Abstract
Non-small cell lung cancer (NSCLC) is a leading cause of cancer-related death worldwide. NSCLC often harbors oncogenic K-RAS mutations that lead to the aberrant activation of several intracellular networks including the phosphoinositol-3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) signaling pathway. Oncogenic K-RAS predicts poor prognosis and resistance to treatment with ionizing radiation (IR). Oncogenic K-Ras expression in the respiratory epithelium is sufficient to initiate NSCLC tumorigenesis, which requires the catalytic subunit of PI3K. Thus, effective inhibition of the PI3K signaling should lead to significant antitumor effects. However, therapy with rapamycin analogs has yielded disappointing results, due in part to compensatory upregulation of AKT. We hypothesized that dual PI3K/mTOR blockade would overcome these limitations. We tested this hypothesis with BEZ235 a novel dual PI3K/mTOR inhibitor that has recently entered clinical development. We found that BEZ235 induces a striking anti-proliferative effect both in transgenic mice with oncogenic K-RAS induced NSCLC and in NSCLC cell lines expressing oncogenic K-RAS. We determined that treatment with BEZ235 was not sufficient to induce apoptosis. However, we found that dual PI3K/mTOR blockade effectively sensitizes NSCLC expressing oncogenic K-RAS to the pro-apoptotic effects of IR both in vitro and in vivo. We conclude that dual PI3K/mTOR blockade in combination with IR may benefit patients with NSCLC expressing oncogenic K-RAS. These findings may have general applicability in cancer therapy, since aberrant activation of PI3K occurs frequently in human cancer.
INTRODUCTION
Non-small cell lung cancer (NSCLC) is a leading cause of cancer-related death. NSCLC often harbors oncogenic K-RAS mutations that lead to the aberrant activation of several intracellular networks including the phosphoinositol-3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) signaling pathway (1, 2).
PI3Ks are heterodimers that consist of the p110 catalytic subunit and p85 adaptor/regulatory subunits. These enzymes catalyze the conversion of phosphatidylinositol biphosphate (PIP2) to phosphatidylinositol triphosphate (PIP3). Thus far, only deregulation of class IA PI3Ks have been implicated in human cancer (2, 3).
In NSCLC, the PI3K signaling pathway is frequently deregulated due to tumor associated mutations affecting its upstream regulators K-RAS or the epidermal growth factor receptor (EGFR) (4). Notably, oncogenic K-RAS mutations confer a poor prognosis in NSCLC and upregulation of the PI3K signaling pathway predicts resistance to cytotoxic therapies due to resistance to apoptosis induction (5). Expression of p110α tumor associated mutations is oncogenic in vitro and in vivo including a mouse model of NSCLC (6–8). Significantly, the p110α subunit of PI3K is required for oncogenic K-Ras-induced NSCLC tumorigenesis in the mouse (9). Moreover, phospho-S6 (P-S6), a downstream target of the PI3K signaling pathway, is upregulated upon oncogenic K-Ras induction in NSCLC of transgenic mice (8).
PIP3 favors AKT membrane translocation and activation of AKT through phosphorylation at threonine 308 and serine 473. This event, in turn, regulates the activity of several downstream targets involved in tumorigenesis, including the mammalian target of rapamycin protein complex (mTORC1) (10). Notably, a rapamycin-insensitive mTOR complex (mTORC2) has been recently identified as the kinase responsible for AKT phosphorylation at serine 473 and consequent activation (11).
Several components of the PI3K signaling pathway have been targeted for development of specific inhibitors capable of antitumor activity. Patients with NSCLC harboring EGFR mutations benefit from treatment with tyrosine kinase inhibitors, such as gefitinib and erlotinib (12–14). However, benefits are usually short-lived due to the development of secondary drug resistance (15, 16). To date, no effective targeted therapy exists for NSCLC tumors that harbor oncogenic K-RAS mutations (17). Rapamycin analogs (rapalogs) have cytostatic properties in preclinical models (18). However, these drugs have had limited success in the clinic because they interrupt negative feedback loops that downregulate PI3K signaling causing paradoxical upregulation of pro-survival signaling pathways (19, 20).
NVP-BEZ235 (BEZ235) is a novel, orally bioavailable dual pan-class I PI3K, mTORC1 and mTORC2 inhibitor, whose structure and pharmacologic properties have been described previously (21). BEZ235 exerts antiproliferative responses in cell lines derived from several malignancies such as breast cancer, glioblastoma and multiple myeloma. Notably, treatment of cancer cells with BEZ235 does not up-regulate AKT activity but does increase the sensitivity of cancer cells to chemotherapy (21–24).
Most recently, it has been reported that treatment with BEZ235 leads to marked tumor regression in NSCLC initiated by p110α H1047R tumor associated mutations, but not significant regression in NSCLC initiated by oncogenic K-Ras. However, BEZ235 administered in combination with a MEK1 inhibitor elicited significant tumor shrinkage in transgenic mice bearing NSCLC initiated by oncogenic K-Ras (8). These results suggest that dual PI3K/mTOR inhibition should be administered in the context of combination therapies that affect multiple pro-survival mechanisms.
Since DNA damage induced by ionizing radiation (IR) potently induces the PI3K signaling pathway to counteract apoptosis (25), we hypothesized that dual PI3K/mTOR blockade would sensitize cancer cells to the pro-apoptotic effects of radiation therapy.
In this study, we characterized the anti-tumor effects exerted by dual PI3K/mTOR inhibition in NSCLC induced by oncogenic K-RAS both in vivo and in vitro. In addition, we demonstrate that the dual PI3K/mTOR pharmacological blockade synergizes with radiation therapy inducing apoptosis in NSCLC expressing oncogenic K-RAS. We propose that this form of combination therapy may lead to important clinical benefits.
MATERIALS AND METHODS
Cell cultures and reagents
Human NSCLC cell lines H23, H460 and H2122 were provided by Dr. A. F. Gazdar (26). Mouse derived NSCLC cell lines LKR10, LKR13 were provided by Dr. Jonathan M. Kurie (18). BEZ235 was obtained from Novartis Pharma (21). LY294002 and Rapamycin were from Biosource. Inhibitors were added to mid-log phase cell cultures at the indicated concentrations. Control cells were incubated with medium containing DMSO at a concentration corresponding to the highest dose used in inhibitor-treated cells. For in vivo studies, BEZ235 was administered by gavage daily in NMP/PEG300 (10:90, v/v) (21). All other chemicals were purchased from Sigma-Aldrich.
Clonogenic survival assays and growth curves
Clonogenic assays were performed as described previously and surviving fractions (SF) were derived using # colonies formed after the treatment / # of cells seeded x plating efficiency (PE) (27). PE is the ratio of colony number to cells seeded. Preliminary experiments showed that the doubling time (DT) of the cells used was 24, 38 and 30 h for H460, H23 and H2122 cells, respectively. Cells were plated in triplicate 14–16h prior to irradiation and/or inhibitor exposure. Cells were irradiated with a Mark I cesium irradiator. Plates were fixed and stained with 0.1% crystal violet 7 to 15 days after treatment. We scored colonies of >50 normal appearing cells and graphed the surviving fraction vs. dose of IR. Survival curves were normalized to drug cytotoxicities. Do and Dq values, reflecting the sensitivities and the shoulder of the survival curves of cells with or without IR exposure, plus or minus inhibitor treatment, were calculated as described (28). Experiments to calculate growth curves were performed as described (29).
Flow cytometric analyses
Cells were allowed to adhere overnight and treated with 50 nM of BEZ235 with or without IR (4 Gy). Cell cycle analysis and determination of apoptosis by propidium iodide staining was performed following a standard procedure with a FC500 Beckman Coulter flow cytometer using the WinMDI V2.8 software (30).
Immunoblotting and antibodies
Western blot (WB) was performed as previously described (29). The following antibodies were obtained from Cell Signaling: Akt, Ser473 phospho-Akt, Ser235/236 phospho-S6, S6, phospho-GSK3β, PARP). Tubulin, HSP-90 were from Sigma-Aldrich, Ki-67 was from Abcam Inc. γ-H2AX from Millipore and 53BP1 from Bethyl labs.
Immunohistochemistry and immunofluorescence
Immunofluorescence and immunohistochemical analyses were performed as described previously (29).
Mouse Studies
Tet-op-K-Ras/CCSP-rtTA (FVB/N) mice were described previously (31). Starting at 8 weeks of age, mice were maintained on doxycycline (doxy)-impregnated food pellets (0,625 g/kg) for 10 weeks. At this point, mice started treatment with BEZ235 or NMP/PEG300 together with doxy diet for additional 2 weeks (Harlan Teklad). Tumor burden was assessed by digital quantification of the area occupied by NSCLC as compared to unaffected lung parenchyma in three representative hematoxylin-and-eosin-stained lung sections/mouse (ImageJ).
Xenograft experiments using H460 NSCLC cells were performed by subcutaneous inoculation of cells into 6-week-old female athymic nude mice (nu/nu). Xenograft tumors of 300 mm3 (7 mice/group) were treated with NMP/PEG300, BEZ235, IR or BEZ235 plus IR. Mice were irradiated with 4 Gy every other day for five treatments using an X-RAD 320 irradiator (Precision X-Ray, Inc., North Branford, CT) to deliver local irradiation to the flank or thigh of lead-shielded mice. BEZ235 was administered daily for seven days. In combination therapy we administered BEZ235 2h prior to IR exposure. Tumor volumes were calculated three times weekly using the formula: (length × width2)/2.
Orthotopic lung growth of H460 tumors was achieved by tail vein injection of 1×106 H460 cells expressing firefly luciferase.
All studies were performed according to the guidelines of the UT Southwestern Institutional Animal Care and Use Committee.
Statistical Analyses
All data presented are the average ± standard deviations of experiments repeated three or more times. Significance was determined using two-tailed unpaired Student's t test or the one-way ANOVA test. Curve fitting for the radiosensitization experiments was performed using the linear-quadratic formula: exp(αD+βD2), which has two components of cell killing: one is proportional to the dose of IR (αD) and the other is proportional to the square of the dose of IR (βD2) (27, 32).
RESULTS
Dual inhibition of PI3K/mTOR leads to a striking anti-tumor effect in transgenic mice with NSCLC induced by oncogenic K-Ras
We used CCSP-rtTA/Tet-op-K-Ras bi-transgenic mice, which express oncogenic K-Ras under the control of the tetracycline operator (Tet-op) and the reverse tetracycline transactivator under the control of the clara cell secretory protein promoter (CCSP-rtTA). In these mice, exposure to doxy faithfully reproduces the events leading to NSCLC tumorigenesis with 100% penetrance (31).
We hypothesized that pharmacologic inhibition of the PI3K signaling pathway would lead to a significant antitumor effect. We tested the antitumor properties of BEZ235, a novel and specific dual pan inhibitor of PI3K and mTORC complexes (i.e. mTORC1 and mTORC2), undergoing clinical testing in cancer patients.
We treated CCSP-rtTA/tet-o-K-Ras mice with doxy for 10 weeks (Fig 1A). At this time point, mice were divided into three cohorts of 8 mice each. A cohort was sacrificed before starting treatment to evaluate baseline NSCLC burden. The treatment cohort received 45 mg/Kg of BEZ235 and the placebo group received NMP/PEG300 by oral gavage together with doxy daily for two additional weeks. We selected this BEZ235 dose and schedule of administration because of a previous study that showed that this dose of BEZ235 achieves maximum intratumoral inhibition of PI3K signaling within 1h of administration and steady-state serum levels between 3 and 5 days (21).
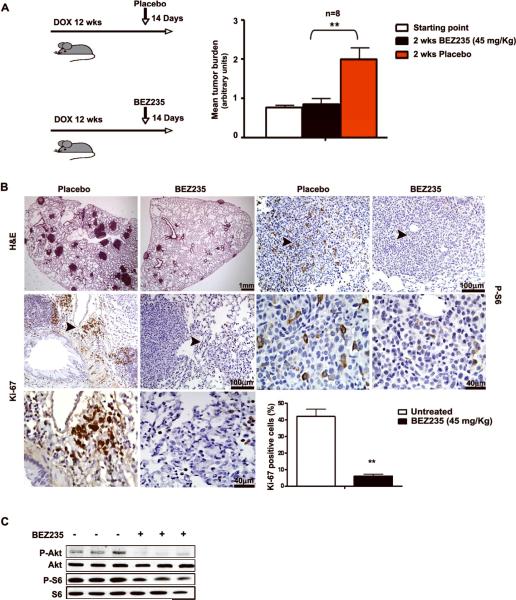
Figure 1. Dual PI3K/mTOR inhibition induces a striking anti-proliferative effect in oncogenic K-Ras induced NSCLC in vivo.
A. Left panel: Treatment scheme: CCSP-rtTA/ Tet-op-K-Ras mice were exposed to either doxy or placebo for 10 weeks. A cohort was sacrificed to estimate baseline tumor burden (indicated by vertical arrows). The other cohorts received either BEZ235 or placebo in addition to doxy for 14 days. Right panel: columns, mean values of tumor burden (n = 8) in the indicated groups of mice (arbitrary units); wks (weeks) **, P<0.01: statistical significant difference between BEZ235 and placebo treated mice; bars, SD. B. Histological evaluation of the effects of dual PI3K/mTOR blockade in onc-K-Ras induced NSCLC. Representative examples of lung sections of CCSP-rtTA/Tet-op-K-Ras mice treated as indicated. H&E: hematoxylin-eosin staining. Sections were stained with the indicated antibodies. Arrows indicate the areas shown magnified in the lower panels. Size of scale bars is indicated. Histogram shows percentage of NSCLC cells positive for Ki-67 in three representative slides (200 nuclei/slide). C. WB of protein extracts obtained from the lungs of six CCSP-rtTA/Tet-op-K-Ras mice treated as indicated at the study endpoint.
As expected, mice exposed to doxy developed multiple large, solid-type adenomas and adenocarcinomas in all lung fields. Pathologic examination of sections stained with hematoxylin-eosin revealed no significant morphologic abnormalities between NSCLC present in mice treated with BEZ235 or placebo. Next, we determined that the tumor burden of mice treated with BEZ235 was less than 50% of the tumor burden of mice treated with placebo and comparable to the tumor burden present in mice of the baseline control group (Fig. 1A and 1B). Treatment with BEZ235 suppressed NSCLC growth, but not the number of NSCLC foci.
We determined that treatment with BEZ235 led to a significant decrease of the level of P-S6 and P-Akt in NSCLC (Fig. 1B and 1C). We also determined that treatment with BEZ235 led to a significant decrease in mitotic activity within NSCLC nodules. In this regard, we found that 40% of NSCLC cells were positive for Ki-67 in control mice, with positive cells preferentially located at the periphery of tumor nodules, while only 5% of NSCLC cells were positive in BEZ235 treated mice (Fig. 1B). We detected no appreciable induction of apoptosis by TUNEL and cleaved caspase 3 staining (data not shown).
Since PI3K signaling controls glucose homeostasis, we assessed glucose levels and body weight in BEZ235 treated and control mice. This analysis revealed no abnormalities. Similarly, we noted no obvious toxicities clinically or by pathological examination of major organs (data not shown). This conclusion is in agreement with published data (8, 21, 23).
Thus, treatment with BEZ235 in vivo caused an efficient inhibition of PI3K signaling in lung tissue without significant compensatory upregulation of P-Akt. This anti-tumor response appeared to be mediated by anti-proliferative effects that occur in the absence of apoptosis.
PI3K/mTOR pathway blockade induces G1 growth arrest in NSCLC cell lines
We tested the properties of BEZ235 in a panel of NSCLC derived cell lines expressing oncogenic K-RAS that are representative of murine and human NSCLC and that have been extensively used for this type of study (26). We used LKR10 and LKR13 cells (derived from NSCLC induced by oncogenic K-Ras in transgenic mice), H23, H460 and H2122 cells (derived from human NSCLC specimens and harbor oncogenic K-RAS mutations) (18, 26). H23 and H2122 cells also harbor deletions/mutations of the p53 tumor suppressor, while H460 cells do not. Finally, the H460 cell line harbors a tumor-associated mutation of the catalytic subunit of PI3K 1.
We found that BEZ235 caused a significant decrease in the level of P-AKT and almost completely inhibited phosphorylation of S6 protein at a concentration between 50 and 150 nM across the different cell lines. These effects were comparable to those exerted by 10 μM of LY294002, a well known-inhibitor of PI3K (Fig. 2A).
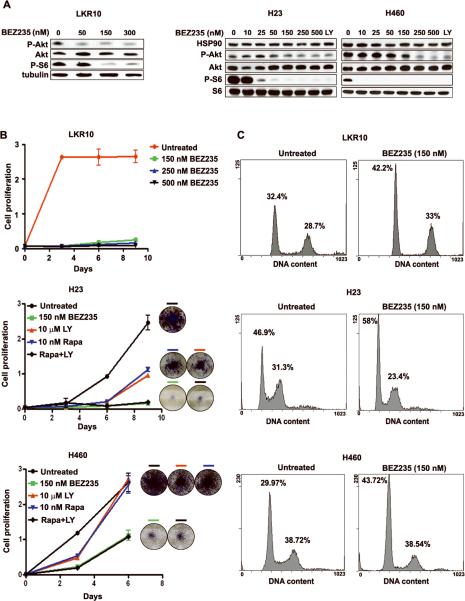
Figure 2. Dual PI3K/mTOR blockade inhibits cell proliferation in NSCLC cells expressing oncogenic K-RAS.
A. BEZ235 inhibits PI3K signaling. Cells were treated as indicated and analyzed by WB. LY: LY294002. B. BEZ235 induces anti-proliferative effects. Color-coded lines indicate growth curves of NSCLC cells treated as indicated. Color-coded bars indicate corresponding tissue culture wells stained at the endpoint of the experiment. Rapa: Rapamycin. Bars, SD C. Cell cycle analyses of NSCLC cells treated for 24h with BEZ235. The percentage of cells in each phase of the cell cycle is provided.
Next, we determined that dual PI3K/mTOR blockade with 150 nM of BEZ235 strikingly inhibited the proliferation of LKR10, LKR13, H23, H460 and H2122 cells (Fig. 2B and Supplemental Fig. 1A). We also compared the effect exerted by BEZ235 to the effects exerted by 10 nM rapamycin (that inhibits mTORC1), 10 μM LY294002 or a combination of the two in H23, H460 and H2122 cells. We concluded that combination treatment with LY294002 together with rapamycin led to anti-proliferative effects that were indistinguishable from responses obtained with BEZ235 (Figure 2B and Supplemental Fig. 1A).
We determined that BEZ235 treatment leads to growth arrest in the G1 phase of the cell cycle with a minor population of apoptotic cells in all NSCLC cells tested. These analyses were performed 24 and 48 h after treatment with equivalent results (Fig. 2C, Supplemental Fig. 1B and data not shown).
Taken together, these data lead to the conclusion that dual PI3K/mTOR blockade with BEZ235 is more effective in inducing anti-proliferative effects than treatment with single agents blocking either PI3K or mTORC1. Moreover, these data strongly suggest that these anti-proliferative effects were mainly due to G1 cell cycle arrest.
Dual PI3K/mTOR blockade sensitizes oncogenic K-RAS expressing NSCLC cells to the pro-apoptotic effects of IR
Our results indicate that dual PI3K/mTOR blockade is not sufficient to efficiently induce apoptosis in NSCLC induced by oncogenic K-Ras in vivo and in NSCLC derived cell lines. Since AKT has been implicated in cell survival responses after IR exposure, we hypothesized that the combination of IR with PI3K/mTOR blockade would lead to pro-apoptotic effects (25, 33, 34). Therefore, we tested whether dual PI3K/mTOR blockade sensitized NSCLC cells expressing oncogenic K-RAS to the anti-proliferative effects of IR.
We performed clonogenic survival assays on H23, H460 and H2122 NSCLC cells exposed to increasing doses of IR (1 to 6 Gy) in the presence of 50 nM BEZ235, the lowest dose that efficiently inhibited colony formation (Fig 3A and Supplemental Fig. 2A). According to preliminary experiments, continuous incubation with BEZ235 at 50 nM completely inhibited colony formation whereas transient exposure for 24h did not cause significant reduction in plating efficiency (Supplemental Table 1). Therefore, we administered BEZ235 2h prior exposure to IR and limited the incubation time to 24h.
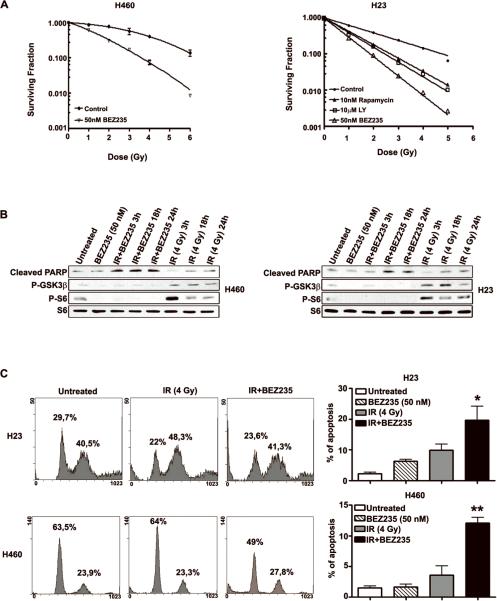
Figure 3. PI3K/mTOR blockade sensitizes NSCLC cells to the pro-apoptotic effects of IR.
A. Graph illustrating clonogenic survival assays of H460 and H23 cells treated as indicated. Colony number was calculated from three replicate plates of three independent experiments; bars, SD. B. WB analysis of H23 and H460 cells treated as indicated. Note that treatment with BEZ235 and IR leads to a significant increase of cleaved PARP (89kDa). C. Cell cycle analysis of H23 and H460 cells treated as indicated. The percentage of cells in each phase of the cell cycle is indicated. Note significant increase of the percentage of sub G1 cells 24h after treatment with BEZ235 in combination with IR. Graph on the right indicates percentage of cells in sub G1. *, P<0.05; **, P<0.01 between untreated and IR+BEZ235.
Analyses of the radiosensitizing effects of BEZ235, indicated that addition of this inhibitor decreased the shoulders (Dq) of the survival curves for all cell lines treated, while concomitantly increasing the radiosensitivities (D0) of exposed cells. Most importantly, the presence of BEZ235 on all irradiated cells increased dose-enhancement ratios (DER) with values ranging between 2.8–1.8 (Supplemental Table 2). These data suggested that addition of BEZ235, inhibits DNA repair and/or augments DNA damage created by IR, possibly by altered cell cycle checkpoint responses.
BEZ235 efficiently inhibited S6 and GSK-3β phosphorylation induced by IR in H460 and H23 cells (Fig. 3B). In addition, BEZ235 induced a significant PARP cleavage, a well-known marker of apoptosis, 3, 18 and 24 h after IR (Fig. 3B). Next, we determined the percentage of sub G1 cells present in H23, H460 and H2122 cell cultures exposed to BEZ235 or to BEZ235 + IR. Also these analyses supported the conclusion that treatment with BEZ235 significantly increased the percentage of irradiated cells undergoing apoptosis (p<0.05) (Fig. 3B, C and Supplemental Fig. 2B).
We detected the same percentage of apoptosis in H23 and H2122 (which express mutant p53) and H460 cells (which express wild type p53). This observation suggests that the response of NSCLC cells to BEZ235 + IR is independent of p53 (Fig. 3B, C and Supplemental Fig. 2B).
Radiosensitization induced by PI3K/mTOR blockade is accompanied by persistence of DNA damage foci
Our data suggested that BEZ235 could facilitate apoptosis inducted by IR by promoting increased or persistent DNA damage. Thus we examined whether BEZ235 affected induction and repair of DNA breaks after IR exposure in H23 and H460 cells. We determined that resolution of γ-H2AX and 53BP1 foci, two well-known markers of DNA double strand break (DSB) damage and repair (when foci decrease), occurred rapidly after treatment with IR (2 Gy). In contrast, treatment with BEZ235 in combination with IR (2 Gy) led to a dramatic persistence of γ-H2AX and 53BP1 foci at 24 h post-IR administration, compared to exposure with IR alone. However, BEZ235 exerted a discordant effect on the number of γ-H2AX and 53BP1 foci 30 minutes after IR in the cell lines we tested. Treatment with BEZ235 alone did not induce γ-H2AX or 53BP1 foci above background levels (Fig. 4A, B and C, Supplemental Fig. 3A, B and Supplemental Figure 4).
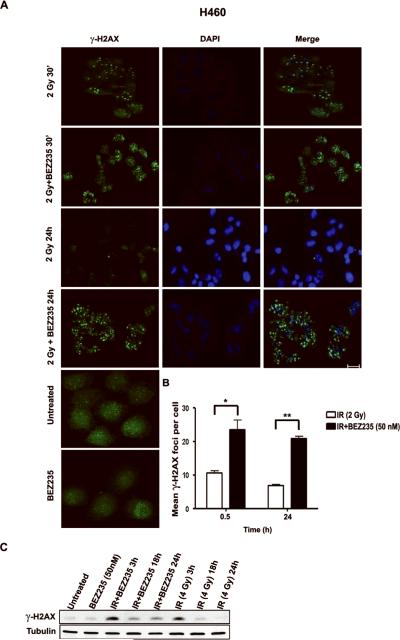
Figure 4. Radiosensitization induced by PI3K/mTOR blockade is accompanied by persistence of DNA damage foci.
A. Detection by immunofluorescence of γ-H2AX foci in H460 cells treated as indicated. Note striking increase in the number of foci 24 h after treatment with BEZ235 in combination with IR; scale bar: 50 μm. B. Histogram represents mean number of γ-H2AX foci/cell. A total of 50 cells were counted in triplicate in three independent experiments; bars, SD; *, P<0.05 and **, P<0.01 between cells treated with IR together with BEZ235 as compared to cells treated with IR. C. WB showing γ-H2AX protein level of cells treated as indicated.
These results indicate that dual PI3K/mTOR blockade with BEZ235 leads to persistent DNA damage induced by IR in oncogenic K-RAS expressing NSCLC cells either because of inhibition of DNA repair or augmentation of damage by cell cycle checkpoint abrogation. These observations also suggest that IR therapy could be exploited to sensitize cancer cells to therapy with dual PI3K/mTOR inhibitors.
The combination of PI3K/mTOR pathway blockade and radiation therapy is an effective antitumor strategyin vivo
We tested the anti-tumor effects of dual PI3K/mTOR blockade in combination with IR in H460 NSCLC xenografts, a well established model of aggressive NSCLC with upregulation of the PI3K/mTOR signalling cascade due to oncogenic K-RAS and p110α mutations (http://Sanger.ac.uk/genetics/CGP/cosmic) (35). Moreover, this cell line is a representative of the cells we used in tissue culture experiments.
We generated 4 cohorts of 7 mice bearing xenografts of 300 mm3 average size. Treatments regimens were as follows: a) placebo; b) 45 mg/Kg BEZ235 daily for 7 days; c) IR treatment alone (five 4 Gy fractions over 10 days); and d) 45 mg/Kg BEZ235 + five 4 Gy fractions over 10 days. Preliminary experiments demonstrated that 45 mg/Kg BEZ235 administered daily for 7 days has limited antiproliferative effects in this xenograft model.
Mice were irradiated 2 h following drug administration since pharmacokinetics studies indicated that within this time frame BEZ235 achieves effective intratumoral concentrations (21). We chose this radiation dose and schedule of administration based on preliminary data that indicated that 20 Gy (administered in fractionated doses every other day) elicits an antitumor response that is not curative in this xenograft model. We chose a fractionated dose to limit overall tissue toxicity and mimic the administration modality used in the clinic (36). Notably, this xenograft volume and ionizing radiation dose is compatible with previous studies involving H460 xenografts (37).
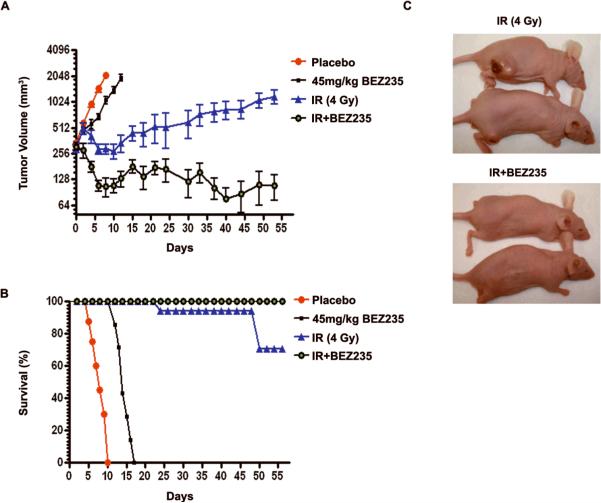
Treatment with BEZ235 in combination with IR, resulted in a greater than 90% reduction in xenograft volume when compared to treatment with IR 45 days after completion of therapy (p<0.001). As expected IR treatment induced an anti-tumor response (p<0.05) as compared to control H460 xenografts. In contrast, single treatment with BEZ235 induced a modest antitumor response that was not statistically significant. All mice in the placebo and BEZ235 treatment groups were euthanized within 10 and 16 days of initiation of treatment, respectively, due to excessive tumor burden. Two mice in the IR treatment group were euthanized 25 and 40 days after initiation of treatment. In contrast, 2 mice of the combination therapy group were apparently tumor free 45 days after initiation of treatment (Fig. 5A, B and C).
Figure 5. Effect of BEZ235 and IR in a xenograft tumor model.
A. Xenograft growth of H460 cells in nude mice treated as indicated. The graph shows xenograft volumes. Each point represents the mean of tumor volume (mm3) of each treatment group (n = 7); bars, SEM; ***P<0.001 between mice treated with IR to mice treated with IR+BEZ235. Mice were sacrificed when tumor volume reached 2000 mm3. B. Kaplan-Meyer curve of H460 xenografts treated as indicated. ***P<0.001 between all survival curves C. Representative pictures of mice 50 days after initiation of treatment.
Combination therapy with BEZ235 and IR was well tolerated in xenograft bearing mice even though we observed a non-statistically significant acute drop in body weight of 5%–10% in the IR and combination treatment groups that resolved 10 days after completion of treatment (data not shown).
We assessed whether combination therapy with BEZ235 and IR induces acute lung toxicity. For this purpose, we generated orthotopic xenografts of H460 cells expressing firefly luciferase. We used luciferase imaging to monitor tumorigenesis. We treated mice with comparable tumor burden with placebo, IR, BEZ235 or BEZ235 in combination with IR.
Histological analysis detected no evidence of acute toxicity in the respiratory epithelium and lung vasculature after treatment with either IR or BEZ235 in combination with IR. As expected, histological analysis of paraffin embedded lung tissue, confirmed that combination therapy leads to a dramatic reduction in tumor burden (supplemental Fig. 5A). Moreover, NSCLC treated with BEZ235 and BEZ235 in combination with IR showed histological changes consistent with IR induced cellular damage such as picnotic nuclei and foamy cellular inclusions (supplemental Fig. 5B).
These results indicate that the dual PI3K/mTOR blockade with BEZ235 leads to significant anti-tumor effects in vivo in the absence of acute lung toxicity (Supplemental Figure 5).
DISCUSSION
Aberrant upregulation of the PI3K signalling pathway occurs commonly in NSCLC. This event is frequently mediated by oncogenic K-RAS mutations. Significantly, expression of oncogenic K-RAS in the respiratory epithelium is also required for maintenance of NSCLC (31). However, no effective inhibitors of oncogenic K-RAS have been developed to date. This is an unmet clinical need since oncogenic K-RAS, the most commonly mutated human proto-oncogene, not only contributes to the tumorigenesis of about 30% of NSCLC, but also confers a poor prognosis and resistance to therapy (34, 38–40).
In the absence of effective K-RAS inhibitors, mTORC1 inhibitors have been tested as anti-cancer drugs in NSCLC. This class of drugs leads to anti-proliferative effects in cell culture. However, rapalogs have demonstrated limited antitumor effects in vivo (18). This lack of effectiveness has been attributed to the inability of rapalogs to inhibit mTORC2 and to the fact that they interrupt several feedback loops ultimately leading to activation of AKT (19, 20). We sought to overcome these limitations with BEZ235, a pan-PI3K inhibitor that also targets the mTORC2 complex.
We found that dual PI3K/mTOR blockade exerts potent anti-proliferative effects but lacks significant pro-apoptotic properties in oncogenic K-RAS induced NSCLC.
These results are surprising since genetic evidence has convincingly demonstrated that the p110α subunit of PI3K is required for oncogenic K-Ras induced NSCLC (9). Therefore, we propose that PI3K function is essential for NSCLC initiation, but not for its maintenance.
Our report provides the first demonstration that dual PI3K/mTOR blockade is an effective strategy to sensitize NSCLC cancer cells expressing oncogenic K-RAS to the pro-apoptotic effects of IR without evidence of acute lung radiation injury. This radiosensitizing effect appears to be independent of p53, a master regulator of the DNA damage response (41). On the contrary, rapalogs in association with IR induce a G2/M block but not significant cell death (42).
Our data suggest that BEZ235 in combination with IR either inhibits DNA repair (seen by decreased Dq values and persistent DNA damage) or augments DNA damage (seen by decreased D0 values, high DER, and enhanced formation of foci due to DSBs). In this setting, apoptosis may be caused by inhibition of pro-survival stress responses (including DNA repair) activated by IR, by abrogation of cell cycle checkpoints or mitotic catastrophe (43). Our observation that BEZ235 induces PARP cleavage at early time points after IR administration, suggests the hypothesis that apoptosis is induced by inhibition of pro-survival responses.
BEZ235 and several other dual PI3K/mTOR inhibitors are being tested in Phase I/II clinical trials. Therefore, our work provides several relevant preclinical data for the design of therapeutic protocols using this novel class of drugs.
In this regard, we add to the evidence that dual PI3K/mTOR inhibition as single therapy leads mainly to cytostatic anti-tumor effects. However, concomitant administration of IR, chemotherapy or a MEK1 inhibitor with dual PI3K/mTOR blockade is sufficient to induce apoptosis (8, 21, 24). Thus, we propose that dual PI3K/mTOR inhibitors should be used in the clinic in combination with agents that either promote apoptosis or that disable pro-survival networks.
We also speculate that PI3K/mTOR blockade in combination with IR may be an effective form of therapy also for NSCLC expressing EGFR or p110α tumor associated mutations and in other malignancies where the PI3K signalling pathway is upregulated independently of oncogenic K-RAS. In this regard, we found that dual PI3K/mTOR blockade induces similar effects in a panel of NSCLC derived cell lines that display upregulation of the PI3K signaling pathway that includes EGFR mutations resistant to tyrosine kinase inhibitors (35) (GK and PPS, unpublished observations).
In the future, it will be of interest to determine the cellular networks responsible for the anti-tumor effects induced by PI3K/mTOR blockade, optimize this form of therapy and identify biomarkers to predict clinical response.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Harold E. Varmus for providing the CC10-rtTA/tet-op-K-Ras tmice. We also thank Dr. Elizabeth Kodym and Junichi Soh (UT Southwestern, Dallas, USA) for insightful suggestions. We are also grateful to Dr. Alice Smith for assistance with Pathologic analysis.
PPS was supported by NIH K08 grant CA 112325, American Cancer Society Institutional Research Grant # 02-196, the Concern Foundation, the Gibson Foundation, Leukemia Texas Inc. GK was supported in part by the graduate program in Molecular, Cellular and Animal Biology, University of Camerino, Italy. The American Italian Cancer Foundation supported AR. DB was supported by DOE grant DE-FG02-06 ER64186. This is manuscript CSCN 047.
Footnotes
DISCLOSURE OF POTENTIAL CONFLICT OF INTEREST S-M. Maira: Stockholder, Novartis. The other authors disclosed no potential conflicts of interest.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–19. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 3.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–30. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 4.Ding L, Getz G, Wheeler DA, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–75. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brognard J, Clark AS, Ni Y, Dennis PA. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer research. 2001;61:3986–97. [PubMed] [Google Scholar]
- 6.Kang S, Bader AG, Vogt PK. Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:802–7. doi: 10.1073/pnas.0408864102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bader AG, Kang S, Vogt PK. Cancer-specific mutations in PIK3CA are oncogenic in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1475–9. doi: 10.1073/pnas.0510857103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engelman JA, Chen L, Tan X, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–6. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta S, Ramjaun AR, Haiko P, et al. Binding of ras to phosphoinositide 3-kinase p110alpha is required for ras-driven tumorigenesis in mice. Cell. 2007;129:957–68. doi: 10.1016/j.cell.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 10.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science (New York, NY. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 12.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:13306–11. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 14.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science (New York, NY. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–92. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 16.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Downward J. Targeting RAS signalling pathways in cancer therapy. Nature reviews. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 18.Wislez M, Spencer ML, Izzo JG, et al. Inhibition of mammalian target of rapamycin reverses alveolar epithelial neoplasia induced by oncogenic K-ras. Cancer research. 2005;65:3226–35. doi: 10.1158/0008-5472.CAN-04-4420. [DOI] [PubMed] [Google Scholar]
- 19.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nature reviews. 2006;6:729–34. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 20.Carracedo A, Ma L, TeruyaFeldstein J, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–74. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maira SM, Stauffer F, Brueggen J, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–63. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 22.Schnell CR, Stauffer F, Allegrini PR, et al. Effects of the dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 on the tumor vasculature: implications for clinical imaging. Cancer research. 2008;68:6598–607. doi: 10.1158/0008-5472.CAN-08-1044. [DOI] [PubMed] [Google Scholar]
- 23.Serra V, Markman B, Scaltriti M, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer research. 2008;68:8022–30. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 24.Eichhorn PJ, Gili M, Scaltriti M, et al. Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer research. 2008;68:9221–30. doi: 10.1158/0008-5472.CAN-08-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bozulic L, Surucu B, Hynx D, Hemmings BA. PKBalpha/Akt1 acts downstream of DNA-PK in the DNA double-strand break response and promotes survival. Molecular cell. 2008;30:203–13. doi: 10.1016/j.molcel.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 26.Phelps RM, Johnson BE, Ihde DC, et al. NCI-Navy Medical Oncology Branch cell line data base. J Cell Biochem Suppl. 1996;24:32–91. doi: 10.1002/jcb.240630505. [DOI] [PubMed] [Google Scholar]
- 27.Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–9. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 28.Boothman DA, Greer S, Pardee AB. Potentiation of halogenated pyrimidine radiosensitizers in human carcinoma cells by beta-lapachone (3,4-dihydro-2,2-dimethyl-2H-naphtho[1,2-b]pyran-5,6-dione), a novel DNA repair inhibitor. Cancer research. 1987;47:5361–6. [PubMed] [Google Scholar]
- 29.Scaglioni PP, Yung TM, Cai LF, et al. A CK2-dependent mechanism for degradation of the PML tumor suppressor. Cell. 2006;126:269–83. doi: 10.1016/j.cell.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 30.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–9. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 31.Fisher GH, Wellen SL, Klimstra D, et al. Induction and apoptotic regression of lung adenocarcinomas by regulation of a K-Ras transgene in the presence and absence of tumor suppressor genes. Genes & development. 2001;15:3249–62. doi: 10.1101/gad.947701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valenzuela MT, Guerrero R, Nunez MI, et al. PARP-1 modifies the effectiveness of p53-mediated DNA damage response. Oncogene. 2002;21:1108–16. doi: 10.1038/sj.onc.1205169. [DOI] [PubMed] [Google Scholar]
- 33.Kim IA, Bae SS, Fernandes A, et al. Selective inhibition of Ras, phosphoinositide 3 kinase, and Akt isoforms increases the radiosensitivity of human carcinoma cell lines. Cancer research. 2005;65:7902–10. doi: 10.1158/0008-5472.CAN-05-0513. [DOI] [PubMed] [Google Scholar]
- 34.Gupta AK, Bakanauskas VJ, Cerniglia GJ, et al. The Ras radiation resistance pathway. Cancer research. 2001;61:4278–82. [PubMed] [Google Scholar]
- 35.Yamamoto H, Shigematsu H, Nomura M, et al. PIK3CA mutations and copy number gains in human lung cancers. Cancer research. 2008;68:6913–21. doi: 10.1158/0008-5472.CAN-07-5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fairchild A, Harris K, Barnes E, et al. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol. 2008;26:4001–11. doi: 10.1200/JCO.2007.15.3312. [DOI] [PubMed] [Google Scholar]
- 37.Iwasa T, Okamoto I, Suzuki M, et al. Radiosensitizing effect of YM155, a novel small-molecule survivin suppressant, in non-small cell lung cancer cell lines. Clin Cancer Res. 2008;14:6496–504. doi: 10.1158/1078-0432.CCR-08-0468. [DOI] [PubMed] [Google Scholar]
- 38.Inoue A, Nukiwa T. Gene mutations in lung cancer: promising predictive factors for the success of molecular therapy. PLoS Med. 2005;2:e13. doi: 10.1371/journal.pmed.0020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huncharek M, Muscat J, Geschwind JF. K-ras oncogene mutation as a prognostic marker in non-small cell lung cancer: a combined analysis of 881 cases. Carcinogenesis. 1999;20:1507–10. doi: 10.1093/carcin/20.8.1507. [DOI] [PubMed] [Google Scholar]
- 40.Pao W, Wang TY, Riely GJ, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2005;2:e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vazquez A, Bond EE, Levine AJ, Bond GL. The genetics of the p53 pathway, apoptosis and cancer therapy. Nat Rev Drug Discov. 2008;7:979–87. doi: 10.1038/nrd2656. [DOI] [PubMed] [Google Scholar]
- 42.Albert JM, Kim KW, Cao C, Lu B. Targeting the Akt/mammalian target of rapamycin pathway for radiosensitization of breast cancer. Mol Cancer Ther. 2006;5:1183–9. doi: 10.1158/1535-7163.MCT-05-0400. [DOI] [PubMed] [Google Scholar]
- 43.Castedo M, Perfettini JL, Roumier T, Andreau K, Medema R, Kroemer G. Cell death by mitotic catastrophe: a molecular definition. Oncogene. 2004;23:2825–37. doi: 10.1038/sj.onc.1207528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.