Abstract
Exercise and cardiorespiratory (CR) fitness may moderate age-related regional brain changes in nondemented older adults (ND). The relationship of fitness to Alzheimer's disease (AD) related brain change is understudied, particularly in the hippocampus which is disproportionately affected in early AD. The role of apolipoprotein E4 (apoE4) genotype in modulating this relationship is also unknown. Nondemented (n=56) and early-stage AD subjects (n=61) over age 65 had MRI and CR fitness assessments. Voxel-based morphometry (VBM) techniques were utilized to identify AD-related atrophy. We analyzed the relationship of CR fitness with white and gray matter within groups, assessed fitness-related brain volume change in areas most affected by AD-related atrophy, and then analyzed differential fitness-brain relationships between apoE4 carriers. Atrophy was present in the medial temporal, temporal, and parietal cortices in subjects with mild AD. There was a significant positive correlation of CR fitness with parietal and medial temporal volume in AD subjects. ND subjects did not have a significant relationship between brain volume and CR fitness in the global or SVC analyses. There was not a significant interaction for fitness × apoE4 genotype in either group. In early-stage AD, cardiorespiratory fitness is associated with regional brain volumes in the medial temporal and parietal cortices suggesting that maintaining cardiorespiratory fitness may modify AD-related brain atrophy.
Keywords: Alzheimer Disease, Cardiorespiratory Fitness, Physical Activity, Hippocampus, Voxel-Based-Morphometry, APOE, Dementia, Aging
Introduction
Accumulating evidence suggests exercise might reduce the deleterious effects of biological and cognitive aging in humans 1, 2. Aerobic exercise in elderly individuals has been shown to enhance efficiency of attentional processes 3, executive-control processes 4, cognition 2, and possibly moderate age-related brain atrophy 1, 5. Healthy elderly with higher fitness levels have less cognitive decline6, 7 and reduced risk for dementia 8, 9 than those with lower fitness levels. A recent randomized trial in subjects at risk for AD demonstrated modest improvement in cognition after 6 months of exercise 10. Morever, brain imaging studies have demonstrated better attention and performance in elderly individuals that were exercising verses those that were not 1. Improving fitness can also lead to both morphological and functional changes in the brains of older animals (reviewed in 11), perhaps by activating neurotrophic factors, enhancing vascularization, or altering expression in a variety of gene transcripts 12. Moreover, exercise appears to stimulate cellular proliferation and dendritic complexity in the dentate gyrus of adult animals 13, 14. Finally, recent imaging studies in aging humans have also revealed reduced atrophy in the temporal and parietal cortices 5, as well as increased cerebral blood volume in the hippocampal dentate gyrus associated with exercise 14.
Cardiorespiratory (CR) fitness might be of therapeutic benefit to individuals with Alzheimer's disease (AD) 15. Exercise in a mouse model of AD inhibited the normal progression of AD-like neuropathology, suggesting that exercise may have disease-modifying effects 16. Exercise training appears to positively affect physical function, cognitive function, and behavioral indices of mood and well-being in individuals with dementia 17. Few studies have looked at the effect of CR fitness on brain volume using imaging, and these have been restricted to cognitively normal older adults 1, 5. We previously assessed the relationship of CR fitness to whole brain volume in AD subjects and found that CR fitness, as defined by peak oxygen consumption (VO2peak), the standard measures of fitness, was positively associated with whole brain and whole white matter volumes in subjects with early AD 18.
In the present study, we utilized novel voxel-based morphometry (VBM) techniques to more closely examine the relationship between CR fitness and regional brain volume in a sample of early AD and nondemented controls previously studied at the whole brain level. We were particularly interested in the relationship between CR fitness and medial temporal lobe structures, such as the hippocampus, which are affected early in the AD process and have demonstrated exercise-related changes in function and structure in animal studies 13, 14, 19. Previous studies have examined the relationship between risk for AD, APOE, and positive effects of exercise, with mixed results 8, 9. Thus we will examine if the relationship between CR fitness and brain volume is influenced by the presence of the Apolipoprotein E E4 allele (apoE4), a risk factor for AD 20 that is associated with structural brain changes 21.
Materials and Methods
Sample and Recruitment
Nondemented (Clinical Dementia Rating (CDR) 0, n=67) and early-stage AD subjects (CDR 0.5 and 1 combined, n=71) aged 65 and over were enrolled in the University of Kansas Brain Aging Project. Participants were recruited from a referral-based memory clinic and by media appeals. Study exclusions have been described previously 18 but included neurological disease other than AD, recent history of cardiovascular disease, history of significant mental illness, diabetes mellitus, or other systemic illness that might impair completion of the study.
Clinical Assessment
The clinical assessment included a semi-structured interview with the participant and with a collateral source knowledgeable about the participant. Diagnostic criteria for AD require the gradual onset and progression of impairment in memory and in at least one other cognitive and functional domain 22. The presence or absence of dementia, and its severity if present, was determined using the CDR 23. These methods have a diagnostic accuracy for AD of 93% 24, are sensitive to detecting the earliest stages of AD by focusing on intraindividual change rather than comparison with group norms 25 and are accurate in identifying the subset of individuals meeting criteria for MCI who have early stage AD 26. Only nondemented (CDR 0) and early stage AD (CDR 0.5 and 1.0) were enrolled. Details on the physical and neurological examinations, neuropsychiatric evaluations and functional activity levels of this group have been discussed previously 18, 27.
Physical Activity Assessment
The participant's level of habitual physical activity was estimated using the Physical Activity Scale in the Elderly (PASE), as described previously 28. The PASE is a reliable and valid measure of physical activity and physical function developed specifically for older individuals 29. The PASE assesses an individual's level of physical activity within the last seven days as an estimate of habitual physical activity. We modified the PASE by administering it to the subject's study partner for both nondemented and AD subjects.
Cardiorespiratory Fitness
Peak oxygen consumption (VO2peak), the standard measure of CR fitness 30, reflects physical activity in addition to other factors, such as age and heredity 31. VO2peak was assessed during a graded treadmill test limited by subject report of fatigue 32, as previously described 18. Expired air was measured for oxygen and carbon dioxide at 15-second intervals using a Parvomedics system. American College of Sports Medicine (ACSM) guidelines 30 were used to determine safety. Blood pressure and the participant's self-assessment of exertion using the 15-point Borg Rating of Perceived Exertion scale 33 were acquired during the last 30 seconds of each 2-minute stage. Participant effort during the treadmill test was assessed with measures of the respiratory exchange ratio (RER). Heart rates were recorded every 15 seconds, with mean heart rate for each subject group listed in Table 1. VO2peak was considered the highest observed value during the test and was normalized by total lean mass as determined by dual energy x-ray absorptiometry (DEXA, [Lunar Prodigy, GE Healthcare, Madison WI]). Test-retest reliability of treadmill testing to determine peak oxygen consumption has been demonstrated in individuals with brain injury or cognitive impairments, including traumatic brain injury 34, stroke 35, and mental retardation 28, 36, 37.
Table 1. Sample Characteristics.
All data represent means (SD), unless otherwise noted.
| Nondemented | Early AD | ||
|---|---|---|---|
| (n=56) | (n=60) | P Value | |
| Age, y | 73.3 (6.2) | 74.3 (6.3) | 0.38 |
| % Female (n) | 58.9 (33) | 61 (37) | 0.77 |
| Education (total years of formal) | 16.4 (2.2) | 15.3 (3.3) | <.05 |
| MMSE | 29.4 (.8) | 26.2 (3.7) | <.001 |
| Total Intracranial Volume, cm3 | 1522.5 (134) | 1486.2 (145.3) | 0.17 |
| Gray matter volume, | 501.8 (43.5) | 447.9 (52) | <.001 |
| White matter volume, cm3 | 444.3 (56) | 419.3 (53.6) | <.05 |
| Whole Brain Volume, cm3 | 946.1 (86.5) | 867.2 (89.9) | <.001 |
| Cardiorespiratory Fitness (VO2peak) | 1.7 (.6) | 1.4 (.4) | <.001 |
| Physical Activity Scale in the Elderly (PASE) | 122 (59.3) | 85.5 (54.5) | <.001 |
| Respiratory Exchange Ratio | 1.10 (.11) | 1.07 (.08) | <.05 |
| Exercise Duration (seconds) | 673.1 (219.6) | 549.8 (176.7) | <.05 |
| Maximum Heart Rate (beats per minute) | 148.9 (15.5) | 139.4 (20.1) | <.001 |
| Rating of Perceived Exertion (6 – 20) | 17.1 (1.7) | 17.03 (2.3) | .78 |
Imaging and Voxel-Based Morphometry
Structural MRI data was acquired using a Siemens 3.0 Tesla Allegra MRI Scanner. High-resolution T1 weighted anatomical images were acquired (magnetization-prepared rapid gradient echo [MPRAGE]; 1×1×1 mm3 voxels, repetition time [TR]=2,500, echo time [TE]=4.38ms, inversion time [TI]=1,100ms, field of view 256×256 with 18% oversample, flip angle=8 degrees) and processed for voxel-based analysis. Every scan was checked for image artifacts and gross anatomical abnormalities. Eleven nondemented and 10 demented subjects were excluded for movement artifact or inhomogeneity that distorted brain matter. Data analysis for 56 ND and 61 AD subjects was performed using the VBM5 toolbox (http://dbm.neuro.uni-jena.de), an extension of the SPM5 algorithms (Wellcome Department of Cognitive Neurology, London, UK) running under MATLAB 7.1 (The MathWorks, Natick, MA, USA) on Linux. Apolipoprotein E genotyping results were obtained using restriction enzyme isotyping, and available for 98 of these subjects with imaging data.
The VBM5 toolbox extends and enhances the unified segmentation approach implemented in SPM5 38 by using a generative model that integrates tissue classification, image registration and MRI inhomogeneity bias correction. This model avoids the “circularity problem” of the optimized VBM procedure in SPM2,39 as the initial image registration does not require a tissue segmentation. We used the Hidden Markov Field (HMRF) model on the estimated tissue maps (3 × 3 × 3). Based on recommendations 38 for aging and diseased populations, estimated tissue probability maps were written without making use of the ICBM tissue priors to avoid a segmentation bias, as these priors are derived from young healthy controls. This approach is a specific feature of the VBM5 toolbox and has been described in detail elsewhere40. The approach is designed to more accurately classify tissues in brains with excess atrophy or abnormal morphology 41, 42 and has been shown to be useful when handling MR imaging data from infants and in cases where the age of the study population is different than the pre-derived priors in SPM5 41, 43. Images were then modulated and saved using affine registration plus non-linear spatial normalization (Wilke, Holland et al. 2008), resulting in final tissue maps of gray matter (GM), white matter (WM) and cerebro-spinal fluid (CSF) which we smoothed with a 10 mm FWHM Gaussian kernel before statistical analysis. Additionally, total GM, WM, CSF and whole brain volumes (GM plus WM) were computed in cm3 using the normalized tissue maps of each study participant.
Statistical Analyses, Demographics
SPSS 16.0 was used for all statistical analysis outside of imaging space. Continuous variables were summarized by means and standard deviations, while categorical variables were summarized by frequency and percent. Continuous demographic and imaging variables were compared in early AD and nondemented groups using ANOVA. Chi-square was used to compare categorical variables between groups.
Imaging Statistics
To analyze brain images in SPM5, we used a full-factorial model (regression and a 2-sample t-test) with independence between subject groups, unequal variance, no grand mean scaling, and centered covariates on the overall mean. We used absolute threshold masking set at 0.10 to restrict each analysis to one tissue type. This model removes the confounding effects due to different brain size between patient and control groups.
First, GM and WM maps of ND and AD subjects were compared to identify areas of AD-related atrophy (p<.05, family-wise error (FWE) corrected) using a 2-sample t-test. Next, the relationship of CR fitness to brain volume was assessed globally within each group using multiple regression, with VO2peak as the variable of interest, and age and gender as confounding variables. The relationship between CR fitness and GM and WM was considered significant at p<0.05 corrected for multiple comparisons (FWE). We then examined the relationship of CR fitness with hippocampal and parahippocampal volumes. The small volume corrections (SVC's), the bilateral hippocampus and bilateral parahippocampus, were derived from the Wake Forest University Pickatlas (http://www.fmri.wfubmc.edu) 44. These medial temporal regions of interest (ROI's) were pre-selected as they are affected early in AD 45 and hippocampal volume loss is considered a valid biomarker of AD neuropathology 46. Additionally, exercise studies in animals suggest that fitness is related to brain volume in the medial temporal lobe 47. In addition we ran a second SVC regression analysis assessing VO2peak in relation to GM and WM controlling for additional confounding variables (physical activity and effort on the treadmill test as measured by RER) in the AD subjects. To correct for multiple comparisons in SVC analyses, results were considered significant at p<0.05 FWE.
Finally, we analyzed a group × ApoE4 interaction for differential fitness-brain relationships (GM and WM) in ApoE4 carriers verses noncarriers in both subject groups. Given few homozygous ApoE4 carriers (n=7), we combined E4 homozygotes and heterozygotes (n=36) into a single group for the AD analysis with noncarriers (n=18). In the ND group there were also too few homozygous ApoE4 carriers (n=3), so we combined them with the 34 heterozygotes (n=15) to compare with the noncarriers (n=29). ApoE4 group was then multiplied by CR fitness (VO2peak) to create an interaction factor for the multiple regressions, done within diagnosis groups. All analyses were covaried for age, gender, and education. Voxels are reported with reference to the Montreal Neurological Institute (MNI) standard space within SPM5 after conversion to the standard space of Talaraich and Tournoux using custom software 48.
Results
Sample Characteristics (Table 1)
The mean age of the cohort (n=117) was 73.8 years (SD=6.3), with no significant difference between ND (n=56) and early-stage AD (n=61) participants. AD subjects had lower CR fitness (VO2peak), education, MMSE performance, and GM and WM volumes (p<.001, Table 1). AD subjects had significantly lower RER, mean heart rate, and exercise duration than the ND group; however there were no differences in ratings of perceived exertion between groups. ApoE4 allele was not related to age, gender, education, MMSE, whole brain volume, GM, WM, and CR fitness.
The role of dementia severity on treadmill testing was examined by assessing the correlation between dementia severity (CDR box score) and measures of effort and VO2peak in the early AD group. Dementia severity was not related to VO2peak (r=-0.32, p=0.81), RER (r=-0.002, p=0.98), peak heart rate (r=0.12, p=0.37), exercise duration (r=-0.09, p=0.50), or rating of perceived exertion (r=-0.84, p=0.52), nor was VO2peak correlated with MMSE in AD subjects when controlling for age and sex (r=.027, p=.842).
Group comparison
First, we used VBM to identify areas of AD related atrophy by comparing GM and WM maps in early AD vs. ND. As expected, we found significantly smaller regional volumes in AD subjects (p<0.05, family-wise error (FWE) corrected) in both GM and WM in medial temporal (hippocampus, parahippocampal gyrus), temporal, and frontal cortices (Table 2, Figure 1). The largest and most significant cluster (k) of GM atrophy was in the left limbic regions, including the hippocampus, insula, left superior temporal and middle temporal cortices (peak at 42, -4, 13, p<0.001 FWE, all coordinates given as x, y, z in Talairach space). GM was lower in AD compared to ND in the bilateral cingulate, bilateral middle frontal gyrus, left inferior temporal gyrus, bilaterally fusiform gyrus, and right supramarginal gyrus. The most significant cluster (k) of WM atrophy was in the left parahippocampal gyrus (peak at -28, -22, -22, p<.001 FWE), followed by decreases in the bilateral parahippocampal/hippocampal cortices, the right superior temporal gyrus, and right posterior cingulate cortex. There were no regions where GM or WM volume was lower in ND compared to AD.
Table 2. Regions of decreased brain volume in AD compared with ND.
| Peak | k | Z | P | ||||
|---|---|---|---|---|---|---|---|
| Type | Region | Coordinate (mm) | Score | Value | |||
| GM | Talairach | x | y | z | FWE | ||
| R Insula (BA 13) | 42 | -4 | 13 | 141815 | Inf | <.001 | |
| (Cluster includes L superior temporal, middle temporal, and hippocampus) | |||||||
| L Cingulate Gyrus (BA 31) | -3 | -40 | 35 | 7618 | 6.77 | <.001 | |
| R Posterior Cingulate | 14 | -59 | 3 | 531 | 5.57 | 0.001 | |
| L Middle Frontal Gyrus | -29 | 26 | 50 | 390 | 5.53 | 0.001 | |
| L Inferior Temporal Gyrus (BA 20) | -31 | -5 | -43 | 821 | 5.25 | 0.003 | |
| R Middle Frontal Gyrus (BA 6) | 29 | -6 | 52 | 181 | 5.22 | 0.003 | |
| R Supramarginal Gyrus (BA 40) | 51 | -48 | 37 | 283 | 5.14 | 0.004 | |
| R Middle Frontal Gyrus | 42 | -34 | 41 | 138 | 5.08 | 0.006 | |
| L Middle Frontal Gyrus | -29 | -7 | 57 | 106 | 5.03 | 0.008 | |
| L Fusiform Gyrus (BA 37) | -45 | -38 | -21 | 37 | 4.81 | 0.02 | |
| L Fusiform Gyrus (BA 19) | -42 | -77 | -20 | 51 | 4.75 | 0.026 | |
| R Fusiform Gyrus | 36 | -77 | -21 | 105 | 4.74 | 0.027 | |
| L Middle Temporal Gyrus (BA 37) | -54 | -59 | -13 | 24 | 4.71 | 0.03 | |
| WM | |||||||
| L Parahippocampal Gyrus | -28 | -22 | -22 | 71 | 5.08 | 0.005 | |
| R Superior Temporal Gyrus | 43 | 3 | -17 | 902 | 5.06 | 0.006 | |
| R Posterior Cingulate | 20 | -54 | 14 | 86 | 4.99 | 0.008 | |
| L Parahippocampal/Hippocampus | -42 | -12 | -19 | 210 | 4.92 | 0.01 | |
| R Posterior Cingulate | 27 | -71 | 7 | 23 | 4.91 | 0.011 | |
| R Parahippocampal/Hippocampus | 28 | -23 | -20 | 31 | 4.77 | 0.02 | |
uncor=uncorrected p value, k= cluster size, L=Left, R=Right, BA=Brodmann's Area, AD=Alzheimer's Disease, GM=Gray Matter volume, WM=White Matter volume. There were no regions that AD had greater GM or WM than ND.
Figure 1.

Statistical maps of the entire brain (A) showing areas where gray matter (A, B) and white matter volume (C) are reduced in patients with Alzheimer's disease compared to nondemented older adults. All shown at a threshold of p<.05 FWE corrected, cluster size (k) > 20. Z scores noted on color charts for gray matter (B) and white matter (C) maps, overlaid on the MNI T1 brain consisting of 154 subjects.
Fitness and Brain Volume
Next, we assessed the relationship of CR fitness with regional brain volumes on a global level (across the entire brain) followed by SVC analyses confined to the hippocampal and parahippocampal regions.
Nondemented Participants
In nondemented participants, CR fitness was not significantly (FWE p<0.05) correlated with gray and white matter regional volumes. There was a trend for a positive relationship of CR fitness and GM in the right inferior frontal gyrus, and WM in the bilateral occipital cortex, lentiform nucleus, and lingual gyrus (p<.001 uncorrected, Table 3, Figure 2). The SVC analysis did not reveal a significant relationship of CR fitness with the hippocampus or parahippocampus in nondemented older adults.
Table 3. GM and WM Regional Correlations with Cardiorespiratory Fitness, Global Results.
| Peak | k | Z | P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Type | Region | Coordinate (mm) | Value | ||||||
| ND | GM | Talairach | x | y | z | FWE | uncor | |||
| R Inferior Frontal Gyrus | 38 | 37 | 6 | 1469 | 3.76 | 0.812 | <.001 | |||
| WM | ||||||||||
| R Inferior Occipital Gyrus | 42 | -77 | -9 | 4448 | 3.68 | 0.771 | <.001 | |||
| L Middle Occipital Gyrus | -28 | -84 | -1 | 5493 | 3.39 | 0.996 | <.001 | |||
| L Lentiform Nucleus Gyrus | -22 | 14 | -3 | 3489 | 3.1 | 0.999 | 0.001 | |||
| L Lingual Gyrus | -7 | -78 | -2 | 1549 | 3.01 | 1 | 0.001 | |||
| AD | GM | |||||||||
| Cerebellum | 0 | -69 | -14 | 16346 | 4 | 0.404 | <.001 | |||
| R Superior Parietal Gyrus | -10 | -71 | 58 | 33822 | 3.98 | 0.426 | <.001 | |||
| R Parahippocampal Gyrus | 23 | 1 | -36 | 5140 | 3.54 | 0.896 | <.001 | |||
| R Inferior Frontal Gyrus | 48 | 49 | 4 | 5655 | 3.42 | 0.959 | <.001 | |||
| R Inferior Frontal Gyrus | 60 | 14 | 22 | 1335 | 3.37 | 0.974 | <.001 | |||
| R Transverse Temporal (BA 42) | 64 | -13 | 13 | 885 | 3.26 | 0.993 | 0.001 | |||
| L Superior Frontal Gyrus | -31 | 47 | 28 | 4004 | 3.18 | 0.998 | 0.001 | |||
| R Middle Occipital Gyrus | 38 | -71 | 6 | 2419 | 3.15 | 0.998 | 0.001 | |||
| R Superior Frontal Gyrus (BA 8) | -4 | 21 | 58 | 7291 | 3.03 | 1 | 0.001 | |||
| L Parahippocampal Gyrus | -19 | -17 | -31 | 1802 | 3.02 | 1 | 0.001 | |||
| WM | ||||||||||
| L Postcentral Gyrus (BA 3) | -59 | -16 | 27 | 84440 | 4.97 | *0.01 | <.001 | |||
| R Postcentral Gyrus (BA 43) | 58 | -9 | 19 | 36123 | 4.85 | *0.018 | <.001 | |||
| R Middle Occipital Gyrus | 48 | -58 | -11 | 1772 | 4.35 | 0.127 | <.001 | |||
| R Precentral Gyrus | 22 | -24 | 54 | 38386 | 4.33 | 0.136 | <.001 | |||
| R Middle Frontal Gyrus | 36 | 18 | 35 | 2952 | 4.06 | 0.325 | <.001 | |||
| L Cingulate Gyrus | -17 | 2 | 49 | 6801 | 3.25 | 0.992 | 0.001 | |||
| R Middle Temporal Gyrus | 57 | -14 | -8 | 3123 | 3.06 | 1 | 0.001 | |||
Regions presented are positively correlated with cardiorespiratory (CR) fitness (VO2peak). No regions negatively correlated with CR fitness. uncorr = uncorrected p value, k = cluster size, L = Left, R = Right, BA = Brodmann's Area, AD = Alzheimer's Disease, ND = Nondemented Elderly, GM = Gray Matter volume, WM = White Matter volume.
regions in bold reached corrected significance (FWE).
Figure 2.
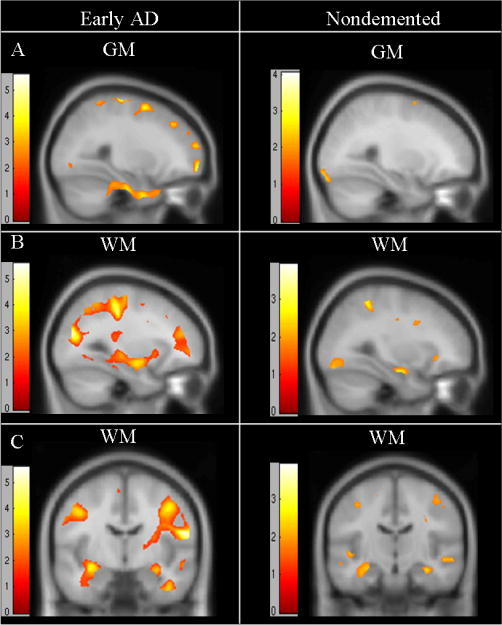
Statistical maps of regions where cardiorespiratory fitness (VO2peak) is positively correlated with brain volume in global analyses of the entire brain (p<.001 uncorrected, Z>2.5). Images represent regions where fitness is associated with gray matter (GM) and white matter (WM) volumes in early AD (left column) and nondemented (right column) subjects. Sagittal views are presented in rows A and B and coronal views in row C. Z scores noted on color charts for each analysis, overlaid on the MNI T1 brain consisting of 154 subjects. Significant regions for each analysis are detailed in Table 3.
Early Alzheimer's Disease
At a global level, CR fitness was positively associated with WM in the bilateral inferior parietal cortex (left at -59, -16, 27, p<0.05 FWE corrected; right at 58, -9, 19, p<0.05 FWE corrected) with positive trends (p<.001 uncorrected) in the frontal, temporal, parietal, and occipital cortices (Table 3, Figure 2). Trends (p<.001) existed in global analyses between CR fitness and GM in the cerebellum, right superior parietal gyrus, inferior and superior frontal cortices, and the temporal and medial temporal cortex (parahippocampal gyrus and uncus). The hypothesis-driven SVC isolating the medial temporal cortex demonstrated a significant positive relationship between CR fitness and WM in the hippocampal region and a significant positive relationship between CR fitness and volume in the parahippocampal gyrus (Table 4, p<0.05 FWE corrected, plotted and detailed in Figure 3). The significant cluster for each result was extracted using the VOI function in SPM5 and the mean volume per cluster for each individual was used to plot the results for visualization purposes in Figure 3. As a secondary analysis, we performed a SVC analysis controlling for RER and PASE and found similar results in AD subjects, demonstrating a significant relationship of fitness with GM and WM in the medial temporal cortex (p=.034 FWE corrected), identical to the results detailed above.
Table 4. GM and WM Limbic Correlations with Cardiorespiratory Fitness.
| Peak | k | Z | P | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Type | Region | Coordinate (mm) | Value | |||||
| ND | GM | Talairach | x | y | z | FWE | uncor | ||
| Hippocampus | N/A | ||||||||
| Parahippocampal | N/A | ||||||||
| WM | |||||||||
| L Hippocampus | 31 | -11 | -20 | 390 | 2.54 | 0.343 | 0.006 | ||
| R Hippocampus | -29 | -17 | -17 | 1193 | 2.26 | 0.514 | 0.012 | ||
| Parahippocampal | N/A | ||||||||
| AD | GM | ||||||||
| Hippocampus | N/A | ||||||||
| L Parahippocampal Gyrus | -19 | -17 | -31 | 1457 | 3.02 | 0.118 | 0.001 | ||
| *R Parahippocampal Gyrus | 23 | 1 | -36 | 2424 | 3.54 | *0.032 | <.001 | ||
| WM | |||||||||
| *L Hippocampus | -32 | -9 | -13 | 2138 | 3.78 | *0.019 | <.001 | ||
| R Hippocampus | 31 | -7 | -17 | 915 | 2.96 | 0.186 | 0.002 | ||
| L Parahippocampal Gyrus | -25 | -43 | -9 | 672 | 3.15 | 0.108 | 0.001 | ||
| R Parahippocampal Gyrus | N/A | ||||||||
Regions presented are positively correlated with cardiorespiratory (CR) fitness (VO2peak), from small volume correction (SVC) analysis localized to the medial temporal lobe. SVC's were bilateral hippocampus and bilateral parahippocampal gyrus ROIs, listed separately (L and R) for statistics breakdown. N/A = no suprathreshold clusters, SVC = small volume correction, uncorr = uncorrected p value, k = cluster size, L = Left, R = Right, BA = Brodmann's Area, AD = Alzheimer's Disease, ND = Nondemented Elderly, GM = Gray Matter volume, WM = White Matter volume.
regions in bold reached corrected significance (FWE).
Figure 3.

Statistical maps and distribution plot from the small volume corrected (SVC) analysis of early AD subjects (p <.05 FWE Corrected). A) Image represents regions where cardiorespiratory fitness is positively associated with hippocampal white matter (WM) volume accompanied by a scatterplot demonstrating the relationship extracted from the left hippocampal cluster at peak (-32, -9, -13). B) Image represents regions where fitness is related to parahippocampal gray matter (GM) volume with scatterplot representing the relationship extracted from the parahippocampal cluster at peak (23, 1, -36). Peak coordinates are significant at p<.05 FWE corrected, and detailed in Table 3. Z scores are noted on the color chart for each analysis, overlaid on the MNI T1 brain consisting of 154 subjects.
Influence of apoE4 on CR fitness – Brain Volume Relationship
Next we examined the role of apoE4 genotype in modifying the relationship between CR fitness and brain volume in ND and AD participants. There were no regions where significant interactions of apoE4 genotype and CR fitness existed in GM and WM in AD or ND at an FWE corrected level p<0.05.
Discussion
Building on our previous work suggesting a relationship between CR fitness and whole brain volume in the earliest clinical stages of AD 18, we used novel methods and identified regionally-specific correlations between increased CR fitness and more regional brain volume in the parietal and medial temporal cortex of individuals with early-stage AD. There was no significant association between CR fitness and regional brain volumes in ND participants. The medial temporal cortex is known to be affected early in the AD course 49, consistent with our findings of lower medial temporal volume in early AD subjects compared to ND (Figure 1). This data suggests that CR fitness, or the maintenance of CR fitness in the early stages of AD, might be associated with a reduction in AD-specific brain atrophy. This relationship between brain volume and CR fitness does not appear to be modified by genetic risk for AD (APOE E4).
Accumulating data primarily generated from animal studies support the biological plausibility that CR fitness could provide disease-modifying benefits in AD. Exercise is associated with increased levels of brain-derived neurotrophic factor 50, enhanced neuronal survival and resistance to brain insults 51, increased vascularization 2, and upregulated neurogenesis in the hippocampus 52. Adult neurogenic zones have been identified in the hippocampus and lateral ventricle walls 53 and physical activity has been correlated with increased neuronal proliferation in the hippocampus and surrounding limbic brain tissue 13, 14, 19. In fact, a recent study in mice suggests that exercise may differentially target the dentate gyrus within the hippocampus and result in local increases in cerebral blood volume, a putative in vivo correlate of adult neurogenesis 14. It has even been suggested that therapeutic stimulation of neurogenesis might contribute to functional ‘repair’ of diseased brain in adults 54. Our findings, while preliminary, suggest that the relationship between CR fitness and medial temporal lobe brain volume are consistent with a fitness-related increased capacity for neuronal proliferation in early AD.
In early AD, CR fitness was positively associated with WM volume in the bilateral inferior parietal cortex (Brodmann's Areas (BA) 3 and 42). This is particularly interesting as BA 3 and 43 are involved in executive processing which may be preferentially enhanced with increased CR fitness through exercise 4. Additionally, strong connections exist between these regions and the hippocampus; the body of the hippocampus and posterior parahippocampal cortex connect with the lateral parietal cortex.55 Our data suggest that CR fitness may be associated with less structural atrophy in early AD within this disease-affected region56, 57 and pathway 58. It is also possible that CR fitness is affecting the vascular burden of the white matter, and that this, in turn, is affecting the VBM results in the white mattet. Still, individuals with a history of clinical stroke, diabetes, and cardiovascular disease were excluded, thus minimizing the role of vascular-related burden on brain change.
Our SVC analysis restricted to the medial temporal lobes demonstrated a relationship between CR fitness and white and gray matter volume. While the hippocampus is largely a gray matter structure, there are four endofolial fiber pathways within the hippocampus 59, and the identified white matter cluster appears to localize to the border of the fimbria and CA3. Despite our usage of the more accurate unified segmentation procedure, it is possible that there is a partial volume effect of gray and white matter segmentation. The stringent correction for multiple comparisons calculated across approximately 6,500 voxels minimizes the possibility that this represents a false-positive. Additionally, there were some assymetries in our findings. Fitness was correlated to parahippocampal gyrus volume on the right, and hippocampal gyrus volume on the left. While we are unable to fully explain these findings, assymetrical patterns in AD atrophy have been reported 60, 61. In addition there were more hippocampal gray and white matter volume decreases in the AD group on the left side, arguing that asymmetries in the CR fitness relationship to brain might reflect the disease-specific changes in this particular subject group.
Our data does not suggest that ApoE4 genotype influences the relationship of CR fitness on atrophy. We hypothesized the presence of an E4 allele might interact with the relationship of fitness on brain volume, based on data showing that the presence of the apoE4 allele has been associated with increased atrophy 62. Prior studies have reported that ApoE4 carriers did not attain the same benefit as noncarriers from physical activity in terms of blood pressure and lipid patterns 63, 64. In addition a previous longitudinal study suggested that the the presence of the ApoE4 allele moderated the benefits of exercise on dementia risk. 8. We found no evidence to suggest the relationship between CR fitness and brain volume was modulated by apoE4 genotype. In line with our results, a longitudinal study measuring the positive effect of self-reported physical activity did not find a mediating effect of ApoE4 genotype on dementia risk 9. Our subject group contained a very low number of E4 homozygotes (n=7 in AD, n=3 in ND). The gene-fitness interaction might have had more power if there was a stronger genetic load of the risk allele in the two analyses. It is also possible that there are other genetic mechanisms mediating the relationship between fitness and brain atrophy, and understanding these will be beneficial to characterizing efficacy of fitness and exercise as a therapeutic option for AD.
Our data can be interpreted as validation of the most recent VBM analytic techniques by replicating previous findings of AD-related atrophy65, 66 in both gray and white matter in medial temporal (hippocampus, parahippocampal gyrus), temporal (superior temporal and middle temporal gyrus), and parietal cortices (p<0.05, FWE). Atrophy in these regions has been shown to be specific to AD as opposed to aging-related changes 65, 67. Comparisons of AD and ND regional brain volume are not novel. However, it was necessary to clarify if this group of very early AD subjects did in fact have significant regional brain atrophy (especially atrophy in the medial temporal cortex) before we could interpret any effect of fitness on “disease-related” brain volume decrease.
The study design is cross-sectional and therefore limits our ability to infer a causal relationship between CR fitness and brain atrophy. Thus, it is possible that AD-related processes may be driving reductions in CR fitness. Our data, however, suggests that individuals with AD performed well on the treadmill tests, and dementia severity (CDR box score) was not associated with cardiorespiratory fitness (VO2peak) or physiologic measures of effort (RER and peak heart rate). Additionally, secondary analyses controlling for participant effort (RER) and physical activity level (PASE), which were both lower in the AD group, did not alter the results of the SVC analysis.
A previous cross-sectional VBM study found evidence that fitness may moderate age-associated atrophy in nondemented older adults. Their analysis, however, assessed the age × VO2peak interaction effect on brain volume while a direct assessment of VO2peak and brain volume, similar to our analysis of interest, did not reveal significant regional relationships with fitness (Colcombe 2003). In addition, another study found an interactive effect of hormonal treatment and VO2peak on brain volume in women while a direct relationship between brain volume and fitness was not evident 68. Our stringent approach of maintaining a rigorous level of statistical significance may have reduced the power to detect fitness-brain associations in nondemented subjects. Additionally, these cross-sectional studies may lack the power to detect direct relationships in nondemented individuals, perhaps related to reduced variability in regional brain atrophy compared to the AD participants. Larger studies with longitudinal outcome measures may provide increased power to detect fitness relationships with brain volume. For instance, a randomized controlled trial of 6 months of exercise in nondemented elderly subjects demonstrated increased brain volume in several regions in those exercising vs. controls 69. Longitudinal studies and randomized controlled trials will be necessary to further define the role of exercise and fitness in promoting brain health.
There are limitations to VBM, including sensitivity to methodological variation in normalization, smoothing kernel, and template. We chose a smoothing kernel of 10mm based on a priori hypotheses about the likely scale of interest. In addition, the use of SVC reduces false negatives that can result from smoothing kernels over 8mm. In this study we used current VBM methods, including unified segmentation without the use of priors to decrease the deleterious effects of template usage in this aging and diseased sample 38. In addition, we compared the global volumes from this new method compared to another version of segmentation (FSL) and found them to be comparable to a previous study in our sample 18. This is one of the first studies in the AD literature to use these newer steps, and first to look at the relationship of CR fitness and medial temporal volume using novel VBM techniques in AD subjects. Finally, further longitudinal and interventional (i.e., exercise trials) are necessary to more precisely define these relationships, in particular the role of exercise in modifying AD-related changes.
Conclusions
This is the first imaging study to characterize the relationship of cardiorespiratory fitness to regional brain volume changes in early Alzheimer's disease. Our data suggest that cardiorespiratory fitness might have a disease-modifying effect in the earliest stages of AD, where increased CR fitness is associated with increased regional brain volumes, particularly in the medial temporal lobes. There is no evidence to suggest this relationship is modified by the presence of the apoE4 risk allele. Longitudinal and interventional studies are necessary to assess the role of exercise to enhance cardiorespiratory fitness as a therapy for attenuating AD-related atrophy.
Acknowledgments
This study was supported by grants R03AG026374 and R21 AG029615 from the National Institutes of Aging and K23NS058252 from the National Institute on Neurological Disorders and Stroke. The University of Kansas General Clinical Research Center (M01RR023940) provided essential space, expertise, and nursing support. The Hoglund Brain Imaging Center is supported by grant C76 HF00201 and Dr. Brooks is supported by NS039123 and AG026482.
Footnotes
Conflict of Interest/Disclosures: There are no financial disclosures relevant to the current study. All other co-authors have no disclosures or conflicts of interest.
References
- 1.Colcombe SJ, Kramer AF, Erickson KI, et al. Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends in Neurosciences. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 3.Kramer AF, Hahn S, Cohen NJ, et al. Ageing, fitness and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- 4.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological Science. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 5.Colcombe SJ, Erickson KI, Raz N, et al. Aerobic Fitness Reduces Brain Tissue Loss in Aging Humans. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2003;58:M176–M180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- 6.Yaffe K, Barnes D, Nevitt M, et al. A Prospective Study of Physical Activity and Cognitive Decline in Elderly Women: Women Who Walk. Arch Intern Med. 2001;161:1703–1708. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- 7.Barnes DE, Yaffe K, Satariano WA, Tager IB. A Longitudinal Study of Cardiorespiratory Fitness and Cognitive Function in Healthy Older Adults. J Am Geriatr Soc. 2003;51:459–465. doi: 10.1046/j.1532-5415.2003.51153.x. [DOI] [PubMed] [Google Scholar]
- 8.Podewils LJ, Guallar E, Kuller LH, et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161:639–651. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- 9.Larson EB, Wang L, Bowen JD, et al. Exercise Is Associated with Reduced Risk for Incident Dementia among Persons 65 Years of Age and Older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 10.Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300:1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 11.Kramer AF, Colcombe SJ, McAuley E, et al. Enhancing brain and cognitive function of older adults through fitness training. J Mol Neurosci. 2003;20:213–221. doi: 10.1385/JMN:20:3:213. [DOI] [PubMed] [Google Scholar]
- 12.Molteni R, Ying Z, Gomez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. Eur J Neurosci. 2002;16:1107–1116. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- 13.Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol. 2005;486:39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- 14.Pereira AC, Huddleston DE, Brickman AM, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pope SK, Shue VM, Beck C. Will a healthy lifestyle help prevent Alzheimer's disease? Annu Rev Public Health. 2003;24:111–132. doi: 10.1146/annurev.publhealth.24.100901.141015. [DOI] [PubMed] [Google Scholar]
- 16.Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary Exercise Decreases Amyloid Load in a Transgenic Model of Alzheimer's Disease. Journal of Neuroscience. 2005;25:4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: A meta-analysis. Archives of Physical Medicine and Rehabilitation. 2004;85:1694–1704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Burns JM, Donnelly JE, A HS, et al. Cardiorespiratory Fitness and Brain Atrophy in Early Alzheimer's Disease. Neurology. 2008 doi: 10.1212/01.wnl.0000317094.86209.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redila VA, Christie BR. Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neuroscience. 2006;137:1299–1307. doi: 10.1016/j.neuroscience.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 20.Martinez M, Campion D, Brice A, et al. Apolipoprotein E epsilon4 allele and familial aggregation of Alzheimer disease. Arch Neurol. 1998;55:810–816. doi: 10.1001/archneur.55.6.810. [DOI] [PubMed] [Google Scholar]
- 21.Bigler ED, Lowry CM, Anderson CV, et al. Dementia, quantitative neuroimaging, and apolipoprotein E genotype. AJNR Am J Neuroradiol. 2000;21:1857–1868. [PMC free article] [PubMed] [Google Scholar]
- 22.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 23.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412b–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 24.Berg L, McKeel DW, Jr, Miller JP, et al. Clinicopathologic Studies in Cognitively Healthy Aging and Alzheimer Disease: Relation of Histologic Markers to Dementia Severity, Age, Sex, and Apolipoprotein E Genotype. Archives of Neurology. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- 25.Storandt M, Grant EA, Miller JP, Morris JC. Longitudinal course and neuropathologic outcomes in original vs revised MCI and in pre-MCI. Neurology. 2006;67:467–473. doi: 10.1212/01.wnl.0000228231.26111.6e. [DOI] [PubMed] [Google Scholar]
- 26.Morris JC, Storandt M, Miller JP, et al. Mild Cognitive Impairment Represents Early-Stage Alzheimer Disease. Archives of Neurology. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 27.Burns JM, Donnelly JE, Anderson HS, et al. Peripheral insulin and brain structure in early Alzheimer disease. Neurology. 2007;69:1094–1104. doi: 10.1212/01.wnl.0000276952.91704.af. [DOI] [PubMed] [Google Scholar]
- 28.Burns JM, Mayo MS, Anderson HS, et al. Cardiorespiratory fitness in early-stage Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22:39–46. doi: 10.1097/WAD.0b013e31815a9ddc. [DOI] [PubMed] [Google Scholar]
- 29.Washburn RA, McAuley E, Katula J, et al. The physical activity scale for the elderly (PASE): evidence for validity. J Clin Epidemiol. 1999;52:643–651. doi: 10.1016/s0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 30.Balady GJ, Berra KA, Golding LA, et al. American College of Sport Medicine's Guidelines for exercise testing and prescription. Sixth. Baltimore: Williams & Wilkins; 2007. [Google Scholar]
- 31.Fleg JL, Morrell CH, Bos AG, et al. Accelerated Longitudinal Decline of Aerobic Capacity in Healthy Older Adults. Circulation. 2005;112:674–682. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- 32.Hollenberg M, Ngo LH, Turner D, Tager IB. Treadmill Exercise Testing in an Epidepiologic Study of Elderly Subjects. Journal of Gerontology: Biological Sciences. 1998;53A:259–267. doi: 10.1093/gerona/53a.4.b259. [DOI] [PubMed] [Google Scholar]
- 33.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 34.Mossberg KA, Greene BP. Reliability of graded exercise testing after traumatic brain injury: submaximal and peak responses. Am J Phys Med Rehabil. 2005;84:492–500. doi: 10.1097/01.phm.0000166883.97562.cd. [DOI] [PubMed] [Google Scholar]
- 35.Dobrovolny CL, Ivey FM, Rogers MA, et al. Reliability of treadmill exercise testing in older patients with chronic hemiparetic stroke. Arch Phys Med Rehabil. 2003;84:1308–1312. doi: 10.1016/s0003-9993(03)00150-3. [DOI] [PubMed] [Google Scholar]
- 36.Fernhall B, Millar AL, Tymeson GT, Burkett LN. Maximal exercise testing of mentally retarded adolescents and adults: reliability study. Arch Phys Med Rehabil. 1990;71:1065–1068. [PubMed] [Google Scholar]
- 37.Pitetti K, M A, Fernhall B. Reliability of a peak performance treadmill test for children and adolescents with and without mental retardation. Adapt Phys Act Q. 2000;17:322–332. [Google Scholar]
- 38.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 39.Good CD, Johnsrude IS, Ashburner J, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 40.Gaser C, Altaye M, Wilke M, Holland SK. Unified segmentation without tissue priors. Neuroimage. 2007;36:S68. [Google Scholar]
- 41.Wilke M, Holland SK, Altaye M, Gaser C. Template-O-Matic: a toolbox for creating customized pediatric templates. Neuroimage. 2008;41:903–913. doi: 10.1016/j.neuroimage.2008.02.056. [DOI] [PubMed] [Google Scholar]
- 42.Meisenzahl EM, Koutsouleris N, Bottlender R, et al. Structural brain alterations at different stages of schizophrenia: A voxel-based morphometric study. Schizophr Res. 2008;104:44–60. doi: 10.1016/j.schres.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 43.Altaye M, Holland SK, Wilke M, Gaser C. Infant brain probability templates for MRI segmentation and normalization. Neuroimage. 2008;43:721–730. doi: 10.1016/j.neuroimage.2008.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 45.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 46.Jack CR, Jr, Dickson DW, Parisi JE, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58:750–757. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tong L, Shen H, Perreau VM, et al. Effects of exercise on gene-expression profile in the rat hippocampus. Neurobiol Dis. 2001;8:1046–1056. doi: 10.1006/nbdi.2001.0427. [DOI] [PubMed] [Google Scholar]
- 48.Honea RA, Meyer-Lindenberg A, Hobbs KB, et al. Is gray matter volume an intermediate phenotype for schizophrenia? A voxel-based morphometry study of patients with schizophrenia and their healthy siblings. Biol Psychiatry. 2008;63:465–474. doi: 10.1016/j.biopsych.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ikeda M, Tanabe H, Nakagawa Y, et al. MRI-based quantitative assessment of the hippocampal region in very mild to moderate Alzheimer's disease. Neuroradiology. 1994;36:7–10. doi: 10.1007/BF00599184. [DOI] [PubMed] [Google Scholar]
- 50.Neeper SA, Gomezpinilla F, Choi J, Cotman C. Exercise and Brain Neurotrophins. Nature. 1995;373:109–109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- 51.Stummer W, Weber K, Tranmer B, et al. Reduced mortality and brain damage after locomotor activity in gerbil forebrain ischemia. Stroke; a Journal Of Cerebral Circulation. 1994;25:1862–1869. doi: 10.1161/01.str.25.9.1862. [DOI] [PubMed] [Google Scholar]
- 52.Chen L, Gong S, Shan LD, et al. Effects of exercise on neurogenesis in the dentate gyrus and ability of learning and memory after hippocampus lesion in adult rats. Neurosci Bull. 2006;22:1–6. [PubMed] [Google Scholar]
- 53.Eriksson PS, Perfilieva E, Bjork-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 54.Galvan V, Bredesen DE. Neurogenesis in the adult brain: implications for Alzheimer's disease. CNS Neurol Disord Drug Targets. 2007;6:303–310. doi: 10.2174/187152707783220938. [DOI] [PubMed] [Google Scholar]
- 55.Burwell RD. The parahippocampal region: corticocortical connectivity. Ann N Y Acad Sci. 2000;911:25–42. doi: 10.1111/j.1749-6632.2000.tb06717.x. [DOI] [PubMed] [Google Scholar]
- 56.Yoshiura T, Mihara F, Koga H, et al. Mapping of subcortical white matter abnormality in Alzheimer's disease using diffusion-weighted magnetic resonance imaging. Acad Radiol. 2006;13:1460–1464. doi: 10.1016/j.acra.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 57.Karas GB, Scheltens P, Rombouts SA, et al. Global and local gray matter loss in mild cognitive impairment and Alzheimer's disease. Neuroimage. 2004;23:708–716. doi: 10.1016/j.neuroimage.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 58.Sandson TA, Felician O, Edelman RR, Warach S. Diffusion-weighted magnetic resonance imaging in Alzheimer's disease. Dement Geriatr Cogn Disord. 1999;10:166–171. doi: 10.1159/000017099. [DOI] [PubMed] [Google Scholar]
- 59.Lim C, Mufson EJ, Kordower JH, et al. Connections of the hippocampal formation in humans: II. The endfolial fiber pathway. J Comp Neurol. 1997;385:352–371. [PubMed] [Google Scholar]
- 60.Whitwell JL, Przybelski SA, Weigand SD, et al. 3D maps from multiple MRI illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to Alzheimer's disease. Brain. 2007;130:1777–1786. doi: 10.1093/brain/awm112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karas GB, Burton EJ, Rombouts SA, et al. A comprehensive study of gray matter loss in patients with Alzheimer's disease using optimized voxel-based morphometry. Neuroimage. 2003;18:895–907. doi: 10.1016/s1053-8119(03)00041-7. [DOI] [PubMed] [Google Scholar]
- 62.Barber R, Gholkar A, Scheltens P, et al. Apolipoprotein E epsilon4 allele, temporal lobe atrophy, and white matter lesions in late-life dementias. Arch Neurol. 1999;56:961–965. doi: 10.1001/archneur.56.8.961. [DOI] [PubMed] [Google Scholar]
- 63.Hagberg JM, Ferrell RE, Katzel LI, et al. Apolipoprotein E genotype and exercise training-induced increases in plasma high-density lipoprotein (HDL)- and HDL2-cholesterol levels in overweight men. Metabolism. 1999;48:943–945. doi: 10.1016/s0026-0495(99)90185-3. [DOI] [PubMed] [Google Scholar]
- 64.Hagberg JM, Ferrell RE, Dengel DR, Wilund KR. Exercise training-induced blood pressure and plasma lipid improvements in hypertensives may be genotype dependent. Hypertension. 1999;34:18–23. doi: 10.1161/01.hyp.34.1.18. [DOI] [PubMed] [Google Scholar]
- 65.Whitwell JL, Shiung MM, Przybelski SA, et al. MRI patterns of atrophy associated with progression to AD in amnestic mild cognitive impairment. Neurology. 2008;70:512–520. doi: 10.1212/01.wnl.0000280575.77437.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kantarci K, Jack CR., Jr Neuroimaging in Alzheimer disease: an evidence-based review. Neuroimaging Clin N Am. 2003;13:197–209. doi: 10.1016/s1052-5149(03)00025-x. [DOI] [PubMed] [Google Scholar]
- 67.Jack CR, Jr, Petersen RC, Xu YC, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Erickson KI, Colcombe SJ, Elavsky S, et al. Interactive effects of fitness and hormone treatment on brain health in postmenopausal women. Neurobiol Aging. 2007;28:179–185. doi: 10.1016/j.neurobiolaging.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 69.Colcombe SJ, Erickson KI, Scalf PE, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]


