Smith G, Antoni MH, Petronis VM, Weiss S, Derhagopian RP. Optimistic personality and psychosocial well-being during treatment predict psychosocial well-being among long-term survivors of breast cancer. Health Psychology. 2005;24(5):508–516. doi: 10.1037/0278-6133.24.5.508. [DOI] [PubMed] [Google Scholar]
- Carver CS, Smith RG, Petronis VM, Antoni MH. Quality of life among long-term survivors of breast cancer: different types of antecedents predict different classes of outcomes. Psycho-Oncology. 2006;15(9):749–758. doi: 10.1002/pon.1006. [DOI] [PubMed] [Google Scholar]
- Corrigan JD, Deming R. Psychometric characteristics of the Community Integration Questionnaire: Replication and extension. Journal of Head Trauma Rehabilitation. 1995;10:41–53. [Google Scholar]
- Falleti MG, Sanfilippo A, Maruff P, Weih L, Phillips K. The nature and severity of cognitive impairment associated with adjuvant chemotherapy in women with breast cancer: A meta-analysis of the current literature. Brain and Cognition. 2005;59:60–70. doi: 10.1016/j.bandc.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Farmer JE, Clark MJ, Sherman AK. Rural versus urban social support seeking as a moderating variable in traumatic brain injury outcome. Journal of Head Trauma Rehabilitation. 2003;18(2):116–127. doi: 10.1097/00001199-200303000-00003. [DOI] [PubMed] [Google Scholar]
- Ferguson RJ, Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole BF, Mott LA. Cognitive-behavioral management of chemotherapy-related cognitive change. Psycho-Oncology. 2007;16(8):772–777. doi: 10.1002/pon.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo MI, Fries E, Ingram KM. The role of disclosure patterns and unsupportive social interactions in the well-being of breast cancer patients. Psycho-Oncology. 2004;13:96–105. doi: 10.1002/pon.717. [DOI] [PubMed] [Google Scholar]
- Freeman JR, Broshek DK. Assessing Cognitive Dysfunction in Breast Cancer: What Are the Tools? Clinical Breast Cancer. 2002;3(Suppl. 3):S91–S99. doi: 10.3816/cbc.2002.s.019. [DOI] [PubMed] [Google Scholar]
- Friedman LC, Kalidas M, Elledge R, Chang J, Romero C, Husain I, Dulay MF, Liscum KR. Optimism, social support and psychosocial functioning among women with breast cancer. Psycho-Oncology. 2006;15:595–603. doi: 10.1002/pon.992. [DOI] [PubMed] [Google Scholar]
- Ganz PA, Desmond KA, Leedham B, Rowland JH, Meyerowitz B, Belin TR. Quality of Life in Long-Term, Disease-Free Survivors of Breast Cancer: A Follow-up Study. Journal of the National Cancer Institute. 2002;94:39–49. doi: 10.1093/jnci/94.1.39. [DOI] [PubMed] [Google Scholar]
- Girard D, Brown J, Burnett-Stolnack M, Hashimoto N, Heir-Wellmer S, Perlman OQ, Seigerman C. The relationship of neuropsychological status and productive outcomes following traumatic brain injury. Brain Injury. 1996;10(9):663–676. doi: 10.1080/026990596124089. [DOI] [PubMed] [Google Scholar]
- Golden CJ. Stroop color and word test. Stoelting; Chicago, IL: 1978. [Google Scholar]
- Griffin SL, Mindt MR, Rankin EJ, Ritchie AJ, Scott JG. Estimating premorbid intelligence: comparison of traditional and contemporary methods across the intelligence continuum. Archives of Clinical Neuropsychology. 2002;17(5):497–507. [PubMed] [Google Scholar]
- Hack TF, Degner LF, Watson P, Sinha L. Do patients benefit from participating in medical decision making? Longitudinal follow-up of women with breast cancer. Psycho-Oncology. 2006;15:9–19. doi: 10.1002/pon.907. [DOI] [PubMed] [Google Scholar]
- Hanks RA, Rapport LJ, Millis SR, Deshpande SA. Measures of executive functioning as predictors of functional ability and social integration in a rehabilitation sample. Archives of Physical Medicine and Rehabilitation. 1999;80(9):1030–1037. doi: 10.1016/s0003-9993(99)90056-4. [DOI] [PubMed] [Google Scholar]
- Helgeson VS, Tomich PL. Surviving Cancer: A Comparison of 5-Year Disease-Free Breast Cancer Survivors with Healthy Women. Psycho-Oncology. 2005;14(4):307–317. doi: 10.1002/pon.848. [DOI] [PubMed] [Google Scholar]
- Holland KD, Hollahan CK. The relation of social support and coping to positive adaptation to breast cancer. Psychology and Health. 2003;18:15–29. [Google Scholar]
- Johnstone B, Callahan CD, Kapila CJ, Bouman DE. The Comparability of the WRAT-R Reading Test and NAART as Estimates of Premorbid Intelligence in Neurologically Impaired Patients. Archives of Clinical Neuropsychology. 1996;11(6):513–519. [PubMed] [Google Scholar]
- Johnstone B, Hexum CL, Ashkanazi G. Extent of cognitive decline in traumatic brain injury based on estimates of premorbid intelligence. Brain Injury. 1995;9(4):377–384. doi: 10.3109/02699059509005777. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological Assessment. Third Edition Oxford University Press Inc.; New York, NY: 1995. [Google Scholar]
- Main DS, Nowels CT, Cavender TA, Etschmaier M, Steiner JF. A qualitative study of work and work return in cancer survivors. Psycho-Oncology. 2005;14:992–1004. doi: 10.1002/pon.913. [DOI] [PubMed] [Google Scholar]
- Meyers CA, Byrne KS, Komaki R. Cognitive deficits in patients with small cell lung cancer before and after chemotherapy. Lung Cancer. 1995;12:231–235. doi: 10.1016/0169-5002(95)00446-8. [DOI] [PubMed] [Google Scholar]
- Micelli G, Caltagirone C, Gainotti G, Masullo C, Silveri MD. Neuropsychological correlated of localized cerebral lesions in nonaphasic brain-damaged patients. Journal of Clinical Neuropsychology. 1981;3:53–63. doi: 10.1080/01688638108403113. [DOI] [PubMed] [Google Scholar]
- Minisini A, Atalay G, Bottomley A, Puglisi F, Piccart M, Biganzoli L. What is the effect of systemic anticancer treatment on cognitive function? The Lancet Oncology. 2004;5:273–282. doi: 10.1016/S1470-2045(04)01465-2. [DOI] [PubMed] [Google Scholar]
- Mols F, Vingerhoets AJ, Coebergh JW, van de Poll-Franse LV. Quality of life among long-term breast cancer survivors: a systematic review. European Journal of Cancer. 2005;41(17):2613–2619. doi: 10.1016/j.ejca.2005.05.017. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute Dexmethylphenidate Reduces Some Symptoms of Chemobrain [On-Line] 2005 Available: http://www.cancer.gov/clinicaltrials/results/chemobrain0605/
- Neisser U, Boodoo G, Bouchard TJ, Boykin AW, Brody N, Ceci SJ, Halpern DF, Leohlin JC, Perloff R, Sternbert RJ, Urbina A. Intelligence: Knowns and unknowns. American Psychologist. 1996;51:77–101. [Google Scholar]
- Osoba D, Hsu M-A, Copley-Merriamn C, Coombs J, Johnson FR, Hauber B, Manjunath R, Pyles A. Stated preferences of patients with cancer for health-related quality of life (HRQOL) domains during treatment. Quality of Life Research. 2006;15:273–283. doi: 10.1007/s11136-005-0580-5. [DOI] [PubMed] [Google Scholar]
- Posner MI, Peterson SE. The Attention System of the Human Brain. Annual Reviews in Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Rao SM, Leo GJ, Ellington L, Nauertz T, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis II. Impact on employment and social functioning. Neurology. 1991;41:692–696. doi: 10.1212/wnl.41.5.692. [DOI] [PubMed] [Google Scholar]
- Reid-Arndt SA. The Potential For Neuropsychology To Inform Functional Outcomes Research With Breast Cancer Survivors. NeuroRehabilitation. 2006;21(1):51–64. [PubMed] [Google Scholar]
- Reitan RM. The relation of the trail making test to organic brain damage. Journal of Consulting Psychology. 1955;16:383–394. doi: 10.1037/h0044509. [DOI] [PubMed] [Google Scholar]
- Rugo HS, Ahles T. The impact of adjuvant therapy for breast cancer on cognitive function: current evidence and directions for research. Seminars in Oncology. 2003;30(6):749–762. doi: 10.1053/j.seminoncol.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Ahles TA, McDonald BC. Mechanisms of chemotherapy-induced cognitive disorders: neuropsychological, pathophysiological, and neuroimaging perspectives. Seminars in Clinical Neuropsychiatry. 2003;8(4):201–216. [PubMed] [Google Scholar]
- Schagen SB, van Dam FSAM, Muller MJ, Boogerd W, Lindeboom J, Bruning PF. Cognitive Deficits after Postoperative Adjuvant Chemotherapy for Breast Carcinoma. Cancer. 1999;85:640–650. doi: 10.1002/(sici)1097-0142(19990201)85:3<640::aid-cncr14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Schagen SB, Muller MJ, Boogerd W, Rosenbrand RM, van Rhijn D, Rodenhuis S, van Dam FSAM. Late effects of adjuvant chemotherapy on cognitive function: a follow up study in breast cancer patients. Annals of Oncology. 2002;13:1387–1397. doi: 10.1093/annonc/mdf241. [DOI] [PubMed] [Google Scholar]
- Schou I, Ekeberg O, Sandvik L, Hjermstad MJ, Ruland CM. Multiple predictors of health-related quality of life in early stage breast cancer. Data from a year follow-up compared with the general population. Quality of Life Research. 2005;14:1813–1823. doi: 10.1007/s11136-005-4344-z. [DOI] [PubMed] [Google Scholar]
- Shacham S. A Shortened Version of the Profile of Mood States. Journal of Personality Assessment. 1983;47(3):305–306. doi: 10.1207/s15327752jpa4703_14. [DOI] [PubMed] [Google Scholar]
- Sheldon K, Bettencourt BA. Psychological Need-Satisfaction and Subjective Well-Being within Social Groups. British Journal of Social Psychology. 2002;41:25–38. doi: 10.1348/014466602165036. [DOI] [PubMed] [Google Scholar]
- Silberfarb PM, Philibert D, Levine PM. Psychosocial Aspects of Neoplastic Disease: II. Affective and Cognitive Effects of Chemotherapy in Cancer Patients. American Journal of Psychiatry. 1980;137:597–601. doi: 10.1176/ajp.137.5.597. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests. Oxford University Press; New York: 1991. [Google Scholar]
- Stanton A, Snider P. Coping with a breast cancer diagnosis: A prospective study. Health Psychology. 1993;12:16–23. doi: 10.1037//0278-6133.12.1.16. [DOI] [PubMed] [Google Scholar]
- Stewart A, Bielajew C, Collins B, Parkinson M, Tomiak E. A Meta-Analysis of the Neuropsychological Effects of Adjuvant Chemotherapy Treatment in Women Treated for Breast Cancer. The Clinical Neuropsychologist. 2006;20:76–89. doi: 10.1080/138540491005875. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP, Hamer L, Palumbo C, Dempster R, Binns M, Levine B, Izukawa D. The effects of focal anterior and posterior brain lesions on verbal fluency. Journal of the International Neuropsychological Society. 1998;4:265–278. [PubMed] [Google Scholar]
- Talley AE, Molix L, Schlegel R, Bettencourt BA. The influence of breast cancer survivors' perceived partner social support and need satisfaction on depressive symptoms: A longitudinal analysis. Psychology and Health. doi: 10.1080/08870440802582682. in press. [DOI] [PubMed] [Google Scholar]
- Tannock IF, Ahles TA, Ganz PA, Van Dam FS. Cognitive impairment associated with chemotherapy for cancer: report of a workshop. Journal of Clinical Oncology. 2003;22(11):2233–2239. doi: 10.1200/JCO.2004.08.094. [DOI] [PubMed] [Google Scholar]
- Taylor EM. Psychological appraisal of children with cerebral deficits. Harvard University Press; Cambridge, MA: 1959. [Google Scholar]
- Tchen N, Juffs HG, Downie FP, Yi Q, Hu J, Chemerynsky I, Clemons M, Crump M, Goss PE, Warr D, Tweedale ME, Tannock IF. Cognitive Function, Fatigue, and Menopausal Symptoms in Women Receiving Adjuvant Chemotherapy for Breast Cancer. Journal of Clinical Oncology. 2003;21:4175–4183. doi: 10.1200/JCO.2003.01.119. [DOI] [PubMed] [Google Scholar]
- Van Dam FSAM, Schagen SB, Muller MJ, Boogerd W, v.d. Wall E, Fortuyn MED, Rodenhuis S. Impairment of Cognitive Function in Women Receiving Adjuvant Treatment for High-Risk Breast Cancer: High Dose Versus Standard-Dose Chemotherapy. Journal of the National Cancer Institute. 1998;90:210–218. doi: 10.1093/jnci/90.3.210. [DOI] [PubMed] [Google Scholar]
- Van Oosterhout AGM, Ganzevles PGJ, Wilmink JT, de Geus BWJ, van Vonderen RGMW, Wijnstra A. Sequelae in Long-Term Survivors of Small Cell Lung Cancer. International Journal of Radiation Oncology. 1996;34:1037–1044. doi: 10.1016/0360-3016(95)02257-0. [DOI] [PubMed] [Google Scholar]
- Visser MRM, Smets EMA. Fatigue, depression and quality of life in cancer patients: how are they related? Supportive Care in Cancer. 1998;6:101–108. doi: 10.1007/s005200050142. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WMS-III administration and scoring manual. The Psychological Corporation; San Antonio, TX: 1997a. [Google Scholar]
- Wechsler D. WAIS-III administration and scoring manual. The Psychological Corporation; San Antonio, TX: 1997b. [Google Scholar]
- Wefel JS, Lenzi R, Theriault RL, Davis RN, Meyers CA. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma. Cancer. 2004;100(11):2292–2299. doi: 10.1002/cncr.20272. [DOI] [PubMed] [Google Scholar]
- Weins AN, Bryan JE, Crossen JR. Estimating WAIS-R FSIQ from National Adult Reading Test - Revised in normal subjects. The Clinical Neuropsychologist. 1993;7:70–84. [Google Scholar]
- Wenzel LB, Fairclough DL, Brady MJ, Cella D, Garrett KM, Kluhsman BC, Crane LA, Marcus AC. Age-related differences in the quality of life of breast carcinoma patients after treatment. Cancer. 1999;86(9):1768–1774. [PubMed] [Google Scholar]
- Wieneke MH, Dienst ER. Neuropsychological Assessment of Cognitive Functioning Following Chemotherapy for Breast Cancer. Psycho-Oncology. 1995;41:61–66. [Google Scholar]
- Wilkinson GS. Wide Range Achievement Test-Revision 3. Jastak Association; Wilmington, DE: 1993. [Google Scholar]
- Willer B, Rosenthal M, Kreutzer JS, Gordon WA, Rempel R. Assessment of community integration following rehabilitation for traumatic brain injury. Journal of Head Trauma Rehabilitation. 1993;8:75–87. [Google Scholar]