Abstract
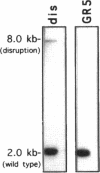
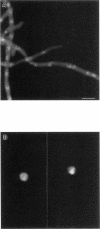
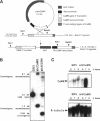
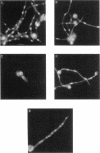
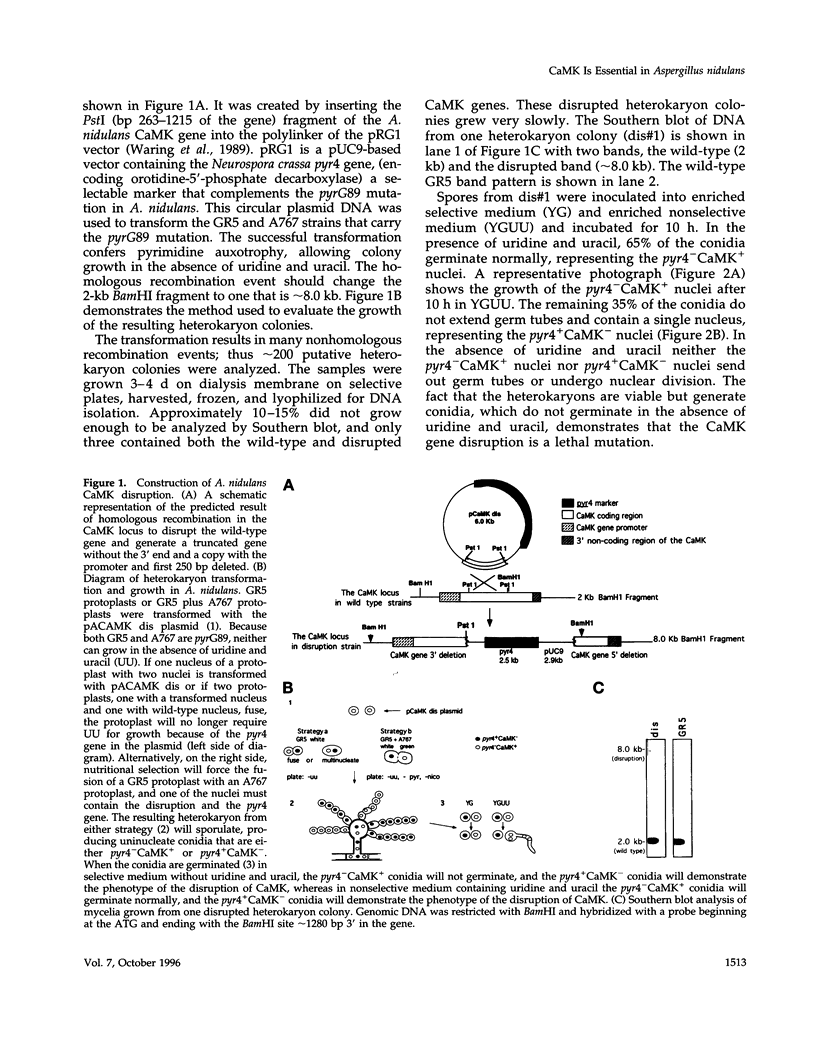
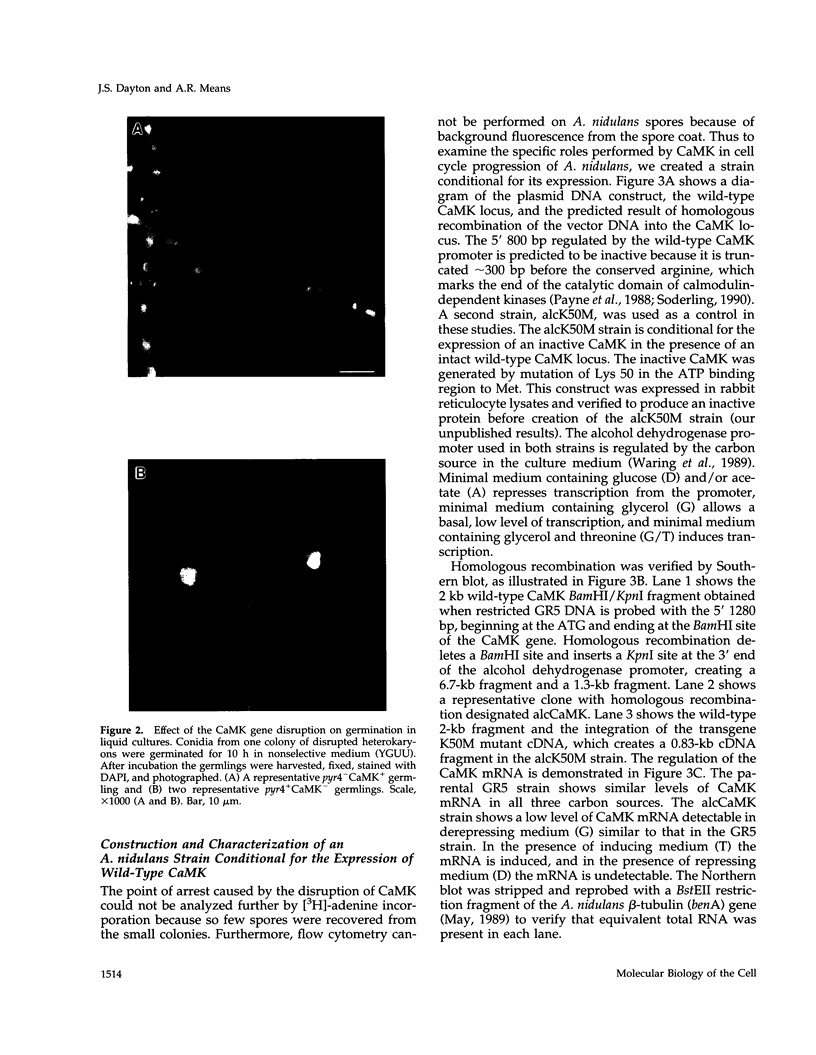
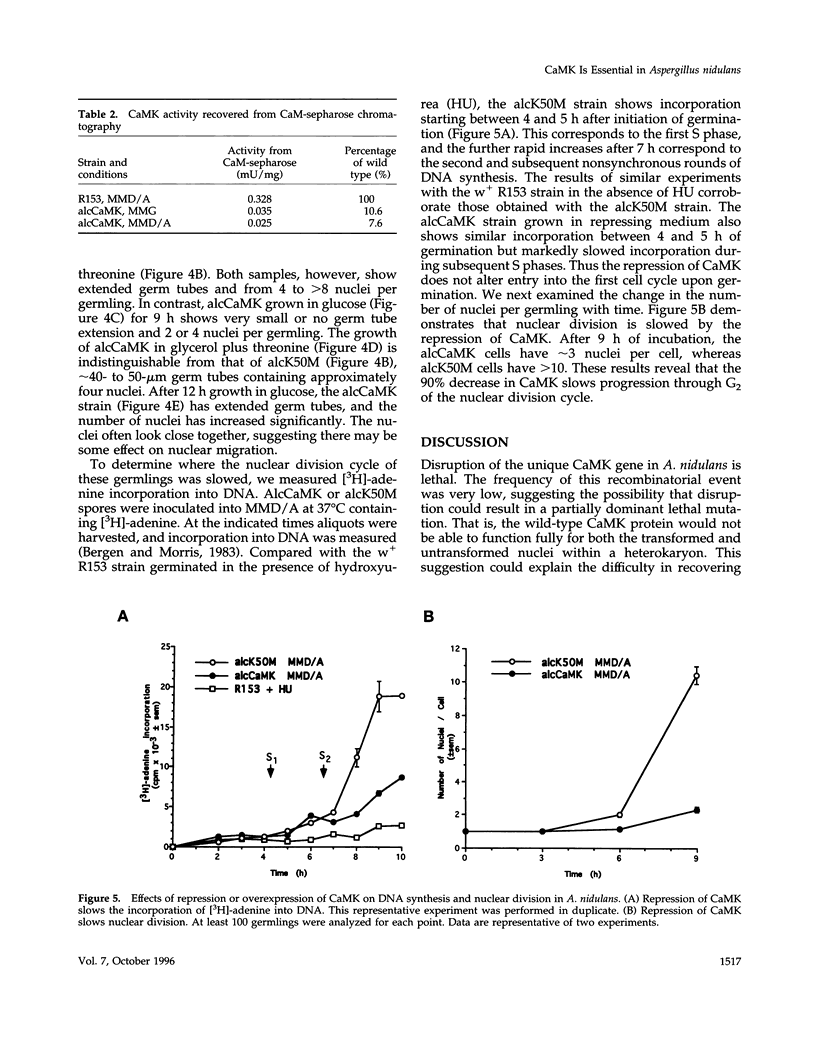
The calmodulin gene has been shown to be essential for cell cycle progression in a number of eukaryotic organisms. In vertebrates and Aspergillus nidulans the calmodulin dependence also requires calcium. We demonstrate that the unique gene encoding a multifunctional calcium/calmodulin-dependent protein kinase (CaMK) is also essential in A. nidulans. This enzyme is required both for the nuclear division cycle and for hyphal growth, because spores containing the disrupted gene arrest with a single nucleus and fail to extend a germ tube. A strain conditional for the expression of CaMK was created. When grown under conditions that resulted in a 90% decrease in the enzyme, both nuclear division and growth were markedly slowed. The CaMK seems to be important for progression from G2 to mitosis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baitinger C., Alderton J., Poenie M., Schulman H., Steinhardt R. A. Multifunctional Ca2+/calmodulin-dependent protein kinase is necessary for nuclear envelope breakdown. J Cell Biol. 1990 Nov;111(5 Pt 1):1763–1773. doi: 10.1083/jcb.111.5.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelt D. C., Fidel S., Farber L. H., Wolff D. J., Hammell R. L. Calmodulin-dependent multifunctional protein kinase in Aspergillus nidulans. Proc Natl Acad Sci U S A. 1988 May;85(10):3279–3283. doi: 10.1073/pnas.85.10.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen L. G., Morris N. R. Kinetics of the nuclear division cycle of Aspergillus nidulans. J Bacteriol. 1983 Oct;156(1):155–160. doi: 10.1128/jb.156.1.155-160.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cherry J. R., Johnson T. R., Dollard C., Shuster J. R., Denis C. L. Cyclic AMP-dependent protein kinase phosphorylates and inactivates the yeast transcriptional activator ADR1. Cell. 1989 Feb 10;56(3):409–419. doi: 10.1016/0092-8674(89)90244-4. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethical guidelines for publications of research. The Council of the Endocrine Society. Endocr Rev. 1993 Feb;14(1):1–2. doi: 10.1210/edrv-14-1-1. [DOI] [PubMed] [Google Scholar]
- Harris S. D., Morrell J. L., Hamer J. E. Identification and characterization of Aspergillus nidulans mutants defective in cytokinesis. Genetics. 1994 Feb;136(2):517–532. doi: 10.1093/genetics/136.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornstein L. B., Gaiso M. L., Hammell R. L., Bartelt D. C. Cloning and sequence determination of a cDNA encoding Aspergillus nidulans calmodulin-dependent multifunctional protein kinase. Gene. 1992 Apr 1;113(1):75–82. doi: 10.1016/0378-1119(92)90671-b. [DOI] [PubMed] [Google Scholar]
- Kulmburg P., Judewicz N., Mathieu M., Lenouvel F., Sequeval D., Felenbok B. Specific binding sites for the activator protein, ALCR, in the alcA promoter of the ethanol regulon of Aspergillus nidulans. J Biol Chem. 1992 Oct 15;267(29):21146–21153. [PubMed] [Google Scholar]
- Lorca T., Cruzalegui F. H., Fesquet D., Cavadore J. C., Méry J., Means A., Dorée M. Calmodulin-dependent protein kinase II mediates inactivation of MPF and CSF upon fertilization of Xenopus eggs. Nature. 1993 Nov 18;366(6452):270–273. doi: 10.1038/366270a0. [DOI] [PubMed] [Google Scholar]
- Lu K. P., Rasmussen C. D., May G. S., Means A. R. Cooperative regulation of cell proliferation by calcium and calmodulin in Aspergillus nidulans. Mol Endocrinol. 1992 Mar;6(3):365–374. doi: 10.1210/mend.6.3.1584213. [DOI] [PubMed] [Google Scholar]
- Mathieu M., Felenbok B. The Aspergillus nidulans CREA protein mediates glucose repression of the ethanol regulon at various levels through competition with the ALCR-specific transactivator. EMBO J. 1994 Sep 1;13(17):4022–4027. doi: 10.1002/j.1460-2075.1994.tb06718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May G. S. The highly divergent beta-tubulins of Aspergillus nidulans are functionally interchangeable. J Cell Biol. 1989 Nov;109(5):2267–2274. doi: 10.1083/jcb.109.5.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGoldrick C. A., Gruver C., May G. S. myoA of Aspergillus nidulans encodes an essential myosin I required for secretion and polarized growth. J Cell Biol. 1995 Feb;128(4):577–587. doi: 10.1083/jcb.128.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B. L., Miller K. Y., Timberlake W. E. Direct and indirect gene replacements in Aspergillus nidulans. Mol Cell Biol. 1985 Jul;5(7):1714–1721. doi: 10.1128/mcb.5.7.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen D. B., Eckstein F. High-efficiency oligonucleotide-directed plasmid mutagenesis. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1451–1455. doi: 10.1073/pnas.87.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmani S. A., Pu R. T., Morris N. R. Mitotic induction and maintenance by overexpression of a G2-specific gene that encodes a potential protein kinase. Cell. 1988 Apr 22;53(2):237–244. doi: 10.1016/0092-8674(88)90385-6. [DOI] [PubMed] [Google Scholar]
- Payne M. E., Fong Y. L., Ono T., Colbran R. J., Kemp B. E., Soderling T. R., Means A. R. Calcium/calmodulin-dependent protein kinase II. Characterization of distinct calmodulin binding and inhibitory domains. J Biol Chem. 1988 May 25;263(15):7190–7195. [PubMed] [Google Scholar]
- Planas-Silva M. D., Means A. R. Expression of a constitutive form of calcium/calmodulin dependent protein kinase II leads to arrest of the cell cycle in G2. EMBO J. 1992 Feb;11(2):507–517. doi: 10.1002/j.1460-2075.1992.tb05081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen C. D., Means R. L., Lu K. P., May G. S., Means A. R. Characterization and expression of the unique calmodulin gene of Aspergillus nidulans. J Biol Chem. 1990 Aug 15;265(23):13767–13775. [PubMed] [Google Scholar]
- Rasmussen C., Garen C., Brining S., Kincaid R. L., Means R. L., Means A. R. The calmodulin-dependent protein phosphatase catalytic subunit (calcineurin A) is an essential gene in Aspergillus nidulans. EMBO J. 1994 Jun 1;13(11):2545–2552. doi: 10.1002/j.1460-2075.1994.tb06544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen C., Rasmussen G. Inhibition of G2/M progression in Schizosaccharomyces pombe by a mutant calmodulin kinase II with constitutive activity. Mol Biol Cell. 1994 Jul;5(7):785–795. doi: 10.1091/mbc.5.7.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen G., Rasmussen C. Calmodulin-dependent protein kinase II is required for G1/S progression in HeLa cells. Biochem Cell Biol. 1995 Mar-Apr;73(3-4):201–207. doi: 10.1139/o95-024. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr Assays of protein kinase. Methods Enzymol. 1983;99:3–6. doi: 10.1016/0076-6879(83)99034-1. [DOI] [PubMed] [Google Scholar]
- Soderling T. R. Protein kinases. Regulation by autoinhibitory domains. J Biol Chem. 1990 Feb 5;265(4):1823–1826. [PubMed] [Google Scholar]
- Tsutsumi-Ishii Y., Tadokoro K., Hanaoka F., Tsuchida N. Response of heat shock element within the human HSP70 promoter to mutated p53 genes. Cell Growth Differ. 1995 Jan;6(1):1–8. [PubMed] [Google Scholar]
- Waldmann R., Hanson P. I., Schulman H. Multifunctional Ca2+/calmodulin-dependent protein kinase made Ca2+ independent for functional studies. Biochemistry. 1990 Feb 20;29(7):1679–1684. doi: 10.1021/bi00459a002. [DOI] [PubMed] [Google Scholar]
- Waring R. B., May G. S., Morris N. R. Characterization of an inducible expression system in Aspergillus nidulans using alcA and tubulin-coding genes. Gene. 1989 Jun 30;79(1):119–130. doi: 10.1016/0378-1119(89)90097-8. [DOI] [PubMed] [Google Scholar]
- Whitaker M., Patel R. Calcium and cell cycle control. Development. 1990 Apr;108(4):525–542. doi: 10.1242/dev.108.4.525. [DOI] [PubMed] [Google Scholar]