Abstract
Aged subjects are more vulnerable to administration of the endotoxin lipopolysaccharide, but research on age-associated sensitivity to other immune stimulants has been limited. The current study examined the effects of administering the superantigen, staphylococcal enterotoxin A (SEA), to young (4-month) and aged (20-month) male C57BL/6J mice on consumption of a novel liquid, cytokine production, corticosterone levels, and expression of central mRNA levels of cytokines and corticotropin releasing hormone. SEA produced exaggerated hypophagia in aged mice, as they showed decreased consumption that persisted for 24hrs. SEA increased hypothalamic mRNA levels of interleukin-1β in the aged, but not the young, mice 2hrs after administration. No differences in cytokine expression were observed 24hrs after SEA. Both age groups showed increased plasma corticosterone levels 2hrs after SEA administration. However, 24hrs after SEA exposure the aged, but not the young, mice showed an augmented corticosterone response to the consumption test. Collectively, these data show that aging may exacerbate the behavioral and neuroinflammatory response to superantigen exposure. Further, the present study suggests that immune activation may result in delayed alterations in stress-induced corticosterone production in aged subjects.
Keywords: Age, SEA, cytokine, corticosterone, interleukin-1β
1. Introduction
The response to infection is not simply limited to activation of immune cells, but rather involves a coordinated response between the central nervous system (CNS) and the endocrine and immune systems (Dantzer, 2004). Immune activation, via the production of cytokines and other inflammatory molecules, induces neural, endocrine, behavioral, and motivational changes, apparently adapted to effectively eliminate infection (Dantzer, 2004). Normal aging is accompanied by progressive alterations in immune function that can result in greater susceptibility to infection (e.g., Staphylococcus aureus and influenza) and delayed recovery (Miller, 1996; Laupland et al., 2003). In addition, research has found that older subjects show exaggerated behavioral and cognitive deficits following immune activation (Sparkman et al., 2004; Godbout et al., 2005; Barrientos et al., 2006; Kohman et al., 2007; Chen et al., 2008; Godbout et al., 2008; Abraham & Johnson, 2009). For example, Godbout et al. [2005] found that 24hrs after administration of lipopolysaccharide (LPS; an endotoxin found in the cell wall of Gram-negative bacteria) there was decreased social behavior and locomotor activity in old mice, but not in young mice. The increased vulnerability to LPS-induced behavioral and cognitive effects in aged subjects has been attributed to an exaggerated inflammatory response within the CNS (Dilger & Johnson, 2008). Microglial cells, a major source of cytokines within the CNS, appear to be in a primed or active state in aged animals even in the absence of an immune stimulus (Rozovsky et al., 1998; Frank et al., 2006; Chen et al., 2008). Further, in response to peripheral immune activation aged animals show higher central levels of the proinflammatory cytokines interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) (Godbout et al., 2005; Barrientos et al., 2006; Chen et al., 2008).
Research on exaggerated neurobehavioral responses in aged subjects following immune activation has predominately employed systemic administration of LPS or infection with E. coli, stimuli which engage the innate arm of the immune system, consisting largely of monocyte and macrophage-mediated responses. However, recent evidence shows that activated T cells also exert neuromodulatory effects (Kipnis & Schwartz, 2005; Urbach-Ross & Kusnecov, 2009). Therefore, whether aged animals show a similar increased behavioral and neuroendocrine sensitivity to T cell activation, is a missing feature of our understanding of immunologically mediated effects on the aging brain.
Staphylococcal enterotoxin A (SEA; produced by Gram-positive bacteria) is a bacterial superantigen that serves as a potent activator of T cells, selectively stimulating the Vβ3+ region of T cell receptors (TCR) in a major histocompatability II dependent manner (Li et al., 1999). The interaction of SEA and other superantigens (such as staphylococcal enterotoxin B, SEB) with specific motifs on the variable region of the TCR β-chain, rapidly stimulates oligoclonal expansion of T cells and the subsequent release of the cytokines interleukin-2 (IL-2), TNF-α, interferon-γ, interleukin-10 (IL-10), and IL-1β (Bette et al., 1993; Rosendahl et al., 1997; Rossi-George et al., 2005; Urbach-Ross et al., 2008). These effects are followed by induction of hypothalamic-pituitary adrenal (HPA) axis activation and neural activation of limbic brain regions (e.g., central amygdala, lateral septum, and paraventricular nucleus of the hypothalamus) (Kusnecov et al., 1999; Goehler et al., 2001; Rossi-George et al., 2005; Serrats & Sawchenko, 2006). In contrast to LPS, administration of SEB, under conditions that activate the HPA axis and autonomic nervous system, fails to induce measurable central levels of proinflammatory cytokine mRNA in the brain (Del Rey et al., 2000).
Behaviorally, superantigen administration leads to a neophobic response to gustatory stimuli, as SEA- or SEB-treated subjects show hypophagia when presented with a novel diet (Kusnecov et al., 1999; Kawashima & Kusnecov, 2002; Rossi-George et al., 2005). However, the reduced consumption does not appear to result from general sickness, as mice show decreased intake of novel, but not familiar, liquid or solid food (Kusnecov et al., 1999; Rossi-George et al., 2005). Previous research by Rossi-George et al. (2005) demonstrated that disrupting the activity of TNF-α, either by administration of an anti-TNF antibody or the use of TNF knockout mice, prevented the SEA-induced hypophagia. Collectively, these reports indicate that superantigen administration induces hypophagia that appears to be specific to novel substances and may involve the production of TNF-α (Kusnecov et al., 1999; Kawashima & Kusnecov, 2002; Rossi-George et al., 2005).
The neurobehavioral impact of bacterial superantigen administration has not been previously investigated in aged subjects. However, there is some evidence that aged subjects are more immunologically sensitive to SEB administration, as aged mice have higher rates of mortality, impaired T cell clonal deletion, and altered cytokine production after an SEB challenge (Aroeira et al., 1994; Kuschnaroff et al., 1997; Castle et al., 1999). Given the extensive characterization of the behavioral, endocrine, and cytokine responses to the superantigen, SEA, (Kusnecov et al., 1999; Urbach-Ross et al., 2008), the current study investigated whether aged mice would show increased sensitivity to SEA administration relative to young mice. It was hypothesized that old mice would show exaggerated hypophagia following SEA administration when compared to young mice. Further, it was hypothesized that concomitant age-related alterations in cytokine production and HPA activation would accompany these behavioral effects.
2. Methods
2.1. Experimental subjects
Subjects were 70 four-month-old and 64 twenty-month-old male C57BL/6J mice that were purchased from The Jackson Laboratory (Bar Harbor, ME) at 6–8 weeks and aged in our colony room at Rutgers. All animals were housed in groups of 3–4 in a standard polycarbonate mouse cage with food and water available ad libitum. Lights were turned on at 0600 and turned off at 1800. Animals were treated in compliance with the Guide for the Care and Use of Laboratory Animals and the experiments were conducted in accordance with a protocol approved by the Institutional Animal Care and Use Committee (IACUC) at Rutgers University.
2.2. Experiment 1: Acute or repeated SEA administration in old and young mice
For each age group, mice were divided into SEA or saline treatment groups. Intraperitoneal (i.p.) injections of SEA (Sigma, St Louis, MO) were given at a dose of 5μg/mouse in 0.9% sterile saline. Mice were evaluated for consumption of a novel liquid (i.e., Prosobee; infant formula; Mead Johnson, Evansville, IN) at 2, 4, 6, and 24hrs after SEA or saline administration. The Prosobee solution was prepared according to the manufacturer’s instructions. During each testing session, mice were individually placed into an opaque cage and given access to the Prosobee solution for 1 hour. Consumption was measured by subtracting the bottle weight (grams) after each session from the pretest weight.
The same mice tested for Prosobee consumption were divided by age and prior treatment condition and given a second i.p. injection of SEA (5μg/mouse) or saline 72 hours after the first injection. The inclusion of a second injection (i.e., SEA-2) resulted in eight treatment groups (i.e., Saline/Saline, Saline/SEA, SEA/Saline, and SEA/SEA for both young and old mice). Two hours after the second injection mice were euthanized via decapitation and brain, blood, and spleen were collected for analysis of transcription levels of corticotropin releasing hormone (CRH), IL-1β, IL-6, and TNF-α in the CNS, splenic and plasma cytokine levels, and plasma corticosterone levels (assay procedures described below). We have previously shown that injection with SEA does not result in detectable splenic or plasma cytokine levels three days later (Urbach-Ross et al., 2008).
2.3. Experiment 2: Response to SEA 24hrs post injection in old and young mice
A separate batch of 32 four-month- and 28 twenty-month-old mice were given an i.p. injection of SEA (5μg/mouse) or an equivalent volume of sterile saline. Twenty-four hours after treatment, half of the mice were given a single testing session to evaluate consumption of a novel liquid (i.e., Prosobee) as described in Experiment 1. The remaining mice stayed in their home cages to control for the effects of the behavioral test. Immediately following consumption testing, mice were euthanized via decapitation and brain, blood, and spleen were collected. Home-cage control mice were sacrificed at the same time as the mice submitted to consumption testing.
2.4. Plasma corticosterone and IL-1β levels
Blood was collected in heparin-treated vacutainer tubes (Becton-Dickinson, Rutherford, NJ), centrifuged (2000rpm for 15min at 4°C), and plasma collected and stored at −70°C until assayed. Plasma corticosterone levels were assessed by a radioimmunoassay (RIA; MP Biomedical, Irvine, CA), according to the manufacturer’s instructions. Samples were run in duplicate and expressed as ng/ml. Plasma IL-1β levels were measured by an enzyme-linked immunosorbant assay (ELISA), according to the manufacturer’s instructions (BD Biosciences, San Diego, CA) and data are expressed as pg/ml. Due to limited sample volume, only IL-1β was assessed in plasma samples.
2.5. Splenic cytokine levels
Spleen samples were homogenized in 1mM phenylmethanesulfonyl fluoride (PMSF) in 0.1M phosphate buffer, centrifuged (4000rpm for 30min at 4°C), and supernatants collected. Cytokine levels were measured by ELISA, according to the manufacturer’s instructions (BD Biosciences, San Diego, CA). Total protein content for the spleen samples were determined by BCA protein assay (Pierce, Rockford, IL), according to the manufacturer’s instructions. Splenic cytokine data are expressed as pg/mg of protein.
2.6. Hypothalamic and amygdala expression of CRH, TNF-α, IL-6, and IL-1β mRNA
The hypothalamus and amygdala were dissected on a chilled glass Petri dish and analyzed for expression of CRH, TNF-α, IL-6, and IL-1β mRNA by quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR). Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions and quantified on a Nanodrop spectrophotometer (Nanodrop, Thermo Scientific, Wilmington, DE). Extracted RNA was converted into cDNA using the High Capacity Reverse Transcription kit (Applied Biosystems, Foster City, CA). Real-time PCR was conducted with primers for the target genes TNF-α, IL-6, IL-1β, and CRH. Primer sequences were obtain from Schnydrig et al. (2007) and Rossi-George et al. (2005). Expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was measured in all samples as the endogenous control. The PCR reactions consisted of 80ng of cDNA, 5pmoles of reverse and forward primers (sequences provided in Table 1), and PCR master mix containing the fluorescent agent SYBR Green (Applied Biosystems, Foster City, CA) and was run on an Applied Biosystems 7900HT PCR instrument. A standard curve was created for each brain region (i.e., hypothalamus and amygdala) and gene (i.e., CRH, TNF-α, IL-6, IL-β, and GAPDH) using tissue from a young mouse that received an i.p. LPS (250μg/kg) injection 3hrs prior to sacrifice. The relative amount of gene expression within each sample was determined by comparing a samples threshold cycle with the standard curve. The data are therefore expressed as arbitrary units based on the standard curve. Expression levels of target genes were normalized against the endogenous control gene GAPDH.
Table 1.
Sequences of forward and reverse primers for RT-PCR.
| Accession | Forward | Reverse | |
|---|---|---|---|
| CRH | NM_205769 | GTTGAATTTCTTGCAACCGGAG | GACTTCTGTTGAGGTTCCCCAG |
| TNF-α | NM_013693 | ATGCTGGGACAGTGACCTGG | CCTTGATGGTGGTGCATGAG |
| IL-6 | NM_031168 | TTCCATCCAGTTGCCTTCTTG | GAAGGCCGTGGTTGTCACC |
| IL-1β | NM_00836 | CCAAAAGATGAAGGGCTGCT | TCATCAGGACAGCCCAGGTC |
| GAPDH | NM_017008 | AACTCCCTCAAGATTGTCAGCAA | GGCTAAGCAGTTGGTGGTGC |
2.7. Statistical analyses
The Prosobee consumption data from Experiment 1 were analyzed by repeated-measures ANOVA (Statview 5.0, SAS, Cary, NC), with SEA treatment and Age as the between-subject variables and Time of session as the within-subject (i.e., repeated measures) variable. As mice were only tested for Prosobee consumption once, the data from Experiment 2 were analyzed by a 2 × 2 factorial with Age and SEA treatment as the between subject variables. The biological measures were evaluated via factorial ANOVAs with Age, SEA treatment, and SEA-2 treatment (i.e., second injection of SEA or saline 72hrs later) as the between-subject factors for Experiment 1. For Experiment 2, Age, SEA treatment, and Test procedure (i.e., consumption or home cage) were the between-subject factors. An alpha level of 0.05 was the criterion for rejection of the null hypothesis. Significant interaction effects were submitted to Bonferroni post hoc tests to protect against familywise error.
3. Results
3.1. Experiment 1: Consumption of a novel liquid diet
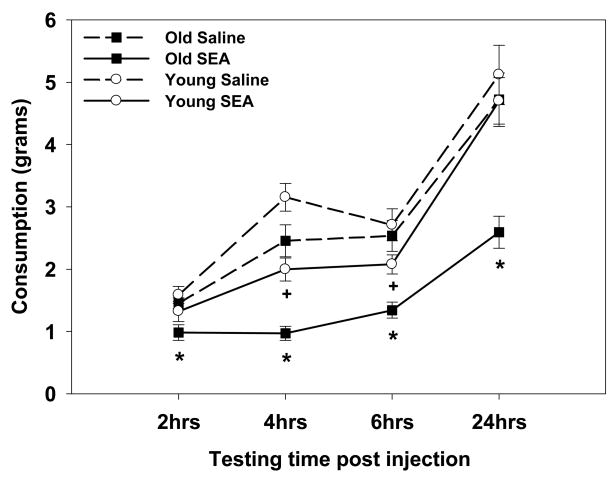
The consumption data revealed that the hypophagic impact of SEA was more pronounced in the aged mice, as SEA administration decreased consumption in the old mice at all of the time-points tested (i.e., 2, 4, 6, and 24hrs post treatment) relative to saline-treated old mice (interaction between Age, SEA, and Time F3, 210=2.83, p=0.03, see Figure 1). For the young mice, SEA administration only decreased consumption 4 and 6hrs after treatment relative to corresponding saline controls (p’s<0.05), but not at the 2 or 24hr time-points. Therefore, SEA administration produced a greater and prolonged magnitude reduction in consumption for the old mice. The increased sensitivity to SEA cannot be accounted for by a general age-related decrease in liquid intake, as the aged saline-treated mice only showed decreased consumption relative to the young saline-treated mice only at the 4hr session (p=0.04), but at no other time points.
Figure 1.
SEA administration in the aged mice decreased consumption 2, 4, 6, and 24hrs later relative to aged saline controls. However, in the young mice SEA administration only decreased consumption 4 and 6hrs following treatment relative to saline-treated mice. Data are expressed as means ± standard error of the means (SEMs). Individual groups had n of 9–10 mice. * indicates a significant difference between old SEA- and saline-treated mice. + indicates a significant difference between young SEA- and saline-treated mice.
3.2. Experiment 1: Plasma corticosterone levels following acute or repeated SEA
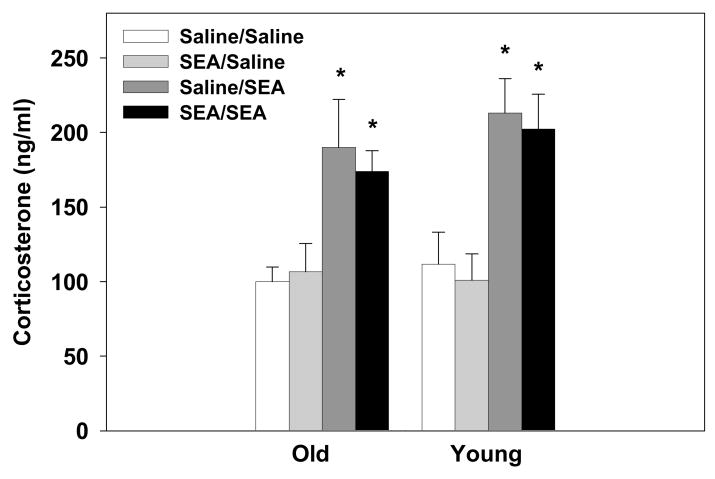
Administration of SEA, 2hrs prior to plasma collection, increased corticosterone levels relative to saline-treated mice (main effect of SEA-2: F1,66=38.14, p=0.0001, see Figure 2). The subject’s age or prior treatment condition (i.e., SEA or saline 72hrs earlier) had no effect on the corticosterone response to SEA. That is, the elevated corticosterone levels were similar in young and old mice after an acute or secondary exposure to SEA.
Figure 2.
SEA administration (2hrs prior) increased plasma corticosterone levels. This response was unaffected by the animal’s age or prior SEA treatment condition. Data are expressed as means ± SEMs. Individual groups had n of 9–10 mice. * indicates a significant difference from saline-treated animals.
3.3. Experiment 1: Central levels of CRH, TNF-α, IL-6, and IL-1β mRNA following acute or repeated SEA
CRH mRNA
There were no significant main effects or interaction effects for CRH mRNA levels in the hypothalamus or the amygdala 2hrs after treatment (i.e., all treatment and age groups had similar CRH mRNA levels; data not shown).
IL-1β mRNA
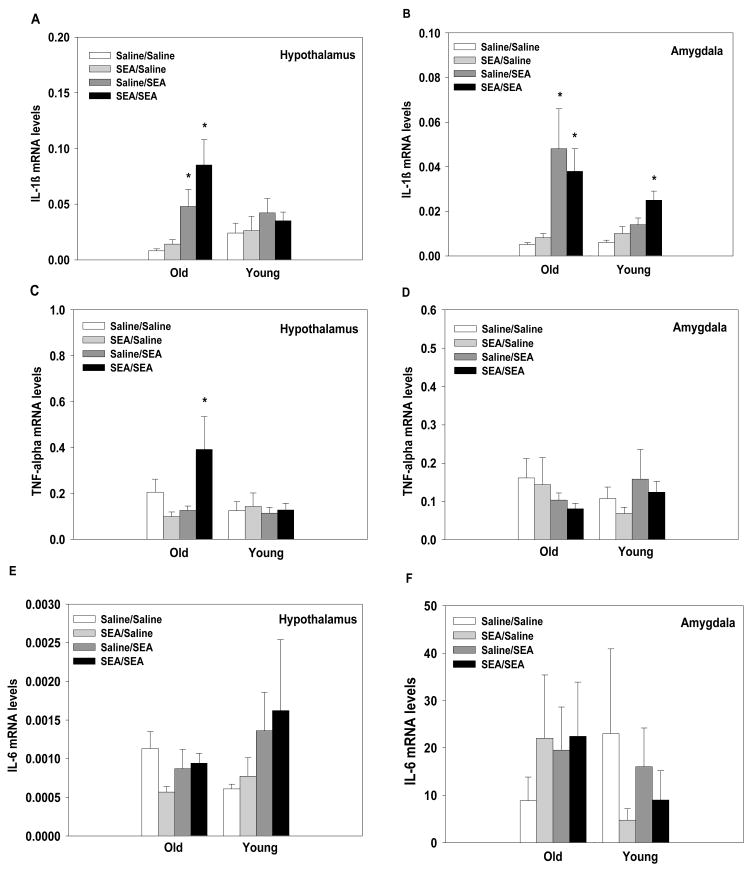
SEA administration significantly elevated hypothalamic levels of IL-1β mRNA in aged, but not young, mice (main effect of SEA-2: F1,66=15.04, p=0.0002; interaction between Age and SEA-2: F1,66=5.68, p=0.02, see Figure 3A). Both age groups showed increased IL-1β mRNA levels in the amygdala in response to acute or repeated SEA administration, but the increase was significantly greater in the aged subjects (main effect of SEA-2: F1,66=24.33, p=0.0001; main effect of Age: F1,66=5.56, p=0.02; interaction between Age and SEA-2: F1,66=6.62, p=0.01, see Figure 3B). These data show that SEA-induced IL-1β expression was enhanced in the aged mice.
Figure 3.
Acute or repeated administration of SEA increased IL-1β mRNA levels in the aged, but not young, mice in the hypothalamus (A). Both young and aged mice showed increased levels of IL-1β mRNA in the amygdala after acute or repeated SEA administration (B). For TNF-α, aged mice showed increased expression in the hypothalamus following repeated SEA administration (C), but no differences were observed in the amygdala (D). IL-6 mRNA levels were not altered in either the hypothalamus (E) or amygdala (F) by SEA administration. The threshold cycle for each sample was converted to arbitrary units based on the standard curve. Data are expressed as means ± SEMs. Individual groups had n of 9–10 mice. * indicates a significant difference from saline controls.
TNF-α mRNA
Analysis of hypothalamic TNF-α mRNA levels showed that aged mice given repeated SEA injections had significantly higher mRNA levels of TNF-α than all other groups (interaction between Age, SEA, and SEA-2: F1,66=4.50, p=0.03, see Figure 3C). No significant differences were found for TNF-α expression within the amygdala (see Figure 3D).
IL-6 mRNA
No significant differences for IL-6 expression were found in the hypothalamus or the amygdala (see Figure 3E and 3F). That is, IL-6 mRNA levels were not altered by the subject’s age or treatment condition.
3.4. Experiment 1: Plasma levels of IL-1β
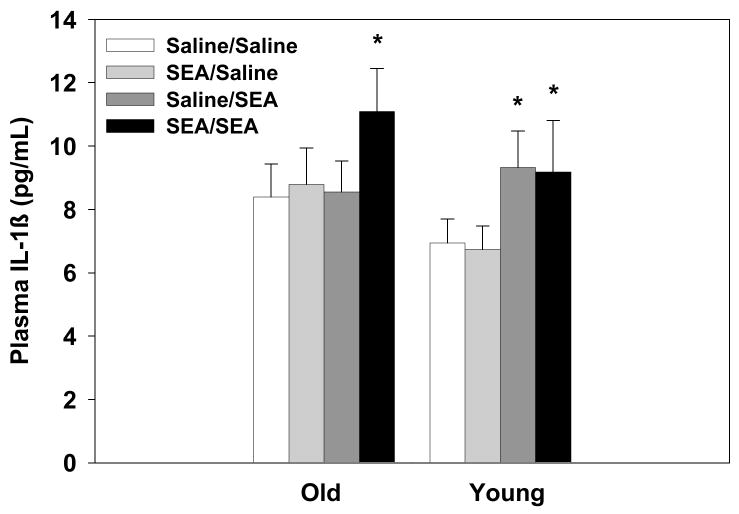
Plasma levels of IL-1β were significantly elevated 2hrs after SEA administration (main effect of SEA-2: F1,66=4.87, p=0.03, see Figure 4). However, the old Saline/SEA treated mice did not show elevated plasma IL-1β levels relative to saline controls.
Figure 4.
A significant main effect of SEA-2 treatment condition showed that administration of SEA 2hrs prior to sample collection increased plasma levels of IL-1β relative to controls. Data are expressed as means ± SEMs. Individual groups had n of 9–10 mice. * indicates a significant difference from saline controls.
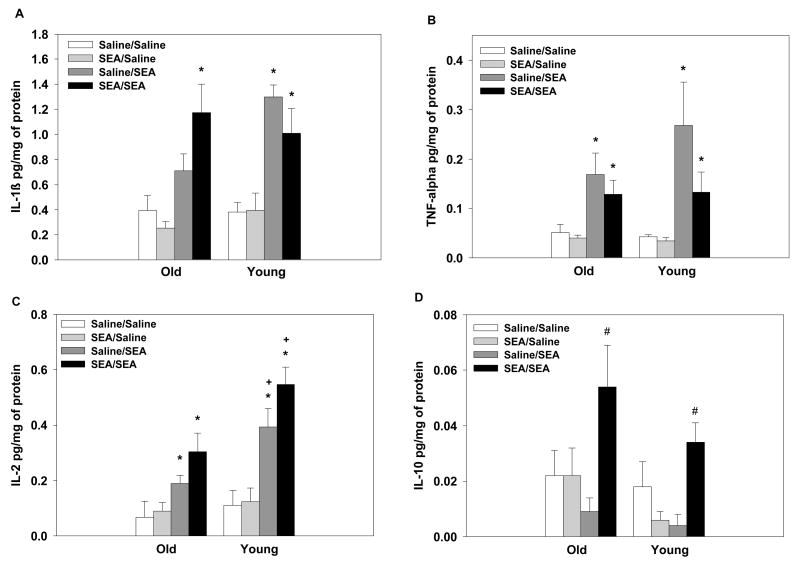
3.5. Experiment 1: Splenic cytokine levels following acute or repeated SEA
IL-1β
SEA administration increased splenic levels of IL-1β relative to saline-treated mice with the exception of the old mice in the Saline/SEA group which did not differ from the old Saline/Saline group (main effect of SEA-2: F1,66=52.04, p=0.0001; interaction between: Age × SEA treatment × SEA-2 treatment interaction: F1,66=5.57, p=0.02, see Figure 5A). Consistent with plasma levels, two injections of SEA (i.e., SEA/SEA group) were needed to significantly increase splenic IL-1β levels in the aged mice (p=0.0006), whereas young mice had elevated IL-1β levels following acute or repeated injections (p’s<0.05).
Figure 5.
Analysis of splenic IL-1β levels (A) revealed that aged mice only showed elevated IL-1β with repeated SEA exposure (i.e., SEA/SEA group; SEA administered 2hrs prior), whereas young subjects showed significantly elevated levels following acute or repeated SEA administration. SEA administration, regardless of the animal’s age or prior treatment condition increased TNF-α levels (B). Aged mice had lower levels of IL-2 following SEA than young mice (C). Elevated IL-10 levels were only observed following repeated SEA exposure, regardless of the animal’s age (D). Data are expressed as means ± SEMs. Individual groups had n of 9–10 mice. * indicates a significant difference from saline controls. + indicates that marked group is significantly different aged mice. # indicates a significant difference from all other treatment groups.
TNF-α
Splenic levels of TNF-α were increased following SEA administration relative to saline-treated mice (main effect of SEA-2: F1,66=24.92, p=0.0001, see Figure 5B). The animal’s age or prior SEA treatment condition did not influence the magnitude of the response to the SEA challenge.
IL-2
SEA administration increased splenic levels of IL-2 relative to saline-treated mice (main effect of SEA-2: F1,66=45.855, p=0.0001). The aged mice had lower basal levels of IL-2 than young mice and showed attenuated levels of IL-2 following SEA administration relative to young mice (main effect of Age: F1,66=11.47, p=0.001; interaction between Age and SEA-2: F1,66=5.70, p<0.01, see Figure 5C).
IL-10
Repeated SEA administration (i.e., SEA/SEA), independent of age, significantly increased levels of IL-10 compared to all other treatment groups (main effect of SEA: F1,66=6.79, p=0.01; interaction between SEA and SEA-2: F1,66=12.54, p=0.0007, see Figure 5D). An acute SEA challenge did not elevate IL-10 levels 2hrs after treatment.
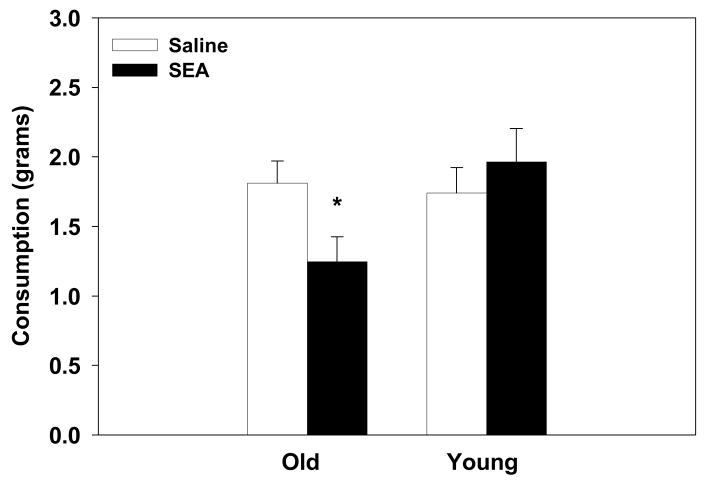
3.6. Experiment 2: Consumption in young and old mice 24hrs post SEA administration
Administration of SEA 24hrs prior to testing decreased consumption in the old mice relative to saline controls (interaction between Age and SEA: (F1,24=3.99, p=0.05, see Figure 6). SEA administration had no effect on consumption rates in the young mice 24hrs later. Further, there was not a significant difference between the old and young saline-treated mice. Consistent with Experiment 1, aged mice showed prolonged hypophagia following SEA administration.
Figure 6.
Administration of SEA 24hrs prior to testing decreased consumption of a novel liquid in the aged, but not in the young mice. Data are expressed as means ± SEMs. Individual groups had n of 7–8 mice. * indicates a significant difference from saline controls.
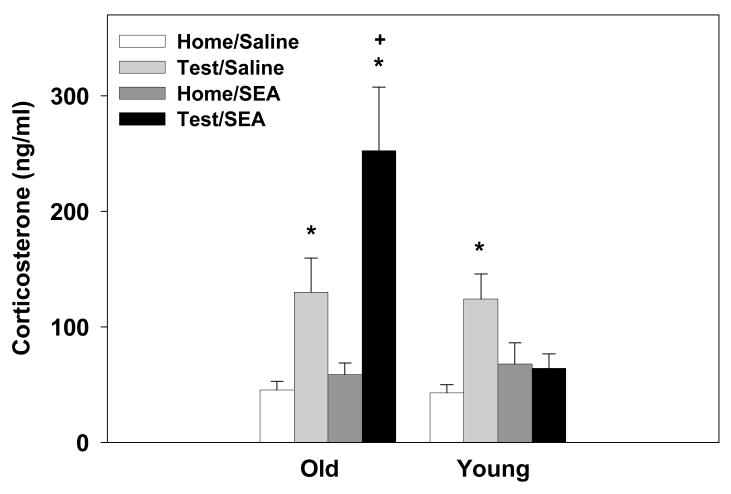
3.7. Experiment 2: Plasma corticosterone levels 24hrs post SEA administration
The old mice that received SEA 24hrs prior had significantly higher corticosterone levels relative to young SEA-treated mice, but only when tested for consumption of Prosobee (main effect of test procedure: F1,52=26.79, p=0.0001; main effect of Age: F1,52=7.50, p=0.008; interaction between Age and SEA: F1,52=6.17, p=0.01; interaction between Age and SEA and Test procedure: F1,52=7.98, p=0.006, see Figure 7). There was no difference between old and young home cage control mice given SEA 24hrs prior. Mice tested for Prosobee consumption, with the exception of the young SEA-treated subjects, had higher plasma corticosterone levels than home cage controls (p’s<0.01). While consumption testing increased corticosterone levels, the response was augmented in the aged mice pretreated with SEA.
Figure 7.
A significant 3-way interaction revealed that administration of SEA 24hrs prior increased plasma corticosterone levels in the aged mice, but not the young mice, that were tested for consumption of a novel liquid. Data are expressed as means ± SEMs. Individual groups had n of 7–8 mice. * indicates a significant difference from saline controls. + indicates a significant difference from Test/Saline group.
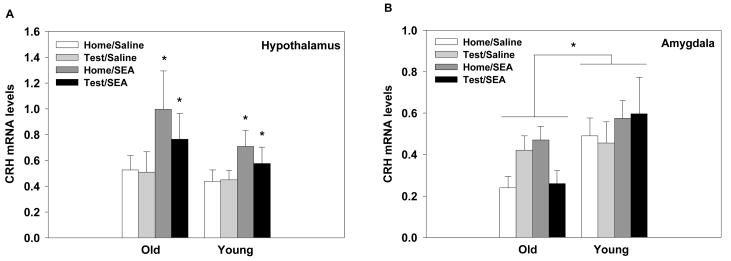
3.8. Experiment 2: Central levels of CRH, TNF-α, IL-6, and IL-1β mRNA 24hrs post SEA administration
Mice treated with SEA 24hrs prior, regardless of Age and Test procedure, had significantly higher levels of CRH gene expression in the hypothalamus compared to saline controls (main effect of SEA: F1,52=6.06, p=0.01, see Figure 8A). For the amygdala, the aged mice overall had lower levels of CRH mRNA relative to young mice (main effect of Age: F1,52=6.99, p=0.01, see Figure 8B). No other significant effects were found.
Figure 8.
Exposure to SEA 24hrs prior, irrespective of age, increased CRH mRNA levels within the hypothalamus (A). Aged mice, regardless of treatment condition, had lower levels of CRH mRNA levels in the amygdala than young mice (B). The threshold cycle for each sample was converted to arbitrary units based on a standard curve. Data are expressed as means ± SEMs. Individual groups had n of 7–8 mice. * indicates a significant difference from saline controls.
No significant effects of Age, SEA treatment, or Test procedure were found for expression of IL-1β, IL-6, and TNF-α mRNA in either the amygdala or hypothalamus (data not shown). That is, all age and treatment groups had equivalent levels of IL-1β, IL-6, and TNF-α mRNA 24hrs after treatment with SEA or saline.
3.9. Experiment 2: Plasma IL-1β levels 24hrs post SEA administration
There were no significant differences in plasma IL-1β levels when assessed 24hrs after treatment (data not shown).
3.10. Experiment 2: Splenic cytokine levels 24hrs after SEA administration
Administration of SEA had no effect on splenic levels of IL-1β or TNF-α 24hrs after treatment, as levels were equivalent for both age and SEA treatment groups (data not shown). For IL-6, the old mice, regardless of treatment condition, had lower splenic levels of IL-6 relative to the young mice (main effect of Age: F1,52=4.53, p=0.03, data not shown). No other significant differences were observed for IL-6. Mice treatment with SEA 24hrs prior had lower splenic IL-10 levels relative to saline controls (main effect of SEA: F1,52=7.60, p=0.008, data not shown). There were no other significant differences found for IL-10 levels.
4. Discussion
It is well recognized that the incidence of infectious disease increases with age (Miller, 1996; Laupland et al., 2003). This poses a particular risk for the development of neurobiological disorders in aging populations, since exposure to infectious pathogens, including viral and bacterial toxins, leads to cognitive and behavioral alterations (Dantzer, 2004; Kusnecov & Goldfarb, 2005). In the current study, young and old animals were exposed to the bacterial T cell superantigen SEA, and behavioral, endocrine, and cytokine responses were assessed. In previous studies using adult animals (approximately 2 months old), SEA activates the HPA axis, increases neuronal activity in limbic brain regions, and augments neophobic reactions to novel gustatory and non-gustatory environmental stimuli (Urbach-Ross & Kusnecov, 2009). Consistent with prior reports (Rossi-George et al., 2005; Urbach-Ross et al., 2008), the younger animals in the current study displayed a neophobic response and HPA axis activation. However, older animals showed greater and more prolonged reductions in consumption, as well as a higher corticosterone response to a psychogenic stressor. Moreover, we report that in response to SEA challenge, central expression of IL-1β mRNA was enhanced in the older animals.
These are the first observations that a bacterial T cell superantigen increases brain IL-1β gene transcription, and in the present context may be relevant to the motivational and HPA axis reactivity of the older animals. Aged mice were more sensitive to the hypophagic effects of SEA, as they showed early onset as well as prolonged duration of the hypophagic response (up to 24hrs); while young mice only showed decreased consumption 4 and 6hrs after SEA. Previous research in adult mice suggests that the reduced consumption may reflect an increase in neophobic behavior rather than a sickness-induced anorexic response, as the hypophagia is specific to a novel diet (Kusnecov et al., 1999; Rossi-George et al., 2005; Urbach-Ross et al., 2008). Though the precise mechanisms of these behavioral effects have not been elucidated, prior studies indicate a dependence on the production of TNF-α and CRH. For example, TNF-α knockout mice fail to display HPA axis activation and novelty induced hypophagia after SEA exposure (Rossi-George et al., 2005) [the HPA axis effects of SEA in young animals appear to be TNF receptor I dependent, Urbach-Ross & Kusnecov, in submission]. Further, central or peripheral CRH receptor I antagonism abrogates the effects of SEA (Kaneta & Kusnecov, 2005). Whether these mechanisms continue to operate in older animals is unknown and will require further investigation, though it is relevant that following acute challenge with SEA both age groups showed a similar increase in splenic levels of TNF-α and no change in central TNF-α mRNA levels. Nonetheless, the aged animals displayed a more pronounced hypophagia when presented with the novel liquid. There is data to suggest that older animals are more sensitive to the apoptotic effects of TNF-α on neuronal systems (Viel et al., 2001; Patel & Brewer, 2008), and that neurodegenerative conditions may arise from greater susceptibility and TNF receptor-mediated sensitivity to TNF-α (Perry et al., 2001; McCoy & Tansey, 2008). Consequently, it will be of value to determine whether in the SEA model there is enhanced reactivity to TNF-α in aged animals.
Although in younger animals SEA-induced gustatory neophobia is TNF-α-dependent (Bernstein, 1996; Rossi-George et al., 2005), it was observed that another hypophagic cytokine, IL-1β, was elevated to a greater extent in older animals. Following SEA administration, IL-1β mRNA was increased in the hypothalamus and amygdala in the aged mice, while young mice only showed increased expression in the amygdala. The findings in the young mice are consistent with other studies showing that acute administration of the superantigen, SEB, fails to increase IL-1β expression in the hypothalamus of young BALB/c mice (Del Rey et al., 2000). Therefore, it is possible that the greater hypothalamic and amygdaloid IL-1β mRNA in aged animals is contributing to the more pronounced and prolonged hypophagic response. This is given credence by a recent report that LPS-induced sickness behavior in aged mice is mediated by central IL-1 (Abraham & Johnson, 2009). Age-related changes in microglial cell activity has been suggested to account for the enhanced neuroinflammatory response following LPS administration (Godbout et al., 2005; Frank et al., 2006; Dilger & Johnson, 2008), however, whether SEA administration activates microglial cells has yet to be explored. Interestingly, mice lacking the IL-1 receptor show a similar hypophagic response to an SEA challenge as compared to wild type controls (Rossi-George et al., 2005). Consequently, elevated IL-1β expression in the brain may exert a more prominent influence on behavior in aged animals.
Aged-related changes in IL-6 have been suggested to contribute to the exaggerated anorexic response in aged mice following LPS, as IL-6 is elevated in the brain concomitantly with the anorexic response 24hrs after treatment (Godbout et al., 2005). However, the present results showed no effect of SEA treatment on central IL-6 expression, whether measured 2 or 24 hrs after treatment. Therefore, following superantigenic T cell stimulation, of the three major proinflammatory cytokines measured in brain, only IL-1β gene expression was higher as a function of aging.
An unexpected, but interesting, observation was that while the SEA-induced increase in central IL-1β expression had dissipated 24hrs after treatment, the hypophagic response was still present in the aged mice 24hrs later. The SEA-injected aged mice may have experienced a prolonged illness or alternatively the earlier SEA challenge may have altered their reaction to the test situation. Several studies have found that stress exposure can sensitize the behavioral and endocrine response to presentation of a second heterotypic stressor (Armario et al., 2004; Belda et al., 2008; Weinberg et al., 2009). Stress exposure and immune activation produce similar effects on endocrine and neural function, suggesting that they may activate common pathways. Indeed, there is evidence to suggest that IL-1β, similar to stress exposure, may potentiate the response to later stressors (Anisman et al., 2003; Schmidt et al., 2003). For example, a single i.p. injection of IL-1β augmented the HPA axis response to an emotional stressor (i.e., 10 min exposure to a novel cage) 3, 11, and 22 days after the injection (Schmidt et al., 2003). Similarly, corticosterone levels of the aged mice were significantly augmented by the behavioral test (as shown in Figure 7). Corticosterone levels of the untested young and old SEA-treated animals did not differ from saline controls, ruling out the possibility of residual or persistent pituitary-adrenal drive due to SEA given 24hrs earlier. Therefore, one could hypothesize that the initial increase in central IL-1β expression in the SEA-treated aged mice may have sensitized the corticosterone response to the novel environment and liquid used to test consummatory behavior.
The possibility that this sensitized corticosterone response involved central CRH was addressed by measuring hypothalamic levels of CRH mRNA. Although acute or repeated administration of SEA did not increase CRH mRNA levels in the amygdala or hypothalamus 2hrs after treatment, hypothalamic expression was elevated in both young and old mice 24hrs later. Since the latter effect was seen in both age groups, it is difficult to reconcile with the augmented corticosterone response of aged animals, though Schmidt et al. (2003) reported a similar effect following IL-1β exposure. In the present study, amygdaloid CRH expression was lower in the aged mice, consistent with prior reports (Kasckow et al., 1999; Pisarska et al., 2000). Further examination revealed that CRH mRNA was markedly lower in SEA-treated and subsequently tested mice relative to the home cage controls, which may reflect stimulus-provoked utilization of the initially elevated CRH mRNA build up in the amygdala. In rats, amygdaloid CRH content fluctuates in the context of food intake (Merali et al., 1998), although CRH release in the amygdala does not appear to mediate suppression of food intake in a novel environment (Merali et al., 2004). Consequently, it was suggested that amygdaloid CRH might increase arousal and orientation to biologically meaningful stimuli (Merali et al., 2004). Nonetheless, in the present circumstances, it remains to be determined whether CRH is enhancing neophobia and promoting increased HPA axis reactivity following T cell activation of aged animals.
Evaluation of age-associated changes in the peripheral immune response to SEA revealed that aged mice showed attenuated splenic levels of IL-2 following SEA administration relative to young mice. These findings are consistent with prior reports that show reduced IL-2 production with age (Fong & Makinodan, 1989; Engwerda et al., 1996; Kirman et al., 1996; Miller, 1996; Pahlavani & Richardson, 1996); however, some reports fail to observe a decrease (Hobbs et al., 1993; Saini & Sei, 1993; Aroeira et al., 1994; Kuschnaroff et al., 1997). The alterations in IL-2 production have been attributed to the age-related decline in T cell proliferation and the shift from predominately naïve to memory T cells, as aged subjects show an overall decrease in the number of T cells that produce IL-2 (Fong & Makinodan, 1989; Hobbs et al., 1993; Aroeira et al., 1994; Miller, 1996; Pahlavani & Richardson, 1996). In addition, plasma and splenic levels of IL-1β were (Pahlavani & Richardson, 1996) attenuated in the aged mice following acute SEA administration, but both age groups showed similar IL-1β levels following repeated SEA injections. As with IL-2, the lower levels of IL-1β may be related to the diminished T cell proliferation in the aged mice. Additionally, these data confirm prior findings (Godbout et al., 2005) that showed dissociation between age-related change in the peripheral and central immune response, as peripheral IL-1β levels were reduced whereas central IL-1β expression was higher in the aged subjects (Miller, 1996; Terao et al., 2002; Godbout et al., 2005). The levels of IL-10 and TNF-α following acute or repeated SEA administration are consistent with prior research in adult mice (Urbach-Ross et al., 2008), in that TNF-α is increased following acute or repeated SEA, whereas IL-10 is only elevated following a secondary SEA challenge, suggesting the anti-inflammatory response is not altered in the aged subjects. Splenic levels of IL-1β, TNF-α and IL-6 were not elevated 24hrs after SEA administration. Together these data confirm the presence of age-associated changes in the peripheral immune response and the dissociation between peripheral and central cytokine activity.
In summary, the present data provide the first evidence that aged mice show an exaggerated and prolonged behavioral response to a bacterial T cell superantigen. The aged mice showed greater reductions in consumption of a novel liquid after SEA administration relative to young mice. Pretreatment with SEA 24hrs prior, increased corticosterone levels in response to the novel testing environment in the aged, but not young, mice. The alterations in corticosterone and consummatory behavior observed in the aged subjects may be related to the increased expression of IL-1β within the CNS. Consistent with previous findings (Del Rey et al., 2000), young mice failed to show an increase in IL-1β, IL-6, or TNF-α in the hypothalamus in response to an acute SEA challenge. Collectively, these findings confirm that aged subjects develop an exaggerated behavioral and neuroinflammatory response to T cell mediated immune activation.
Acknowledgments
Work was supported by grants MH60706, NIEHS P30 ES05022 and NIEHS Training grant 5T32 E507148.
Abbreviations
- ACTH
adrenocorticotropin
- CNS
central nervous system
- CRH
corticotropin releasing hormone
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HPA
hypothalamic-pituitary adrenal
- IL-1β
interleukin-1β
- IL-2
interleukin-2
- IL-6
interleukin-6
- IL-10
interleukin-10
- LPS
lipopolysaccharide
- qRT-PCR
quantitative real-time reverse transcription-polymerase chain reaction
- SEA
staphylococcal enterotoxin A
- SEB
staphylococcal enterotoxin B
- SEM
standard error of the mean
- TCR
T cell receptors
- TNF-α
tumor necrosis factor-α
References
- Abraham J, Johnson RW. Central inhibition of interleukin-1beta ameliorates sickness behavior in aged mice. Brain Behav Immun. 2009;23:396–401. doi: 10.1016/j.bbi.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisman H, Merali Z, Hayley S. Sensitization associated with stressors and cytokine treatments. Brain Behav Immun. 2003;17:86–93. doi: 10.1016/s0889-1591(02)00100-9. [DOI] [PubMed] [Google Scholar]
- Armario A, Marti O, Valles A, Dal-Zotto S, Ons S. Long-term effects of a single exposure to immobilization on the hypothalamic-pituitary-adrenal axis: neurobiologic mechanisms. Ann N Y Acad Sci. 2004;1018:162–172. doi: 10.1196/annals.1296.019. [DOI] [PubMed] [Google Scholar]
- Aroeira LS, Williams O, Lozano EG, Martinez AC. Age-dependent changes in the response to staphylococcal enterotoxin B. Int Immunol. 1994;6:1555–1560. doi: 10.1093/intimm/6.10.1555. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27:723–732. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Belda X, Fuentes S, Nadal R, Armario A. A single exposure to immobilization causes long-lasting pituitary-adrenal and behavioral sensitization to mild stressors. Horm Behav. 2008;54:654–661. doi: 10.1016/j.yhbeh.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Bernstein IL. Neural mediation of food aversions and anorexia induced by tumor necrosis factor and tumors. Neurosci Biobehav Rev. 1996;20:177–181. doi: 10.1016/0149-7634(95)00046-h. [DOI] [PubMed] [Google Scholar]
- Bette M, Schafer MK, van Rooijen N, Weihe E, Fleischer B. Distribution and kinetics of superantigen-induced cytokine gene expression in mouse spleen. J Exp Med. 1993;178:1531–1539. doi: 10.1084/jem.178.5.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle SC, Uyemura K, Crawford W, Wong W, Klaustermeyer WB, Makinodan T. Age-related impaired proliferation of peripheral blood mononuclear cells is associated with an increase in both IL-10 and IL-12. Exp Gerontol. 1999;34:243–252. doi: 10.1016/s0531-5565(98)00064-3. [DOI] [PubMed] [Google Scholar]
- Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun. 2008;22:301–311. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur J Pharmacol. 2004;500:399–411. doi: 10.1016/j.ejphar.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Del Rey A, Randolf A, Pitossi F, Rogausch H, Besedovsky HO. Not all peripheral immune stimuli that activate the HPA axis induce proinflammatory cytokine gene expression in the hypothalamus. Ann N Y Acad Sci. 2000;917:169–174. doi: 10.1111/j.1749-6632.2000.tb05381.x. [DOI] [PubMed] [Google Scholar]
- Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J Leukoc Biol. 2008;84:932–939. doi: 10.1189/jlb.0208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engwerda CR, Fox BS, Handwerger BS. Cytokine production by T lymphocytes from young and aged mice. J Immunol. 1996;156:3621–3630. [PubMed] [Google Scholar]
- Fong TC, Makinodan T. In situ hybridization analysis of the age-associated decline in IL-2 mRNA expressing murine T cells. Cell Immunol. 1989;118:199–207. doi: 10.1016/0008-8749(89)90369-9. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006;27:717–722. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, J OC, Castanon N, Kelley KW, Dantzer R, Johnson RW. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology. 2008;33:2341–2351. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RP, Hansen MK, Kleiner JL, Maier SF, Watkins LR. Staphylococcal enterotoxin B induces fever, brain c-Fos expression, and serum corticosterone in rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1434–1439. doi: 10.1152/ajpregu.2001.280.5.R1434. [DOI] [PubMed] [Google Scholar]
- Hobbs MV, Weigle WO, Noonan DJ, Torbett BE, McEvilly RJ, Koch RJ, Cardenas GJ, Ernst DN. Patterns of cytokine gene expression by CD4+ T cells from young and old mice. J Immunol. 1993;150:3602–3614. [PubMed] [Google Scholar]
- Kaneta T, Kusnecov AW. The role of central corticotropin-releasing hormone in the anorexic and endocrine effects of the bacterial T cell superantigen, Staphylococcal enterotoxin A. Brain Behav Immun. 2005;19:138–146. doi: 10.1016/j.bbi.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Kasckow JW, Regmi A, Mulchahey JJ, Plotsky PM, Hauger RL. Changes in brain corticotropin-releasing factor messenger RNA expression in aged Fischer 344 rats. Brain Res. 1999;822:228–230. doi: 10.1016/s0006-8993(98)01365-1. [DOI] [PubMed] [Google Scholar]
- Kawashima N, Kusnecov AW. Effects of staphylococcal enterotoxin A on pituitary-adrenal activation and neophobic behavior in the C57BL/6 mouse. J Neuroimmunol. 2002;123:41–49. doi: 10.1016/s0165-5728(01)00486-6. [DOI] [PubMed] [Google Scholar]
- Kipnis J, Schwartz M. Controlled autoimmunity in CNS maintenance and repair: naturally occurring CD4+CD25+ regulatory T-Cells at the crossroads of health and disease. Neuromolecular Med. 2005;7:197–206. doi: 10.1385/NMM:7:3:197. [DOI] [PubMed] [Google Scholar]
- Kirman I, Zhao K, Tschepen I, Szabo P, Richter G, Nguyen H, Weksler ME. Treatment of old mice with IL-2 corrects dysregulated IL-2 and IL-4 production. Int Immunol. 1996;8:1009–1015. doi: 10.1093/intimm/8.7.1009. [DOI] [PubMed] [Google Scholar]
- Kohman RA, Tarr AJ, Byler SL, Boehm GW. Age increases vulnerability to bacterial endotoxin-induced behavioral decrements. Physiol Behav. 2007;91:561–565. doi: 10.1016/j.physbeh.2007.03.032. [DOI] [PubMed] [Google Scholar]
- Kuschnaroff LM, Goebels J, Valckx D, Heremans H, Matthys P, Waer M. Increased mortality and impaired clonal deletion after staphylococcal enterotoxin B injection in old mice: relation to cytokines and nitric oxide production. Scand J Immunol. 1997;46:469–478. doi: 10.1046/j.1365-3083.1997.d01-153.x. [DOI] [PubMed] [Google Scholar]
- Kusnecov AW, Goldfarb Y. Neural and behavioral responses to systemic immunologic stimuli: a consideration of bacterial T cell superantigens. Curr Pharm Des. 2005;11:1039–1046. doi: 10.2174/1381612053381602. [DOI] [PubMed] [Google Scholar]
- Kusnecov AW, Liang R, Shurin G. T-lymphocyte activation increases hypothalamic and amygdaloid expression of CRH mRNA and emotional reactivity to novelty. J Neurosci. 1999;19:4533–4543. doi: 10.1523/JNEUROSCI.19-11-04533.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laupland KB, Church DL, Mucenski M, Sutherland LR, Davies HD. Population-based study of the epidemiology of and the risk factors for invasive Staphylococcus aureus infections. J Infect Dis. 2003;187:1452–1459. doi: 10.1086/374621. [DOI] [PubMed] [Google Scholar]
- Li H, Llera A, Malchiodi EL, Mariuzza RA. The structural basis of T cell activation by superantigens. Annu Rev Immunol. 1999;17:435–466. doi: 10.1146/annurev.immunol.17.1.435. [DOI] [PubMed] [Google Scholar]
- McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merali Z, Khan S, Michaud DS, Shippy SA, Anisman H. Does amygdaloid corticotropin-releasing hormone (CRH) mediate anxiety-like behaviors? Dissociation of anxiogenic effects and CRH release. Eur J Neurosci. 2004;20:229–239. doi: 10.1111/j.1460-9568.2004.03468.x. [DOI] [PubMed] [Google Scholar]
- Merali Z, McIntosh J, Kent P, Michaud D, Anisman H. Aversive and appetitive events evoke the release of corticotropin-releasing hormone and bombesin-like peptides at the central nucleus of the amygdala. J Neurosci. 1998;18:4758–4766. doi: 10.1523/JNEUROSCI.18-12-04758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- Pahlavani MA, Richardson A. The effect of age on the expression of interleukin-2. Mech Ageing Dev. 1996;89:125–154. doi: 10.1016/0047-6374(96)01725-3. [DOI] [PubMed] [Google Scholar]
- Patel JR, Brewer GJ. Age-related differences in NFkappaB translocation and Bcl-2/Bax ratio caused by TNFalpha and Abeta42 promote survival in middle-age neurons and death in old neurons. Exp Neurol. 2008;213:93–100. doi: 10.1016/j.expneurol.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RT, Collins JS, Wiener H, Acton R, Go RC. The role of TNF and its receptors in Alzheimer’s disease. Neurobiol Aging. 2001;22:873–883. doi: 10.1016/s0197-4580(01)00291-3. [DOI] [PubMed] [Google Scholar]
- Pisarska M, Mulchahey JJ, Welge JA, Geracioti TD, Jr, Kasckow JW. Age-related alterations in emotional behaviors and amygdalar corticotropin-releasing factor (CRF) and CRF-binding protein expression in aged Fischer 344 rats. Brain Res. 2000;877:184–190. doi: 10.1016/s0006-8993(00)02606-8. [DOI] [PubMed] [Google Scholar]
- Rosendahl A, Hansson J, Antonsson P, Sekaly RP, Kalland T, Dohlsten M. A mutation of F47 to A in staphylococcus enterotoxin A activates the T-cell receptor Vbeta repertoire in vivo. Infect Immun. 1997;65:5118–5124. doi: 10.1128/iai.65.12.5118-5124.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi-George A, Urbach D, Colas D, Goldfarb Y, Kusnecov AW. Neuronal, endocrine, and anorexic responses to the T-cell superantigen staphylococcal enterotoxin A: dependence on tumor necrosis factor-alpha. J Neurosci. 2005;25:5314–5322. doi: 10.1523/JNEUROSCI.0687-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozovsky I, Finch CE, Morgan TE. Age-related activation of microglia and astrocytes: in vitro studies show persistent phenotypes of aging, increased proliferation, and resistance to down-regulation. Neurobiol Aging. 1998;19:97–103. doi: 10.1016/s0197-4580(97)00169-3. [DOI] [PubMed] [Google Scholar]
- Saini A, Sei Y. Age-related impairment of early and late events of signal transduction in mouse immune cells. Life Sci. 1993;52:1759–1765. doi: 10.1016/0024-3205(93)90464-e. [DOI] [PubMed] [Google Scholar]
- Schmidt ED, Aguilera G, Binnekade R, Tilders FJ. Single administration of interleukin-1 increased corticotropin releasing hormone and corticotropin releasing hormone-receptor mRNA in the hypothalamic paraventricular nucleus which paralleled long-lasting (weeks) sensitization to emotional stressors. Neuroscience. 2003;116:275–283. doi: 10.1016/s0306-4522(02)00555-9. [DOI] [PubMed] [Google Scholar]
- Schnydrig S, Korner L, Landweer S, Ernst B, Walker G, Otten U, Kunz D. Peripheral lipopolysaccharide administration transiently affects expression of brain-derived neurotrophic factor, corticotropin and proopiomelanocortin in mouse brain. Neurosci Lett. 2007;429:69–73. doi: 10.1016/j.neulet.2007.09.067. [DOI] [PubMed] [Google Scholar]
- Serrats J, Sawchenko PE. CNS activational responses to staphylococcal enterotoxin B: T-lymphocyte-dependent immune challenge effects on stress-related circuitry. J Comp Neurol. 2006;495:236–254. doi: 10.1002/cne.20872. [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Martin LA, Calvert WS, Boehm GW. Effects of intraperitoneal lipopolysaccharide on Morris maze performance in year-old and 2-month-old female C57BL/6J mice. Behav Brain Res. 2004;159:145–151. doi: 10.1016/j.bbr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Terao A, Apte-Deshpande A, Dousman L, Morairty S, Eynon BP, Kilduff TS, Freund YR. Immune response gene expression increases in the aging murine hippocampus. J Neuroimmunol. 2002;132:99–112. doi: 10.1016/s0165-5728(02)00317-x. [DOI] [PubMed] [Google Scholar]
- Urbach-Ross D, Crowell B, Kusnecov AW. Relationship of varying patterns of cytokine production to the anorexic and neuroendocrine effects of repeated Staphylococcal enterotoxin A exposure. J Neuroimmunol. 2008;196:49–59. doi: 10.1016/j.jneuroim.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbach-Ross D, Kusnecov AW. Impact of superantigenic molecules on central nervous system function. Front Biosci. 2009;14:4416–4426. doi: 10.2741/3537. [DOI] [PubMed] [Google Scholar]
- Viel JJ, McManus DQ, Smith SS, Brewer GJ. Age- and concentration-dependent neuroprotection and toxicity by TNF in cortical neurons from beta-amyloid. J Neurosci Res. 2001;64:454–465. doi: 10.1002/jnr.1097. [DOI] [PubMed] [Google Scholar]
- Weinberg MS, Bhatt AP, Girotti M, Masini CV, Day HE, Campeau S, Spencer RL. Repeated ferret odor exposure induces different temporal patterns of same-stressor habituation and novel-stressor sensitization in both hypothalamic-pituitary-adrenal axis activity and forebrain c-fos expression in the rat. Endocrinology. 2009;150:749–761. doi: 10.1210/en.2008-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]