Abstract
In an experience-sampling study that bridged laboratory, ecological, and individual-differences approaches to mind-wandering research, 72 subjects completed an executive-control task with periodic thought probes (reported by McVay & Kane, 2009) and then carried PDAs for a week that signaled them 8 times daily to report immediately whether their thoughts were off-task. Subjects who reported more mind wandering during the laboratory task endorsed more mind-wandering experiences during everyday life (and were more likely to report worries as off-task thought content). We also conceptually replicated laboratory findings that mind wandering predicts task performance: subjects rated their daily-life performance to be impaired when they reported off-task thoughts, with greatest impairment when subjects’ mind wandering lacked meta-consciousness. The propensity to mind-wander appears to be a stable cognitive characteristic and seems to predict performance difficulties in daily life, just as it does in the laboratory.
The study of mind wandering provides a novel means to explore fundamental issues of consciousness. For example, the commonplace experience of moving one’s eyes across a page without comprehending a thing suggests the startling conclusion that we are sometimes unaware of our own conscious experience; if we “knew” our thoughts were elsewhere, we would return to reading or drop the charade (Schooler, Reichle, & Halpern, 2004). Moreover, despite controversy about the causal functions of consciousness (e.g., Morsella, 2005; Rosenthal, 2008; Wegner, 2002), field and laboratory studies of human performance (e.g., Reason, 1990; Smallwood et al., 2004) indicate that errors increase when people report experiencing task-unrelated thoughts (TUTs). Mind wandering thus co-occurs with events of scientific and practical interest. It is also beginning to figure into general theories of executive control, metacognition, and the “default-mode” brain network (e.g., Bar, 2007; Buckner & Carroll, 2007; Burgess, Dumontheil, & Gilbert, 2007; Mason et al., 2007; Schooler, 2002; Smallwood & Schooler, 2006); we have argued, for example, that unwanted mind-experiences represent momentary failures of goal maintenance that reflect, in part, enduring individual differences in executive control (Kane et al., 2007; McVay & Kane, 2009).
Like most areas of cognitive investigation, mind-wandering research is dominated by laboratory and neuroimaging methods; here, subjects engage an ongoing task that is periodically interrupted for them to report or categorize their current thoughts (e.g., as on- or off-task; Giambra, 1995; Mason et al. 2007; Smallwood et al., 2007). Such thought-probe responses appear to be valid: TUT reports vary systematically with experimental manipulations, such as memory load, stimulus pacing, and task practice (e.g., Antrobus, Singer, & Greenberg, 1966; Teasdale, Proctor, Lloyd, & Baddeley, 1993), TUTs show a reliable neural signature (e.g., Mason et al., 2007), and task errors can increase by 25% during TUTs versus on-task thoughts (McVay & Kane, 2009; Schooler et al., 2004). As well, individual differences in TUT rates are reliable across different primary tasks and across substantial test-retest lags (Giambra, 1995; Grodsky & Giambra, 1990–91) and they are predicted by objective cognitive-ability assessments (McVay & Kane, 2009).
Unlike some heavily investigated cognitive phenomena, however, mind wandering seems ubiquitous in everyday life. Perhaps for this reason, researchers have also investigated TUTs in ecologically valid contexts, by inserting thought probes into normal classroom activities (e.g., Cameron & Giuntoli, 1972; Geerligs, 1995) or by electronically paging (“beeping”) subjects to answer questions about their thoughts, emotions, and environmental context during unconstrained daily activities (e.g., Hurlburt, 1979). As in laboratory studies, daily-life mind wandering occurs frequently and it varies reliably with context: Subjects report TUTs at 30 – 40% of probes, overall (e.g., Klinger & Cox, 1987–88), but they occur more often during classroom lectures than discussions (e.g., Schoen, 1970), and less often during enjoyable activities and happy moods (e.g., Kane et al., 2007).
The goal of the present work was to bridge the controlled and ecological approaches to mind-wandering research by asking whether people who experience more (or fewer) TUTs during a challenging laboratory task also experience more (or fewer) TUTs in daily life. If mind wandering reflects, in part, executive-control failure (Kane et al., 2007; McVay & Kane, 2009) and if executive-control capabilities are domain-general (e.g., Engle & Kane, 2004), then subjects’ TUT rates in the lab should predict those outside the lab. To test this prediction, we administered a daily-life, experience-sampling protocol to subjects who had previously completed a laboratory assessment of mind wandering during an executive-control, go/no-go task, the Sustained Attention to Response Task (SART; Robertson, Manly, Andrade, Baddeley, & Yiend, 1997; Smallwood et al., 2004). McVay and Kane (2009) reported that these subjects experienced frequent mind wandering, high error rates, quite variable response times to “go” trials, and significant associations among the three.
Our secondary goal was to examine the relation between mind wandering and performance in daily life, a relation that is well established in the laboratory (e.g., McVay & Kane, 2009; Schooler et al., 2004; Smallwood et al., 2007). We therefore asked subjects to evaluate their performance of ongoing activities on the same occasions that we probed their thoughts. We expected that, in life, as in the lab, subjects would report performing less well when mind wandering than during on-task thinking.
Methods
Laboratory SART
The SART presented 1810 words for go/no-go responses based on a perceptual (letter case) or semantic (animals vs. foods) discrimination. Subjects pressed a key for non-target “go” stimuli (e.g., lowercase words) and withheld responses to infrequent (11%) target “no-go” stimuli (e.g. uppercase words; for details, see McVay & Kane, 2009).
Thought-probe screens followed 60% of the no-go targets and presented the question, “What were you just thinking about?” with the response options: 1) the task; 2) own task performance; 3) everyday stuff; 4) current state of being; 5) personal worries; 6) daydreams; 7) other. We instructed subjects to report what they had been thinking immediately before the probe, and the experimenter initially elaborated on the response-option meanings. For all analyses, options 3–7 were considered TUTs.
Subjects
Of 244 undergraduates (aged 18 – 35) who completed the laboratory SART, 72 subsequently completed the experience-sampling study (ESM). Subjects began the 7-day ESM collection period between 1 and 63 days (M = 16; SD = 13) after the laboratory session, based on their availability. This sub-sample had similar laboratory TUT rates to the full sample (both Ms = .55), and similar levels of SART performance [M signal-detection accuracy (dL) = 3.73 vs. 3.35, respectively; M go-trial response time (RT) variability (RTSD) = 151 ms vs. 158 ms, respectively]; for analyses of the full sample, see McVay & Kane (2009).1
Experience-Sampling Protocol
Palm Pilot PDAs using iESP software (Intel, 2004; Barrett & Barrett, 2004), presented questionnaires and collected data during subjects’ daily-life activities. A “beep” signaled subjects to complete 8 daily questionnaires, between noon and midnight, for 7 full days. The signals occurred once randomly during each 90 min block. During a 60-min training session, the experimenter instructed subjects to take immediate stock of their thoughts upon the beep and to report on only these thoughts; the experimenter also familiarized subjects with the PDAs, questionnaires, and mind-wandering examples. PDA signal blocks began immediately after subjects left the session (yielding an additional, partial day of data collection). Subjects completing ≥70% of the questionnaires were entered into a lottery for a retailer gift card.
The PDA questionnaire first asked subjects whether their current thoughts had wandered from their activity (“yes” = 1; “no” = 0). If so, they answered 5 questions about those thoughts; all subjects also answered 18 questions about their mental and environmental context (all on a 1 – 7 scale; see Table 1 and Table 2).
Table 1.
Mean ratings (1–7) for perceived thought control and content when subjects reported daily-life mind wandering.
| Questionnaire Prompt | M±SD |
|---|---|
| I was aware my mind was wandering in the moments before the beep. | 4.40±0.95 |
| I allowed my thoughts to wander on purpose. | 4.06±0.96 |
| I was thinking about personal concerns or things I need to do. | 4.29±1.00 |
| I was daydreaming or fantasizing about something. | 3.81±1.25 |
| I was worrying about something. | 3.37±1.05 |
Table 2.
Contextual predictors of daily-life mind-wandering episodes.
| b±SE | t(71) | P | Prep | |
|---|---|---|---|---|
| Negative: | ||||
| I was doing this activity successfully. | −.541±.049 | −10.949 | .000 | .99 |
| I was trying to concentrate on what I was doing. | −.577±.057 | −10.135 | .000 | .99 |
| I like what I'm doing right now. | −.177±.024 | −7.421 | .000 | .99 |
| I am trying hard at what I’m doing right now. | −.155±.029 | −5.366 | .000 | .99 |
| I feel happy right now. | −.145±.033 | −4.358 | .000 | .99 |
| I'm good at what I'm doing right now. | −.124±.030 | −4.132 | .000 | .99 |
| What I'm doing right now is important. | −.083±.021 | −3.163 | .003 | .98 |
| It takes a lot of mental effort to do this activity. | −.077±.027 | −2.912 | .005 | .98 |
| Positive: | ||||
| I would prefer to do something else right now. | .168±.022 | 7.507 | .000 | .99 |
| What I'm doing right now is boring. | .173±.026 | 6.555 | .000 | .99 |
| There is a lot going on around me right now. | .063±.026 | 2.451 | .017 | .95 |
| I feel anxious right now. | .084±.032 | 2.259 | .027 | .94 |
| I feel tired right now. | .073±.032 | 2.259 | .027 | .94 |
| What I'm doing right now is stressful. | .050±.024 | 2.092 | .040 | .93 |
| Non-significant: | ||||
| What I'm doing now is mentally challenging. | −.043±.027 | −1.625 | .108 | .87 |
| What I'm doing now is related to schoolwork. | .029±.019 | 1.483 | .142 | .85 |
| What I'm doing right now is unusual for me. | −.007±.027 | −0.276 | .783 | .63 |
| I’m interacting with other people right now. | −.002±.019 | −0.123 | .903 | .54 |
Note: Parallel analyses from Kane et al. (2007) considered the contextual predictors of on-task thinking, and so the signs of the b and t values here are reversed compared to that study.
Statistical Analyses
Experience-sampling data have a hierarchical structure in which responses (Level-1 data) are nested within subjects (Level-2 data) and are best analyzed with multilevel or hierarchical linear modeling (HLM; Raudenbush & Bryk, 2002). We used HLM to examine within- and between-subject predictors of two daily-life outcomes: mind wandering and subjective evaluation of activity performance. All Level-1, within-subject variables (e.g., self-reported happiness) were standardized within subjects (group centered). The mind-wandering variable was dichotomous (on-task vs. TUT), which violates the normality assumption of HLM; we therefore used an HLM model for binary outcomes (using a log transformation and Bernoulli sampling distribution, a special case of the binomial distribution where the values are 0 and 1; see Raudenbush & Bryk, 2002) to evaluate Level-1 and Level-2 effects on TUTs. We analyzed three Level-2, between-subject, predictors of thought and performance (grand-mean centered): SART-session TUT rate, dL, and RTSD.
Results
We report non-directional NHSTs (α = .05) with prep values. On average, subjects completed 45.6 (SD = 10.8, range = 20–65) usable questionnaires, which were uncorrelated with daily-life [r(72) = −.04] and laboratory [r(72) = −.13] TUT rates.
Mind Wandering in Daily Life
Our initial analyses examined the frequency and nature of daily-life mind wandering. Consistent with previous findings, subjects reported TUTs at 30% of the beeps, on average, with considerable variation among subjects (SD: 15%; range: 6% – 75%). Table 1 presents mean ratings of thought content and awareness when subjects reported mind wandering; replicating Kane et al. (2007), subjects generally reported more personal-concern content than fantasy, and more fantasy than worry (but these categories were not mutually exclusive, as concern-related content might intrude into worries or daydreams; see Klinger, 1971). Also replicating the findings from Kane et al. (2007), mind wandering varied significantly with context (Table 2), increasing with stress, boredom, sleepiness, or chaotic environments, and decreasing with concentration, effort, successful performance, enjoyable tasks, or happiness. However, in contrast to Kane et al., TUTs decreased during more important and more effortful activities and did not increase during schoolwork.3 In general, these findings are congruent with Klinger’s “current concerns” theory, which argues that thoughts about one’s current goals will be especially likely to intrude into consciousness when they are cued by the environment, particularly during less important or less goal-relevant tasks (Klinger, 1971; 1999; see Kane et al., 2007 for further discussion of contextual predictors of mind wandering).
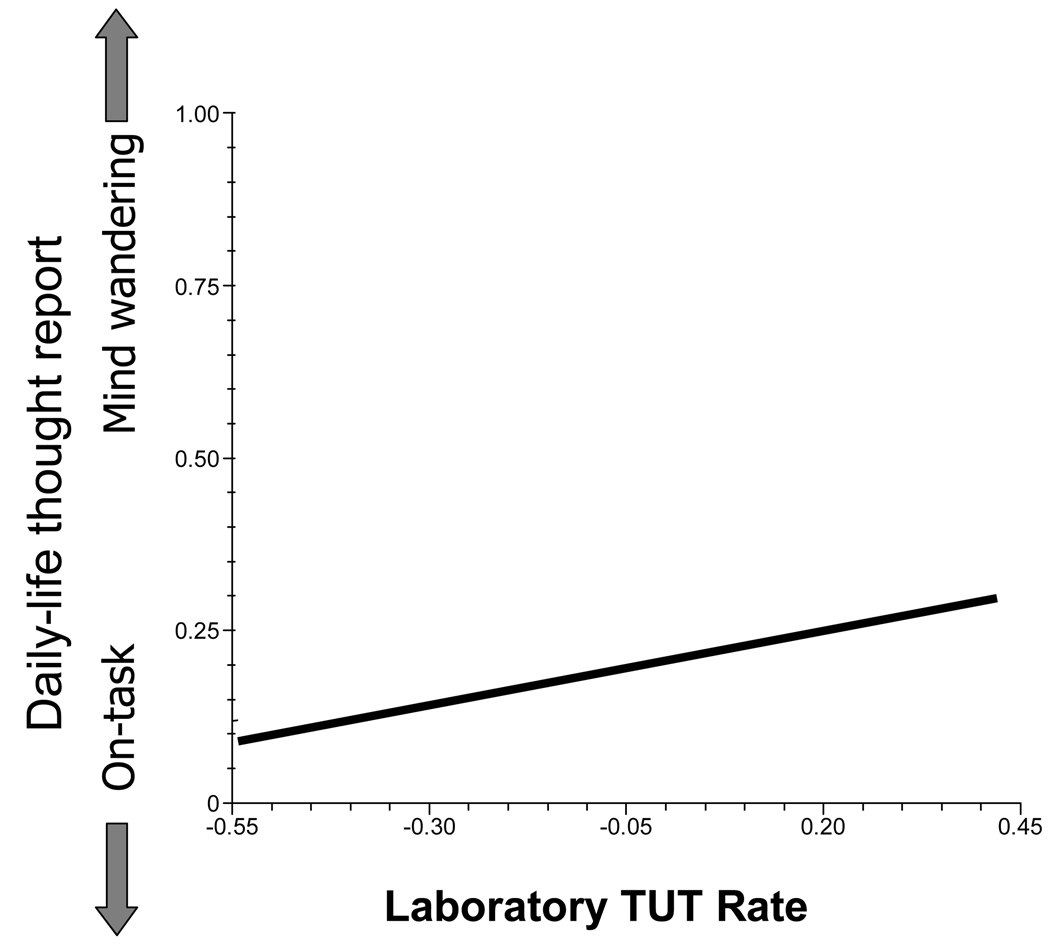
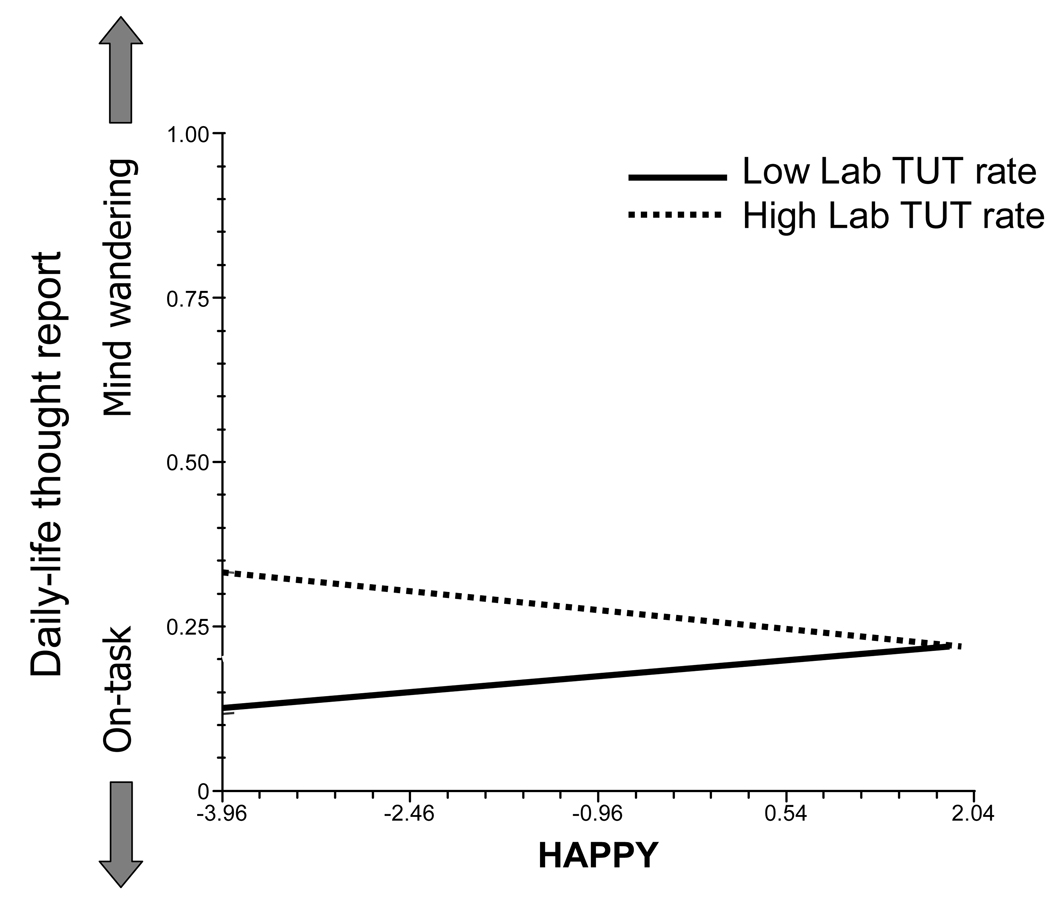
For our primary question – about the stability of off-task thinking – we evaluated the effects of our three laboratory variables on daily-life mind wandering using a model that included the significant Level-1 context variables from Table 2. As hypothesized, subjects with higher laboratory TUT rates also reported more daily-life TUTs, b = 1.29, SE = 0.61, t(68) = 2.12, prep = .93 (see Figure 1). Note also that happiness moderated this effect (see Figure 2), such that when subjects were happier than usual it minimized the mind-wandering differences between subjects with high versus low laboratory TUT rates, b = −0.65, SE = 0.20, t(68) = −3.26, prep = .99. Neither SART accuracy (dL) nor RT variability (RTSD) significantly predicted daily-life TUTs, both ts(68) < 1.85.
Figure 1.
The relation between daily-life mind wandering and the propensity to experience task-unrelated thoughts (TUTs) during a lab task. Values on the y-axis represent the mind-wandering dependent variable, scored on each questionnaire as either 1 (for mind wandering) or 0 (for on-task thoughts). Values on the x-axis represent grand-mean centered laboratory TUT rate.
Figure 2.
The relation between daily-life mind wandering and self-reported happiness as a function of the propensity to experience task-unrelated thoughts (TUTs) during a lab task. Lines depict the means of the within-person slopes for subjects in the top and bottom quartiles for laboratory TUT rate. Values on the y-axis represent the mind-wandering dependent variable, scored on each questionnaire as either a 1 (for mind wandering) or 0 (for on-task thoughts). Values on the x-axis represent group-centered ratings for happiness (“I feel happy right now.”).
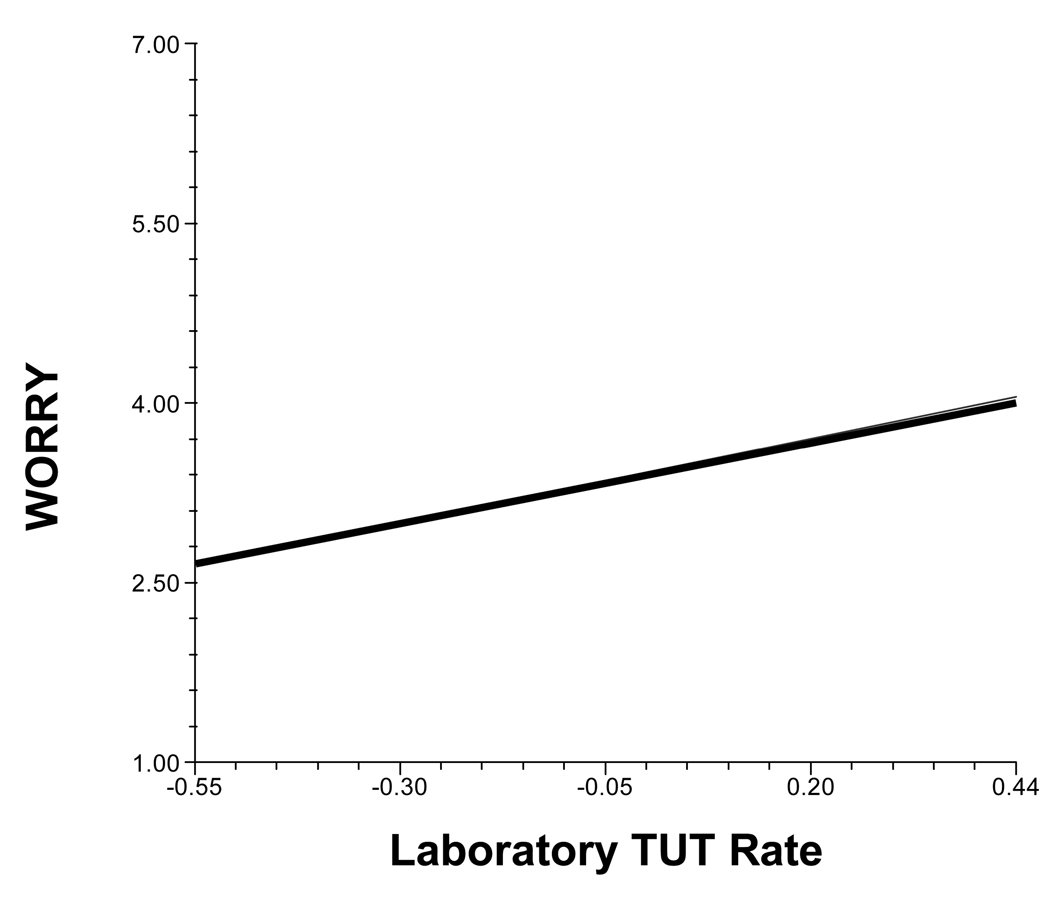
In a more exploratory vein, we examined whether laboratory TUT rate predicted daily-life mind-wandering content. In fact, it predicted worrying (see Figure 3): When subjects’ daily-life thoughts were off-task, those who had experienced more lab TUTs reported more worried content than did those who had experienced fewer lab TUTs, b = 1.49, SE = 0.53, t(68) = 2.84, prep = .97. Lab TUT rate did not predict any other mind-wandering qualities.
Figure 3.
The relation between daily-life worry and the propensity to experience task-unrelated thoughts (TUTs) during a lab task. Values on the y-axis represent ratings for worry content (only completed on occasions where subjects reported mind wandering), scored on each questionnaire on a 1 – 7 scale (higher values indicate more worrying). Values on the x-axis represent grand-mean centered laboratory TUT rate.
Performance in Daily-Life Activities
Before we address the critical question of whether daily-life thoughts predicted performance, Table 3 shows that contextual factors predicted subjects’ self-reported success at daily-life tasks. Subjects reported performing better when engaged in important or enjoyable activities, when concentrating or expending effort, or when happy. They reported performing worse when engaged in stressful, unappealing, or boring activities, when in chaotic environments, or when anxious or tired.
Table 3.
Significant contextual predictors of daily-life performance ratings.
| b±SE | t(71) | p | prep | |
|---|---|---|---|---|
| Positive: | ||||
| Trying to concentrate | .349±.025 | 14.024 | .000 | .99 |
| Like activity | .206±.018 | 11.493 | .000 | .99 |
| Good at activity | .243±.025 | 9.595 | .000 | .99 |
| Important activity | .126±.017 | 7.283 | .000 | .99 |
| Feeling happy | .192±.026 | 7.368 | .000 | .99 |
| Trying hard | .129±.022 | 5.957 | .000 | .99 |
| Negative: | ||||
| Prefer something else | −.115±.019 | −6.452 | .000 | .99 |
| Boring activity. | −.131±.020 | −6.436 | .000 | .99 |
| Stressful activity | −.099±.023 | −4.391 | .000 | .99 |
| A lot going on around me | −.053±.018 | −2.905 | .005 | .98 |
| Feeling tired | −.038±.014 | −2.647 | .010 | .97 |
| Feeling anxious | −.048±.022 | −2.201 | .031 | .94 |
Of central importance, and as predicted, subjects’ performance ratings were lower while mind wandering than while mentally on-task, b = −1.15, SE = .10, t(71) = −11.69, prep > .99. We also found that mind-wandering awareness and content predicted perceived performance. When subjects were aware that they had been mind wandering, they believed they had been performing better than when they had been mind wandering without awareness, b = .094, SE = .040, t(71) = 2.34, prep = .95. This finding mirrors laboratory reports of increased task errors during mind-wandering episodes that lack “meta-consciousness” (Smallwood, McSpadden, et al., 2007; 2008). Finally, with respect to mind-wandering content, when subjects experienced daily-life TUTs their self-reported performance was worse with more worry- or fantasy-based content [for worry, b = −.072, SE = .027, t(71) = −2.67, prep = .97; for fantasy, b = −.105, SE = .026, t(71) = − 4.02, prep > .99]; neither purposeful mind wandering nor variation in personal-concern content predicted performance ratings (ts < 1.28, ps > .21).
Discussion
We found that the propensity to mind-wander is a stable cognitive characteristic, representing an individual difference that is reliable across time, activities, and contexts. Subjects with higher TUT rates during a laboratory executive-control task experienced more off-task thoughts during their unconstrained, everyday activities. This relation held even though we measured daily-life TUTs several weeks after the initial lab assessment. Our TUT-rate findings also differed in an important way from our previous results regarding laboratory measures of working-memory capacity (WMC), which are correlated with, but not isomorphic to, laboratory TUT rates (Kane et al., 2007): Whereas WMC variation predicted daily-life mind wandering differences only in cognitively demanding contexts (such as those perceived as requiring concentration), laboratory TUT-rate variation predicted daily-life mind wandering across virtually all contexts. Thus, whatever variables, aside from WMC, contribute to high levels of mind wandering during laboratory tasks (e.g., personality, emotion, psychopathology, goals, recent life events), they assert their influence very broadly across people’s everyday lives and activities.
Unexpectedly, we found that mood moderated the lab-to-life TUT relationship, with happiness being an equalizer. When people reported being especially happy in the moment, their laboratory TUT rate no longer predicted well whether they were presently mind wandering. We will not make too much of this unanticipated result. However, given that unhappiness exacerbated mind-wandering differences between lab-TUTers and lab-non-TUTers, and that lab-TUTers were especially likely to be worrying when they did mind-wander in daily life, future mind-wandering research should draw upon allied work in the clinical and personality domains, on phenomena such as rumination and worry and their covariation with depression and anxiety (e.g., Nolen-Hoeksema, Wisco, & Lyubomirsky, 2008; Watkins, 2008; Sarason, Pierce, & Sarason, 1996; Smallwood, Fitzgerald, Miles, & Phillips, 2009; Smallwood, O’Connor, Sudbery, & Obansawin, 2007).
Our study conceptually replicated, in daily life, the common laboratory finding that task performance suffers on occasions when people report off-task versus on-task thought (e.g., McVay & Kane, 2009; Schooler et al., 2004; Smallwood, Baracia, Lowe, & Obansawin, 2003; Smallwood, McSpadden, & Schooler, 2008; Smallwood, Riby, Heim, & Davies, 2006; Smallwood et al., 2004, 2007). That is, subjects rated their ongoing activity performance to be worse when mind wandering than when mentally focused. Were these subjective reports valid? We believe so, although we could not measure subjects’ performance objectively outside the laboratory. We see evidence for validity in that subjects’ performance ratings varied systematically with contextual variables, meta-consciousness, and thought content, despite it being unlikely that subjects shared folk beliefs about the effects of metaconsciousness on performance, or about the effects of fantasy versus personal-concern thought content on performance. If we are right that the thought-performance relation is real, then the consistency with which our study and others find that 30 – 40% of daily-life thoughts are off-task (Hurlburt, 1979; Klinger, 1978–79; Klinger & Cox, 1987–88; Kane et al., 2007) suggests a need to take mind wandering seriously as an impediment to learning, productivity, and public safety (e.g., Reason, 1990; Smallwood, Fishman, & Schooler, 2007; Wiegmann et al., 2005).3
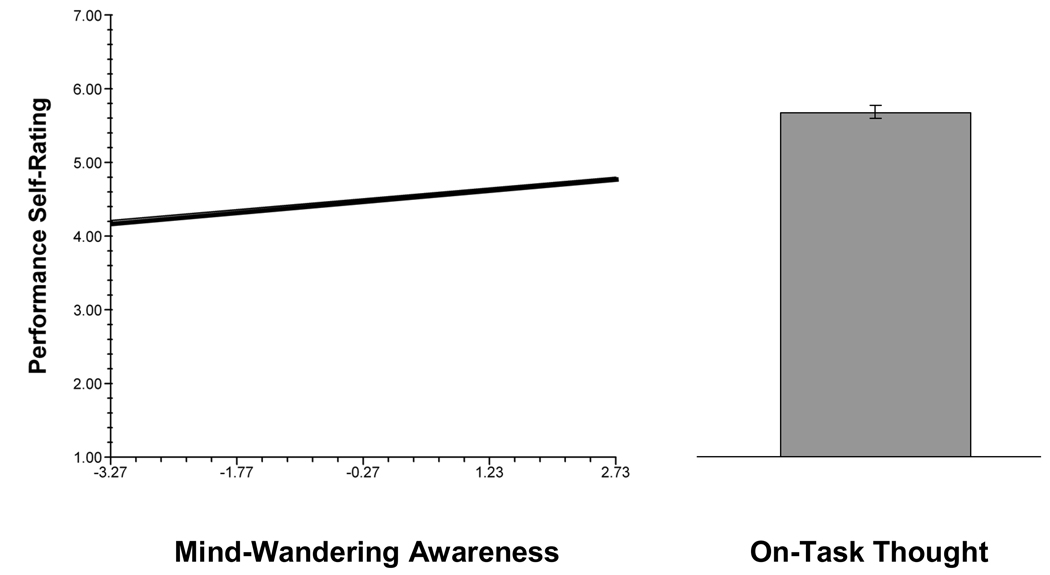
As far as we know, our study is the first to demonstrate a relation between meta-consciousness and human performance outside the laboratory: Subjects performed their daily-life activities more poorly when they had been less aware, before the beep, of their TUTs. Our findings thus support the Smallwood and Schooler (2006; Smallwood et al., 2007) argument that mind wandering without meta-awareness of one’s mind wandering (or, “zoning out”) is particularly harmful to task performance. At the same time, our data, like theirs, suggest that mind wandering with awareness can have negative consequences. Figure 4 shows that, whereas increased awareness of mind wandering was associated with higher self-rated performance, even the highest levels of meta-awareness during mind wandering predicted considerably lower performance ratings than did on-task thoughts; thus, awareness of off-task thought does not necessarily trigger enough (or timely enough) conscious focus to prevent errors. Further laboratory and daily-life research should address the performance consequences of mind wandering with and without awareness (see also Smallwood et al., 2007; 2008).
Figure 4.
The relation between daily-life performance and thought. Values on the y-axis represent ratings for success in performing one’s ongoing activity, scored on each questionnaire on a 1 – 7 scale (higher values indicate better performance). The line graph depicts the relation between performance and subjects’ awareness that they had been mind wandering; values on the x-axis represent group-centered ratings for awareness (“I was aware my mind was wandering in the moments before the beep.”). The bar graph depicts the M performance rating on occasions when subjects reported on-task thoughts.
Indeed, we suggest that mind wandering, in general, should become a phenomenon of choice in studies of conscious awareness, metacognition, executive control, and individual differences therein (see Smallwood & Schooler, 2006). Unlike other theoretically diagnostic outcome variables, such as action slips, RT variability, and perseverative errors, mind wandering is readily observable (albeit indirectly) in a wide variety of laboratory tasks and ecological settings. Moreover, technological advances are increasingly allowing researchers to triangulate self-report, behavioral, and neuroimaging data as a means to better measure and understand mind wandering and other subjective experiences (e.g., Christoff, Gordon, Smallwood, Smith, & Schooler, in press; Mason et al., 2007; Smallwood, Beach, Schooler, & Handy, 2008). We contend that ecological and individual-differences investigations of mind wandering, like the present one (see also Kane et al., 2007), should similarly contribute to building comprehensive theories of conscious awareness and control.
Acknowledgements
We thank Nina Powell and Amethyst Royal for data-collection assistance.
Portions of this work were supported by a UNCG Regular Faculty Grant awarded to Michael Kane and an NIH Ruth L. Kirschstein National Research Service Award (NRSA) Individual Pre-doctoral Fellowship granted to Jennifer McVay, Award Number F31MH081344 from the National Institute of Mental Health (NIMH). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIMH.
Footnotes
McVay and Kane (2009) also collected working-memory capacity (WMC) data from these subjects, but the current sample was too small to detect WMC effects on daily-life mind wandering (it was only about half that of Kane et al., 2007).
The significant effects on mind wandering in the current study for the “effort” posed by current activities may have differed here from Kane et al. (2007) because we modified this prompt between studies. In the current study, the prompt asked whether one’s activity was mentally effortful, whereas Kane et al. did not use the “mentally” qualifier (thus conflating physical and mental effort).
We recognize the possibility that, although mind wandering is often detrimental to immediate task performance, it may serve the adaptive function of allowing people to keep their broader life goals mentally accessible (e.g., Klinger, 1971; Singer, 1968; Singer & Singer, 2006).
References
- Antrobus JS, Singer JL, Greenberg S. Studies in the stream of consciousness: Experimental enhancement and suppression of spontaneous cognitive processes. Perceptual and Motor Skills. 1966;23:399–417. [Google Scholar]
- Bar M. The proactive brain: using analogies and associations to generate predictions. Trends in Cognitive Science. 2007;11:280–289. doi: 10.1016/j.tics.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Barrett DJ. [Retrieved February 2004];The Experience Sampling Program (Version 2.0) [Computer software] 2004 from http://www2.bc.edu/_barretli/esp/
- Buckner RL, Carroll DC. Self-projection and the brain. Trends in Cognitive Sciences. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends in Cognitive Sciences. 2007;11:290–298. doi: 10.1016/j.tics.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Cameron P, Giuntoli D. Consciousness sampling in the college classroom or Is anybody listening? Intellect. 1972;101:63–64. [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle RW, Kane MJ. Executive attention, working memory capacity, and a two-factor theory of cognitive control. In: Ross B, editor. The Psychology of Learning and Motivation. New York: Academic Press; 2004. pp. 145–199. [Google Scholar]
- Geerligs T. Students’ thoughts during problem-based small-group discussions. Instructional Science. 1995;22:269–278. [Google Scholar]
- Giambra LM. A laboratory method for investigating influences on switching attention to task-unrelated imagery and thought. Consciousness and Cognition. 1995;4:1–21. doi: 10.1006/ccog.1995.1001. [DOI] [PubMed] [Google Scholar]
- Grodsky A, Giambra LM. The consistency across vigilance and reading tasks of individual differences in the occurrence of task-unrelated and task-related images and thoughts. Imagination, Cognition and Personality. 1990–91;10:39–52. [Google Scholar]
- Hurlburt RT. Random sampling of cognitions and behavior. Journal of Research in Personality. 1979;13:103–111. [Google Scholar]
- Intel Corporation. [Retrieved April 2004];iESP [Computer software] 2004 from http://seattleweb.intel-research.net/projects/ESM/iESP.html.
- Kane MJ, Brown LE, McVay JC, Silvia PJ, Myin-Germeys I, Kwapil TR. For whom the mind wanders, and when: An experience-sampling study of working memory and executive control in daily life. Psychological Science. 2007;18:614–621. doi: 10.1111/j.1467-9280.2007.01948.x. [DOI] [PubMed] [Google Scholar]
- Klinger E. Structure and functions of fantasy. New York: Wiley; 1971. [Google Scholar]
- Klinger E. Dimensions of thought and imagery in normal waking states. Journal of Altered States of Consciousness. 1978–79;4:97–113. [Google Scholar]
- Klinger E. Thought flow: Properties and mechanisms underlying shifts in content. In: Singer Jefferson A, Salovey Peter., editors. At play in the fields of consciousness: Essays in honor of Jerome L. Singer. Mahwah, NJ: Lawrence Erlbaum Associates; 1999. pp. 29–50. [Google Scholar]
- Klinger E, Cox WM. Dimensions of thought flow in everyday life. Imagination, Cognition and Personality. 1987–88;7:105–128. [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: The default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, Kane MJ. Conducting the train of thought: Working memory capacity, goal neglect, and mind wandering in an executive-control task. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2009;35:196–204. doi: 10.1037/a0014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsella E. The function of phenomenal states: Supramodular interaction theory. Psychological Review. 2005;112:1000–1021. doi: 10.1037/0033-295X.112.4.1000. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Perspectives on Psychological Science. 2008;3:400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. 2nd ed. Newbury Park, CA: Sage; 2002. [Google Scholar]
- Reason JT. Human error. Cambridge, England: Cambridge University Press; 1990. [Google Scholar]
- Robertson IH, Manly T, Andrade J, Baddeley BT, Yiend J. ‘Oops!’: Performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia. 1997;35:747–758. doi: 10.1016/s0028-3932(97)00015-8. [DOI] [PubMed] [Google Scholar]
- Rosenthal DM. Consciousness and its function. Neuropsychologia. 2008;46:829–840. doi: 10.1016/j.neuropsychologia.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Sarason IG, Pierce GR, Sarason BR. Cognitive interference: Theories, methods, and findings. Mahwah, NJ: Erlbaum; 1996. [Google Scholar]
- Schoen JR. Use of consciousness sampling to study teaching methods. Journal of Educational Research. 1970;9:387–390. [Google Scholar]
- Schooler JW. Re-representing consciousness: Dissociations between experience and meta-consciousness. Trends in Cognitive Sciences. 2002;6:339–344. doi: 10.1016/s1364-6613(02)01949-6. [DOI] [PubMed] [Google Scholar]
- Schooler JW, Reichle ED, Halpern DV. Zoning out while reading: Evidence for dissociations between experience and metaconsciousness. In: Levin D, editor. Thinking and seeing: Visual metacognition in adults and children. Cambridge, MA: MIT Press; 2004. pp. 203–226. [Google Scholar]
- Singer JL. The importance of daydreaming. Psychology Today. 1968;1:18–27. [Google Scholar]
- Singer JL, Singer DG. Preschoolers' imaginative play as precursor of narrative consciousness. Imagination, Cognition and Personality. 2006;25:97–117. [Google Scholar]
- Smallwood J, Baracia SF, Lowe M, Obonsawin MC. Task-unrelated-thought whilst encoding information. Consciousness and Cognition. 2003;12:452–484. doi: 10.1016/s1053-8100(03)00018-7. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Beach E, Schooler JW, Handy TC. Going AWOL in the brain: Mind wandering reduces cortical analysis of external events. Journal of Cognitive Neuroscience. 2008;20:458–469. doi: 10.1162/jocn.2008.20037. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Davies JB, Heim D, Finnigan F, Sudberry MV, O Connor RC, Obonsawin MC. Subjective experience and the attentional lapse. Task engagement and disengagement during sustained attention. Consciousness and Cognition. 2004;4:657–690. doi: 10.1016/j.concog.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Fishman DJ, Schooler JW. Counting the cost of an absent mind: Mind wandering as an underrecognized influence on educational performance. Psychonomic Bulletin & Review. 2007;14:230–236. doi: 10.3758/bf03194057. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Fitzgerald A, Miles LK, Phillips LH. Shifting moods, wandering minds: Negative moods lead the mind to wander. Emotion. 2009;9:271–276. doi: 10.1037/a0014855. [DOI] [PubMed] [Google Scholar]
- Smallwood JM, McSpadden M, Schooler JW. The lights are on but no one’s home: Meta-awareness and the decoupling of attention when the mind wanders. Psychonomic Bulletin and Review. 2007;14:527–533. doi: 10.3758/bf03194102. [DOI] [PubMed] [Google Scholar]
- Smallwood J, McSpadden M, Schooler JW. When attention matters: The curious incidence of the wandering mind. Memory & Cognition. 2008;36:1144–1150. doi: 10.3758/MC.36.6.1144. [DOI] [PubMed] [Google Scholar]
- Smallwood J, O’Connor RC, Sudbery MV, Obansawin MC. Mind-wandering and dysphoria. Cognition and Emotion. 2007;21:816–842. [Google Scholar]
- Smallwood J, Riby L, Heim D, Davies JD. Encoding during the attentional lapse: Accuracy of encoding during the semantic SART. Consciousness and Cognition. 2006;15:218–231. doi: 10.1016/j.concog.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Smallwood JM, Schooler JW. The restless mind. Psychological Bulletin. 2006;132:946–958. doi: 10.1037/0033-2909.132.6.946. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Proctor L, Lloyd CA, Baddeley AD. Working memory and stimulus-independent thought: Effects of memory load and presentation rate. European Journal of Cognitive Psychology. 1993;5:417–433. [Google Scholar]
- Watkins ER. Constructive and unconstructive repetitive thought. Psychological Bulletin. 2008;134:163–206. doi: 10.1037/0033-2909.134.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner DM. The illusion of conscious will. Cambridge, MA: MIT Press; 2002. [Google Scholar]