Abstract
Background
Some clinical studies have suggested that chest compressions prior to defibrillation improve survival in cardiac arrest due to prolonged ventricular fibrillation (VF)(i.e., within the Circulatory Phase). Animal data have also supported this conclusion, and we have previously demonstrated that pre-shock chest compressions increase the VF median frequency and improve the likelihood of a return of spontaneous circulation (ROSC) in normal swine. We hypothesized that chest compressions prior to defibrillation in a swine model of acute myocardial ischemia would also increase VF median frequency and improve resuscitation outcome.
Methods and Results
Twenty six swine were subjected to balloon occlusion of the left anterior descending coronary artery for two hours. The balloon was removed and VF was induced and untreated for 8 minutes. Swine were then treated with up to 3 stacked defibrillation shocks (N=13, shock first group) or three minutes of chest compressions prior to shock (N=13, pre-shock CPR group). In the pre-shock CPR group, median frequency was increased from 7.0 ± 0.8 Hz to 13.9 ± 1.6 Hz after chest compressions, P=0.002. Despite the improved median frequency in the pre-shock CPR group, 24-hour survival with favorable neurological status was significantly worse in the pre-shock CPR group (1/13) compared with the shock first group (8/13, P=0.01).
Conclusions
In a swine model of prolonged VF in acute myocardial ischemia, 24-hour survival with favorable neurological status was more likely when defibrillation was performed first without preceding chest compressions. Myocardial substrate is an important factor in determining the optimal resuscitation strategy.
Keywords: cardiopulmonary resuscitation, myocardial infarction, heart arrest, ventricular fibrillation, defibrillation
Introduction
More than 150,000 Americans suffer a cardiac arrest each year, mostly in the prehospital setting, with approximately 60,000 cases attributed to ventricular fibrillation (VF) (1) Patients with VF are more likely to survive an arrest compared to patients with other rhythms (1), and successful resuscitation from VF is time-dependent. (2, 3, 4, 5) Unfortunately, emergency medical service providers are generally not on site until more than 5 minutes after collapse from VF, and defibrillation from such prolonged VF typically results in a non-perfusing rhythm (pulseless electrical activity or asystole). (3,4,6, 7, 8) Animal and human data indicate that successful resuscitation from these non-perfusing rhythms depends upon prompt, effective cardiopulmonary resuscitation (CPR) (3,4,6,7). Since shocks alone frequently result in successful resuscitation from short duration VF, whereas pre-shock or post-shock circulatory support is generally necessary for prolonged VF, Weisfeldt and Becker proposed that short duration VF (<5 minutes) is the Electrical Phase of VF and that longer duration VF (5–15 minutes) is the Circulatory Phase. (2,5)
Some animal and human data indicate that pre-shock chest compressions can improve outcome from Circulatory Phase VF compared with a “shock first” strategy (3,4,6,7, 9, 10, 11). Other human data do not support these findings (12). Importantly, it is currently unknown whether underlying myocardial substrate, such as acute myocardial ischemia and infarction, may influence the effectiveness of the strategy of pre-shock chest compressions compared with a strategy of shock first. We hypothesized that a strategy of pre-shock chest compressions would improve 24-hour survival with favorable neurological outcome from Circulatory Phase VF compared with a shock first strategy in a swine model of prolonged VF after acute myocardial ischemia. We also hypothesized that pre-shock chest compressions would increase the VF waveform median frequency and improve the likelihood of achieving a return of spontaneous circulation (ROSC).
Methods
Experimental protocols were approved by the University of Arizona Institutional Animal Care and Use Committee. Twenty-six domestic female swine (27 ± 1 kg) were anesthetized with 5% isoflurane in 100% oxygen delivered by a nose cone, followed by oral endotracheal intubation. A surgical plane of anesthesia was maintained with 1.5 to 3% isoflurane in air until the electrical induction of VF. An infrared capnometer (47210A, Hewlett Packard Co., Palo Alto, CA) and pneumotachometer (3700, Hans Rudolph, Kansas City, MO) were placed in-line to measure end-tidal concentration of carbon dioxide and minute ventilation, respectively. Mechanical ventilation was supplied via a rate- and volume-regulated ventilator (Narkomed 2A, North American Drager, Telford, PA) using an initial rate of 12 breaths per minute and a tidal volume of 15 ml/kg. Rate and tidal volume were adjusted to maintain an end-tidal carbon dioxide level of 40± 4 mmHg. The lowest concentration of anesthetic which prevented movement during surgical instrumentation was used. Vascular introducer sheaths (5–7 F, Cordis Corp., Miami, FL) were placed in the right internal and external jugular veins and right carotid artery by a sterile cutdown technique. Micromanometer-tip, Millar solid state pressure transducers (MCP-500, Millar Instruments, Houston, TX) were placed in the descending aorta and right atrium. A Swan-Ganz thermodilution (Baxter Healthcare Corp, Irvine, CA) catheter was placed in the pulmonary artery to determine cardiac output (CO) by thermodilution. A pressure transducer was periodically placed in the left ventricle to measure dP/dt. Correct catheter placement was verified by fluoroscopy. Electrocardiogram, right atrial pressure, and aortic pressure were continuously recorded (P3P Ponemah Physiology Platform, Data Sciences International, St Paul, MN). Hemoglobin was measured with a blood gas analyzer (IL-1306 with 482 cooximeter, Instrumentation Laboratories, Lexington, MA). A pacing catheter electrode was placed into the right ventricle to induce VF with a 100 Hz alternating current. Ventricular fibrillation was confirmed by the ECG waveform and a precipitous decline in aortic pressure. Ventilation was discontinued and a continuous ECG was obtained for the duration of untreated VF. Electrocardiographic signals (lead II) were filtered over a bandpass of 0.5–30 Hz. Median frequency and VF amplitude was computed from the power spectrum obtained by a Fast Fourier Transform analysis over a 5-second interval with the same techniques as previously utilized (9, 10). This analysis was obtained at 8 minutes of VF and prior to the first defibrillation shock.
Acute myocardial ischemia was induced by placement of a coronary balloon catheter in the left anterior descending artery just beyond the second diagonal branch. The balloon was inflated and radioopaque contrast was injected to confirm total occlusion of the vessel. The balloon was kept inflated for two hours. After this period of time, the balloon was removed and VF was induced with a 100 Hz alternating current from the pacing catheter in the right ventricle.
The resuscitation protocol was initiated after 8 minutes of untreated VF (Figure 1). Resuscitation commenced with either 3 minutes of uninterrupted chest compressions (100 compressions/min) and rescue breathing with mechanical ventilation with 100% oxygen followed by defibrillation (pre-shock CPR) or immediate shock (shock first). Up to 3 sequential defibrillation shocks were delivered (stacked shocks) with the first two shocks at 200 J and the third and any subsequent defibrillation shocks at 300 J (monophasic waveform). If VF persisted, chest compressions were continued for one minute before the next defibrillation attempt. Mechanical ventilation with 100% oxygen was continued throughout the resuscitation. Return of spontaneous circulation (ROSC) was defined as an unassisted pulse with a peak systolic aortic pressure greater than 50 mm Hg and a pulse pressure of at least 20 mmHg lasting for at least one minute. Resuscitation was continued until ROSC was achieved, and was terminated if ROSC could not be achieved by 25 minutes. Epinephrine (0.02mg/kg) was administered at 20 minutes post-arrest if defibrillation or pulseless electrical activity had not been successfully terminated. Swine that were successfully resuscitated were observed in the laboratory for one hour, and then returned to the observation pen for an additional 24 hours of surveillance and assessment of neurological status. Neurological status was evaluated by swine cerebral performance categories (9,10). Category 1 was assigned to animals with normal levels of consciousness, gait, and feeding behavior, response to an approaching human and response to human restraint. Category 2 was assigned to animals with mild dysfunction in any of these areas, category 3 to more severe dysfunction including an inability to stand walk or eat. Category 4 was assigned to animals in coma with minimal response to noxious stimuli, and category 5 was assigned to dead animals. Categories 1 and 2 were regarded as a favorable neurological state. The following outcome variables were assessed: a) termination of VF with the first shock b) ROSC achieved with the first set of shocks c) ROSC achieved after the first set of shocks with up to 3 minutes of post shock chest compressions d) ROSC achieved by the end of the resuscitation protocol e) 24-hour survival and f) 24-hour survival with favorable neurological state.
Figure 1.
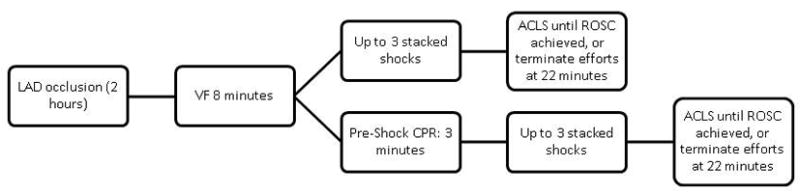
Resuscitation Protocol. VF was untreated for 8 minutes in all acute MI swine. Following this period swine underwent either a defibrillation shock (up to 3 stacked shocks, shock first group) or chest compressions for 3 minutes prior to the first defibrillation shock (pre-shock CPR group). Epinephrine was administered if resuscitation continued beyond 20 minutes. If ROSC was achieved, the animal was monitored for 1 hour, followed by observation in a holding pen for 24 hours, after which time the neurological assessment was performed (see text).
Statistics
Continuous variables were evaluated by Student’s t-test or ANOVA and reported as mean ± standard deviation. Repeated observations were assessed by a paired Student’s t-test. Comparison of discrete variables was accomplished by a Fisher’s exact test. The authors had full access to the data and take responsbility for its integrity. All authors have read and agree to the manuscript as written.
Results
A total of 26 acute MI swine were studied; 13 animals in the pre-shock CPR group and 13 animals in the shock first group. Baseline characteristics are provided in Table 1. Right atrial pressure was higher in the pre-shock CPR group (Table 1). However, there were no significant differences in weight, aortic pressure, cardiac output, or left ventricular dP/dt between pre-shock CPR and shock first subgroups.
Table 1.
Baseline Characteristics
| Shock first (N=13) | Pre-Shock CPR (N=13) | |
|---|---|---|
| Weight, kg | 31.0 ± 4.5 | 31.0 ± 4.1 |
| Hemoglobin, g/dL | 8.6 ± 1.5 | 8.7 ± 1.8 |
| AoS, mm Hg | 81.2 ± 13.8 | 81.1 ± 7.3 |
| AoD, mm Hg | 58.3 ± 14.6 | 60 ± 8.8 |
| RA mm Hg* | 8.0 ± 2.8 | 12.5 ± 4.9 |
| CO L/min | 3.8 ± 1.0 | 3.5 ±0.5 |
| dP/dt max | 1131 ± 389 | 1042 ± 108 |
| dP/dt min | −1072 ± 233 | −1083 ± 153 |
Defib, defibrillation; CPR, cardiopulmonary resuscitation; AoS, systolic aortic pressure; AoD, diastolic aortic pressure; RA, right atrial pressure; CO, cardiac output, dP/dt max, maximum dP/dt, dP/dt min, minimum dP/dt. Data is given as mean ± standard deviation.
P=0.009
The median frequency at 8 minutes of untreated VF was similar in the pre-shock CPR group (7.0 ± 0.8 Hz) and shock first group (9.1 ± 0.7 Hz, P=NS). The three minutes of pre-shock CPR improved the VF median frequency in the pre-shock CPR group to 13.9 ± 1.6 Hz, P=0.002. Therefore, before the first defibrillation shock attempt, VF frequency was higher in animals with pre-shock CPR (13.9 ± 1.6 Hz versus 9.1 ± 0.7 Hz in the shock first group, P=0.01). The VF amplitude was similar in the two groups at 8 minutes of untreated VF (46.0 ± 1.4 mV shock first group versus 45.0 ± 0.8 mV pre-shock CPR group), and after 3 minutes of pre-shock CPR the amplitude increased to 49.1 ± 2.4 mV (P=NS).
Twenty four-hour survival with favorable neurological outcome was significantly more likely to occur in shock first animals: 8 of 13 compared to 1 of 13 animals in the pre-shock CPR group (P=0.01, Table 2). Other outcome variables were not significantly different between groups but tended to favor the shock first group of animals. Termination of VF with the first shock (with or without a perfusing rhythm to follow) occurred in 11 of 13 shock first animals and 6 of 13 pre-shock CPR animals. With up to 3 minutes of chest compressions after the first shock, a perfusing rhythm was restored in 5 of 13 shock first animals and in 1 of 13 pre-shock CPR animals. There was also a trend for improved survival at 24 hours in the shock first group (8 of 13) compared with the pre-shock CPR group (3 of 13, P=0.11).
Table 2.
Resuscitation Outcome
| Outcome | Shock first (N=13) | Pre-shock CPR(N=13) | P value |
|---|---|---|---|
| Termination of VF with first shock | 11 | 6 | 0.097 |
| ROSC, first shocks | 0 | 1 | 1.0 |
| ROSC, first shocks plus 3 min CC | 5 | 1 | 0.160 |
| Any ROSC | 11 | 9 | 0.645 |
| 24 hour survival | 8 | 3 | 0.111 |
| Good Neuro | 8 | 1 | 0.011 |
VF, ventricular fibrillation; CPR, cardiopulmonary resuscitation; ROSC, first shocks, return of spontaneous circulation after the first set of shocks; ROSC, first set of shocks plus 3 min CC, ROSC with the first set of shocks with up to 3 minutes of post shock chest compressions; any ROSC – return of spontaneous circulation after completion of resuscitation protocol; Good Neuro – neurological class I or 2 after 24 hours.
Of the 11 animals that survived 24 hours, 8 animals required 1 shock only during resuscitation, and 3 animals required multiple shocks (range 4 to 10 shocks), including shocks needed to treat recurrent VF. Only one animal that survived 24 hours (in the pre-shock CPR group) required epinephrine. Animals that survived 24 hours required an average of 5.7 minutes of resuscitation (range 1 to 11.25 minutes).
Discussion
These data demonstrate that 24-hour survival with favorable neurological outcome was substantially more likely with a shock first strategy compared with a pre-shock CPR strategy in a swine model of prolonged VF after acute myocardial ischemia. These findings are in stark contrast to previous results from our laboratory and other laboratories showing that pre-shock CPR is a superior strategy for prolonged (Circulatory Phase) VF among swine without acute myocardial ischemia. Although VF waveform frequency improved after 3 minutes of pre-shock chest compressions in these swine with VF after acute myocardial ischemia, the VF waveform frequency improvements did not translate into higher rates of initial successful resuscitation or 24-hour survival.
In previous swine studies without pre-arrest acute myocardial ischemia, we have shown that pre-shock CPR improved VF median frequency and improved myocardial readiness for successful resuscitation and thereby improved the response to initial defibrillation attempts compared with a shock first strategy in both 10-minute untreated VF (9) and 8-minute untreated VF models (10). These previous findings were consistent with earlier animal studies by others also indicating that pre-shock CPR could improve initial defibrillation success compared with a shock first strategy for prolonged VF of 8 minutes, but not for VF 5 minutes (11,13,14). These animal studies suggest that between 5 and 8 minutes the myocardium without pre-arrest ischemia transitions from the Electrical to Circulatory Phase, where chest compressions prior to defibrillation are beneficial.
In contrast, we find in animals with pre-arrest myocardial ischemia, 24-hour survival with favorable neurological outcome was significantly decreased with a pre-shock CPR treatment strategy. Furthermore, these animals rarely attained ROSC with the first shocks regardless of treatment strategy: 1 of 13 animals with pre-shock CPR and 0 of 13 animals with shock first. Why did animals with pre-arrest acute myocardial ischemia have worse outcomes with a pre-shock CPR strategy in contrast to the animals without pre-arrest myocardial ischemia that conversely had benefited from the pre-shock CPR? Our data cannot answer this important question. It is possible that in acute myocardial ischemia the onset of the Circulatory Phase is shifted to a later time, such that at 8 minutes swine may still be within the Electrical Phase. Perhaps the pre-arrest balloon dilation of the coronary arteries and acute myocardial ischemia provided a preconditioning that allowed the myocardium to remain in the Electrical phase longer and to respond more favorably to the initial defibrillation attempts. The benefits of the additional three minutes of pre-shock CPR in preparing the myocardium for successful initial response to defibrillation may have been overshadowed by the benefits of preconditioning, and the adverse effects of three more minutes before the first shock may have been unmasked.
Another possibility is that the difference in outcomes in these two experiments after 8 minutes of untreated VF is related to the duration of chest compressions, which was increased from 90 seconds in the previous studies without myocardial ischemia to 3 minutes in this study with pre-arrest myocardial ischemia. However, we have previously demonstrated benefits of pre-shock CPR after 3 minutes of chest compressions (9). In that study we compared pre-shock CPR versus shock first following 10 minutes of untreated VF. In addition, human studies have demonstrated improved outcomes after either 90 seconds or three minutes of pre-shock CPR compared with shock first (15, 16). Therefore, we do not believe that the longer duration of CPR with the acute myocardial infarction model is responsible for the differences in outcome compared with our previous pre-shock CPR study after 8 minutes of untreated VF without myocardial ischemia.
It has been proposed that ischemic preconditioning results in protection of the myocardium from subsequent ischemic insults by activation of a transcription factor, hypoxia-inducible factor (HIF-1) (17). In a murine model of repetitive coronary occlusion to achieve ischemic preconditioning, activation of HIF-1 was associated with cardioprotection with smaller infarct sizes from subsequently induced ischemia, whereas the suppression of HIF-1 abolished this cardioprotective effect (15). Activation of this transcription factor or some other factor during myocardial ischemia in our swine model may have maintained the myocardium in the electrical phase during VF and improved the likelihood of successful defibrillation to a perfusing rhythm.
Clinical Significance
What is the clinical relevance of this novel observation that pre-shock CPR can have adverse effects in the setting of acute myocardial ischemia? Landmark studies in Seattle (15) and Norway(16) have demonstrated that chest compressions prior to the first defibrillation shock can improve outcomes from out-of-hospital VF when emergency services providers arrive greater than four (15) or five (16) minutes after the initial emergency service call. These two clinical studies and the previous animal investigations are the basis for the current paradigm: pre-arrest CPR is recommended for prolonged VF (2,5).
In a more recent randomized controlled trial, Jacobs and colleagues (12) found no significant difference in achieving ROSC among the patients who were randomized to pre-shock CPR or to shock first. Survival to hospital discharge was also not significantly different between pre-shock CPR and shock first patients. Unlike the two other studies, there was no tendency of differential results with brief duration VF versus prolonged duration VF. However, none of these three clinical studies addressed the issue of concomitant coronary obstruction with acute myocardial ischemia or infarction. The clinical significance of this swine study of acute myocardial ischemia is that it offers a possible explanation for the contradictory results of human studies, if the population studied by Jacobs included more patients with acute myocardial ischemia. In addition, these data suggest that a shock first strategy may be superior to a pre-shock CPR strategy for patients with clear evidence of pre-arrest acute myocardial infarction even when the duration of VF is greater than 5 minutes.
VF Waveform
The VF waveform data from our swine acute myocardial infarction study raise another important question: does the myocardial substrate need to be considered for appropriate interpretation of the VF waveform. Animal and human data have established that higher VF median frequencies are associated with successful defibrillation to ROSC (9,10, 18, 19 ,20, 21, 22, 23, 24, 25, 26). However, the higher median frequencies attained in this study with pre-shock CPR did not translate into an improved outcome. Perhaps the optimal VF waveform for successful defibrillation and resuscitation with favorable neurological outcome is dependent on myocardial substrate, in particular the presence of pre-arrest myocardial ischemia. Consistent with this concept are previous observations indicating that VF waveforms and responses to shocks are influenced by myocardial substrate (27, 28,29). The proportion of sudden cardiac death due to ventricular fibrillation in the setting of acute myocardial infarction is unclear, with autopsy studies demonstrating acute coronary thrombosis in 80% of young victims (30), while other studies have suggested lower percentages of 50% (31) or even 15% (32). Of patients with acute myocardial infarction, it is estimated that approximately 20% develop VF within the first 15 hours (33). Among patients that do survive to hospital admission, 48% were found to have acute coronary occlusion and successful angioplasty was an independent predictor of survival (34). Thus, a model of pre-arrest acute myocardial ischemia and infarction is a clinically relevant model to investigate, particularly if the majority of sudden cardiac arrest due to VF is related to myocardial ischemia. Further investigation is warranted to determine the influence of resuscitation interventions and myocardial substrate upon the predictability of defibrillation. We speculate that the predictability of successful resuscitation based on a VF waveform will require additional consideration of the duration of VF, the previous resuscitation efforts that have occurred, and the underlying myocardial substrate of the fibrillating myocardium.
Limitations
In our experimental model, VF was induced electrically in young healthy swine after removal of the balloon catheter. This model allowed for reperfusion of the vessel at the time of VF induction. This process is presumably relevant to clinical scenarios, such as myocardial ischemia resulting from coronary vasospasm or other acute coronary syndromes with post-arrest coronary artery perfusion. However, this method of temporary coronary occlusion with a balloon catheter may not replicate the clinical situation of acute coronary occlusion due to plaque rupture and coronary thrombosis. In the latter situation, coronary reperfusion may not occur without pharmacologic or percutaneous coronary intervention, although VF arrest may occur following such reperfusion. We chose to deflate the balloon prior to VF induction in order that ongoing cardiogenic shock related to myocardial infarction would not confound our results, particularly with regard to assessing 24 hour neurological outcome. Also, this model may result in preconditioning that might not occur in many patients with acute VF cardiac arrests. However, it is possible that preconditioning occurs in humans who have an acute myocardial infarction prior to VF. Despite these limitations, animal models with acute myocardial ischemia have advantages in representing clinical VF compared with non-ischemic models.
We also acknowledge that by performing statistical tests to assess multiple outcomes there is a chance for a type I statistical error, and that the P values that we obtain should ideally be adjusted. However, adequately correcting for this “multiple comparisons-like” effect is not feasible, as the statistical test used, the Fisher exact test, is already a highly conservative test and a standard Bonferroni correction would likely yield an overly aggressive correction for this uncertainty. Importantly, the most clinically relevant outcome, 24-hour survival with favorable neurological outcome, was substantially more likely in the shock first group. In addition, the next most important endpoint, 24-hour survival, occurred much more commonly in the shock first group, even though the differences did not reach statistical significance.
Conclusions
In this swine model of prolonged VF with pre-arrest acute myocardial ischemia, the 24-hour survival rate with favorable neurological outcome was better with a strategy of shock first compared to pre-shock chest compressions. This study suggests that myocardial substrate is an important factor in determining the optimum resuscitation strategy. The clinical relevance of these findings warrants further investigation.
Commentary
Population studies have shown inconsistent results concerning the utility of delaying the first defibrillation shock to perform chest compressions first in cardiac arrest due to prolonged ventricular fibrillation. Animal studies in normal swine, however, have shown that pre-shock chest compressions improves the likelihood of attaining a perfusing rhythm after defibrillation. It is also known that an altered myocardial substrate, such as acute myocardial ischemia due to coronary occlusion may complicate resuscitation. This study investigates whether pre-shock chest compressions improve resuscitation outcome in the state of acute myocardial ischemia in a swine model of cardiac arrest due to ventricular fibrillation. In swine subjected to balloon occlusion of the left anterior descending artery prior to the induction of ventricular fibrillation, 24-hour survival with favorable neurological status was significantly worse in swine that receive chest compressions prior to the first defibrillation compared to swine treated with shocks first. This study suggests that a shock first strategy may be superior to a pre-shock chest compression strategy for patients with clear evidence of pre-arrest acute myocardial infarction even when the duration of VF is greater than 5 minutes. These findings warrant further investigation.
Acknowledgments
This work was funded by NIH grant, NHLBI R01 HL71694-01, AHA grant 0855587G, and a grant from the Arizona Disease Control Research Commission.
Footnotes
Conflict of Interest Disclosures
Dr. Robert Berg has research grants from the NIH to study post-shock chest cmpressions in swine after prolonged ventricular fibrillation and also receives research funding from Laerdal. There are no other disclosures.
References
- 1.Rea TD, Eisenberg MS, Sinibaldi G, White RD. Incidence of EMS treated out-of-hospital cardiac arrest in the United States. Resuscitation. 2004;63:17–24. doi: 10.1016/j.resuscitation.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 2.Weisfeldt ML, Becker LB. Resuscitation after cardiac arrest: a 3-phase time-sensitive model. JAMA. 2002;288:3035–3038. doi: 10.1001/jama.288.23.3035. [DOI] [PubMed] [Google Scholar]
- 3.Valenzuela TD, Kern KB, Clark LL, Berg RA, Berg MD, Berg DD, Hilwig RW, Otto CW, Newburn D, Ewy GA. Interruption of chest compressions during emergency medical systems resuscitation. Circulation. 2005;112:1259–1265. doi: 10.1161/CIRCULATIONAHA.105.537282. [DOI] [PubMed] [Google Scholar]
- 4.Kern KB, Valenzuela TD, Clark LL, Berg RA, Hilwig RW, Berg MD, Otto CW, Newburn D, Ewy GA. An alternative approach to advancing resuscitation science. Resuscitation. 2005;64:261–268. doi: 10.1016/j.resuscitation.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Gilmore CM, Rea TD, Becker LJ, Eisenberg MS. Three-phase model of cardiac arrest: time-dependent benefit of bystander cardiopulmonary resuscitation. Am J Cardiol. 2006;98:497–499. doi: 10.1016/j.amjcard.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 6.Niemann JT, Stratton SJ, Cruz B, Lewis RJ. Outcome of out-of-hospital postcountershock asystole and pulseless electrical activity versus primary asystole and pulseless electrical activity. Crit Care Med. 2001;29:2366–2370. doi: 10.1097/00003246-200112000-00020. [DOI] [PubMed] [Google Scholar]
- 7.White RD, Russell JK. Refibrillation, resuscitation and survival in out-of-hospital sudden cardiac arrest victims treated with biphasic automated external defibrillators. Resuscitation. 2002;55:17–23. doi: 10.1016/s0300-9572(02)00194-6. [DOI] [PubMed] [Google Scholar]
- 8.Iwami T, Kawamura T, Hiraide A, Berg RA, Hayashi Y, Nishiuchi T, Kajino K, Yonemoto N, Yukioka H, Sugimoto H, Kakuchi H, Sase K, Yokoyama H, Nonogi H. Effectiveness of bystander-initiated cardiac-only resuscitation for patients with out-of-hospital cardiac arrest. Circulation. 2007;116:2900–2907. doi: 10.1161/CIRCULATIONAHA.107.723411. [DOI] [PubMed] [Google Scholar]
- 9.Berg RA, Hilwig RW, Kern KB, Ewy GA. Precountershock Cardiopulmonary Resuscitation improves ventricular fibrillation median frequency and myocardial readiness for successful defibrillation from prolonged ventricular fibrillation: a randomized, controlled swine study. Annals of Emerg Med. 2002;40:563–570. doi: 10.1067/mem.2002.129866. [DOI] [PubMed] [Google Scholar]
- 10.Berg RA, Hilwig RW, Ewy GA, Kern KB. Precountershock cardiopulmonary resuscitation improves initial esponse to defibrillation from prolonged ventricular fibrillation: a randomized, controlled swine study. Crit Care Med. 2004;32:1352–1357. doi: 10.1097/01.ccm.0000127780.01362.e5. [DOI] [PubMed] [Google Scholar]
- 11.Niemann JT, Cairns CB, Sharma J, Lewis RJ. Treatment of prolonged ventricular fibrillation: Immediate countershock versus high-dose epinephrine and CPR preceding countershock. Circulation. 1992;85:281–287. doi: 10.1161/01.cir.85.1.281. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs IG, Finn JC, Oxer HF, Jelinek GA. CPR before defirillation in out-of-hospital cardiac arrest: A randomized trial. Emerg Med Australia. 2005;17:39–45. doi: 10.1111/j.1742-6723.2005.00694.x. [DOI] [PubMed] [Google Scholar]
- 13.Yakaitis RW, Ewy GA, Otto CW, Taren DL, Moon TE. Influence of time and therapy on ventricular defibrillation in dogs. Crit Care Med. 1980;8:157–163. doi: 10.1097/00003246-198003000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Niemann JT, Cruz B, Garner D, Lewis RJ. Immediate countershock versus cardiopulmonary resuscitation before countershock in a 5-minute swine model of ventricular fibrillation arrest. Ann Emerg Med. 2000;36:543–546. doi: 10.1067/mem.2000.109441. [DOI] [PubMed] [Google Scholar]
- 15.Cobb LA, Fahrenbruch CE, Walsh TR, Copass MK, Olsufka M, Breskin M, Hallstrom AP. Influence of cardiopulmonary resuscitation prior to defibrillation in patients with out-of-hospital ventricular fibrillation. JAMA. 1999;281:1182–1188. doi: 10.1001/jama.281.13.1182. [DOI] [PubMed] [Google Scholar]
- 16.Wik L, Hanse TB, Fylling F, Steen T, Vaagenes P, Auestad BH, Steen PA. Delaying defibrillation to give basic cardiopulmonary resuscitation to patients with out-of-hospital ventricular fibrillation. A randomized trial. JAMA. 2003;289:1389–1395. doi: 10.1001/jama.289.11.1389. [DOI] [PubMed] [Google Scholar]
- 17.Eckle T, Kohler D, Lehmann R, El Kasmi KC, Eltaschig HK. Hypoxia-Inducible Factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation. 2008;118:166–175. doi: 10.1161/CIRCULATIONAHA.107.758516. [DOI] [PubMed] [Google Scholar]
- 18.Brown CG, Griffith RF, Van Ligten P, Hoekstra J, Nejman G, Mitchell L, Dzwonczyk R. Median frequency: a new parameter for predicting defibrillation success rate. Ann Emerg Med. 1991;20:787–789. doi: 10.1016/s0196-0644(05)80843-1. [DOI] [PubMed] [Google Scholar]
- 19.Strohmenger HU, Lindner KH, Keller A, Lindner IM, Pfenninger EG. Spectral analysis of ventricular fibrillation and closed-chest cardiopulmonary resuscitation. Resuscitation. 1996;33:155–161. doi: 10.1016/s0300-9572(96)01003-9. [DOI] [PubMed] [Google Scholar]
- 20.Noc M, Weil MH, Tan W, Sun S, Pernat A, Bisera J. Electrocardiographic prediction of the success of cardiac resuscitation. Crit Care Med. 1999;27:708–714. doi: 10.1097/00003246-199904000-00021. [DOI] [PubMed] [Google Scholar]
- 21.Achleitner U, Wenzel V, Strohmenger H-U, Krismer AC, Lurie KG, Linder KH, Amann A. The effects of repeated doses of vasopressin or epinephrine on ventricular fibrillation in a porcine model of prolonged cardiopulmonary resuscitation. Anesthesia and Analgesia. 2000;90:1067–1075. doi: 10.1097/00000539-200005000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Marn-Pernat A, Weil MH, Tang W, Pernat A, Bisera J. Optimizing timing of ventricular defibrillation. Crit Care Med. 2001;29:2360–2365. doi: 10.1097/00003246-200112000-00019. [DOI] [PubMed] [Google Scholar]
- 23.Eftestol T, Sunde K, Aase SO, Husoy JH, Steen PA. Predicting outcome of defibrillation by spectral characterization and non-parameteric classification of ventricular fibrillation in patients with out-of-hospital cardiac arrest. Circulation. 2000;102:1523–1529. doi: 10.1161/01.cir.102.13.1523. [DOI] [PubMed] [Google Scholar]
- 24.Brown CG, Dzwonczyk R. Signal Analysis of the Human Electrocardiogram During Ventricular Fibrillation: Frequency and Amplitude Parameters as Predictors of Successful Countershock. Ann Emerg Med. 1996;27:184–188. doi: 10.1016/s0196-0644(96)70346-3. [DOI] [PubMed] [Google Scholar]
- 25.Strohmenger H-U, Eftestol T, Sunde K, Wenzel V, Mair M, Ulmer H, Lindner KH, Steen PA. The predictive value of ventricular fibrillation electgrocardiogram signal frequency and amplitude variables in patients with out-of-hospital cardiac arrest. Anesth Analg. 2001;93:1428–1433. doi: 10.1097/00000539-200112000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Callaway CW, Menegazzi JJ. Waveform analysis of ventricular fibrillation to predict defibrillation. Curr Opin Crit Care. 2005;11:192–199. doi: 10.1097/01.ccx.0000161725.71211.42. [DOI] [PubMed] [Google Scholar]
- 27.Indik JH, Donnerstein R, Berg R, Hilwig R, Berg M, Kern K. Ventricular Fibrillation Frequency Characteristics are Altered in Acute Myocardial Infarction. Critical Care Medicine. 2007;35:1133–1138. doi: 10.1097/01.CCM.0000259540.52062.99. [DOI] [PubMed] [Google Scholar]
- 28.Indik JH, Donnerstein RL, Hilwig RW, Zuercher M, Feigelman J, Kern KB, Berg MD, Berg RA. The influence of myocardial substrate upon ventricular fibrillation waveform: A swine model of acute and post-myocardial infarction. Critical Care Medicine. 2008;36:2136–2142. doi: 10.1097/CCM.0b013e31817d798c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berg RA, Kern KB, Hilwig RW, Ewy GA. Assisted ventilation during bystander CPR in a swine acute myocarial infarction model does not improve outcome. Circulation. 1997;96:4364–4371. doi: 10.1161/01.cir.96.12.4364. [DOI] [PubMed] [Google Scholar]
- 30.Schmermund A, Schwartz RS, Adamzik M, Sangiorgi G, Pfeifer EA, Rumberger JA, Burke AP, Farb A, Virmani R. Coronary atherosclerosis in unheralded sudden coronary death under age 50: histo-pathologic comparison with ‘healthy’ subjects dying out of hospital. Atherosclerosis. 2001;155:499–508. doi: 10.1016/s0021-9150(00)00598-0. [DOI] [PubMed] [Google Scholar]
- 31.Engdahl J, Holmberg M, Karlson BW, Luepker R, Herlitz J. The epidemiology of out-of-hospital ‘sudden’ cardiac arrest. Resuscitation. 2002;52:235–245. doi: 10.1016/s0300-9572(01)00464-6. [DOI] [PubMed] [Google Scholar]
- 32.Fornes P, Lecomte D, Nicolas G. Sudden out-of-hospital coronary death in patients with no previous cardiac history. An analysis of 221 patients studied at autopsy. J of Forensic Sciences. 1993;38:1084–1091. [PubMed] [Google Scholar]
- 33.O’Doherty M, Tayler DI, Quinn E, Vincent R, Chamberlain DA. Five hundred patients with myocardial infarction monitored within one hour of symptoms. Br Med J (Clin Red Ed) 1983;286:1405–1408. doi: 10.1136/bmj.286.6375.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spaulding CM, Joly L-M, Rosenberg A, Monchi M, Weber SN, Dhainaut J-FA, Carli P. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. New Engl J Med. 1997;336:1629–1633. doi: 10.1056/NEJM199706053362302. [DOI] [PubMed] [Google Scholar]


