Abstract
Although early studies on mild cognitive impairment (MCI) focused on memory dysfunction; more recent studies suggest that MCI is clinically heterogeneous. The objective of this study is to examine patterns of cerebral perfusion in anmestic (N=12) and non-amnestic (N=12) single-domain MCI patients from four a priori regions of interest (ROI): middle and superior frontal cortex, posterior cingulate, and precuneus, to compare them relative to healthy controls (N=12), and to correlate perfusion with neuropsychological measures. Relative to controls, all MCI patients had hypoperfusion in the posterior cingulate, bilaterally. MCI patients with executive dysfunctions also showed hypoperfusion in bilateral middle frontal cortex and the left precuneus relative to controls and in the left middle frontal cortex, left posterior cingulate, and left precuneus relative to amnestic MCI patients. Perfusion in the posterior cingulate correlated positively with memory performance while perfusion in all four a priori ROIs, predominately on the left side, correlated with executive function performance. The finding that single-domain MCI patients with prominent deficits in different cognitive domains exhibited different patterns of hypoperfusion relative to controls supports the existence of distinct subgroups of MCI. These data further suggest that cognitive impairment in MCI is related to cerebral hypoperfusion.
Introduction
Although early studies on mild cognitive impairment (MCI) focused primarily on patients with memory dysfunction who were at risk for developing Alzheimer disease (AD),1 recent studies suggest that MCI is a clinically heterogeneous syndrome.2 In 2003, an international working group proposed subgroups of MCI based on patterns of cognitive impairment (i.e., amnestic and non-amnestic, single or multiple domain).3, 4
Metabolic neuroimaging methods, such as positron emission tomography (PET), single photon emission computed tomography (SPECT), and arterial spin labeling magnetic resonance imaging (ASL-MRI) have been used to understand early brain changes in MCI patients. The majority of studies have shown decreases in metabolism and/or cerebral perfusion in the temporoparietal, posterior cingulate, and medial temporal brain regions of patients with amnestic MCI.5-8 However, no study has investigated cerebral perfusion in non-amnestic presentations of MCI. Thus, the goal of this study is to examine the patterns of cerebral perfusion in two groups of single-domain MCI patients, those with isolated memory impairments (i.e., amnestic MCI, aMCI) and those with isolated executive function impairments (i.e., dysexecutive MCI, dMCI). Based on previous PET, SPECT, and ASL-MRI findings, we hypothesized that aMCI patients would show perfusion abnormalities in the medial parietal cortex. We further hypothesized that dMCI patients would show perfusion abnormalities in the frontal cortex based on our recent findings of left prefrontal cortex atrophy in dMCI patients.9 Finally, we explored the relationship between perfusion and performance on neuropsychological tests.
Methods
Participants and Diagnostic Procedure
Subjects were recruited from the University of California, San Francisco Memory and Aging Clinic, the Memory Disorders Clinic at the San Francisco Veterans Affairs Medical Center, the California Pacific Medical Center, or from a community screening clinic (where subjects responded to a newspaper advertisement). MCI patients were diagnosed after an extensive clinical evaluation including a detailed history, physical and neurological examination, including the Unified Parkinson's Disease Rating Scale–part III motor scale (UPDRS-III),10 neuropsychological screening, and interview with a study partner (who knew the subject for at least 10 years). The one-hour neuropsychological screening battery assessed multiple domains of cognition, including memory, executive function, language, and visuospatial skills.11 The interview with the study partner involved the Clinical Dementia Rating (CDR)12 to evaluate functional abilities and the Neuropsychiatric Inventory (NPI)13 to evaluate behavior. Screening for depression was done using the self-reported, 30-item Geriatric Depression Scale (GDS)14 and an interview with the study partner. Diagnosis was determined by consensus involving the neurologist, neuropsychologist, and nurse using only the diagnostic information described above. Healthy controls were recruited through the community screening clinic and had a CDR of 0 and a Mini-Mental State Examination (MMSE)15 score ≥ 28.
Subjects were excluded if they met criteria for dementia (DSM-IV),16 had a history of a neurological disorder, current psychiatric illness, head trauma with loss of consciousness greater than 10 minutes, severe sensory deficits, substance abuse, or were taking medications that affect cognition (e.g., butyryl- or acetyl-cholinesterase inhibitor). In addition, subjects with significant vascular lesions on brain MRI, defined as a Longstreth17 grade ≥4 (out of 8), were excluded.
We further classified MCI patients according to the predominant domain(s) of cognitive impairment using the screening neuropsychological battery described above and the recently proposed MCI diagnostic scheme.4 Two single-domain MCI groups were included in this study: aMCI and dMCI. We employed a 10th percentile cut-off (1.28 standard deviations) previously used in other studies of non-amnestic MCI patients18 to determine the primary cognitive domain of impairment. Patients were classified as dMCI with relatively focal executive dysfunction, which was operationally defined as scores at or below the 10th percentile of control performance on at least one of four screening tests of executive function (i.e., modified Trailmaking Test B, modified Stroop interference, number of D words in one minute, or abstractions).19 In addition, patients with dMCI had to score within the normal range (i.e., within one SD from the norm) on tests of other cognitive domains. These included memory (i.e., 20-minute delayed recall on California Verbal Learning Test (CVLT)20 and 10-minute recall of modified Rey-Osterrieth figure), language (i.e., 15-item Boston Naming Test21) and visuospatial skills (i.e., copy of modified Rey-Osterrieth figure and Number Location subtest from The Visual Object and Space Perception Battery (VOSP).22 In contrast, patients were classified as aMCI if scores were at or below the 10th percentile on the screening tests of memory (described above) and within the normal range on tests of other cognitive domains (e.g., executive function, language, and visuospatial skills). Scores of the two MCI groups on the neuropsychological screening battery are summarized in Table 1. The study sample included 12 dysexecutive MCI (dMCI), 12 amnestic MCI (aMCI), and 12 healthy controls.
Table 1. Screening Neuropsychological Test Results of the two MCI groups. Mean and (standard deviation).
| aMCI | dMCI | ||
|---|---|---|---|
| Memory | CVLT Long Delay free recall (max. 16) | 7.2 (4.1) | 8.8 (3.1) |
| Modified Rey-Osterrieth figure recall (max. 17) | 6.8 (2.4) | 11.0 (2.7) | |
| Executive Function | Modified Trailmaking Test B (max. 120 sec) | 37.1 (21.9) | 48.8 (28.0) |
| Modified Stroop Interference (number in one minute) | 42.4 (9.6) | 37.9 (8.9) | |
| Letter fluency (D words in one minute) | 15.3 (2.7) | 12.5 (4.8) | |
| Abstractions (max. 6) | 4.8 (0.6) | 4.3 (1.2) | |
| Visuo-spatial | Copy of modified Rey-Osterrieth figure (max. 17) | 15.6 (1.0) | 15.5 (1.3) |
| VOSP Number Location (max. 10) | 8.5 (1.5) | 9.0 (1.7) | |
| Language | Modified Boston Naming Test (max. 15) | 13.4 (2.3) | 13.8 (1.2) |
| Syntax Comprehension (max. 5) | 5.0 (0.0) | 4.8 (0.4) | |
| Other | Calculations (max. 5) | 4.8 (0.4) | 4.6 (0.8) |
Abbreviations: CVLT = California Verbal Learning Test, VSOP = Visual Object and Space Perception Battery
Additional Neuropsychological Testing
To further characterize memory and executive function, we administered additional neuropsychological tests that were not used in diagnosis. These included the immediate and 30-minute delayed recall trails of the Wechsler Memory Scale (WMS) - Visual Reproductions,23 the Delis Kaplan Executive Function System (DKEFS)24 Design Fluency (switching condition), Trailmaking (number letter condition), and Stroop (switching condition) tests, and the Wechsler Adult Intelligence Scale (WAIS) – III Digit Symbol test. We also examined additional data from the CVLT that were not used in diagnosis (i.e., recognition trial). In addition, the Functional Activities Questionnaire (FAQ) 25 was used to document independent activities of daily living. The apolipoprotein E (APOE) genotype was obtained from the Alzheimer's Disease Research Center.
Structural and Arterial-Spin Labeling MRI acquisition
Within three months of the diagnostic visit, subjects were scanned on a 1.5T Siemens Vision scanner. Structural MRI included the following: 2D FLASH MRI along three orthogonal directions to obtain scout views of the brain for initial positioning of MRI slices, double spin echo (DSE) sequence to obtain proton density and T2-weighted MRIs, TR/TE1/TE2 = 5000/20/85 ms, 51 contiguous axial slices (3mm) covering the entire brain and angulated -10 degrees from the AC-PC line; 1.0 × 1.25mm2 inplane resolution, and volumetric T1-weighted gradient echo MRI (MPRAGE) of entire brain, TR/TE/TI = 11/4/850 ms, 12° flip angle sequence with a spatially isotropic resolution of 1.0 mm3. ASL-MRI were acquired with the Double Inversions with Proximal Labeling of Both Tag and Control Images (DIPLOMA-II)26 method with a single-shot gradient-echo planar imaging (GRE-EPI) sequence (TR 2500 ms, TE 15 ms, post arterial spin labeling pulse time = 1500 ms, FOV 260 mm, matrix 128 × 128 mm, covering7 slices above the AC-PC line, slice thickness 8 mm, slice gap 2 mm). A single shot gradient echo EPI scan (TR 2500 ms, TE 15 ms, FOV 260 mm, 128 × 128 mm, 24 slices, slice thickness 5 mm, slice gap 2 mm) covering the whole head was also obtained to facilitate coregistration of perfusion and structural images. Details of the ASL-MR acquisition have previously been published.7
Spatial Processing
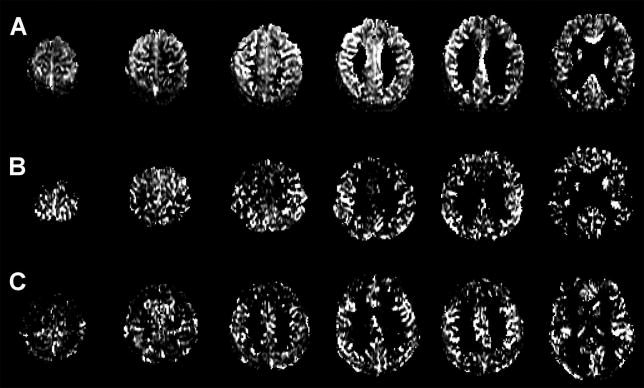
ASL-perfusion data was preprocessed using Statistical Parametric Mapping (SPM2; Wellcome Department of Cognitive Neurology, London, UK) running under MATLAB 7.1 (Mathworks, Natick, MA) Matlab (The Mathworks, Inc, Natick, Massachusetts, USA). For each subject, the perfusion-weighted image was coregistered with the T1-weighted image by using the mutual information coregistration algorithm within SPM2. Because direct coregistraion of perfusion-weighted and T1-weighted images was not reliable due to the poor signal-to-noise ratio and the lack of structural features of the perfusion-weighted images, a stepwise coregistration was performed by first registering separately the labeled and unlabeled ASL-MRI images to the echo-planar reference image for each subject and then registering the echo-planar reference image to the T1-weighted image. Once all four images had been coregistered, a perfusion-weighted image was calculated by subtracting the labeled from unlabeled ASL-MR images. Perfusion intensity was adjusted for instrumental variability by correcting for receiver gain and coil loading. Each subject's perfusion-weighted image was corrected for partial volume effects (PVE)27 based on the probabilistic tissue segmentation maps of gray matter (GM), white matter (WM), and cerebral spinal fluid (CSF) from each subject's T1-weighted image, segmented with Expectation-Maximization Segmentation (EMS).28 First, to account for GM/WM PVE, an estimate of global WM perfusion was computed by extracting the mean perfusion of the centrum semiovale (a mainly uniform WM region) and then by scaling the probabilistic WM map (from EMS segmentation) with this value, smoothed to the resolution of the perfusion image. This virtual WM perfusion image was then subtracted from the original perfusion image. Next, the probabilistic GM map (from EMS segmentation) was resampled to the resolution of the perfusion image and converted to a binary mask thresholded at 20% probability to exclude voxels with small amounts of GM. Next, the perfusion image was multiplied with this binary GM mask to remove any non-brain voxels. The final perfusion images were spatially normalized to a study specific T1 template, created using the EMS segmented GM, WM, and CSF maps of all the study subjects, and smoothed with a 10 mm Gaussian kernel. Representative perfusion images for one control subject, one amnestic and one dysexecutive MCI patient are shown in Figure 1.
Figure 1.
Representative ASL MR perfusion images after partial volume correction from a (A) control subject, (b) aMCI patient, and (c) dMCI patient.
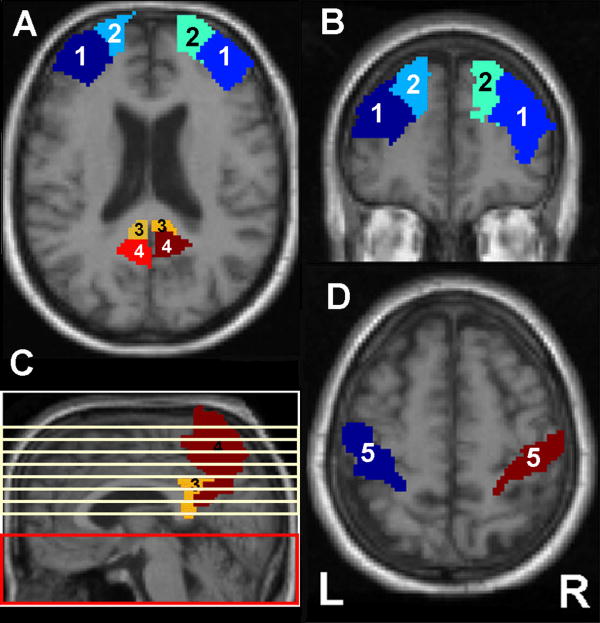
Based on our hypothesis that dMCI patients would have perfusion abnormalities in the frontal cortex while aMCI patients would have perfusion abnormalities in the medial parietal cortex, we identified four a priori regions of interest (ROI). These included the superior and middle frontal cortex, posterior cingulate gyrus, and precuneus. The ROIs were anatomically defined by hand on a single brain matched to the MNI/ICBM template27 (see Figure 2). We used the MarsBaR29 (http://marsbar.sourceforge.net/) toolbox in SPM2 to extract raw perfusion values from these ROIs in each hemisphere of the brain.
Figure 2.
A. Example of middle frontal (1), superior frontal (2), posterior cingulate (3), and precuneus (4) ROIs on an axial image. B. Example of middle (1) and superior (2) frontal ROIs on a coronal image. C. Example of posterior cingulate (3) and precuneus (4) ROIs on a sagittal image. The red box indicates the brain region where pulsed arterial spin labeling (ASL) occurred while the yellow lines represent the 7 slices where ASL MR data was acquired. D. Example of postcentral ROI (5) where mean perfusion values were extracted for use as covariates to adjust for global variations of perfusion.
References of global perfusion were calculated for each subject by extracting data from an ROI that included portions of the postcentral gyrus bilaterally (see Figure 2) and calculating the mean perfusion of these voxels. The postcentral gyrus was chosen because it allowed us to account for global intersubject variability while minimizing the disease effects of that variability.
Voxel-based morphometry analysis
We analyzed the T1-weighted images with voxel-based morphometry (VBM) in order to rule out any morphological differences that might confound the perfusion results. To identify regions of atrophy, we compared aMCI and dMCI patients to control subjects using VBM, implemented in SPM2 running under MATLAB 7.1. The VBM analysis was based on modulated probabilistic tissue segmentation maps of GM from each subject's T1-weighted image that had been segmented with EMS. In order to achieve comparable statistics between the VBM and ASL-perfusion analyses, we blurred the resolution of the GM maps to that of the ASL perfusion images and applied the same isotropic Gaussian smoothing kernel of 10mm FWHM. Next, we fitted a general linear model at each voxel, with one variable of interest (group) and three confounds of no interest (gender, age and total intracranial volume, which was calculated by summing across the modulated GM, WM, and CSF images). The resulting statistical parametric map (SPM) was thresholded at voxel-wise p < 0.001 and a cluster-wise threshold of 7 contiguous voxels.
Statistical Analyses
Statistical analyses were conducted using the Statistical Package for the Social Sciences (SPSS) 16.0. Multiple analysis of variance (MANOVA) was used to evaluate group differences in neuropsychological measures. Post hoc comparisons were performed using the Tukey HSD method with p<0.05. Multiple analysis of covariance (MANCOVA) was used to evaluate group differences in the perfusion values extracted from the a priori ROIs, with age, gender, and mean postcentral gyrus perfusion entered as covariates. We included gender as a covariate because previous research reported gender difference in cerebral blood flow within our regions of interest.30 Planned contrasts were used to compare perfusion differences between the groups. Partial correlation, controlling for age, gender, and mean postcentral gyrus perfusion, were used to examine the relationship between perfusion and neuropsychological test scores. Correlations were performed on all 36 subjects.
Finally, we used logistic regression analysis to examine the power of perfusion values extracted from the a priori ROIs for discriminating dMCI and aMCI patients from controls and from each other in terms of sensitivity, specificity, and overall correct classification. Furthermore, we performed receiver operator characteristic (ROC) analysis in order to express group separation in terms of area under the ROC curves (AUC).
Results
Table 2 summarizes the demographic and clinical characteristics of the MCI patients and healthy controls. There were no significant group differences in age or years of education, although there was a trend (p=0.09) for aMCI patients to be older than dMCI and controls. A χ2 test of independence revealed no differences in the male-to-female ratios among the study groups (χ2 = 2.82, df =3, p=0.24). There was a main group effect for CDR sum of boxes score (F2,33=8.86, p=0.001). Tukey's post-hoc test revealed that both MCI groups had significantly higher CDR box scores than controls (both p≤0.004). One dMCI and one aMCI patient did not have CDR scores. Eighty-two percent of the aMCI and 73% of the dMCI patients had a CDR = 0.5, and the remainder of MCI subjects had a CDR = 0. The aMCI patients with a CDR=0 had objective memory impairment on the screening cognitive testing and complained about memory deficits, but their study partners did not endorse observing memory deficits. Fifty-five percent of the aMCI, and 36% of the dMCI patients had at least one APOE ε4 allele. In contrast, only 8% of the controls had an APOE ε4 allele. We did not have APOE ε4 allele information for one dMCI and one aMCI patient.
Table 2. Demographic and Clinical Information. Mean and (standard deviation).
| Controls | aMCI | dMCI | |
|---|---|---|---|
| N | 12 | 12 | 12 |
| Age (years) | 65.3 (6.1) | 69.6 (8.0) | 62.3 (9.2) |
| Male/Female Ratio | 4/8 | 5/7 | 8/4 |
| Education (years) | 17.3 (1.6) | 16.8 (2.3) | 17.4 (2.4) |
| MMSE (max. 30) | 29.8 (0.6) | 28.8 (1.4) | 29.2 (0.9) |
| Percent with CDR = 0 | 100% | 18% | 27% |
| Percent with CDR = 0.5 | 0% | 82% | 73% |
| CDR sum of boxes (max. 18) | 0.0 (0.0) | 1.0 (0.9)^ | 1.0 (0.8)^ |
| Geriatric Depression Scale (max. 30) | 4.7 (4.8) | 5.2 (4.5) | 6.9 (65.5) |
| FAQ | 0.0 (0.0) | 1.3 (1.6) | 1.8 (2.9) |
| Frequency of APOE ε4 allele carriers | 8.3% | 55% | 36% |
Abbreviations: MMSE = Mini Mental Status Exam, CDR = Clinical Dementia Rating, APOE = apolipoprotein, FAQ = Functional Activities Questionnaire
different from controls, Tukey HSD post-hoc, p≤0.004
Experimental Neuropsychological Test Results
Table 3 shows the results of the additional neuropsychological tests not used to classify the MCI patients. As expected, dMCI patients performed worse than controls and aMCI patients on all of tests of executive function (p≤0.02) while aMCI patients performed significantly worse than controls and dMCI patients on CVLT recognition (p=0.0002) and worse than controls on the 30-minute delayed recall of Visual Reproductions (p=0.01).
Table 3. Experimental Neuropsychological Test Results. Mean and (standard deviation).
| F-statistics1 | Controls | aMCI | dMCI | ||
|---|---|---|---|---|---|
| Memory | CVLT Recognition - Hits (max. 16) | 10.82*** | 15.8 (0.5) | 10.3 (4.3)^ | 13.3 (2.3)^§ |
| Visual Reproductions immediate recall, scaled | 2.63† | 11.8 (4.5) | 7.6 (4.1) | 9.2 (3.5) | |
| Visual Reproductions, 30 min. delayed recall, scaled | 5.91* | 13.2 (3.3) | 8.4 (4.1)^ | 9.5 (2.9)^ | |
| Executive Function | DKEFS-Trailmaking test, Number Letter, scaled | 10.85*** | 13.3 (1.1) | 11.6 (2.2) | 8.9 (3.2)^§ |
| DKEFS-Design Fluency, switching, scaled | 9.40** | 12.6 (2.2) | 12.8 (2.1) | 9.0 (2.8)^§ | |
| DKEFS-Stroop, inhibition, scaled | 17.27*** | 12.8 (2.3) | 12.1 (1.7) | 8.1 (2.4)^§ | |
| DKEFS-Stroop, set shifting, scaled | 5.34* | 11.9 (1.8) | 11.8 (2.5) | 9.0 (3.0)^§ | |
| WAIS-III Digit Symbol – scaled | 11.35*** | 13.3 (2.4) | 12.8 (2.9) | 8.8 (2.3)^§ | |
Abbreviations: MMSE = Mini-Mental State Examination, CVLT = California Verbal Learning Test, DKEFS = Delis Kaplan Executive Function System, WAIS = Wechsler Adult Intelligence Scale
degrees of freedom = 2,35
p=0.05,
p≤0.01,
p=0.001,
p<0.0001
different from controls, Tukey HSD post-hoc, p<0.05
different from aMCI, Tukey HSD post-hoc, p<0.05
Group perfusion differences
Table 4 shows the perfusion values extracted from the a priori ROIs. Relative to controls, aMCI patients had hypoperfusion in the posterior cingulate, bilaterally, while dMCI patients had hypoperfusion in the middle frontal and posterior cingulate ROIs, bilaterally, and in the left precuneus ROI. Dysexecutive MCI patients also had hypoperfusion in the left middle frontal, left posterior cingulate, and left precuneus ROIs relative to aMCI.
Table 4. Raw perfusion values from a priori ROIs. Mean and (standard deviation).
| Region | Hemisphere | F-statistics1 | Controls | aMCI | dMCI |
|---|---|---|---|---|---|
| Middle frontal | Left | 3.70* | 5.8 (2.0) | 4.7 (1.7) | 3.5 (1.6)^§ |
| Right | 3.62* | 6.2 (2.4) | 4.9 (2.0) | 3.9 (1.7)^ | |
| Superior frontal | Left | 1.33 | 4.5 (1.3) | 4.2 (1.6) | 3.3 (1.5) |
| Right | 1.46 | 5.2 (2.1) | 4.4 (1.8) | 3.7 (1.6) | |
| Posterior cingulate | Left | 5.27* | 13.9 (4.2) | 10.3 (3.9)^ | 8.4 (3.4)^§ |
| Right | 3.39† | 12.7 (3.4) | 9.1 (4.4)^ | 8.4 (4.4) | |
| Precuneus | Left | 4.80* | 7.2 (1.9) | 6.9 (1.3) | 5.1 (1.8)^§ |
| Right | 2.73 | 8.3 (2.3) | 6.7 (1.5) | 6.3 (2.2) | |
| Postcentral Gyrus | -- | -- | 9.7 (3.6) | 10.1 (3.8) | 7.0 (2.7) |
degrees of freedom = 2,35
p=0.05,
p<0.05
different from controls, planned contrasts, p<0.05
different from aMCI, planned contrasts, p<0.05
VBM results
Although we corrected each subject's perfusion-weighted image for partial volume effects because we previously found GM atrophy in the left prefrontal cortex of dMCI patients relative to controls9, we performed VBM analysis on the T1-weighted images to rule out morphological differences that might confound the perfusion results. To achieve comparable statistics between the VBM and ASL-perfusion analyses, we blurred the GM maps to the resolution of the ASL perfusion images. At a voxel-wise threshold of p<0.001 and a cluster-wise threshold of 7 contiguous voxels, the whole-brain VBM analysis revealed no significant differences between dMCI and controls. The only significant GM density differences between aMCI and controls were in the left medial and ventral temporal cortex. Importantly, there were no significant differences between either MCI group or controls in the brain region where ASL-perfusion images had been acquired.
Correlation between perfusion, neuropsychological and behavioral measures
Table 5 summarizes the significant correlations between perfusion and neuropsychological measures. Perfusion in the both left and right posterior cingulate correlated positively with performance on CVLT recognition trials (i.e., hits) while perfusion in the right middle frontal ROI, left posterior cingulate, and precuneus, bilaterally, correlated positively with performance on executive function tasks.
Table 5. Relationship between perfusion and neuropsychological test scores.
| Neuropsychological test | Perfusion ROI | Correlation Coefficient | p-value |
|---|---|---|---|
| CVLT Recognition - Hits | L posterior cingulate | 0.36 | 0.04 |
| R posterior cingulate | 0.37 | 0.04 | |
| DKEFS-Trailmaking, Number-Letter | R middle frontal | 0.36 | <0.05 |
| DKEFS-Stroop, set shifting | L precuneus | 0.35 | 0.04 |
| WAIS-III Digit Symbol | R middle frontal | 0.36 | 0.04 |
| L posterior cingulate | 0.36 | 0.04 | |
| L precuneus | 0.43 | 0.01 | |
| R precuneus | 0.38 | 0.03 | |
Abbreviations: CVLT = California Verbal Learning Test, DKEFS = Delis Kaplan Executive Function System, WAIS = Wechsler Adult Intelligence Scale
Classification between amnestic and dysexecutive MCI patients and control subjects
Table 6 summarizes the sensitivity, specificity, and overall correct classification from using perfusion values extracted from the a priori ROIs for separating aMCI and dMCI from controls and each other. Also listed in Table 6 are the areas under the curve (AUC) from a receiver operator characteristic (ROC) analysis. Combining perfusion values extracted from the left middle frontal, left posterior cingulate, and left precuneus ROIs produced the best separation between dMCI and controls. In contrast, perfusion values extracted from the right posterior cingulate ROI alone resulted in the best separation between aMCI and controls. Perfusion values extracted from the left precuneus ROI alone resulted in the best separation between dMCI and aMCI.
Table 6. Classification between dysexecutive and amnestic MCI patients and cognitively normal controls with ASL-perfusion.
| dMCI from Controls | aMCI from Controls | dMCI from aMCI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Overall | AUC | Sensitivity | Specificity | Overall | AUC | Sensitivity | Specificity | Overall | AUC | |
| L mid Frontal | 0.75 | 0.75 | 0.75 | 0.81 | 0.54 | 0.55 | 0.54 | 0.62 | 0.67 | 0.67 | 0.67 | 0.70 |
| R mid Frontal | 0.75 | 0.75 | 0.75 | 0.80 | 0.62 | 0.64 | 0.63 | 0.67 | 0.58 | 0.58 | 0.58 | 0.60 |
| L post cingulate | 0.77 | 0.82 | 0.79 | 0.85 | 0.58 | 0.58 | 0.58 | 0.72 | 0.75 | 0.75 | 0.75 | 0.65 |
| R post cingulate | 0.75 | 0.75 | 0.75 | 0.79 | 0.67 | 0.67 | 0.67 | 0.73 | 0.64 | 0.62 | 0.63 | 0.57 |
| L precuneus | 0.69 | 0.73 | 0.71 | 0.78 | 0.50 | 0.50 | 0.50 | 0.54 | 0.69 | 0.73 | 0.71 | 0.81 |
| L mid Frontal + L post cingulate | 0.77 | 0.82 | 0.79 | 0.89 | 0.58 | 0.58 | 0.58 | 0.71 | 0.89 | 0.64 | 0.70 | 0.67 |
| L + R post cingulate | 0.73 | 0.89 | 0.79 | 0.88 | 0.67 | 0.67 | 0.67 | 0.73 | 0.89 | 0.62 | 0.64 | 0.63 |
| L mid Frontal + L post cingulate + L precuneus | 0.83 | 0.83 | 0.83 | 0.88 | 0.64 | 0.62 | 0.63 | 0.73 | 0.69 | 0.73 | 0.71 | 0.77 |
Sensitivity, specificity, and overall classification by logistic regression analysis and area under receiver operator characteristic curve (AUC) by receiver operator characteristic analysis.
Comment
The first major finding of this study is that MCI patients who exhibited prominent deficits in different, single cognitive domains exhibited different patterns of regional hypoperfusion, as detected by ASL-MRI. MCI patients with memory deficits had hypoperfusion in the posterior cingulate, bilaterally, relative to controls, consistent with previous SPECT, PET, and ASL-MRI studies.5-8 In contrast, MCI patients with executive function deficits exhibited hypoperfusion in the middle frontal cortex and posterior cingulate, bilaterally, as well as in the left precuneus relative to controls. Relative to aMCI patients, dMCI patients had hypoperfusion in the left middle frontal, left posterior cingulate, and left precuneus.
We recently found GM atrophy in the left prefrontal cortex of dMCI patients relative to controls.9 The fact that dMCI patients exhibit hypoperfusion in the frontal cortex, even after accounting for partial volume effects, suggests that perfusion reductions in dMCI are not an artifact of underlying structural variations, which we confirmed with VBM analysis. These results also suggest that ASL-MRI has potential to aid in the diagnosis of different MCI subtypes. In support of this, logistic regression analysis showed that combining perfusion values extracted from the left middle frontal, left posterior cingulate, and left precuneus ROIs produced the best separation between dMCI and controls while perfusion values extracted from the left precuneus ROI resulted in the best separation between dMCI and aMCI patients.
Consistent with a previous neuropathological study where we showed high concentrations of early neurofibrillarly tangles in the posterior cingulate gyrus of a dMCI patient,31 we found bilateral posterior cingulate hypoperfusion in dMCI patients relative to controls in the present study. Compared to aMCI patients and controls, dMCI patients also exhibited hypoperfusion in the left precuneus, a polymodal sensory brain region that has been proposed to be involved in “high-level integration between posterior association processes and anterior executive functions”32 such as attentional set-shifting.33
The second major finding of this study is that perfusion in the posterior cingulate, bilaterally, correlated positively with performance on CVLT recognition trial. This finding is consistent with a number of clinical cases that have reported of amnestic syndromes associated with posterior cingulate lesions34-36 as well as functional neuroimaging studies that have linked posterior cingulate activity to successful memory retrieval.37
The third major finding of this study is that perfusion in all four a priori ROIs, correlated with executive function performance. Specifically, there was significant, positive correlation between perfusion in the right middle frontal ROI and performance on the Trailmaking and Digit Symbol tests, which is consistent with the role of the prefrontal cortex as the major substrate of executive function that has been put forth by theoretical models 38, neuropsychological,39 and human lesion40 studies. A recent study also reported significant correlations between right middle frontal glucose metabolism and trail making performance in patients with very mild AD.41 We also found significant correlations between left precuneus perfusion and performance on the Stroop (shifting condition) and Digit Symbol tests. As discussed earlier, the precuneus has been associated with attentional set-shifting33, which is required to complete both the Digit Symbol and Stroop shifting tasks. Finally, we found a significant correlation between posterior cingulate perfusion and performance on Digit Symbol task. Although the posterior cingulate is not a brain region traditionally associated with executive function, a recent study showed that metabolite ratios from the posterior cingulate gyrus were associated with a measure of executive function in patients with MCI.42 Furthermore, Bracco and colleagues 41 also noted significant correlations between medial parietal glucose metabolism and executive function performance in patients with mild AD.
Despite some similar results between this study and that of Bracco et al., it is noteworthy that we found correlations between executive function test scores and frontal and medial parietal perfusion in entire sample of controls and MCI patients (who, together, had a mean MMSE of 29). In contrast, Bracco et al. only found significant correlations between executive function and prefrontal metabolism in very mild AD patients (who had a mean MMSE of 24). In contrast, the relationship between executive function and glucose metabolism was more widely distributed in patients with mild AD (mean MMSE of 20), extending to the posterior brain regions. Bracco et al. attributed this to the influence of disease severity. The discrepant findings may partially be explained by the difference in methodology. In the current study, we used partial correlations, correcting for age, gender, and mean postcentral gyrus perfusion, to examine the relationship between neuropsychological test scores and raw perfusion values, as determined by ASL-MRI, extracted from a priori ROIs. In contrast, Bracco et al. used SPM to examined voxel-wise correlations between glucose metabolism and raw executive function test scores (i.e., time to completion) separately in patients with very mild and mild AD.
This study has several limitations. First, our implementation of ASL-MRI did not cover more inferior regions of the brain, such as the inferior frontal and medial temporal cortex that are involved in dysexecutive and amnestic MCI. Therefore, more brain regions may show hypoperfusion and correlations between perfusion and cognitive deficits, which we could not detect. Second, the ASL-MRI measurements of perfusion were based on a simple model of water perfusion in which an instantaneous exchange of water from intra- to extra-vascular space was assumed. Moreover, the computations of perfusion did not include variable arterial transit times and variations in the T1 relaxation of the water labels. To the extent that these factions were systematically different between controls, aMCI and dMCI patients, the measurements may have over- or underestimated cerebral perfusion. Finally, the number of subjects in each group was relatively small; therefore, these results may not generalize well to broader MCI populations. These limitations notwithstanding, the fact that we found different patterns of hypoperfusion in dysexecutive and amnestic MCI patients supports the existence of two distinct subgroups of single domain MCI.
Acknowledgments
Supported by NIH NIA R01 AG022538, R01 AG010897, P50 AG0300601
Footnotes
Disclosures: The authors report no conflicts of interest.
References
- 1.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 2.Gauthier S, Reisberg B, Zaudig M, et al. Mild Cognitive Impairment. The Lancet. 2006;367:1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- 3.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment - beyond controversies, towards a consensus: report of the International Working Group on mild cognitive impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–192. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 5.Huang C, Wahlund LO, Svensson L, et al. Cingulate cortex hypoperfusion predicts Alzheimer's disease in mild cognitive impairment. BMC Neurol. 2002;2:9–14. doi: 10.1186/1471-2377-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chetelat G, Desgranges B, de la Sayette V, et al. Mild cognitive impairment: Can FDG-PET predict who is to rapidly convert to Alzheimer's disease? Neurology. 2003;60:1374–1377. doi: 10.1212/01.wnl.0000055847.17752.e6. [DOI] [PubMed] [Google Scholar]
- 7.Johnson NA, Jahng GH, Weiner MW, et al. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology. 2005;234:851–859. doi: 10.1148/radiol.2343040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caroli A, Testa C, Geroldi C, et al. Cerebral perfusion correlates of conversion to Alzheimer's disease in amnestic mild cognitive impairment. J Neurol. 2007;12:1698–1707. doi: 10.1007/s00415-007-0631-7. [DOI] [PubMed] [Google Scholar]
- 9.Pa J, Boxer A, Chao LL, et al. Voxel-based morphometry reveals differential patterns of atrophy in dysexecutive and amnestic MCI subgroups. Ann Neurology [Google Scholar]
- 10.Fahn S, Elton RL Committee m. o. t. U. D. Recent Developments in Parkinson's Disease. Florham Park, NJ: Macmillan Health-care Information; 1987. pp. 153–163. [Google Scholar]
- 11.Kramer JH, Jurik J, Sha SJ, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol. 2003;16:211–218. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 13.Cummings JL, Mega M, Gray K, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 14.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of Geriatric Depression Rating Scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psych Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.APA. Diagnostic and Statistical Manual of Mental Disorders - (DSM-IV) Washington, D.C: American Psychiatric Association; 1994. [Google Scholar]
- 17.Longstreth WT, Jr, Arnold AM, Beauchamp NJJ, et al. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2005;36:56–61. doi: 10.1161/01.STR.0000149625.99732.69. [DOI] [PubMed] [Google Scholar]
- 18.Alladi S, Arnold R, Mitchell J, et al. Mild cognitive impairment: applicability of research criteria in a memory clinic and characterization of cognitive profile. Psychol Med. 2006;36:507–515. doi: 10.1017/S0033291705006744. [DOI] [PubMed] [Google Scholar]
- 19.Kramer JH, Jurik J, Sha SJ, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol. 2003 Dec;16:211–218. doi: 10.1097/00146965-200312000-00002. 2003. [DOI] [PubMed] [Google Scholar]
- 20.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. (2nd) 2000 doi: 10.1037//0022-006x.56.1.123. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan E, Goodglass H, Wintraub S. The Boston Naming Test. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- 22.Warrington EK, James M. The Visual Object and Space Perception Battery. Bury St Edmunds: Thames Valley Test Company; 1991. [Google Scholar]
- 23.Wechsler D. WMS - III Technical Manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 24.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System. 2001 doi: 10.1017/S1355617704102191. [DOI] [PubMed] [Google Scholar]
- 25.Pfeffer RI, Kurosaki TT, Hurrah CHJ, et al. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 26.Jahng GH, Zhu XP, Matson GB, et al. Improved perfusion-weighted MRI by a novel double inversion with proximal labeling of both tagged and control acquisitions. Magn Reson Med. 2003;49:307–314. doi: 10.1002/mrm.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller-Gartner HW, Links JM, Prince JL, et al. Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects. J Cereb Blood Flow Metab. 1992;12:571–583. doi: 10.1038/jcbfm.1992.81. [DOI] [PubMed] [Google Scholar]
- 28.Van Leemput K, Maes F, Vandermeulen D, Suetens P. Automated model-based tissue classification of MR images of the brain. IEEE Trans Med Imaging. 1999;18:897–908. doi: 10.1109/42.811270. [DOI] [PubMed] [Google Scholar]
- 29.Tzurio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 30.Li ZJ, Matsuda H, Asada T, et al. Gender difference in brain perfusion 99mTc-ECD SPECT in aged healthy volunteers after correction for partial volume effects. Nucl Med Commun. 2004;25:999–1005. doi: 10.1097/00006231-200410000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Johnson JK, Vogt BA, Kim R, et al. Isolated executive impairment and associated frontal neuropathology. Dement Geriatr Cogn Disord. 2004;17:360–367. doi: 10.1159/000078183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 33.Nagahama Y, Okada T, Katsumi Y, et al. Transient neural activity in the medial superior frontal gyrus and precuneus time locked with attention shift between object features. Neuroimage. 1999;10:193–199. doi: 10.1006/nimg.1999.0451. [DOI] [PubMed] [Google Scholar]
- 34.Kasahata N. A case of verbal amnesia due to left retrosplenial lesion. Jap J Stroke. 1994;16:290–295. [Google Scholar]
- 35.Takahashi N, Kawamura M, Shiota J, et al. Pure topographic disorientation due to right retrosplenial lesion. Neurology. 1997;49:464–469. doi: 10.1212/wnl.49.2.464. [DOI] [PubMed] [Google Scholar]
- 36.Gainotti G, Almonti S, Di Betta AM, Silveri MC. Retrograde amnesia in a patient with retrosplenial tumour. Neurocase. 1998;4:519–526. [Google Scholar]
- 37.Cabeza R, Nyberg L. Neural bases of learning and memory: functional neuroimaging evidence. Curr Opin Neurol. 2000;13:415–421. doi: 10.1097/00019052-200008000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Shallice T. The allocation of processing resources. From neuropsychology to mental structure. Cambridge: Cambridge University Press; 1988. [Google Scholar]
- 39.Fuster JM. Synposis of function and dysfunction of the frontal lobe. Acta Psychiatr Scand Suppl. 1999;395:51–57. doi: 10.1111/j.1600-0447.1999.tb05983.x. [DOI] [PubMed] [Google Scholar]
- 40.Shallice T, Burgess PW. Deficits in strategy application following frontal lobe damage in man. Brain. 1991;114:727–741. doi: 10.1093/brain/114.2.727. [DOI] [PubMed] [Google Scholar]
- 41.Bracco L, Bessi V, Piccini C, et al. Metabolic correlates of executive dysfunction. Different patterns in mild and very mild Alzheimer's disease. J Neurol. 2007;254:1052–1065. doi: 10.1007/s00415-006-0488-1. [DOI] [PubMed] [Google Scholar]
- 42.Griffith HR, Hollander JA, Okonkwo O, et al. Executive function is associated with brain proton magnetic resonance spectroscopy in amnestic mild cognitive impairment. J Clin Exp Neuropsychol. 2007;29:599–609. doi: 10.1080/13803390600826595. [DOI] [PubMed] [Google Scholar]