Abstract
Animal shape and size is controlled with amazing precision during development. External factors such as nutrient availability and crowding can alter overall animal size, but individual body parts scale reproducibly to match the body even with challenges from a changing environment. How is such precision achieved? Here, we review selected research from the last few years in Drosophila - arguably the premier genetic model for study of animal growth - that sheds light on how body and tissue size are regulated by forces intrinsic to individual organs. We focus on two topics currently under intense study: the influence of pattern regulators on organ and tissue growth, and the role of local competitive interactions between cells in tissue homeostasis and final size.
Keywords: Organ and tissue size, imaginal disc, cell competition, pattern regulation, cell-cell interactions
Introduction
Animal growth is subject to external and internal control systems
Three major cellular processes contribute to tissue growth and must be coordinated: cell division, cellular growth (mass accumulation), and cell survival. Each of these cellular processes is controlled by a specific and highly conserved regulatory pathway that is employed in response to the specific needs of any given tissue. In turn, these regulatory pathways, which include the cellular toolkits that control the cell cycle, protein synthesis, and apoptosis, are modulated by three inputs: those arising from the animal’s external environment (nutrients, temperature, crowding), those operating systemically in the animal (hormones, neuronal signals), and those that arise from within individual tissues (patterning cues). The sensing and processing of nutrients is essential for animal growth, and is mediated by signaling from the highly conserved Insulin/IGF Receptor (InR) and TOR (Target Of Rapamycin) pathways (extensively reviewed elsewhere)(Grewal 2008, Wullschleger et al 2006). The level of engagement of the InR and TOR pathways establish the basal growth rate based on the resources available, and also ultimately set the size of each organ and the animal as a whole. Growth and body size are also influenced by physiological signals such as hormones and other systemic factors (reviewed in Edgar 2006, Nijhout 2008).
The influence on growth of the third regulatory system - that encoded autonomously within each organ - is considerably less well understood. Growth regulation by an organ- intrinsic program first became evident from transplantation, regeneration, and grafting experiments carried out many years ago. Salamander eyes or limbs, when exchanged between a smaller and a larger species, take on a size characteristic of the donor (Twitty & Schwind 1931). Likewise, when multiple thymus glands are transplanted into one developing mouse, each gland grows to its normal size (Metcalf 1963). In Drosophila, immature imaginal discs, the primordial cells of the adult, grow at their normal rate and stop growing at the correct final size even when transplanted into the growth-permissive environment of an adult female fly abdomen (Bryant & Simpson 1984). These and similar examples of the intrinsic regulatory ability of organs abound in the literature, but we know very little about the nature of this program. The generally accepted view is that the core of this program is formed by a small number of evolutionarily conserved developmental signaling pathways - BMP/TGF-beta, Wnt, Hh, Notch, and EGF - which regulate patterning of both invertebrates and vertebrates. Most, if not all, of these pathways are also required for animal growth. However, despite the extensive discoveries made about their control of pattern formation, the mechanism by which these pathways control growth remains elusive, and is currently the subject of intense research. Some of the important issues that remain unsolved include which pathways are the key mediators of growth, the identity of the growth effectors, how information is integrated from specific tissues into differences in shape, and the mechanism by which pattern-directed growth is integrated with nutrient sensing and animal physiology.
Drosophila imaginal discs: a model for organ growth
Most of the research on growth in Drosophila utilizes the imaginal discs, the proliferating larval epithelial cells that form the adult appendages of the fly. Imaginal discs have provided an excellent developmental model for many years, and are unsurpassed for studying the relationship between pattern and growth due to their structural simplicity and the wealth of genetic tools available. Disc cells are fueled by nutrients provided from larval feeding, and using conserved cell cycle and growth regulatory factors proliferate rapidly from the beginning of larval development until its end four days later (Figure 1). Differentiation of disc cells is postponed until the larva molts into the pupal stage of development, but the cells acquire their fates in tight coordination to their growth and proliferation.
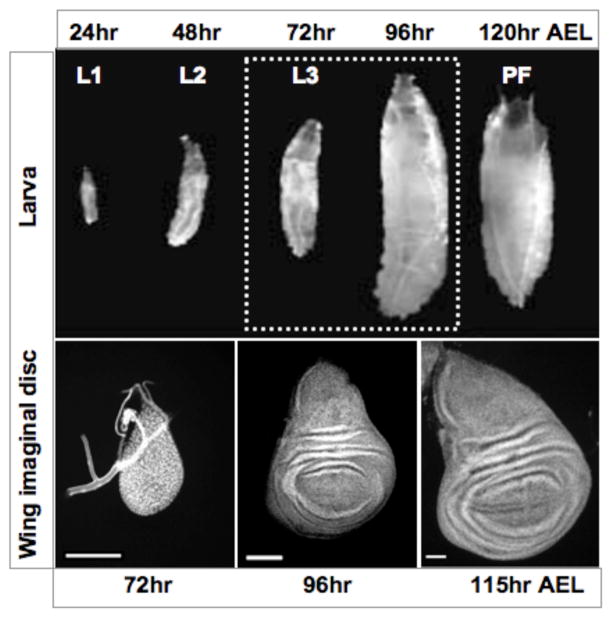
Figure 1. The development of Drosophila imaginal discs.
Larval development begins at 24 hours after egg laying (AEL). Top panels, larvae from each of the three instars are shown above. Larval instars are designated by L1, L2, and L3 and the approximate time of molting is noted. Puparium formation (PF) begins at approximately 120 hours AEL (in animals raised at 25°C). L3 larvae are noted by dotted box. Bottom panels, L3 wing discs dissected from L3 larvae at the times indicated below. The wing disc grows rapidly and increases its size through cell proliferation, and patterning of cell fates occurs simultaneously. At wandering stage the growth of the wing disc slows, and the larva forms a puparium in response to hormones. The final size of the wing is largely determined by the time the animal enters pupal development.
Each imaginal disc starts its life as cluster of essentially similar cells with an identity (wing, eye, head, leg) acquired in response to positional cues in the embryo. Once the hatched larva begins to feed, disc growth occurs rapidly; the wing disc, for example, expands from 50 to 50,000 cells in four days. This rapid growth phase is tightly integrated with the patterning process, ensuring that the appropriate fates are assigned as the tissue expands. Early in their development discs are subdivided into spatially distinct, stable units called compartments, which are developmental fields of cells sharing common ancestry and adhesive properties. Compartments are genetically determined by the activity of selector genes, and the compartmental identity of a cell is inherited through all subsequent divisions so that cells remain with their ancestors and cells in opposite compartments never mix. Signaling between compartments establishes the Anterior-Posterior (A-P) and Dorsal-Ventral (D-V) “organizers”, special cells at the boundaries which express the long-range morphogens Decapentaplegic (Dpp) and Wingless (Wg), respectively (Figure 2). Compartments can grow relatively independently of each other, and thus can be thought of as units of growth (Blair 1995).
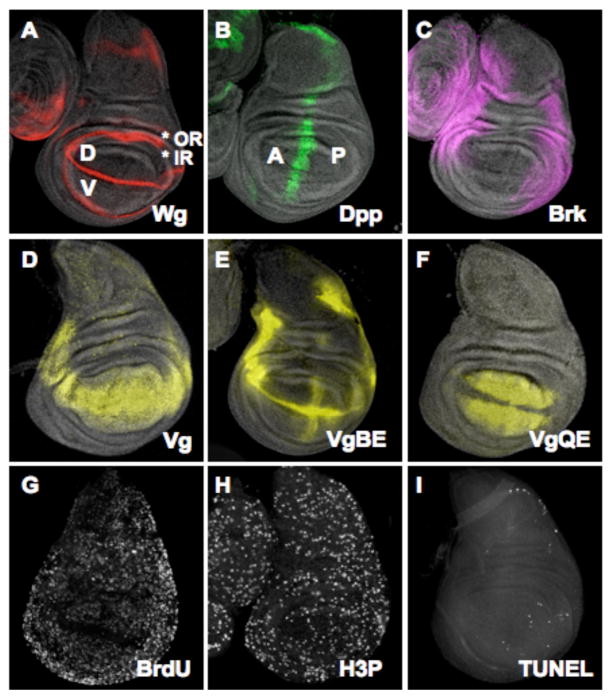
Figure 2. Cell proliferation and patterning in the wing disc.
A) Wingless (Wg; red) is expressed a stripe of cells at the dorsal (D)-ventral (V) boundary of the wing disc. Wg is secreted and forms a gradient across the disc. In addition to expression along the boundary, Wg is expressed in two concentric rings, the inner ring (IR) and outer ring (OR). The IR separates the distal cells (within the IR), which will form the wing blade, from the proximal cells that will form the hinge, pleura and thorax of the fly
B) Dpp (green) is expressed in a stripe of anterior of cells next to the anterior (A)-posterior (P) boundary of the disc, which is established in the embryo. Dpp is also secreted and diffuses across the disc in a graded manner (not evident in this image)
C) Brinker (Brk), a transcriptional repressor of Dpp target genes, is expressed in a gradient that is complementary to Dpp. See text for more information
D) Vestigial (Vg), the wing selector gene, is activated in most cells of the wing pouch (the presumptive wing blade). Vg expression is transcriptionally controlled through enhancers that integrate information from Notch, Wg, and Dpp
E) Expression of Vg at the D-V boundary is controlled by the “Boundary Enhancer” (VgBE). This enhancer is regulated by Notch activity and is activated midway through L2
F) In most distal cells of the wing disc Vg is regulated through the “Quadrant Enhancer” (VgQE), which is regulated by both Wg and Dpp, and also requires input from Vg itself (see text for details)
G) BrdU is incorporated into cells in S phase of the cell cycle, and appears uniform across the proliferating wing disc from L1 through most of L3. Late in L3, cells along the D-V boundary exit the cell cycle as part of a proneural program and do not incorporate BrdU (shown as a stripe of black across the middle of the disc)
H) Mitotic cells, marked by an antibody that recognizes a mitosis-specific phosphorylation of Histone 3, show a distribution similar to cells in S phase (G)
I) Cells undergoing apoptosis are labeled by TUNEL assay. Cell death in the wing disc is minimal and non-patterned during most of larval development.
Pattern regulators also control growth
Wg and Dpp provide the primary pattern organizing activities of imaginal discs and are expressed in orthogonal, overlapping gradients that inform disc cells about their identity and position in the wing disc. Genetic analysis has clearly demonstrated that both Dpp, a member of the TGF-beta/BMP family, and Wg, the founding member of the Wnt family of proteins, are required for the specification of cell fates and for the growth, survival, and proliferation of cells within developing organs. The downstream effectors activated by signaling mediated by Wg or Dpp depend on the identity of the organ and the location of affected cells within each organ. Loss of Dpp or Wg results in growth defects and loss of the wing (Couso et al 1993, Zecca et al 1995). Although it is not known how either factor regulates growth, the reduced growth observed in each mutant is partially due to cell death (Giraldez & Cohen 2003, Johnston & Sanders 2003). Wing disc cells unable to transduce Wg signaling induce the pro-apoptotic gene, hid and die via apoptosis (Giraldez & Cohen 2003, Johnston & Sanders 2003). Cells lacking Dpp activity also die, but probably as an indirect consequence of loss of epithelial integrity, as they are pushed out of the epithelium and activate the Jun-N-terminal kinase (JNK) stress pathway. Delamination (and proliferation) still occurs in the absence of JNK activity, suggesting that cell death is collateral damage (Gibson & Perrimon 2005, Shen & Dahmann 2005). Wg or Dpp can promote growth when activated in ectopic locations within the wing disc, but have little effect (or restrain growth) in regions where they are normally expressed (Basler & Struhl 1994, Giraldez & Cohen 2003, Johnston & Schubiger 1996, Martin-Castellanos & Edgar 2002, Zecca et al 1995). These and other genetic studies illustrate the tight linkage between the patterning process and growth, and have led to the generally held belief that they each regulate growth.
Despite the clear connections in their regulation, we have very little mechanistic knowledge of how patterning contributes to regulation of growth. During the rapid growth phase of the wing disc cell division appears to be stochastic, and no pattern to its regulation is obvious until the end of disc development. Cell death is also unpatterned, and minimal (Figure 2). As Wg and Dpp are expressed in discrete locations at compartment boundaries (Wg is also in two proximal rings surrounding the center of the wing disc), the prevailing view is that their contribution to disc growth is indirect (Figure 2).
The mechanism that underlies the disc intrinsic growth regulatory program incorporates certain properties. Neither cell number nor cell divisions are counted, as demonstrated by manipulation of cell division rates in the wing disc. Although a faster division rate increases cell number, the size of the wing remains unchanged because cell size is reduced; likewise, wing size is not altered by a slower division rate because each cell is larger (Neufeld et al 1998). Increasing the ploidy of some wing cells also does not substantially affect the shape and size of the wing, because the wings contain fewer cells but each is larger (Weigmann 1997). Furthermore, mosaic analysis indicates that the contribution of any cell’s descendants to the adult wing can vary widely without consequences to wing size, demonstrating an impressive inherent flexibility in wing growth (Morata & Ripoll 1975). This plasticity is allowed and promoted by a process called cell competition (discussed below). These and similar observations argue that the dimensions of the wing, and by inference many organs, are measured by a mechanism that senses its total mass or volume rather than counting cells.
Growth effectors downstream of patterning
Several growth promoting factors, including PI3K, Ras, Myc, Cyclin D/Cdk4, and Yorkie (Yki), are essential for wing growth and could be regulated, directly or indirectly, by a dimension-sensing mechanism (de la Cova & Johnston 2006, Edgar 2006, Reddy & Irvine 2008). Below, we discuss two growth regulators that have particular relevance to the linkage between pattern and growth, dMyc, the Drosophila homolog of the c-myc proto-oncogene, and Yki, the nuclear transducer of the conserved Hippo/Warts (Hpo/Wts) tumor suppressor pathway.
Growth regulation by dMyc
dMyc is a member of a highly conserved family of basic-helix-loop-helix (bHLH) transcription factors with critical roles growth regulation (de la Cova & Johnston 2006). Functional studies and expression profiling have determined that the primary role of dMyc is to promote cellular growth. dMyc controls growth by regulating ribosome biogenesis and uses RNA Polymerases I, II and III- dependent transcription to carry out this function (Grewal et al 2004). dMyc also regulates the cell cycle, by controlling Cyclin E, Cyclin D, and the E2F/RBF axis (Duman-Scheel et al 2004, Johnston et al 1999, Prober & Edgar 2000), and apoptosis (by regulating the proapoptotic genes hid and reaper (de la Cova et al 2004, Montero et al 2008), thereby influencing cell, tissue, and overall body size (de la Cova & Johnston 2006). Remarkably, dMyc controls each of these aspects of growth by both cell-autonomous and non-cell-autonomous mechanisms, putting dMyc in a critical position in tissue homeostasis (discussed below) (de la Cova et al 2004, Moreno & Basler 2004, Senoo-Matsuda & Johnston 2007).
Mutations that eliminate dm (diminutive, the gene that encodes dMyc) function in the fly are lethal, and mutant animals die during the first larval instar (Pierce et al 2004). Weak alleles lead to adults with small body size and cell size, slow cell cycles and moderately delayed development (Johnston et al 1999). Interestingly, dMyc expression is under developmental regulation by Wg. Late in wing disc development, dm mRNA expression is repressed by Wg activity in cells at the D/V boundary. Loss of dm expression is necessary for these cells to undergo a transient cell cycle arrest (Duman-Scheel et al 2004, Johnston et al 1999). In addition, expression of a constitutively active Dpp receptor can increase dMyc protein expression (Prober & Edgar 2000), possibly by post-transcriptional means as dm mRNA is not substantially affected under these conditions (Moreno & Basler 2004).
The absolute requirement for dMyc in cell and tissue growth, and its regulation by Wg and Dpp suggests that dMyc functions as a growth effector downstream of these signaling pathways. Over the course of wing disc development, dMyc expression undergoes dynamic changes that correspond to the specification of regional fates (C. Wu and L. A. Johnston, unpublished data). However, how and when pattern regulators deploy dMyc during wing development is unknown. Understanding how dMyc is regulated during development is important for a thorough understanding of its function in growth control, and its role in connecting growth to pattern formation. An important aspect of dMyc’s responsibility in tissue growth is its role in cell competition and is discussed below in a separate section (de la Cova & Johnston 2006).
Growth regulation by the Hpo/Wts pathway
A recently discovered Hpo/Wts pathway also controls the three major aspects of growth by suppressing the activity of the transcriptional co-activator, Yki. The core of this pathway consists of two kinases, Hpo and Warts (Wts), that when active, suppress growth (Figure 3C). The Sterile-20 family kinase Hpo, facilitated by a scaffold protein called Salvador (Sav), phosphorylates and activates the nuclear Dbf2-related (NDR) family kinase, Wts. Wts, in turn, phosphorylates the transcriptional co-activator Yki, the nuclear transducer of the pathway, and prevents its translocation from the cytoplasm to the nucleus (Dong et al 2007, Harvey et al 2003, Huang et al 2005, Kango-Singh et al 2002, Pantalacci et al 2003, Tapon et al 2002, Udan et al 2003, Wu et al 2003). Yki is the critical effector of Hpo/Wts signaling and its loss is cell lethal. The phosphorylation of Yki by Wts is potentiated by the Mob1-related protein Mats, which, like Sav, is thought to act as a scaffold (Lai et al 2005, Wei et al 2007). Inactivation of Hpo, Wts, Mats, or Sav unleashes Yki from the cytoplasm and culminates in overgrowth of several Drosophila epithelial tissues.
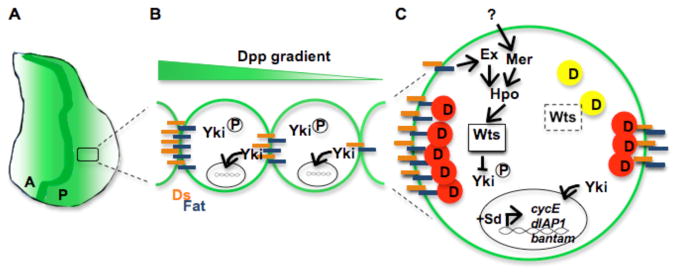
Figure 3. A model for regulation of wing growth by Dpp, Fat, and Hpo/Wts.
(A) Dpp is secreted from a stripe of cells along the anterior edge of the A-P boundary and moves across the disc to form a gradient of signaling activity (shown in green)
(B) The gradient of Dpp signaling activity influences the expression of Fat regulators, Ds and Fj (not shown). Ds (orange bars) modulates Fat signaling by polarizing its distribution (as shown, blue bars) or its activity within a cell. This polarization would allow for differences in Fat activity within the cell. Yki is retained in the cytoplasm by high Fat and Wts activity, but allowed to enter the nucleus where there are fewer Ds-Fat complexes
(C) Polarized Fat activity locally inactivates Dachs (D), and thereby increases the stability and activity of Wts, which phosphorylates Yki and causes its retention in the cytoplasm by 14-3-3-proteins (left side of cell). Wts activity is also regulated by Hpo, which can integrate inputs relayed by Ex (itself regulated by Fat) and Mer (regulated by an unknown receptor). A local reduction in Ds-Fat complexes allows D to accumulate, causing degradation of Wts (right side of cell). A regional pool of unphosphorylated Yki is thus produced, which translocates to the nucleus; together with Sd, Yki promotes the expression of downstream target genes such as cycE, diap1 and bantam.
The overgrowth results from the Yki-dependent activation of several genes required for cell growth, survival and proliferation, including bantam, a microRNA that prevents translation of Hid as well as unknown growth inhibitory factors; the inhibitor of apoptosis diap1; and the G1 cyclin, cyclin E (Huang et al 2005, Nolo et al 2006, Thompson & Cohen 2006). Other targets of the pathway include genes encoding factors that feedback on the pathway and influence its activity, such as Four-jointed (Fj) and Expanded (Ex) (Cho et al 2006, Hamaratoglu et al 2006), and genes involved in patterning, such as wg and Serrate, encoding a Notch ligand, although these targets are not activated in all tissues (Cho et al 2006, Mao et al 2006). Mammalian orthologs of each of the core components of the Hpo/Wts pathway exist and can functionally replace their respective Drosophila counterparts, and their mutations are associated with tumorigenesis in mammals (Chan et al 2005, Tamaskovic et al 2003).
Control of Hpo/Wts at the cell surface
Hpo/Wts activity is regulated at two levels: at the membrane interface between neighboring cells and within the nucleus. The core kinases of the pathway are linked to cell-cell signaling in the local extracellular environment, which provides spatial and temporal control of signaling. The atypical cadherin Fat (Ft), previously implicated as growth suppressor, stimulates Hpo/Wts activity from the cell membrane. Fat activity itself is regulated by another cadherin, Dachsous (Ds), with which it forms heterodimeric bridges between neighboring cells (Bennett & Harvey 2006, Cho et al 2006, Silva et al 2006, Willecke et al 2006) (Figure 3). Ds is expressed in a gradient that declines from proximal to distal cells, thus the extent of the Ds/Fat association on a cell is determined by its location along the proximal-distal axis of the disc. Fj is expressed in an opposing gradient, from distal to proximal cells (Cho et al 2006, Clark et al 1995, Ishikawa et al 2008). Fj is a Golgi protein kinase and phosphorylates cadherin domains in both Fat and Ds (Ishikawa et al 2008). The interaction between Fat and Ds appears to be that of a receptor-ligand pair that activates Hpo/Wts signaling. Interestingly, Ds is required in both signal sending and receiving cells, suggesting that it might function as both ligand and receptor (Casal et al 2006, Willecke et al 2008).
Fat activity in any given cell is controlled first, by how much Ds is present, and second, by phosphorylation of both Ds and Fat by Fj. All of the effects of Fat have been shown genetically to be due to Dachs, a nonconventional myosin located at the apical cortex of the cell (Mao et al 2006). Fat regulates the intracellular location of Dachs, and Dachs binds to and influences the intracellular location of the Wts kinase, as well as its stability (Cho & Irvine 2004). Inhibition of Dachs by Fat stabilizes Wts and allows its phosphorylation of Yki (Figure 3C).
Ex and Merlin (Mer), two members of a family of proteins that contain the 4.1, Ezrin, Radixin, Moesin (FERM) domain and link transmembrane proteins to the actin cytoskeleton, have also been implicated in the Hpo/Wts pathway (Hamaratoglu et al 2006, Silva et al 2006). How these FERM proteins promote the interaction between Hpo and Wts remains unclear. Mer and Ex are in close association with the membrane, but are intracellular and require interaction with transmembrane receptors to transmit signals to the Hpo/Wts pathway. Some data support a model in which Fat transmits information to Hpo and Wts through Ex, whereas other experiments suggest that Ex and Mer activate Hpo and Wts in a parallel pathway (Pan 2007). Importantly, signaling input to Fat and Ds is also provided by Wg (Cho et al 2006) (Jaiswal et al 2006), Notch and Dpp (Cho et al 2006, Rogulja et al 2008).
Nuclear control of Hpo/Wts activity
A second mechanism for spatial and temporal specificity of Hpo/Wts activity occurs in the nucleus. As a co-activator, Yki does not bind DNA and requires a DNA binding partner to activate target gene expression. The TEAD DNA binding protein, Scalloped (Sd), has been shown to carry out this function (Goulev et al, Wu et al 2008, Zhang et al 2008). This important finding is somewhat puzzling, however, as genetic studies indicate that although sd is expressed in numerous tissues, it is required for cell proliferation only in the wing; by contrast, yki appears to be required for cell proliferation in most tissues (Liu et al 2000). Even within the wing disc, Sd is not uniformly expressed, but is present at low levels in proximal cells and high levels in distal cells where the wing selector gene, vestigial (vg) is also expressed. Intriguingly, Sd also functions as the DNA binding component for transcriptional activation of target genes required for wing formation by Vg (Halder et al 1998, Paumard-Rigal et al 1998, Simmonds et al 1998). Vg and Yki appear to function independently, but share a subset of targets (Wu et al 2008). YAP, the mammalian homolog of Yki, functions as a co-activator for several transcriptional regulators in addition to TEAD proteins (Pan 2007), and thereby promotes the selective activation of target genes (Strano et al 2005). It is thus highly likely that other DNA binding proteins partner with Yki to confer temporal or regional specificity to the complex. Since the striking effects of Yki activity on growth are only partially explained by the few targets that have been identified in the fly other targets must exist. Identification of additional transcriptional partners for Yki should help to unveil new target genes.
Mechanistic links to pattern regulation
How are these growth regulating factors and pathways be influenced by the developmental signals that control organ and tissue dimensions? As discussed above, the two major disc pattern regulators, Wg and Dpp, are morphogens. The concentration of a morphogen perceived by a cell identifies its distance from the morphogen source, thus assigning it a position in the developmental field. Pattern regulation by Wg and Dpp is concentration-dependent: cells sensing a high concentration activate a different set of targets than those receiving lower levels, yielding a nested set of target gene expression patterns. If the concentration-dependent target gene activation applies to growth targets as well, the dose sensed by a cell should determine its growth rate, and patterned cell proliferation might be expected. However, this is not the case (Figure 2G, H), suggesting that morphogens control growth differently than they control pattern. One idea proposed long ago is that the slope of a morphogen gradient is sensed locally by individual cells, and stimulates cell proliferation when a certain steepness is perceived (Day & Lawrence 2000). Since the slope is the same in cells within the gradient, this would lead to uniform proliferation of the cells, as is observed (Figure 2G, H).
This model has not been easy to address experimentally, but recent experiments provide evidence that proliferation of wing disc cells is indeed controlled by a local comparison of differences in morphogen activity between cells. Juxtaposition of cells with high and low Dpp activity transiently stimulates cell proliferation. Moreover, flattening the activity gradient by hyper-activating Dpp uniformly across the disc does not alter proliferation of cells in the center of the disc, where endogenous Dpp activity is high, but stimulates it in lateral regions where it is normally low. Thus, each cell compares its level of Dpp signaling with its neighbors. If the difference is great enough, cell proliferation is stimulated; if no difference is perceived, cells do not proliferate. As medial and lateral cells in the disc respond differently to Dpp hyperactivation, it was proposed that the behavior of the two populations of cells reflects their position in the wing disc and therefore the level of Dpp activity they normally perceive (Rogulja & Irvine 2005). The inference from these results is that information imparted by the Dpp gradient is interpreted by cells in two different ways: its concentration is measured by each cell and converted to pattern information, whereas uniform growth is achieved though local comparisons of Dpp activity across the gradient. The net result is that tissue expansion is intimately coupled to tissue patterning.
Linkage of the Dpp gradient with Hpo/Wts signaling
How is the steepness of a morphogen gradient translated molecularly into information that tells a cell to proliferate or not to proliferate? A recent study found that regulation of the Hpo/Wts growth-suppressing pathway allows cells to convert the Dpp activity gradient into a growth response.
Irvine and colleagues noticed that the localization of Dachs, a protein that is required for the growth-suppressing function of Fat, is polarized with respect to the A/P and D/V compartment boundaries, where Wg and Dpp are expressed. On the assumption that this polarization reflects input from the morphogens, they examined the role of Dpp in regulating the activity of the Hpo/Wts pathway (Rogulja et al 2008). Using transient expression methods to either hyper-activate or suppress Dpp signaling in clones of cells, the researchers showed that Dpp activity modulates Fat activity by controlling how much of Ds and Fj are expressed, and their localization (Figure 3) (Rogulja et al 2008). These observations suggest that the Dpp activity information is transduced by Fat via Dachs to the Hpo/Wts pathway core, suppressing its activity and increasing expression of Yki target genes and cell proliferation. Building on previous data from their own and other groups, the authors propose a model in which the graded expression of Fj and Ds (and possibly other regulators of Fat) tunes the activity of the Hpo/Wts pathway. Polarized Fat activity is inferred from the observed polarized localization of Dachs and Wts. In principle, because of its graded expression, a higher level of Ds on the proximal side of a cell will polarize Fat activity and lead to signal transduction that is localized within the cell (Figure 3). This may be countered by polarized expression of Fj, which is graded in the opposite direction from Ds. Dpp-dependent Fat activity that is biased to one side of a cell thus provides a mechanism for propagating information about the Dpp gradient from cell to cell.
One appealing aspect of the idea that Dpp regulates the activity of Hpo/Wts signaling is that it provides a molecular mechanism for how the slope of morphogen gradient is read by an intracellular signaling pathway that controls cell division, cell survival and cellular growth. Another is that it explains how a complete complement of patterned cell fates is linked to the cessation of growth at the end of wing development. Expansion of the tissue extends the Dpp gradient and gradually flattens it; attenuation of local differences in Dpp activity will gradually increase Hpo/Wts activity throughout the disc and its growth will ultimately stop. Interestingly, Notch activation leads to similar effects on Hpo/Wts activity, suggesting that modulation of the activity of Fat and Hpo/Wts represents a general mechanism of linking pattern gradients to growth (Rogulja et al 2008).
Repressing a repressor of growth: Dpp versus Brinker
An alternative growth regulatory mechanism, whereby Dpp exerts its influence by inhibiting a repressor of its own activity, has also gained experimental support (Martin et al 2004, Schwank et al 2008). Activation of target genes by Dpp signaling is antagonized by Brinker (Brk), a transcriptional repressor (Campbell & Tomlinson 1999, Jazwinska et al 1999, Marty et al 2000, Minami et al 1999). In addition, Dpp regulates Brk expression: high Dpp activity represses Brk expression, but low Dpp activity allows it, and results in repression of several Dpp target genes (Affolter & Basler 2007). The net result is that Dpp and Brk exist in inverse activity (and expression) gradients in the wing disc (Figure 2). Thus Dpp-dependent target gene activation is controlled in an interesting feedback relationship. If brk is absent, as in brk null mutants, Dpp patterning targets are activated, even in the absence of Dpp (Campbell & Tomlinson 1999, Marty et al 2000). In brk mutants, wing disc growth is expanded along the A/P axis (Campbell & Tomlinson 1999, Marty et al 2000, Muller et al 2003), just like discs in which Dpp is hyperactivated uniformly (Rogulja & Irvine 2005, Schwank et al 2008). Strikingly, proliferation of lateral cells is enhanced and of medial cells is reduced, even in the complete absence of both dpp and brk (Schwank et al 2008). These observations contradict the idea that the disparate proliferative response of medial and lateral cells to uniform Dpp signaling are encoded in the Dpp gradient itself, as postulated previously (Rogulja & Irvine 2005). Rather, they suggest that the intrinsic growth potential of lateral cells is different from that of medial cells, and that these differences are independent of Dpp. These differences are then dampened by the combinatorial action of Brk and Dpp, allowing even proliferation across the disc.
Taken together, these data argue that Brk - not Dpp - plays the primary role in wing growth. By this logic the sole responsibility of Dpp is to de-repress Brk targets. The idea that a gradient of Dpp is only required to refine a reverse gradient of Brk is not compatible with the gradient slope model. More work is plainly needed to clarify the relationship between Brk and Dpp in growth control and how this influences the Hpo/Wts growth regulatory pathway.
Growth regulation by the wing selector, Vestigial
Additional growth regulatory input to distal cells of the wing disc comes from the wing selector, Vg. Vg is critical for specification of wing blade fates and for its growth, and its graded expression in distal wing disc cells requires the activity of Notch, Wg and Dpp (Figure 2). vg expression is under the control of two enhancers, the vg boundary enhancer (vgBE) and the vg quadrant enhancer (vgQE), whose activity is temporally controlled (Kim et al 1997, Williams et al 1994). The vg BE is activated in response to Notch signaling in cells at the D/V boundary (Figure 2) (Williams et al 1994). Subsequently, the vgQE is activated due to input from both Wg and Dpp, resulting in broad expression of Vg in the blade primordium (Kim et al 1997, Klein & Arias 1998). The vgBE and vgQE enhancers thus integrate signaling from three of the major signaling pathways in wing development (Figures 2 and 4). vg mutant animals are viable, but lack the cells of the wing disc that give rise to the blade (the wing pouch). Clones of cells lacking vg do not survive well and cannot be induced to grow even when given a growth advantage with the Minute technique. Howver, Vg does not directly control cell proliferation or survival, as vg mutant cells delaminate from the epithelium, and like cells lacking Dpp activity, activate the JNK stress pathway but continue to proliferate (Baena-Lopez & Garcia-Bellido).
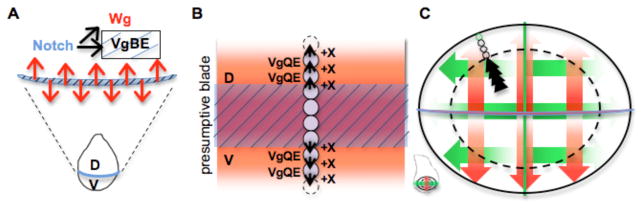
Figure 4. Expansion of the wing blade primordium by a Vestigial feed-forward circuit.
(A) Shortly after specification in early L2 the wing primordium is subdivided into Dorsal and Ventral compartments. Short-range Delta/Serrate-Notch signaling across the D-V boundary induces cells straddling the boundary to express Wg and Vg, the latter driven by the Vg Boundary Enhancer (BE)
(B) By early third instar, D-V border cells send a Vg-dependent signal (X) that is required, together with Wg, to activate the Vg quadrant enhancer (QE) in adjacent cells, thereby upregulating Vg expression. Reiterative cycles of this short-range feed-forward signaling system expands the population of Vg-expressing cells in the presumptive wing blade
(C) In response to both Wg (red) and Dpp (green) the QE mediates the feed-forward Vg auto-regulation and allows expansion of the wing primordium. Besides contributing to Vg autoregulation, Dpp stimulates proliferation of presumptive wing pouch cells through its effects on Hpo/Wts signaling (Figure 3), thereby sustaining the cell population from which wing cells can be recruited.
A molecular circuit was recently elucidated that recruits cells into the vg expression domain, expanding the distal region of the wing disc (Zecca & Struhl 2007a, Zecca & Struhl 2007b). Following establishment of the D/V boundary, cells expressing vg send a short-range signal that, in combination with Wg activity, induces neighboring cells to express vg (Zecca & Struhl 2007b). This establishes a non-autonomous circuit of vg auto-regulation that is reiterated cell to cell in a feed-forward circuit, recruiting neighboring cells into the wing blade primordium. The range of the feed-forward circuit of vg auto-regulation is likely to be contact-mediated as only one or very few cells are recruited at a time. The feed-forward circuit is clearly mediated by the vgQE in response to Wg. However, Dpp activity also regulates this enhancer (Williams et al 1994), suggesting the Vg auto-regulatory circuit requires combinatorial input from Dpp and Wg (Figure 4). The identity of the short-range signal that recruits cells to the Vg-expression domain is unknown.
A clue to the identity of the signal may be found in experiments with mis-expression of vg, which indicate that vg influences cell proliferation both cell autonomously and non-cell autonomously. The effect of vg expression is context-dependent: in distal cells (that already express Vg) it suppresses proliferation (Liu et al 2000) (Baena-Lopez & Garcia-Bellido 2006). In contrast, proliferation of proximal cells is stimulated by ectopic expression of vg, and this is accompanied by non-autonomous induction of Fj and Wg. Both of these outcomes could be interpreted as implicating the Hpo/Wts pathway (Baena-Lopez & Garcia-Bellido 2006, Liu et al 2000, Zecca & Struhl 2007b). In the former, since both Vg and Yki require Sd to activate target gene expression perhaps the high levels of Vg compete with Yki for Sd, suppressing Yki target gene activation. In the latter, Wg is known to stimulate proliferation of proximal cells (Johnston & Sanders 2003, Neumann & Cohen 1996), and Fj suppresses association of Fat and Ds, which could increase Yki activity; also both Fj and Wg are targets of the Hpo/Wts pathway (Cho et al 2006). This pathway was proposed to participate in the feed-forward circuit of Vg auto-regulation (Zecca & Struhl 2007b). The evidence points to Hpo/Wts signaling as a good candidate for a critical mediator between patterning and growth in the wing.
Shape and size regulation by Hox proteins
Organ identity and the physical traits associated with it are determined by the activity of Hox genes, evolutionarily conserved DNA binding proteins that are the major regulators of axial patterning in all animals (Mann & Carroll 2002). The suppression of wing development and specification of haltere fate in Drosophila by the Ultrabithorax (Ubx) is a classic example of how Hox genes can superimpose organ-specific information on universally used developmental fields (i.e., those governed by Wg, Dpp and Hh signaling) to control structure-specific morphology (Lewis 1978). In the absence of Ubx, haltere cells develop wing identities and grow with the characteristics of wing cells, but how Ubx function governs haltere development was not understood. Initially it was found that Ubx represses the expression of Wg and several Wg and Dpp target genes (Weatherbee et al 1998). More recently, it became clear that regulation of haltere size and shape occurs through several mechanisms that operate together, primarily to reduce Dpp activity. In the haltere disc, Ubx reduces dpp transcription relative to its expression in the wing disc and also limits the diffusion of Dpp by increasing the levels of its receptor, Tkv. At the same time, Ubx down-regulates the expression of the glypican division abnormally delayed (dally) in the haltere, which also contributes to the reduction of Dpp diffusion. By limiting the expression and activity domains of Dpp, Ubx thus restricts the size of the haltere (Crickmore & Mann 2006, de Navas et al 2006, Makhijani et al 2007). The regulation of organ dimensions by Hox control of spatial and quantitative aspects of morphogen signaling suggests a general mechanism that could alter organ shape and size during evolution.
The role of local competitive interactions in tissue homeostasis and growth
Global growth regulatory cues from patterning morphogens, tissue selectors and Hox proteins are clearly important for growth, but the data point to local cell interactions as an important mechanism for communicating this information. Tissue growth is strongly influenced by local homeostatic interactions that are not observably regulated by global pattern signals (Johnston 2009). Imaginal disc cells do not divide a fixed number of times; rather, they monitor their own growth and survival status relative to their neighbors, and divide more or less according to this information. This kind of cell-to-cell communication gives cells control over their immediate environment and promotes the plasticity and fitness of the tissue. Mechanisms exist that permit cells to perceive stress or potential danger and rapidly respond by altering their growth or survival, and it has been postulated that these mechanisms contribute to the organ-intrinsic growth regulatory program (Bryant & Simpson 1984, de la Cova et al 2004, Johnston & Gallant 2002). We will discuss tissue plasticity and homeostasis in the context of two such mechanisms, cell competition and morphogenetic apoptosis.
Homeostasis through cell competition
Cell competition is emerging as an important contributor to tissue growth and homeostasis. This process results when neighboring cells within a growing tissue sense metabolic or growth-rate differences and respond with stereotypic behavior: apoptosis is triggered in the weaker cells, while the stronger cells are stimulated to proliferate (Johnston 2009). Cell competition was first discovered in Drosophila wings that were mosaic for Minute (M/+) and wildtype (+/+) cells. During growth, the +/+ cells filled large territories in the wing at the expense of the slow-growing M/+ cells. Importantly, M/+ cells, which carry a mutation in one of several genes encoding ribosomal proteins (rp genes) (Marygold et al 2005), are viable in homotypic conditions and only when in the presence of +/+ cells are the M/+ cells at a disadvantage. This process was called cell competition because the cells competed for contribution to the adult wing (Morata & Ripoll 1975a). Cell competition is thus defined as cell-cell interactions that lead to reciprocal and inter-dependent changes in the growth of each cell. The stronger, “winner” cell actively causes the death of the weaker, “loser” cell, and the winner then proliferates to fill in the vacated space.
Subsequent studies demonstrated several special properties are associated with cell competition. It occurs between cells in close proximity (de la Cova et al 2004, Simpson & Morata 1981), and never crosses the boundaries of developmental compartments, so that cells on one side of a boundary are insulated from competitive elimination by “winner” cells on the other side (de la Cova et al 2004, Garcia-Bellido et al 1973). Cell competition only occurs in growing tissues, but not all growing tissues exhibit competition (de la Cova et al 2004, Simpson 1981). Post-mitotic cells are protected from competition-induced death, as are proximal cells of the wing disc, which lose dMyc expression at later stages of wing growth but are not out-competed by distal cells, which express dMyc at high levels (Neto-Silva, Wu and Johnston, unpublished). Competition is associated with cells that grow at different rates, but a cell-cell difference in growth rate is not sufficient to induce competition (de la Cova et al 2004, Johnston 2009). For example, although M/+ cells compete with +/+ cells, and cells that express different levels of dMyc compete against each other, neighboring differences in PI3K activity or Cyclin D/Cdk4 activity do not lead to cell competition (de la Cova et al 2004).
As M/+ cells are rp-deficient and dMyc regulates many genes required for ribosome biogenesis, including rp genes, Rp regulation may form the basis of a common mechanism (Figure 5) (Johnston 2009). Whether this is the case remains to be determined, as there are differences as well as similarities in their respective competitive behavior. For example, competition between M/+ and +/+ cells appears to be restricted to close-range interactions (Li & Baker 2007), whereas competition between cells expressing different levels of dMyc can occur up to 10 cell diameters away (de la Cova et al 2004, Senoo-Matsuda & Johnston 2007). Additionally, genome-wide expression analysis of “winners” and “losers” from dMyc-induced competition and competition between M/+ and +/+ cells indicates that although “losers” have a number of gene expression changes in common, “winner” cells from each competitive context have little in common (de la Cova et al, ms. in preparation).
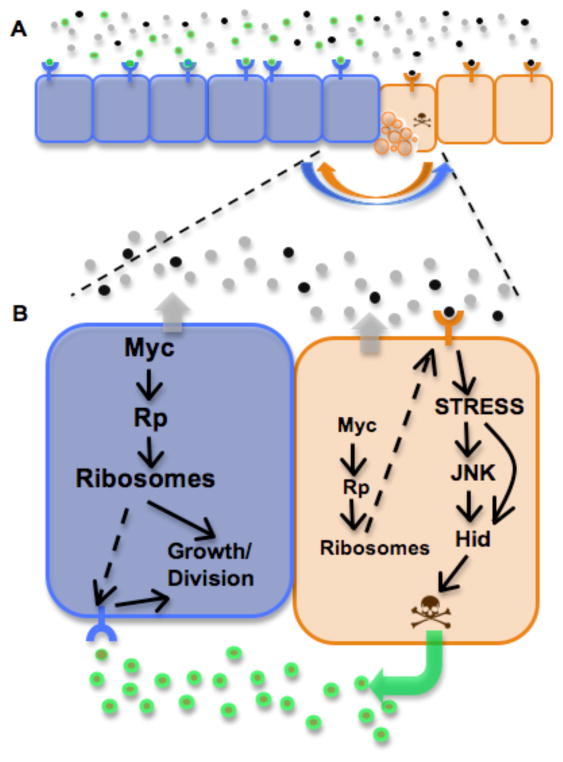
Figure 5. Tissue homeostasis through cell competition.
A) Interactions between neighboring cells in a growing epithelium allow mutual assessment of metabolic state. If no metabolic difference is detected between neighboring cells, all cells survive and reproduce to populate the tissue. Detection of a metabolic difference between neighboring cells leads to competition: the relatively more robust state of ‘winner’ cells (blue) gives them a growth advantage, but signals exchanged between the populations lead to death of their relatively less robust neighbors (‘loser’ cells, orange)
B) A model of cell competition. We postulate that cells continually monitor their own metabolic state (effectively a measure of ribosome function) and that of their neighbors. This mutual sensing may be through the production of soluble factors by both cells that report ribosome function. Cells with lower levels of dMyc or Rp have reduced ribosome function (“losers”) and are sensed by neighbor cells with higher dMyc or Rp levels and thus higher ribosome function (“winners”). Winner cells relay a death signal to loser cells, which respond by activating the JNK stress pathway, induce expression of the proapoptotic factor Hid, and commit suicide. Winner cells are protected from these death signals by an unknown mechanism. Winner cells are stimulated to proliferate more in response to signals produced by dying loser cells (green arrow). The extracellular factors produced during cell competition can diffuse across the epithelium and modulate growth and cell survival decisions up to 10 cell diameters from the competitive boundary (A). Although JNK activity is induced in loser cells, it is not required for their death.
Molecular mechanisms of cell competition
A longstanding puzzle is what cells compete for during the process of cell competition. Several models have been put forth, but a consensus view is still lacking. One idea is that cells compete for Dpp. When residing next to +/+ cells, some M/+ cells up-regulate Brk, the repressor of Dpp activity, and this leads to JNK activation and cell death. This sequence of events also occurs in cells mutant for tkv, and therefore suggested a model in which M/+ cells capture less Dpp ligand, reducing signal transduction and leading to their elimination (Moreno et al 2002). While this model was appealing, given the status of Dpp in disc development, it did not stand up to further tests. In other experiments, Dpp signaling was not altered (de la Cova et al 2004), and more importantly, a direct genetic test demonstrated that cell competition is not prevented in the complete absence of Dpp signaling (Tyler et al 2007). Thus although Dpp is critical for disc patterning and growth, its role in the cell competition is relatively minor.
Death effectors of competition
The death-promoting factor, Hid is an effector of loser cell death. Hid is induced in winner and loser cells during competition, and is absolutely required for the death of loser cells (de la Cova et al 2004). Indeed, loser cells are especially sensitive to Hid function, as removing only one copy of the hid gene prevents more than 90% of loser cell death (de la Cova et al 2004). Why winner cells - which also express Hid - are not susceptible to its pro-apoptotic function is curious. Preliminary work indicates that winner cells maintain their survival under these conditions through a unique genetic program that requires the p53 transcription factor (C. de la Cova, N. Senoo-Matsuda and L. Johnston, unpublished). The JNK pathway, which can induce the expression of several pro-death factors including Hid and Reaper, is another potential death effector. The JNK pathway is induced under competitive conditions in both winner and loser cells, as it is during many stress-inducing conditions. However, although eliminating JNK activity prevents some loser cells from dying, it is unable to block most of the effects of competition (de la Cova et al 2004, Tyler et al 2007).
Interestingly, the death of loser cells may actively contribute to the stimulation of winner cell proliferation. Expression of the baculovirus caspase inhibitor, P35, in the disc prevents elimination of M/+ cells by +/+ winner cells, and also prevents the additional growth of the winner cells (Li & Baker 2007). genes with roles in cell engulfment are also required for loser death and winner growth during competition. Mutations that block engulfment in otherwise wildtype cells prevent loss of nearby M/+ cells; in addition, under these conditions ‘winners’ no longer overgrow (Li & Baker 2007).
Competition mediated by diffusible factors
How do cells with different growth rate potentials recognize each other and communicate the need for corrective measures? Recent evidence from in vitro studies suggests that cells use short range signaling to define their status (Senoo-Matsuda & Johnston 2007). Conditioned medium (CM) from co-cultures of competing populations of cells expressing different levels of dMyc contains activities that induce death of naïve wildtype cells, but stimulate proliferation of naïve cells that express extra dMyc. These results, and others in which filters were used to physically separate competing co-cultures demonstrated that the activities arise from soluble factors, and their production does not require direct contact between the competing populations. However, mutual recognition by each of the two cell populations is critical, as serial conditioning of media with separate cultures of cells did not create active CM (Senoo-Matsuda & Johnston 2007). If these in vitro assays and competition assays in vivo are truly similar, the data strongly argue against a mechanism whereby cells merely struggle for growth or survival factors. Instead, both cell types produce soluble factors that instruct each about their status and also how they should respond to differences (Figure 5).
Does Hpo/Wts signaling play a role in competition?
A genetic screen for mutations that suppress cell competition identified several genes involved in the growth-repressing Hpo/Wts pathway. Mutations in wts, hpo, ex and ft all lead to overgrowth, and suppress competition between +/+ cells and M/+ cells (Tyler et al 2007). In addition, clones of cells mutant for hpo, wts, ft, or sav leads to death of neighboring +/+ cells, suggestive of cell competition, although growth assays are needed to verify whether this is the case (Tyler et al 2007). In contrast, mutations in other members of the pathway, including fj, ds, and atrophin, a transcriptional repressor that binds to Fat (Fanto et al 2003), were not able to rescue M/+ cells from elimination during competition (Tyler et al 2007).
Cell competition contributes to size control
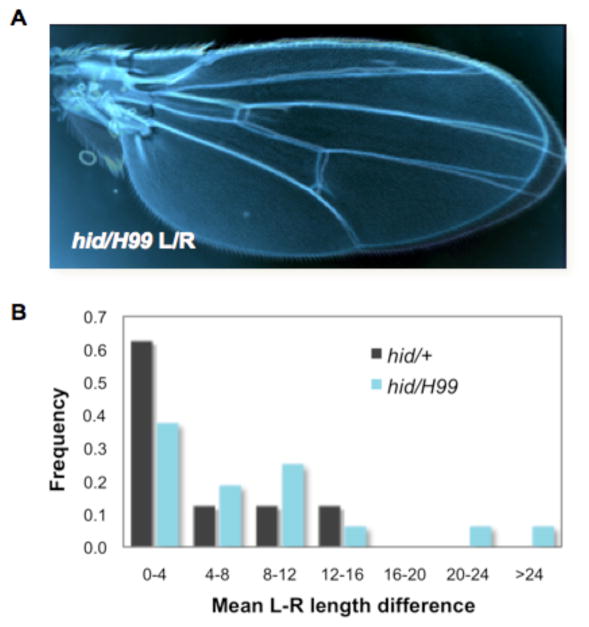
Cell competition provides tissues with plasticity and allows rapid restoration of homeostasis when growth or metabolic differences arise. How does competition contribute to organ growth? In flies, competition buffers tissues from deviations of their size norm. Cells expressing different levels of dMyc are unable to compete if they have only one wildtype copy of hid. As a consequence these wing discs unable to regulate their size properly, and overgrow (de la Cova et al 2004). Even in the absence of competition induced experimentally, hid-induced cell death is critical for maintaining normal wing size, and interestingly, wings entirely mutant for hid lose bilateral size symmetry (Figure 6). As this could significantly impair flight and thus ultimately survival, a mechanism for ensuring symmetrical appendage size is likely to be under selective pressure during evolution. Indeed, fluctuating asymmetry, the randomization of wing size, is a measure of developmental instability (Parsons 1992). It is not known whether loss of cell competition is responsible for the bilateral asymmetry of hid mutant flies, but the possibility is intriguing. Given the prominent role of Hid in cell competition and it’s role in buffering size changes when growth is challenged, competition may provide a mechanism for minimizing noise and errors that occur during development.
Figure 6. Bilateral symmetry of wing size requires Hid-induced cell death.
Mutations that remove Hid function (in this case using a hid null mutation over a small deletion (H99) that removes hid and two other pro-apoptotic genes) leads to bilateral asymmetry of wing size. Loss of Hid-induced death, which causes >90% of cell competition-induced apoptosis, subtly but significantly alters wing growth and causes fluctuating asymmetry, an indicator of developmental instability
A) Left and right wings from an individual fly that is null for the hid gene
B) The frequency of asymmetry of left and right wings increases significantly in animals that lack hid. Graph plots the mean length difference (in pixels) between left (L) and right (R) wing sizes from 8 hid/+ and 12 hid/H99 individual female flies. L and R wing size are matched in more than 60% of hid/+ animals. In contrast, in the complete absence of Hid (hid/H99) L and R wings are frequently mis-matched in size.
A conserved processes of homeostasis
Cell competition appears to be conserved and also occurs in mammals, although few examples have been documented. The best characterized occurs in mice carrying a mutation of the Belly spot and tail (Bst) gene, which encodes RpL24 and like Drosophila Minutes, causes slow growth and developmental delay (Oliver et al 2004). Injection of +/+ embryonic stem (ES) cells into Bst/+ blastocysts led to competition that eliminated the Bst/+ cells (Oliver et al 2004). Another example occurs in a rat model of liver regeneration. Fetal rat liver stem/progenitor cells will repopulate an unmodified host liver through a mechanism that looks much like cell competition. Wildtype host cells within one to two cell diameters of the donor cells are instructed to die and the liver is reconstituted by the fetal donor cells, which proliferate faster than the host cells (Oertel et al 2006). These mammalian examples suggest competitive interactions by a mechanism similar to that in Drosophila imaginal discs, and also demonstrate the potential for cell competition in a therapeutic role in transplantation.
Morphogenetic apoptosis
A related homeostatic mechanism called morphogenetic apoptosis eliminates cells with incorrect positional values, such as those induced by disparities in the Dpp morphogen gradient (Adachi-Yamada & O’Connor 2002). A discontinuity in the Dpp or Wg gradient leads to JNK-dependent apoptosis in cells on each side of the discontinuity. The gradient is then restored by proliferation of the remaining cells (Figure 7) (Adachi-Yamada & O’Connor 2002). There are clear parallels between cell competition and morphogenetic apoptosis, but there are critical differences, and the relationship between the two processes is not fully understood. Most notably, while compartment boundaries provide complete insulation from cell competition, morphogenetic apoptosis is not restricted by these boundaries, and indeed the effects are observed frequently across compartments. Furthermore, JNK activity is essential for morphogenetic apoptosis (Adachi-Yamada et al 1999, Adachi-Yamada & O’Connor 2002), but although induced, it is not required either for cell death induced by cell competition or for competition-induced growth (de la Cova et al 2004, Tyler et al 2007). Finally, the extent to which both growth and tissue size are affected by morphogenetic apoptosis is unknown and awaits further research.
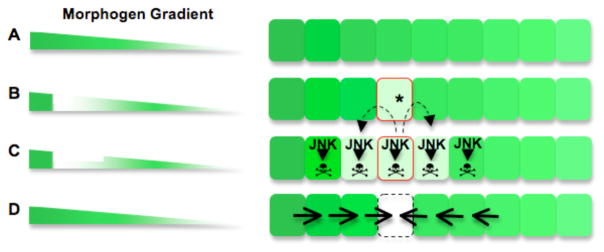
Figure 7. Morphogenetic Apoptosis.
A) Morphogenetic apoptosis eliminates cells that disrupt the continuity of a morphogen gradient. Left, an uninterrupted Dpp gradient. Right, the field of cells along this gradient
B) One cell within the gradient acquires a mutation (*) that disrupts the continuity of the gradient
C) Proliferation of the mutated cell widens the discontinuity. The discrepancy in graded morphogen expression activates the JNK stress pathway in the mutant cells and in adjacent wildtype cells, resulting in cell death and clearance from the epithelium. This process does not occur in the absence of JNK activity
D) Neighboring wildtype cells proliferate, reconstituting a continuous gradient.
A major goal is to understand not only the molecular mechanism of cell competition but also how it is connected to the organ intrinsic size control program. Although we don’t yet know how the homeostatic processes are mechanistically related to size control, the evidence suggests that cell death -particularly that induced via the pro-death factor, Hid - is critical for fine-tuning wing size (de la Cova et al 2004). The finding that Hpo/Wts signaling is modulated by Dpp activity (and possibly also Notch and Vg) to control local cell proliferation and survival raises the intriguing possibility that this pathway is also used in tissue homeostatic processes.
Concluding remarks
In this review we have examined recent literature in Drosophila that explores the control of organ and tissue growth. Although it has long been realized that pattern formation contributes to organ growth during development, only recently have experiments began to address the molecular mechanisms underlying the process. Although we have discussed only work from Drosophila, organ growth and tissue homeostasis are universal processes that utilize highly conserved regulatory pathways and factors. Given this conservation, it is probably safe to assume that many - if not all - of the molecular mechanisms that are found in the fly will be also relevant to how tissues grow in mammals.
A prominent theme in much of the recent Drosophila work is the importance of local cell interactions in control of cell proliferation and cell survival. Important quests for future research include the identification of molecular mediators of cell competition. The recent work clearly demonstrates that the process of competition is carried out in a series of steps: first cells locally sense a difference between their metabolic state and their neighbors, possibly through the production of soluble factors that communicate this information. These factors lead to signaling that activates stress pathways and the apoptotic suicide of loser cells, and may deploy an engulfment program in winner cells. Finally, winner cell proliferation is stimulated and fills in space left by loss of loser cells (Johnston 2009). What are the molecular sensors, receptors, mediators and effectors of these processes? How is cell competition employed during development? Is it used during regeneration, as suggested by the rat liver transplantation experiments? How are cell competition and the related process, morphogenetic apoptosis, connected to the global size-sensing mechanism of an organ?
The identification of the many components of the Hpo/Wts pathway is an exciting development in the filed of growth regulation, and impacts both developmental control of growth and cancer biology. Its regulation by cadherin-mediated cell-cell interactions, influenced by the Dpp morphogen gradient, makes it likely that it is influenced by many developmental signaling pathways and thus plays a key role in defining organ size and shape. Input from diverse pathways could provide additional temporal instructions for tissue growth, as well as spatial control within tissues and between different organs. The discovery of transcriptional activators that partner with Yki is an important quest for future research, as is filling in the missing components that link pattern regulation and growth regulation.
Finally, although the role of Dpp in wing growth is still unclear, the work reviewed here promises to stimulate new studies. Similarly, the regulation of shape and size by selector genes is an area in need of additional work. An important and still mysterious aspect of growth is how the organ-intrinsic regulatory program is integrated with growth influenced by systemic cues and the external environment. The answers to questions arising from the work discussed in this review will be important for a thorough understanding of how organ size and shape is controlled.
Acknowledgments
RMNS and BSW contributed equally to this review. We thank C. de la Cova for data in Figure 5. We apologize to our colleagues whose work we could not review or cite due to space constraints. Work in our laboratory has received support from the NIH, the Rita Allen Foundation, the Uehara Memorial Foundation, and a doctoral grant to RMNS from Fundacao para a Ciencia e a Tecnologia, Portugal.
Imaginal disc development. Drosophila larval development is divided into three instars separated by cuticle molts. Larval development begins at 24 hours after egg laying (AEL). The first (L1) and second (L2) instars are each 24 hours in length, whereas L3 lasts for 48 hours. During these 4 days the wing disc grows rapidly, increasing from approximately 50 to 50,000 cells, and is simultaneously patterned so that each cell acquires a specific cell fate. In response to hormonal cues near the end of L3, the larva wanders away from the food source to find a spot for pupariation. Growth of imaginal discs slows at this point, and most cells accumulate in G2 phase of the cell cycle. After the animal forms a pupa wing disc cells undergo two nearly synchronous divisions before finally exiting the cell cycle. Metamorphosis occurs during the pupal stage and proceeds for five days, culminating in eclosion of the adult fly.
Morphogens are secreted molecules that are produced from a local source and spread though a tissue in a gradient that specifies positional information. The amount of the morphogen perceived by each cell provides it with information about its distance from the source. Distinct, concentration-dependent responses are elicited across the target field of cells and activate patterning target genes. In the wing disc, Wg and Dpp are long-range morphogens, produced by cells at the D-V and A-P compartment boundaries, respectively (Figure 2) and form orthogonal and partially overlapping gradients. Cells grow and differentiate according to the specific positional information they receive from the combination of these morphogens.
Selector proteins regulate the identity of segments, compartments, or organs and appendages, and are transcription factors that control the activation of numerous genes required for realization and maintenance of a particular identity. Examples of selectors include Hox proteins, which regulate segmental identity, the eye-specific selector, Eyeless/Pax-6, the wing selector, Vestigial, and Engrailed and Apterous, which control the identity of the posterior and dorsal compartments, respectively.
Compartments are lineage-restricted, non-overlapping, developmental fields of cells sharing common ancestry and adhesive properties, which are genetically determined by the combination of active and inactive selector genes. Compartment boundaries are crucial for setting up and maintaining organizing centers, where morphogens are produced and secreted.
Minute mutations (M) are carried by a class of Drosophila mutants with lesions at several different loci encoding ribosomal proteins. M mutations are recessive lethal but cause a dominant inhibition of growth, presumably due to impaired ribosome function and reduced protein synthesis. When growing in proximity to wild-type cells in mosaics, the survival of M/+ cells is compromised, and the wild-type cells grows at the expense of its M/+ neighbor, eventually eliminating M/+ cells from an entire compartment. The “M technique” exploits these reciprocal effects on growth rates to increase the size of otherwise poor-growing mutant clones. In the M technique, mitotic recombination generates a clone mutant for a gene X, but lacking the M/+ mutation. As surrounding cells are X/+ and M/+, they are at a disadvantage and the X/X mutant clones are able to grow larger.
Cell competition is a context-dependent process in which cells acquire “winner” or “loser” identity in a mixed (heterotypic) environment, but are viable and form adult animals in a homotypic environment. Competition is characterized by death of the relatively weaker cells (losers) and extra proliferation of the relatively stronger cells (winners). Importantly, loser cell death is triggered by interactions with winner cells (Figure 5). This contrasts cell competition with compensatory proliferation that occurs after cells are killed by physical damage or surgery. Cells are insulated from competition by compartment boundaries. Cell competition contributes to control of organ size.
Morphogenetic apoptosis describes a mechanism for correcting discontinuities in morphogen gradients by eliminating cells with inappropriate morphogen activity (Figure 7).
LITERATURE CITED
- Adachi-Yamada T, Fujimura-Kamada K, Nishida Y, Matsumoto K. Distortion of proximodistal information causxes JNK-dependent apoptosis in Drosophila wing. Nature. 1999;400:166–9. doi: 10.1038/22112. [DOI] [PubMed] [Google Scholar]
- Adachi-Yamada T, O’Connor MB. Morphogenetic apoptosis: a mechanism for correcting discontinuities in morphogen gradients. Dev Biol. 2002;251:74–90. doi: 10.1006/dbio.2002.0821. [DOI] [PubMed] [Google Scholar]
- Affolter M, Basler K. The Decapentaplegic morphogen gradient: from pattern formation to growth regulation. Nat Rev Genet. 2007;8:663–74. doi: 10.1038/nrg2166. [DOI] [PubMed] [Google Scholar]
- Baena-Lopez LA, Garcia-Bellido A. Control of growth and positional information by the graded vestigial expression pattern in the wing of Drosophila melanogaster. Proc Natl Acad Sci U S A. 2006;103:13734–9. doi: 10.1073/pnas.0606092103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler K, Struhl G. Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Cell. 1994;76:89–102. doi: 10.1038/368208a0. [DOI] [PubMed] [Google Scholar]
- Bennett FC, Harvey KF. Fat cadherin modulates organ size in Drosophila via the Salvador/Warts/Hippo signaling pathway. Curr Biol. 2006;16:2101–10. doi: 10.1016/j.cub.2006.09.045. [DOI] [PubMed] [Google Scholar]
- Blair SS. Compartments and appendage development in Drosophila. BioEssays. 1995;17:299–309. doi: 10.1002/bies.950170406. [DOI] [PubMed] [Google Scholar]
- Bryant P, Simpson P. Instrinsic and extrinsic control of growth in developing organs. Quarterly Review of Biology. 1984;59:3887–415. doi: 10.1086/414040. [DOI] [PubMed] [Google Scholar]
- Campbell G, Tomlinson A. Transducing the Dpp morphogen gradient in the wing of Drosophila: regulation of Dpp targets by brinker. Cell. 1999;96:553–62. doi: 10.1016/s0092-8674(00)80659-5. [DOI] [PubMed] [Google Scholar]
- Casal J, Lawrence PA, Struhl G. Two separate molecular systems, Dachsous/Fat and Starry night/Frizzled, act independently to confer planar cell polarity. Development. 2006;133:4561–72. doi: 10.1242/dev.02641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan EH, Nousiainen M, Chalamalasetty RB, Schafer A, Nigg EA, Sillje HH. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 2005;24:2076–86. doi: 10.1038/sj.onc.1208445. [DOI] [PubMed] [Google Scholar]
- Cho E, Feng Y, Rauskolb C, Maitra S, Fehon R, Irvine KD. Delineation of a Fat tumor suppressor pathway. Nat Genet. 2006;38:1142–50. doi: 10.1038/ng1887. [DOI] [PubMed] [Google Scholar]
- Cho E, Irvine KD. Action of fat, four-jointed, dachsous and dachs in distal-to-proximal wing signaling. Development. 2004;131:4489–500. doi: 10.1242/dev.01315. [DOI] [PubMed] [Google Scholar]
- Clark HF, Brentrup D, Schneitz K, Bieber A, Goodman C, Noll M. Dachsous encodes a member of the cadherin superfamily that controls imaginal disc morphogenesis in Drosophila. Genes Dev. 1995;9:1530–42. doi: 10.1101/gad.9.12.1530. [DOI] [PubMed] [Google Scholar]
- Couso JP, Bate M, Martinez-Arias A. A wingless-dependent polar coordinate system in Drosophila imaginal discs. Science. 1993;259:484–9. doi: 10.1126/science.8424170. [DOI] [PubMed] [Google Scholar]
- Crickmore MA, Mann RS. Hox control of organ size by regulation of morphogen production and mobility. Science. 2006;313:63–8. doi: 10.1126/science.1128650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day SJ, Lawrence PA. Measuring dimensions: the regulation of size and shape. Development. 2000;127:2977–87. doi: 10.1242/dev.127.14.2977. [DOI] [PubMed] [Google Scholar]
- de la Cova C, Abril M, Bellosta P, Gallant P, Johnston LA. Drosophila myc regulates organ size by inducing cell competition. Cell. 2004;117:107–16. doi: 10.1016/s0092-8674(04)00214-4. [DOI] [PubMed] [Google Scholar]
- de la Cova C, Johnston LA. Myc in model organisms: a view from the flyroom. Semin Cancer Biol. 2006;16:303–12. doi: 10.1016/j.semcancer.2006.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Navas LF, Garaulet DL, Sanchez-Herrero E. The ultrabithorax Hox gene of Drosophila controls haltere size by regulating the Dpp pathway. Development. 2006;133:4495–506. doi: 10.1242/dev.02609. [DOI] [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–33. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman-Scheel M, Johnston LA, Du W. Repression of dMyc expression by Wingless promotes Rbf-induced G1 arrest in the presumptive Drosophila wing margin. Proc Natl Acad Sci U S A. 2004;101:3857–62. doi: 10.1073/pnas.0400526101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar BA. How flies get their size: genetics meets physiology. Nat Rev Genet. 2006;7:907–16. doi: 10.1038/nrg1989. [DOI] [PubMed] [Google Scholar]
- Fanto M, Clayton L, Meredith J, Hardiman K, Charroux B, et al. The tumor-suppressor and cell adhesion molecule Fat controls planar polarity via physical interactions with Atrophin, a transcriptional co-repressor. Development. 2003;130:763–74. doi: 10.1242/dev.00304. [DOI] [PubMed] [Google Scholar]
- Garcia-Bellido A, Ripoll P, Morata G. Developmental compartmentalisation of the wing disk of Drosophila. Nat New Biol. 1973;245:251–3. doi: 10.1038/newbio245251a0. [DOI] [PubMed] [Google Scholar]
- Gibson MC, Perrimon N. Extrusion and death of DPP/BMP-compromised epithelial cells in the developing Drosophila wing. Science. 2005;307:1785–9. doi: 10.1126/science.1104751. [DOI] [PubMed] [Google Scholar]
- Giraldez AJ, Cohen SM. Wingless and Notch signaling provide cell survival cues and control cell proliferation during wing development. Development. 2003;130:6533–43. doi: 10.1242/dev.00904. [DOI] [PubMed] [Google Scholar]
- Goulev Y, Fauny JD, Gonzalez-Marti B, Flagiello D, Silber J, Zider A. SCALLOPED interacts with YORKIE, the nuclear effector of the hippo tumor-suppressor pathway in Drosophila. Curr Biol. 2008;18:435–41. doi: 10.1016/j.cub.2008.02.034. [DOI] [PubMed] [Google Scholar]
- Grewal SS. Insulin/TOR signaling in growth and homeostasis: A view from the fly world. Int J Biochem Cell Biol. 2008 doi: 10.1016/j.biocel.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Halder G, Polaczyk P, Kraus ME, Hudson A, Kim J, et al. The Vestigial and Scalloped proteins act together to directly regulate wing-specific gene expression in Drosophila. Genes Dev. 1998;12:3900–9. doi: 10.1101/gad.12.24.3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, et al. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol. 2006;8:27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- Harvey KF, Pfleger CM, Hariharan IK. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 2003;114:457–67. doi: 10.1016/s0092-8674(03)00557-9. [DOI] [PubMed] [Google Scholar]
- Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–34. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Ishikawa HO, Takeuchi H, Haltiwanger RS, Irvine KD. Four-jointed is a Golgi kinase that phosphorylates a subset of cadherin domains. Science. 2008;321:401–4. doi: 10.1126/science.1158159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal M, Agrawal N, Sinha P. Fat and Wingless signaling oppositely regulate epithelial cell-cell adhesion and distal wing development in Drosophila. Development. 2006;133:925–35. doi: 10.1242/dev.02243. [DOI] [PubMed] [Google Scholar]
- Jazwinska A, Kirov N, Wieschaus E, Roth S, Rushlow C. The Drosophila gene brinker reveals a novel mechanism of Dpp target gene regulation. Cell. 1999;96:563–73. doi: 10.1016/s0092-8674(00)80660-1. [DOI] [PubMed] [Google Scholar]
- Johnston LA. Competitive interactions between cells: death, growth and geography. Science. 2009 doi: 10.1126/science.1163862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LA, Gallant P. Control of growth and body size in Drosophila. BioEssays. 2002;24:54–64. doi: 10.1002/bies.10021. [DOI] [PubMed] [Google Scholar]
- Johnston LA, Prober DA, Edgar BA, Eisenman RN, Gallant P. Drosophila myc regulates cellular growth during development. Cell. 1999;98:779–90. doi: 10.1016/s0092-8674(00)81512-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LA, Sanders AL. Wingless promotes cell survival but constrains growth during Drosophila wing development. Nat Cell Biol. 2003;5:827–33. doi: 10.1038/ncb1041. [DOI] [PubMed] [Google Scholar]
- Johnston LA, Schubiger G. Ectopic expression of wingless in imaginal discs interferes with decapentaplegic expression and alters cell determination. Development. 1996;122:3519–29. doi: 10.1242/dev.122.11.3519. [DOI] [PubMed] [Google Scholar]
- Kango-Singh M, Nolo R, Tao C, Verstreken P, Hiesinger PR, et al. Shar-pei mediates cell proliferation arrest during imaginal disc growth in Drosophila. Development. 2002;129:5719–30. doi: 10.1242/dev.00168. [DOI] [PubMed] [Google Scholar]
- Kim J, Johnson K, Chen HJ, Carroll S, Laughon A. Drosophila Mad binds to DNA and directly mediates activation of vestigial by Decapentaplegic. Nature. 1997;388:304–8. doi: 10.1038/40906. [DOI] [PubMed] [Google Scholar]
- Klein T, Arias AM. Different spatial and temporal interactions between Notch, wingless, and vestigial specify proximal and distal pattern elements of the wing in Drosophila. Dev Biol. 1998;194:196–212. doi: 10.1006/dbio.1997.8829. [DOI] [PubMed] [Google Scholar]
- Lai ZC, Wei X, Shimizu T, Ramos E, Rohrbaugh M, et al. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell. 2005;120:675–85. doi: 10.1016/j.cell.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Lewis EB. A gene complex controlling segmentation in Drosophila. Nature. 1978;276:565–70. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- Li W, Baker NE. Engulfment is required for cell competition. Cell. 2007;129:1215–25. doi: 10.1016/j.cell.2007.03.054. [DOI] [PubMed] [Google Scholar]
- Liu X, Grammont M, Irvine KD. Roles for scalloped and vestigial in regulating cell affinity and interactions between the wing blade and the wing hinge. Dev Biol. 2000;228:287–303. doi: 10.1006/dbio.2000.9939. [DOI] [PubMed] [Google Scholar]
- Makhijani K, Kalyani C, Srividya T, Shashidhara LS. Modulation of Decapentaplegic gradient during haltere specification in Drosophila. Dev Biol. 2007;302:243–55. doi: 10.1016/j.ydbio.2006.09.029. [DOI] [PubMed] [Google Scholar]
- Mann RS, Carroll SB. Molecular mechanisms of selector gene function and evolution. Curr Opin Genet Dev. 2002;12:592–600. doi: 10.1016/s0959-437x(02)00344-1. [DOI] [PubMed] [Google Scholar]
- Mao Y, Rauskolb C, Cho E, Hu WL, Hayter H, et al. Dachs: an unconventional myosin that functions downstream of Fat to regulate growth, affinity and gene expression in Drosophila. Development. 2006;133:2539–51. doi: 10.1242/dev.02427. [DOI] [PubMed] [Google Scholar]
- Martin FA, Perez-Garijo A, Moreno E, Morata G. The brinker gradient controls wing growth in Drosophila. Development. 2004;131:4921–30. doi: 10.1242/dev.01385. [DOI] [PubMed] [Google Scholar]
- Martin-Castellanos C, Edgar BA. A characterization of the effects of Dpp signaling on cell growth and proliferation in the Drosophila wing. Development. 2002;129:1003–13. doi: 10.1242/dev.129.4.1003. [DOI] [PubMed] [Google Scholar]
- Marty T, Muller B, Basler K, Affolter M. Schnurri mediates Dpp-dependent repression of brinker transcription. Nat Cell Biol. 2000;2:745–9. doi: 10.1038/35036383. [DOI] [PubMed] [Google Scholar]
- Marygold SJ, Coelho CM, Leevers SJ. Genetic analysis of RpL38 and RpL5, two minute genes located in the centric heterochromatin of chromosome 2 of Drosophila melanogaster. Genetics. 2005;169:683–95. doi: 10.1534/genetics.104.034124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. The autonomous behavior of normal thymus grafts. Aust J Exp Biol Med Sci. 1963;41:437–47. doi: 10.1038/icb.1963.64. [DOI] [PubMed] [Google Scholar]
- Minami M, Kinoshita N, Kamoshida Y, Tanimoto H, Tabata T. brinker is a target of Dpp in Drosophila that negatively regulates Dpp-dependent genes. Nature. 1999;398:242–6. doi: 10.1038/18451. [DOI] [PubMed] [Google Scholar]
- Montero L, Muller N, Gallant P. Induction of apoptosis by Drosophila Myc. Genesis. 2008;46:104–11. doi: 10.1002/dvg.20373. [DOI] [PubMed] [Google Scholar]
- Morata G, Ripoll P. Minutes: mutants of drosophila autonomously affecting cell division rate. Dev Biol. 1975a;42:211–21. doi: 10.1016/0012-1606(75)90330-9. [DOI] [PubMed] [Google Scholar]
- Morata G, Ripoll P. Minutes: mutants of Drosophila autonomously affecting cell division rate. Developmental Biology. 1975b;42:211–21. doi: 10.1016/0012-1606(75)90330-9. [DOI] [PubMed] [Google Scholar]
- Moreno E, Basler K. dMyc transforms cells into super-competitors. Cell. 2004;117:117–29. doi: 10.1016/s0092-8674(04)00262-4. [DOI] [PubMed] [Google Scholar]
- Moreno E, Basler K, Morata G. Cells compete for Decapentaplegic survival factor to prevent apoptosis in Drosophila wing development. Nature. 2002;416:755–9. doi: 10.1038/416755a. [DOI] [PubMed] [Google Scholar]
- Muller B, Hartmann B, Pyrowolakis G, Affolter M, Basler K. Conversion of an extracellular Dpp/BMP morphogen gradient into an inverse transcriptional gradient. Cell. 2003;113:221–33. doi: 10.1016/s0092-8674(03)00241-1. [DOI] [PubMed] [Google Scholar]
- Neufeld T, de la Cruz AF, Johnston LA, Edgar BA. Coordination of growth and cell division in the Drosophila wing. Cell. 1998;93:1183–93. doi: 10.1016/s0092-8674(00)81462-2. [DOI] [PubMed] [Google Scholar]
- Neumann CJ, Cohen SM. Distinct mitogenic and cell fate specification functions of wingless in different regions of the wing. Development. 1996;122:1781–9. doi: 10.1242/dev.122.6.1781. [DOI] [PubMed] [Google Scholar]
- Nijhout HF. Size matters (but so does time), and it’s OK to be different. Developmental Cell. 2008;15:491–2. doi: 10.1016/j.devcel.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Nolo R, Morrison CM, Tao C, Zhang X, Halder G. The bantam microRNA is a target of the hippo tumor-suppressor pathway. Curr Biol. 2006;16:1895–904. doi: 10.1016/j.cub.2006.08.057. [DOI] [PubMed] [Google Scholar]
- Oertel M, Menthena A, Dabeva MD, Shafritz DA. Cell competition leads to a high level of normal liver reconstitution by transplanted fetal liver stem/progenitor cells. Gastroenterology. 2006;130:507–20. doi: 10.1053/j.gastro.2005.10.049. quiz 90. [DOI] [PubMed] [Google Scholar]
- Oliver ER, Saunders TL, Tarle SA, Glaser T. Ribosomal protein L24 defect in belly spot and tail (Bst), a mouse Minute. Development. 2004;131:3907–20. doi: 10.1242/dev.01268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D. Hippo signaling in organ size control. Genes Dev. 2007;21:886–97. doi: 10.1101/gad.1536007. [DOI] [PubMed] [Google Scholar]
- Pantalacci S, Tapon N, Leopold P. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat Cell Biol. 2003;5:921–7. doi: 10.1038/ncb1051. [DOI] [PubMed] [Google Scholar]
- Parsons PA. Fluctuating asymmetry: a biological monitor of environmental and genomic stress. Heredity. 1992;68 ( Pt 4):361–4. doi: 10.1038/hdy.1992.51. [DOI] [PubMed] [Google Scholar]
- Paumard-Rigal S, Zider A, Vaudin P, Silber J. Specific interactions between vestigial and scalloped are required to promote wing tissue proliferation in Drosophila melanogaster. Dev Genes Evol. 1998;208:440–6. doi: 10.1007/s004270050201. [DOI] [PubMed] [Google Scholar]
- Pierce SB, Yost C, Britton JS, Loo LW, Flynn EM, et al. dMyc is required for larval growth and endoreplication in Drosophila. Development. 2004;131:2317–27. doi: 10.1242/dev.01108. [DOI] [PubMed] [Google Scholar]
- Prober DA, Edgar BA. Ras1 promotes cellular growth in the Drosophila wing. Cell. 2000;100:435–46. doi: 10.1016/s0092-8674(00)80679-0. [DOI] [PubMed] [Google Scholar]
- Reddy BV, Irvine KD. The Fat and Warts signaling pathways: new insights into their regulation, mechanism and conservation. Development. 2008;135:2827–38. doi: 10.1242/dev.020974. [DOI] [PubMed] [Google Scholar]
- Rogulja D, Irvine KD. Regulation of cell proliferation by a morphogen gradient. Cell. 2005;123:449–61. doi: 10.1016/j.cell.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Rogulja D, Rauskolb C, Irvine KD. Morphogen control of wing growth through the Fat signaling pathway. Dev Cell. 2008;15:309–21. doi: 10.1016/j.devcel.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwank G, Restrepo S, Basler K. Growth regulation by Dpp: an essential role for Brinker and a non-essential role for graded signaling levels. Development. 2008;135:4003–13. doi: 10.1242/dev.025635. [DOI] [PubMed] [Google Scholar]
- Senoo-Matsuda N, Johnston LA. Soluble factors mediate competitive and cooperative interactions between cells expressing different levels of Drosophila Myc. Proc Natl Acad Sci U S A. 2007;104:18543–8. doi: 10.1073/pnas.0709021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Dahmann C. Extrusion of cells with inappropriate Dpp signaling from Drosophila wing disc epithelia. Science. 2005;307:1789–90. doi: 10.1126/science.1104784. [DOI] [PubMed] [Google Scholar]
- Silva E, Tsatskis Y, Gardano L, Tapon N, McNeill H. The tumor-suppressor gene fat controls tissue growth upstream of expanded in the hippo signaling pathway. Curr Biol. 2006;16:2081–9. doi: 10.1016/j.cub.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Simmonds AJ, Brook WJ, Cohen SM, Bell JB. Distinguishable functions for engrailed and invected in anterior-posterior patterning in the Drosophila wing. Nature. 1995;376:424–7. doi: 10.1038/376424a0. [DOI] [PubMed] [Google Scholar]
- Simpson P. Growth and cell competition in Drosophila. J Embryol exp Morph. 1981;65:77–88. [Google Scholar]
- Simpson P, Morata G. Differential mitotic rates and patterns of growth in compartments in the Drosophila wing. Developmental Biology. 1981;85:299–308. doi: 10.1016/0012-1606(81)90261-x. [DOI] [PubMed] [Google Scholar]
- Strano S, Monti O, Pediconi N, Baccarini A, Fontemaggi G, et al. The transcriptional coactivator Yes-associated protein drives p73 gene-target specificity in response to DNA Damage. Mol Cell. 2005;18:447–59. doi: 10.1016/j.molcel.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Tamaskovic R, Bichsel SJ, Hemmings BA. NDR family of AGC kinases--essential regulators of the cell cycle and morphogenesis. FEBS Lett. 2003;546:73–80. doi: 10.1016/s0014-5793(03)00474-5. [DOI] [PubMed] [Google Scholar]
- Tapon N, Harvey KF, Bell DW, Wahrer DC, Schiripo TA, et al. salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110:467–78. doi: 10.1016/s0092-8674(02)00824-3. [DOI] [PubMed] [Google Scholar]
- Thompson BJ, Cohen SM. The Hippo pathway regulates the bantam microRNA to control cell proliferation and apoptosis in Drosophila. Cell. 2006;126:767–74. doi: 10.1016/j.cell.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Twitty VC, Schwind JL. The growth of eyes and limbs transplanted heteroplastically between two species of Amblystoma. J Exp Zool. 1931;59:61–86. [Google Scholar]
- Tyler DM, Li W, Zhuo N, Pellock B, Baker NE. Genes affecting cell competition in Drosophila. Genetics. 2007;175:643–57. doi: 10.1534/genetics.106.061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udan RS, Kango-Singh M, Nolo R, Tao C, Halder G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat Cell Biol. 2003;5:914–20. doi: 10.1038/ncb1050. [DOI] [PubMed] [Google Scholar]
- Weatherbee SD, Halder G, Kim J, Hudson A, Carroll S. Ultrabithorax regulates genes at several levels of the wing-patterning hierarchy to shape the development of the Drosophila haltere. Genes Dev. 1998;12:1474–82. doi: 10.1101/gad.12.10.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Shimizu T, Lai ZC. Mob as tumor suppressor is activated by Hippo kinase for growth inhibition in Drosophila. Embo J. 2007;26:1772–81. doi: 10.1038/sj.emboj.7601630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigmann K, Cohen SM, Lehner CF. Cell cycle progression, growth and patterning in imaginal discs despite inhibition of cell division after inactivation of Drosophila Cdc2 kinase. Development. 1997;124:3555–63. doi: 10.1242/dev.124.18.3555. [DOI] [PubMed] [Google Scholar]
- Willecke M, Hamaratoglu F, Kango-Singh M, Udan R, Chen CL, et al. The fat cadherin acts through the hippo tumor-suppressor pathway to regulate tissue size. Curr Biol. 2006;16:2090–100. doi: 10.1016/j.cub.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Willecke M, Hamaratoglu F, Sansores-Garcia L, Tao C, Halder G. Boundaries of Dachsous Cadherin activity modulate the Hippo signaling pathway to induce cell proliferation. Proc Natl Acad Sci U S A. 2008;105:14897–902. doi: 10.1073/pnas.0805201105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JA, Paddock SW, Vorwerk K, Carroll SB. Organization of wing formation and induction of a wing-patterning gene at the dorsal/ventral compartment boundary. Nature. 1994;368:299–305. doi: 10.1038/368299a0. [DOI] [PubMed] [Google Scholar]
- Wu S, Huang J, Dong J, Pan D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114:445–56. doi: 10.1016/s0092-8674(03)00549-x. [DOI] [PubMed] [Google Scholar]
- Wu S, Liu Y, Zheng Y, Dong J, Pan D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell. 2008;14:388–98. doi: 10.1016/j.devcel.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Zecca M, Basler K, Struhl G. Sequential organizing activities of engrailed, hedgehog and decapentaplegic in the Drosophila wing. Development. 1995;121:2265–78. doi: 10.1242/dev.121.8.2265. [DOI] [PubMed] [Google Scholar]
- Zecca M, Struhl G. Control of Drosophila wing growth by the vestigial quadrant enhancer. Development. 2007a;134:3011–20. doi: 10.1242/dev.006445. [DOI] [PubMed] [Google Scholar]
- Zecca M, Struhl G. Recruitment of cells into the Drosophila wing primordium by a feed-forward circuit of vestigial autoregulation. Development. 2007b;134:3001–10. doi: 10.1242/dev.006411. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ren F, Zhang Q, Chen Y, Wang B, Jiang J. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev Cell. 2008;14:377–87. doi: 10.1016/j.devcel.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]