Abstract
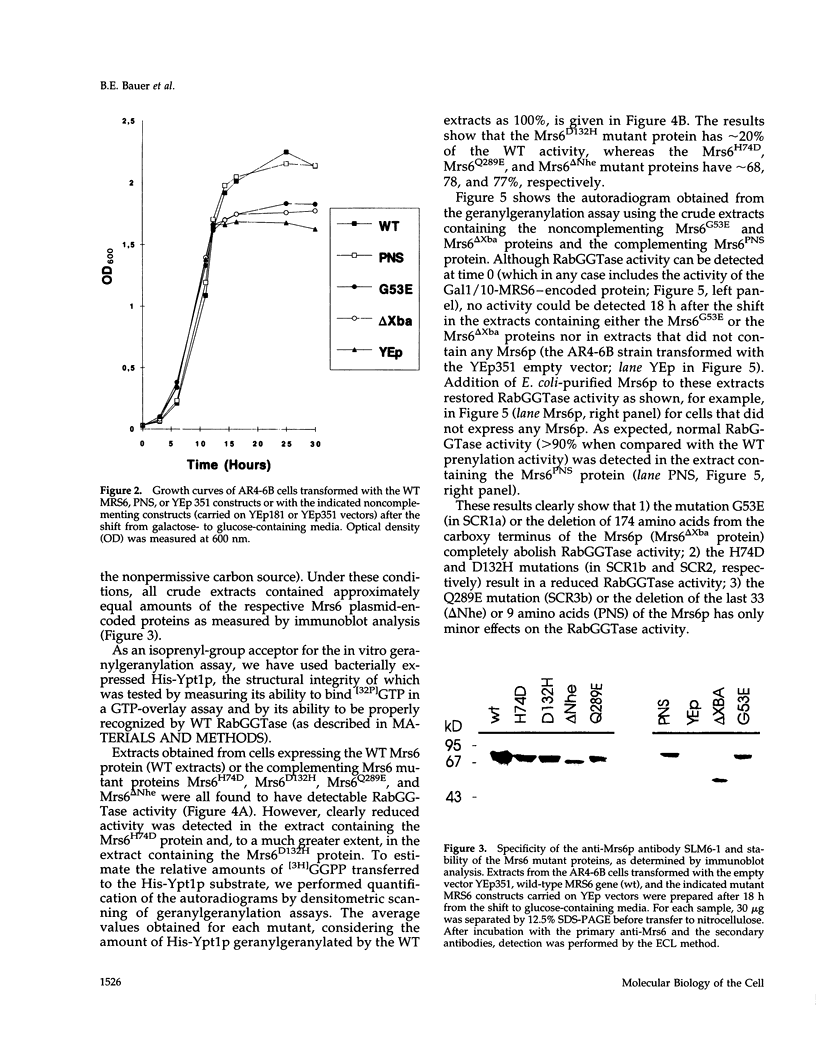
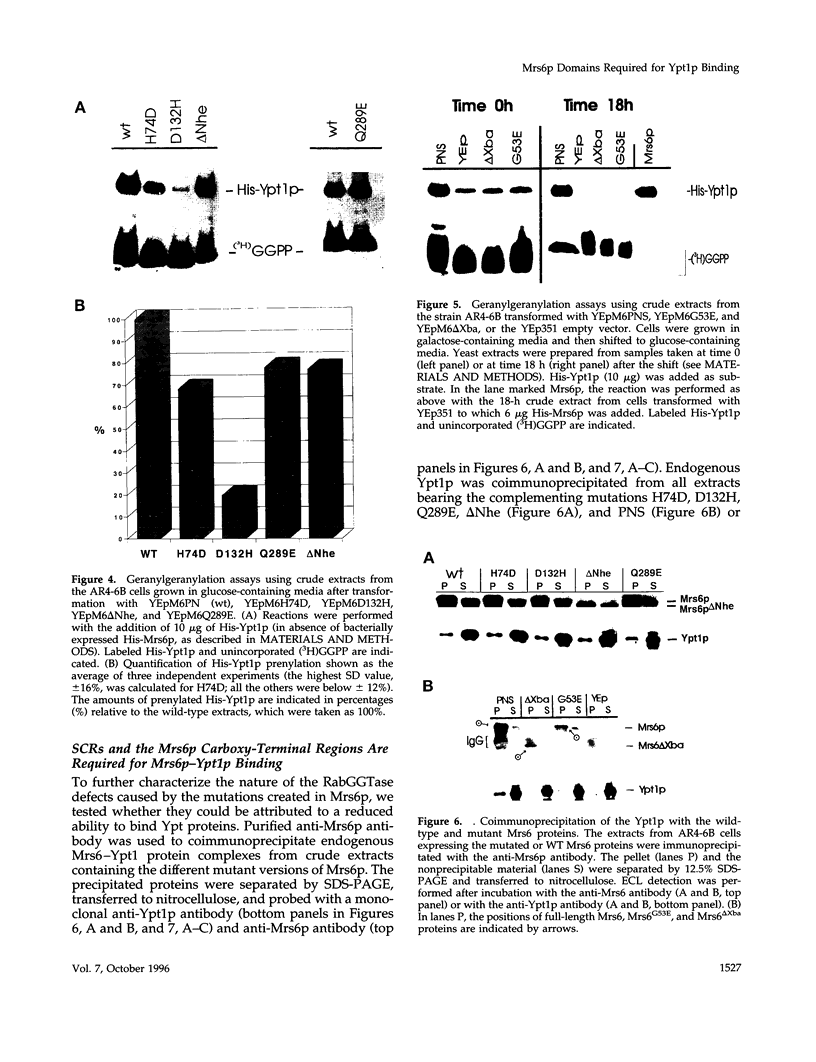
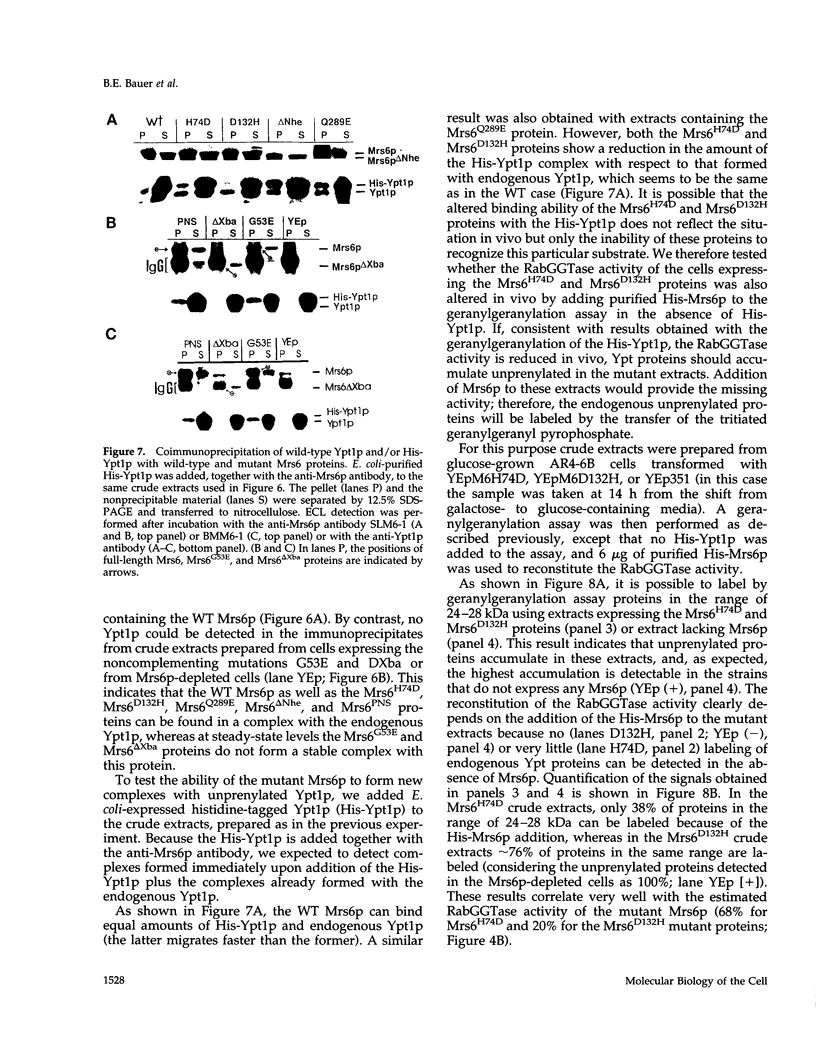
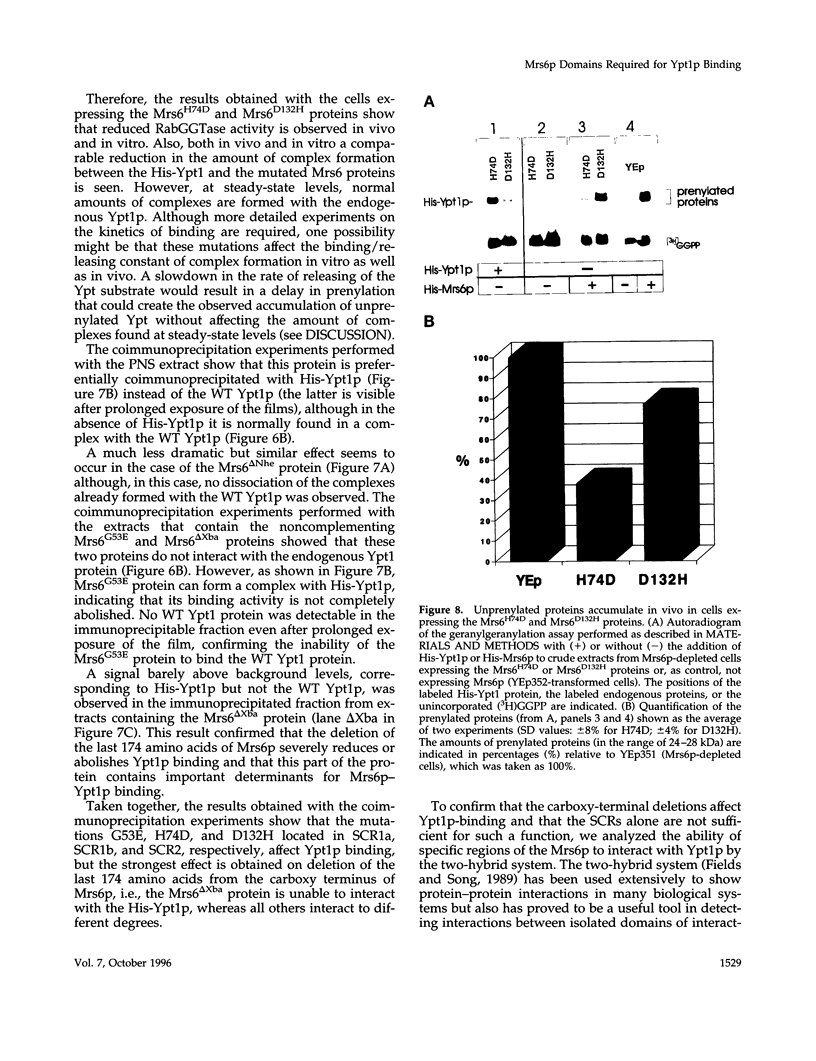
The Rab escort protein (REP) is an essential component of the heterotrimeric enzyme Rab geranylgeranyl transferase that modifies the carboxy-terminal cysteines of the Ras-like small G proteins belonging to the Rab/Ypt family. Deletions in the human CHM locus, encoding one of the two REPs known in humans, result in a retinal degenerative syndrome called choroideremia. The only known yeast homologue of the choroideremia gene product is encoded by an essential gene called MRS6. Besides three structurally conserved regions (SCRs) previously detected in the amino-terminal half of REPs and RabGDIs, three other regions in the carboxy-terminal domain (RCR 1-3) are here identified as being characteristic of REPs alone. We have performed the first mutational analysis of a REP protein to experimentally define the regions functionally important for Rab/Ypt protein binding, making use of the genetic system of the yeast Saccharomyces cerevisiae. This analysis has shown that the SCRs are necessary but not sufficient for Ypt1p binding by the yeast REP, the carboxy-terminal region also being required.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandrov K., Horiuchi H., Steele-Mortimer O., Seabra M. C., Zerial M. Rab escort protein-1 is a multifunctional protein that accompanies newly prenylated rab proteins to their target membranes. EMBO J. 1994 Nov 15;13(22):5262–5273. doi: 10.1002/j.1460-2075.1994.tb06860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J. B., Walberg M. W., Edwards M. C., Elledge S. J. Finding prospective partners in the library: the two-hybrid system and phage display find a match. Trends Biochem Sci. 1995 Dec;20(12):511–516. doi: 10.1016/s0968-0004(00)89119-7. [DOI] [PubMed] [Google Scholar]
- Amberg D. C., Basart E., Botstein D. Defining protein interactions with yeast actin in vivo. Nat Struct Biol. 1995 Jan;2(1):28–35. doi: 10.1038/nsb0195-28. [DOI] [PubMed] [Google Scholar]
- Andres D. A., Seabra M. C., Brown M. S., Armstrong S. A., Smeland T. E., Cremers F. P., Goldstein J. L. cDNA cloning of component A of Rab geranylgeranyl transferase and demonstration of its role as a Rab escort protein. Cell. 1993 Jun 18;73(6):1091–1099. doi: 10.1016/0092-8674(93)90639-8. [DOI] [PubMed] [Google Scholar]
- Araki S., Kaibuchi K., Sasaki T., Hata Y., Takai Y. Role of the C-terminal region of smg p25A in its interaction with membranes and the GDP/GTP exchange protein. Mol Cell Biol. 1991 Mar;11(3):1438–1447. doi: 10.1128/mcb.11.3.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito-Moreno R. M., Miaczynska M., Bauer B. E., Schweyen R. J., Ragnini A. Mrs6p, the yeast homologue of the mammalian choroideraemia protein: immunological evidence for its function as the Ypt1p Rab escort protein. Curr Genet. 1994 Dec;27(1):23–25. doi: 10.1007/BF00326574. [DOI] [PubMed] [Google Scholar]
- Bennett M. K., Scheller R. H. The molecular machinery for secretion is conserved from yeast to neurons. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2559–2563. doi: 10.1073/pnas.90.7.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguski M. S., McCormick F. Proteins regulating Ras and its relatives. Nature. 1993 Dec 16;366(6456):643–654. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brennwald P., Novick P. Interactions of three domains distinguishing the Ras-related GTP-binding proteins Ypt1 and Sec4. Nature. 1993 Apr 8;362(6420):560–563. doi: 10.1038/362560a0. [DOI] [PubMed] [Google Scholar]
- Cremers F. P., Armstrong S. A., Seabra M. C., Brown M. S., Goldstein J. L. REP-2, a Rab escort protein encoded by the choroideremia-like gene. J Biol Chem. 1994 Jan 21;269(3):2111–2117. [PubMed] [Google Scholar]
- Cremers F. P., Molloy C. M., van de Pol D. J., van den Hurk J. A., Bach I., Geurts van Kessel A. H., Ropers H. H. An autosomal homologue of the choroideremia gene colocalizes with the Usher syndrome type II locus on the distal part of chromosome 1q. Hum Mol Genet. 1992 May;1(2):71–75. doi: 10.1093/hmg/1.2.71. [DOI] [PubMed] [Google Scholar]
- Cremers F. P., van de Pol D. J., van Kerkhoff L. P., Wieringa B., Ropers H. H. Cloning of a gene that is rearranged in patients with choroideraemia. Nature. 1990 Oct 18;347(6294):674–677. doi: 10.1038/347674a0. [DOI] [PubMed] [Google Scholar]
- Donnelly P., Menet H., Fouanon C., Herbert O., Moisan J. P., Le Roux M. G., Pascal O. Missense mutation in the choroideremia gene. Hum Mol Genet. 1994 Jun;3(6):1017–1017. doi: 10.1093/hmg/3.6.1017. [DOI] [PubMed] [Google Scholar]
- Dunn B., Stearns T., Botstein D. Specificity domains distinguish the Ras-related GTPases Ypt1 and Sec4. Nature. 1993 Apr 8;362(6420):563–565. doi: 10.1038/362563a0. [DOI] [PubMed] [Google Scholar]
- Espenshade P., Gimeno R. E., Holzmacher E., Teung P., Kaiser C. A. Yeast SEC16 gene encodes a multidomain vesicle coat protein that interacts with Sec23p. J Cell Biol. 1995 Oct;131(2):311–324. doi: 10.1083/jcb.131.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro-Novick S., Jahn R. Vesicle fusion from yeast to man. Nature. 1994 Jul 21;370(6486):191–193. doi: 10.1038/370191a0. [DOI] [PubMed] [Google Scholar]
- Fields S., Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989 Jul 20;340(6230):245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- Fujimura K., Tanaka K., Nakano A., Toh-e A. The Saccharomyces cerevisiae MSI4 gene encodes the yeast counterpart of component A of Rab geranylgeranyltransferase. J Biol Chem. 1994 Mar 25;269(12):9205–9212. [PubMed] [Google Scholar]
- Garrett M. D., Zahner J. E., Cheney C. M., Novick P. J. GDI1 encodes a GDP dissociation inhibitor that plays an essential role in the yeast secretory pathway. EMBO J. 1994 Apr 1;13(7):1718–1728. doi: 10.1002/j.1460-2075.1994.tb06436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz R. D., Sugino A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988 Dec 30;74(2):527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Adami G. R., Wei N., Keyomarsi K., Elledge S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993 Nov 19;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Hill J. E., Myers A. M., Koerner T. J., Tzagoloff A. Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast. 1986 Sep;2(3):163–167. doi: 10.1002/yea.320020304. [DOI] [PubMed] [Google Scholar]
- Jedd G., Richardson C., Litt R., Segev N. The Ypt1 GTPase is essential for the first two steps of the yeast secretory pathway. J Cell Biol. 1995 Nov;131(3):583–590. doi: 10.1083/jcb.131.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Ferro-Novick S. Identification of yeast component A: reconstitution of the geranylgeranyltransferase that modifies Ypt1p and Sec4p. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4377–4381. doi: 10.1073/pnas.91.10.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin S., Altschul S. F. Methods for assessing the statistical significance of molecular sequence features by using general scoring schemes. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2264–2268. doi: 10.1073/pnas.87.6.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian J. P., Stone S., Jiang Y., Lyons P., Ferro-Novick S. Ypt1p implicated in v-SNARE activation. Nature. 1994 Dec 15;372(6507):698–701. doi: 10.1038/372698a0. [DOI] [PubMed] [Google Scholar]
- Magee T., Newman C. The role of lipid anchors for small G proteins in membrane trafficking. Trends Cell Biol. 1992 Nov;2(11):318–323. doi: 10.1016/0962-8924(92)90172-j. [DOI] [PubMed] [Google Scholar]
- Merry D. E., Jänne P. A., Landers J. E., Lewis R. A., Nussbaum R. L. Isolation of a candidate gene for choroideremia. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2135–2139. doi: 10.1073/pnas.89.6.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagiec E. E., Bernstein A., Whiteheart S. W. Each domain of the N-ethylmaleimide-sensitive fusion protein contributes to its transport activity. J Biol Chem. 1995 Dec 8;270(49):29182–29188. doi: 10.1074/jbc.270.49.29182. [DOI] [PubMed] [Google Scholar]
- Ragnini A., Teply R., Waldherr M., Voskova A., Schweyen R. J. The yeast protein Mrs6p, a homologue of the rabGDI and human choroideraemia proteins, affects cytoplasmic and mitochondrial functions. Curr Genet. 1994 Oct;26(4):308–314. doi: 10.1007/BF00310494. [DOI] [PubMed] [Google Scholar]
- Sanford J. C., Pan Y., Wessling-Resnick M. Properties of Rab5 N-terminal domain dictate prenylation of C-terminal cysteines. Mol Biol Cell. 1995 Jan;6(1):71–85. doi: 10.1091/mbc.6.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalk I., Zeng K., Wu S. K., Stura E. A., Matteson J., Huang M., Tandon A., Wilson I. A., Balch W. E. Structure and mutational analysis of Rab GDP-dissociation inhibitor. Nature. 1996 May 2;381(6577):42–48. doi: 10.1038/381042a0. [DOI] [PubMed] [Google Scholar]
- Schmitt H. D., Wagner P., Pfaff E., Gallwitz D. The ras-related YPT1 gene product in yeast: a GTP-binding protein that might be involved in microtubule organization. Cell. 1986 Nov 7;47(3):401–412. doi: 10.1016/0092-8674(86)90597-0. [DOI] [PubMed] [Google Scholar]
- Seabra M. C., Brown M. S., Goldstein J. L. Retinal degeneration in choroideremia: deficiency of rab geranylgeranyl transferase. Science. 1993 Jan 15;259(5093):377–381. doi: 10.1126/science.8380507. [DOI] [PubMed] [Google Scholar]
- Seabra M. C., Goldstein J. L., Südhof T. C., Brown M. S. Rab geranylgeranyl transferase. A multisubunit enzyme that prenylates GTP-binding proteins terminating in Cys-X-Cys or Cys-Cys. J Biol Chem. 1992 Jul 15;267(20):14497–14503. [PubMed] [Google Scholar]
- Seabra M. C., Ho Y. K., Anant J. S. Deficient geranylgeranylation of Ram/Rab27 in choroideremia. J Biol Chem. 1995 Oct 13;270(41):24420–24427. doi: 10.1074/jbc.270.41.24420. [DOI] [PubMed] [Google Scholar]
- Soni R., Carmichael J. P., Murray J. A. Parameters affecting lithium acetate-mediated transformation of Saccharomyces cerevisiae and development of a rapid and simplified procedure. Curr Genet. 1993 Nov;24(5):455–459. doi: 10.1007/BF00351857. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kaibuchi K., Kikuchi A., Kawata M. Small GTP-binding proteins. Int Rev Cytol. 1992;133:187–230. doi: 10.1016/s0074-7696(08)61861-6. [DOI] [PubMed] [Google Scholar]
- Ullrich O., Horiuchi H., Bucci C., Zerial M. Membrane association of Rab5 mediated by GDP-dissociation inhibitor and accompanied by GDP/GTP exchange. Nature. 1994 Mar 10;368(6467):157–160. doi: 10.1038/368157a0. [DOI] [PubMed] [Google Scholar]
- Waldherr M., Ragnini A., Jank B., Teply R., Wiesenberger G., Schweyen R. J. A multitude of suppressors of group II intron-splicing defects in yeast. Curr Genet. 1993 Oct;24(4):301–306. doi: 10.1007/BF00336780. [DOI] [PubMed] [Google Scholar]
- Waldherr M., Ragnini A., Schweyer R. J., Boguski M. S. MRS6--yeast homologue of the choroideraemia gene. Nat Genet. 1993 Mar;3(3):193–194. doi: 10.1038/ng0393-193. [DOI] [PubMed] [Google Scholar]
- de Mendez I., Adams A. G., Sokolic R. A., Malech H. L., Leto T. L. Multiple SH3 domain interactions regulate NADPH oxidase assembly in whole cells. EMBO J. 1996 Mar 15;15(6):1211–1220. [PMC free article] [PubMed] [Google Scholar]
- van Bokhoven H., Schwartz M., Andréasson S., van den Hurk J. A., Bogerd L., Jay M., Rüther K., Jay B., Pawlowitzki I. H., Sankila E. M. Mutation spectrum in the CHM gene of Danish and Swedish choroideremia patients. Hum Mol Genet. 1994 Jul;3(7):1047–1051. doi: 10.1093/hmg/3.7.1047. [DOI] [PubMed] [Google Scholar]
- van Bokhoven H., van den Hurk J. A., Bogerd L., Philippe C., Gilgenkrantz S., de Jong P., Ropers H. H., Cremers F. P. Cloning and characterization of the human choroideremia gene. Hum Mol Genet. 1994 Jul;3(7):1041–1046. doi: 10.1093/hmg/3.7.1041. [DOI] [PubMed] [Google Scholar]
- van den Hurk J. A., van de Pol T. J., Molloy C. M., Brunsmann F., Rüther K., Zrenner E., Pinckers A. J., Pawlowitzki I. H., Bleeker-Wagemakers E. M., Wieringa B. Detection and characterization of point mutations in the choroideremia candidate gene by PCR-SSCP analysis and direct DNA sequencing. Am J Hum Genet. 1992 Jun;50(6):1195–1202. [PMC free article] [PubMed] [Google Scholar]
- von Bokhoven H., von Genderen C., Molloy C. M., van de Pol D. J., Cremers C. W., von Aarem A., Schwartz M., Rosenberg T., Geurts van Kessel A. H., Ropers H. H. Mapping of the choroideremia-like (CHML) gene at 1q42-qter and mutation analysis in patients with Usher syndrome type II. Genomics. 1994 Jan 15;19(2):385–387. doi: 10.1006/geno.1994.1077. [DOI] [PubMed] [Google Scholar]