Abstract
There are currently no FDA-approved treatments for frontotemporal lobar degeneration (FTLD). The objectives of this study were to explore the tolerability of memantine treatment in FTLD and to monitor for possible effects on behavior, cognition and function. 43 individuals who met clinical criteria for FTLD (21 with frontotemporal dementia [FTD], 13 with semantic dementia [SD] and 9 with progressive nonfluent aphasia [PA]) received 26 weeks of open label treatment with memantine at a target dose of 20 mg daily. Concurrent treatment with acetylcholinesterase inhibitors was prohibited. Cognitive and functional outcome measures included the MMSE, ADAS-cog, CDR-sum of boxes, NPI, Frontal Behavior Inventory (FBI), Executive Interview (EXIT25), Texas Functional Living Scale (TFLS), Geriatric Depression Scale (GDS) and UPDRS-motor scale. Most subjects were able to tolerate the target dose of memantine. A transient improvement was observed on the total NPI score primarily in the FTD group. Variable declines were observed on the ADAS-cog, EXIT25, FBI, NPI, TFLS and UPDRS scores. The FTD and SD groups declined on most of the cognitive and behavioral outcome measures, but remained stable on the UPDRS, whereas the PA group remained relatively stable on the ADAS-cog, NPI and TFLS, but declined on the UPDRS. Memantine was well tolerated in these subjects. Future placebo-controlled trials of memantine in FTLD are warranted and may have greater power to detect behavioral and cognitive effects if focused on the FTD and SD clinical syndromes.
Keywords: Frontotemporal dementia, semantic dementia, progressive nonfluent aphasia, memantine, open-label treatment study
Introduction
Frontotemporal lobar degeneration (FTLD) is a common cause of dementia, particularly in younger individuals with disease onset at ages less than 65. 1 FTLD represents at least three clinical syndromes which are distinct from Alzheimer's Disease (AD). The most common, frontotemporal dementia (FTD), presents with behavioral changes and a dysexecutive syndrome, whereas the other two syndromes, semantic dementia (SD) and progressive nonfluent aphasia (PA) feature language dysfunction and variable behavioral abnormalities. 2
The clinical and pathological heterogeneity of FTLD creates particular challenges for determining optimal pharmacological management. Currently, there are no FDA-approved treatments for FTLD, although a number of medications that affect the function of specific neurotransmitter systems have been studied and may offer modest benefits for the behavioral and cognitive sequelae of this disorder. 3 There is selective vulnerability of serotonergic neurons in FTLD, and a meta-analysis of SSRI treatment studies suggests that these medications offer modest benefits for improving the behavioral symptoms of FTLD. Although commonly used to control behavior in FTLD, antipsychotic agents may be of limited utility in FTLD due to enhanced sensitivity to extrapyramidal side effects.4 Less success has been reported in treating the cognitive symptoms of FTLD. Unlike AD, cholinergic neurons are relatively preserved in FTD, and studies using acetylcholinesterase inhibitors have produced mixed results. One open-label study suggested that rivastigmine was well-tolerated in FTD,5 and a recent placebo-controlled study demonstrated in a potential benefit of galantamine in primary progressive aphasia,6 however experience from a number a FTLD-specialty clinics suggests that acetylcholinesterase inhibitors frequently worsen behavior in FTLD patients with more severe behavioral impairments. 1, 7
Aberrant glutamatergic neurotransmission has been implicated in neuronal dysfunction from a variety of neurodegenerative disorders including FTLD. 3, 8 Memantine is an uncompetitive antagonist of the N-methyl-D-aspartate (NMDA) receptor that stabilizes neurobehavioral decline in moderate to severe AD, 9, 10 and has recently been reported to improve behavior in three FTD patients.11 Phosphorylation and degradation of the tau protein, abnormalities of which are associated with some forms of FTLD, are powerfully regulated by NMDA receptor activation. 12
The relative efficacy of memantine for neuropsychiatric symptoms of AD, initial experience in FTD, as well as the possible involvement of NMDA-receptor mediated cellular dysfunction in a variety of neuropathological variants of FTLD, regardless of clinical syndrome, suggests that memantine may be useful in treating the symptoms of FTLD. To investigate the effects of memantine treatment in FTLD subjects, 43 individuals who met research criteria for one of the three FTLD neurobehavioral syndromes 2 were studied for 26 weeks during open-label memantine treatment with the FDA-approved dose for AD.
Methods
Study Design
This was a 26 week, open-label study of memantine HCl in tablet form. Subjects who met enrollment criteria were titrated to the maximal FDA-approved dose of memantine for Alzheimer's disease, 10mg twice daily, over a period of four weeks. Individuals who could not tolerate 20mg of memantine daily were allowed to remain on the highest tolerated dose. The primary objective of the study was to assess the tolerability of memantine in FTLD over 26 weeks of open-label treatment. The secondary objectives of the study were to explore whether memantine treatment was associated with a stabilization or improvement in the neuropsychiatric, cognitive, motor or functional impairments associated with FTLD. Secondary outcome measures are listed in detail below. After completing the first 26 weeks of the study, all subjects were given the option to receive an additional 26 weeks of open label memantine treatment.
National Clinical Trial identification number: NCT00187525.
Patient Selection
Subjects were recruited from the Memory and Aging Center at the University of California, San Francisco, or the Department of Neurology at the University of Texas Southwestern Medical Center. All aspects of the study were approved by the Institutional Review Boards at each site.
All subjects met criteria for one of the three core FTLD clinical syndromes: FTD, SD or PA. 2 Age was between 40 and 80 years. Because of the relative insensitivity of the Mini Mental State Exam (MMSE) 13 to the executive and behavioral deficits of FTLD, subjects were enrolled if they had an MMSE score greater than 15 or a clinical dementia rating (CDR)14 of less than 3. All subjects had to have a reliable informant with frequent contact who could accompany them to all study visits. Subjects with concurrent FTD and amyotrophic lateral sclerosis (ALS) were allowed to participate if they did not have bulbar symptoms that would be expected to lead to death or an inability to complete the study procedures within six months of the baseline visit. Participants had an MRI or CT scan of the brain within one year of screening consistent with an FTLD diagnosis.
Exclusion criteria
Potential participants were excluded from the study if they 1) had insufficient English fluency to complete the study procedures, 2) evidence of another neurological or psychiatric disorder which would preclude a diagnosis of FTLD; 3) had used acetylcholinesterase inhibitors, antipsychotic agents, mood stabilizers (such as lithium and valproate) or benzodiazepines (other than temazepam or zolpidem) within 4 weeks of baseline; 3) had a clinically significant medical disease within the past year; 4) had brain MRI or CT findings not consistent with an FTLD diagnosis (eg. a large cortical stroke, extensive white matter disease); 5) had other laboratory or ECG abnormalities judged to be clinically significant by the study investigators.
Procedures
Study visits included a screening visit, a baseline visit and follow up visits at weeks 4, 8, 16, 26. Although subjects were excluded if they had used antipsychotic medications within 4 weeks of baseline, participants were allowed to start (or restart) if needed after the baseline visit. Study assessments included the Alzheimer's Disease Assessment Scale-Cognitive (ADAS-cog; performed at baseline, week 16 and week 26)15, the Clinical Dementia Rating scale, sum of boxes score (CDR-SOB; performed at baseline and week 26)14, the Executive Interview (EXIT25; performed at baseline and week 26)16, the Frontal Behavioral Inventory (FBI; performed at baseline and weeks 8, 16 and 26)17, the Geriatric Depression Scale (GDS; performed at baseline and week 26)18, the Mini Mental State Exam (MMSE; performed at baseline and week 26) 13, the Neuropsychiatric Inventory, total score (NPI; performed at baseline, weeks 4, 8, 16 and 26) 19, the Texas Functional Living Scale (TFLS; Performed at baseline, weeks 8, 16 and 26) 20) and the Unified Parkinson's Disease Rating Scale – motor (UPDRS; performed at baseline, weeks 8, 16 and 26) 21.
Safety Assessments
Adverse event information was collected at all study visits and follow-up phone calls, including early termination visits by asking whether there were any changes in health since the last visit. Baseline and 26 week laboratory (cell blood counts, routine chemistries, urinalysis) and ECG data were reviewed by the investigators to monitor for treatment-related changes. Adverse events were rated as mild, moderate or severe based on standard definitions.
Statistical Analysis
Baseline demographic and outcome measure values were compared with Chi square or univariate ANOVA statistics with Tukey post-hoc analysis. Changes in secondary outcomes over time and differences were explored by plotting mean ± standard error for each FTLD subtype at each time point during which data were collected. Statistical analyses were performed using SPSS for Windows (SPSS, Chicago, Version 15).
Results
Enrolment and baseline demographics
A total of 43 subjects were enrolled in the study and 37 subjects completed 26 weeks of open-label treatment (Table 1). There were 21 subjects with FTD, 13 individuals with SD and 9 with PA. Both the FTD and SD groups were predominantly male, whereas the PA group was predominantly female (Chi-square = 9.834; p = 0.007). There were no differences in education or disease duration between subject groups. Three of the FTD subjects, none of the SD subjects and one of the PA subjects withdrew prior to completing 26 weeks of treatment. One FTD subject completed 26 weeks of treatment but did not return for the final study visit. Three of the withdrawals were due to AEs, one was due to a caregiver issue and one was due to worsening of disease precluding further measurements.
Table 1. Subject Demographics.
| FTD | SD | PA | Total | |
|---|---|---|---|---|
| Enrolled | 21 | 13 | 9 | 43 |
| Age ± SEM (yrs.) | 60.3±1.8 | 66.3±2.1 | 70.1±2.4 | 64.2±1.3 |
| Gender (M/F) | 15/6 | 11/2 | 2/7* | 28/15 |
| Education (yrs.) | 15.5±0.7 | 16.6±0.9 | 15.1±0.9 | 15.7±0.4 |
| Disease duration (yrs.) | 4.0±0.6 | 4.0±0.6 | 4.6±0.6 | 4.2±0.3 |
| Completed 26 wks | 16† | 13 | 8 | 37 |
| Withdrawals | 4 | 0 | 1 | 5 |
| SSRI Use (Number [%]) | 9 (42.3) | 7 (53.8) | 3 (33.3) | 19 (44.2) |
| Prior antipsychotic use | 4 | 2 | 0 | 6 |
| Antipsychotic started during study | 1 | 1 | 0 | 2 |
| Mean daily dose (mg) | 20 | 18.5±3.0 | 17.5±4.0 | 18.6±2.3 |
| Adverse events | 4 | 0 | 4 | 8 |
p = 0.007, Chi square;
one subject completed 26 weeks of treatment but did not return for the final visit.
Adverse events
Three FTD subjects experienced mild adverse events (AE's) and one experienced a severe AE (severe constipation leading to an emergency room visit) that were judged to be possibly medication-related. Four PA subjects experienced mild AEs. There were no AEs reported in the SD group (Table 2). The most common adverse event was confusion, reported in 4 subjects.
Table 2. Treatment Emergent Non-Serious Adverse Events.
| Diagnosis | Organ System | Description | Action Taken/Outcome |
|---|---|---|---|
| FTD | CNS | Mild Confusion, near-syncopal episode | None. Patient and caregiver elected to remain on 10 mg BID. |
| PA | CNS | Confusion, more difficulty with ADL's | Dose decreased without change in symptoms. Patient remained on 15 mg QD for the duration of study. |
| PA | CNS, GI | Confusion, nausea | Dose decreased to 10 mg QD. Symptoms resolved after 48 hrs. |
| PA | CNS, GI | Confusion, nausea, right-sided headache | Dose decreased to 15 mg QD with improvement |
| FTD | Immune, CNS | Allergic reaction with facial swelling, psychosis | Medication stopped. Patient withdrawn. |
| FTD | Immune | Rash | Medication stopped. Patient withdrawn. |
| PA | CNS | Excessive somnolence | Medication stopped. Patient withdrawn. |
Behavioral Scales
NPI
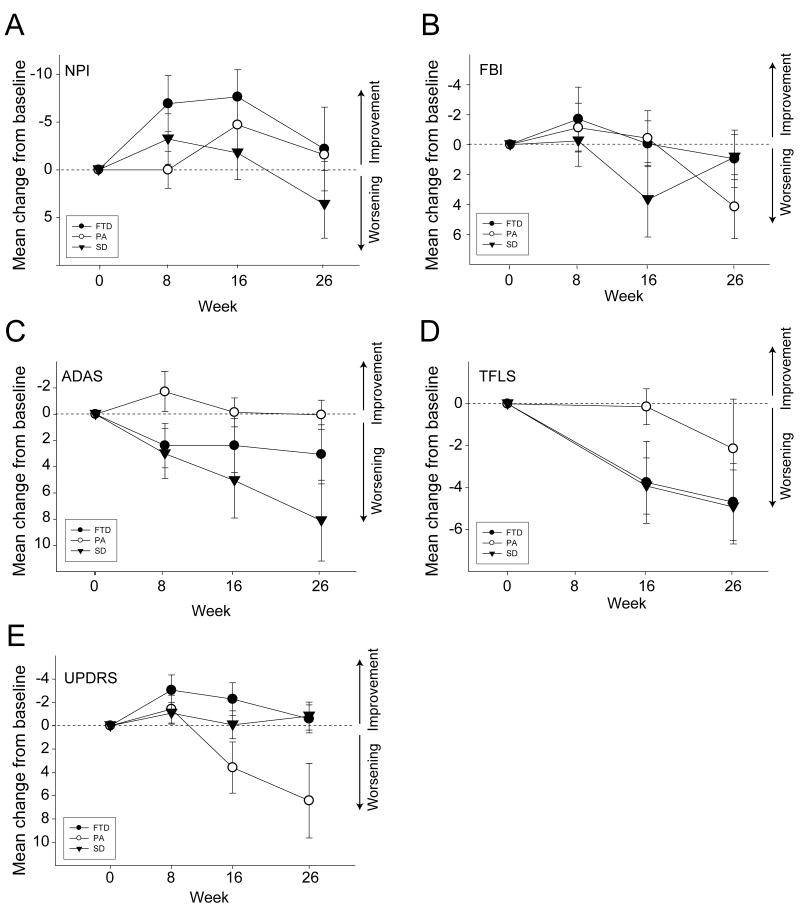
Baseline NPI scores differed between groups (F[2,36]=3.194; p=0.054), and were higher in the FTD group than the PA (p=0.047)(Table 2). A transient decrease (improvement) in the total NPI score, that was maximal at week 16 but declined to baseline at week 26, was observed when data were combined in all three groups (Figure 1A). In all subjects combined, changes from baseline were (change from baseline ± SEM) -2.71±0.85 points (p= 0.001; 95% CI: -4.37,-1.04) at 4 weeks, increased to -4.40±1.43 (p=0.002; 95% CI:-7.20,-1.61) at 8 weeks; further increased to -4.76±1.84 (p=0.01; 95% CI: -8.36,-1.16) at 16 weeks, but declined back to baseline at 26 weeks 0.48±1.80 (p = 0.27; 95% CI: -3.05, 4.01).
Figure 1. Longitudinal Changes in Secondary Outcome Measures.
A. Mean (±SEM) change from baseline in Neuropsychiatric Inventory (NPI) total score in each patient group.
B. Mean (±SEM) change from baseline in Frontal Behavior Inventory (FBI) in each patient group.
C. Mean (±SEM) change from baseline on the ADAS-cog in each patient group.
D. Mean (±SEM) change from baseline on the ADAS-cog in each patient group.
E. Mean (±SEM) change from baseline on the TFLS in each patient group.
FBI
At baseline, FBI scores differed between the three patient groups (F[2,35]=3.434, p = 0.044). The FTD group had a higher mean FBI score than the PA group (p=0.036). There was a non-significant trend towards higher FBI scores in the SD than PA groups (p=0.128). The longitudinal FBI data are shown in Figure 1B. For all subjects, the overall estimate of change in FBI from baseline to week 26 was 2.43±1.17 (p=0.038, 95% CI=0.14, 4.73).
Cognitive Scales
ADAS-cog
There were no differences in baseline ADAS-cog scores between groups (F[2,35]=0.13; p=0.878; Table 3). The largest decline in ADAS-cog performance was observed in the SD group. From baseline to week 26, SD subjects' ADAS-cog scores increased by 8.1±3.1 points (p = 0.001; 95%CI= 1.3, 14.9). There was a non-significant trend towards an increase in ADAS-cog score in the FTD group of 3.1±2.3 points (p=0.156; 95%CI: -1.7, 7.8), whereas the PA group remained relatively stable (0.1±1.1 points change, p=0.942, 95% CI: -2.7, 2.8).
Table 3. Change in Cognitive and Functional Scores (Week 26 – Baseline).
| FTD | SD | PA | Total | P value* | Post-hoc† | ||
|---|---|---|---|---|---|---|---|
| ADAS-cog | |||||||
| Baseline | 19.9±4.0 | 22.5±3.3 | 21.8±3.6 | 21.1±2.2 | 0.878 | ||
| 26 Weeks | 23.0±4.9 | 30.6±4.0 | 21.9±3.7 | 25.3±3.0 | 0.474 | ||
| Change | 3.1±2.3 | 8.1±3.1 | 0.1±1.1 | 4.2±1.6 | 0.150 | ||
| CDR-SOB | |||||||
| Baseline | 7.17±0.8 | 4.75±0.7 | 2.33±0.8 | 5.41±0.6 | 0.002 | FTD > PA | |
| 26 Weeks | 7.40±1.0 | 4.88±0.7 | 3.49±1.4 | 5.77±0.6 | 0.047 | FTD > PA | |
| Change | 0.23±0.6 | 0.13±0.5 | 1.2±0.7 | 0.39±0.3 | 0.517 | ||
| EXIT25 | |||||||
| Baseline | 14.9±2.0 | 13.9±2.4 | 16.0±3.3 | 14.8±1.3 | 0.866 | ||
| 26 Weeks | 15.9±2.9 | 18.6±2.9 | 14.4±3.0 | 16.4±1.8 | 0.665 | ||
| Change | 0.94±1.7 | 4.67±1.8 | -1.63±1.1 | 1.61±1.0 | 0.066 | SD > FTD | |
| FBI | |||||||
| Baseline | 26.5±3.6 | 23.5±3.4 | 10.3±3.4 | 22.3±2.5 | 0.044 | FTD > PA | |
| 26 Weeks | 28.0±3.0 | 24.3±4.2 | 16.7±5.2 | 24.6±2.3 | 0.109 | ||
| Change | 1.4±1.9 | 0.83±1.5 | 5.0±2.3 | 1.9±1.1 | 0.523 | ||
| GDS | |||||||
| Baseline | 7.7±1.7 | 7.3±2.1 | 2.8±0.8 | 6.7±1.1 | 0.254 | ||
| 26 Weeks | 6.5±1.4 | 8.2±2.2 | 2.6±1.0 | 6.1±1.0 | 0.178 | ||
| Change | -1.2±1.2 | 0.8±1.2 | -0.2±0.7 | 0.5±0.8 | 0.575 | ||
| MMSE | |||||||
| Baseline | 23.4±1.8 | 20.8±1.9 | 24.8±1.4 | 22.8±1.0 | 0.350 | ||
| 26 Weeks | 21.0±2.5 | 19.0±2.5 | 23.3±2.4 | 20.8±1.5 | 0.574 | ||
| Change | -2.4±1.1 | -1.8±1.4 | -1.5±1.1 | 2.0±0.7 | 0.866 | ||
| NPI | |||||||
| Baseline | 27.7±4.8 | 19.5±4.1 | 9.1±3.6 | 21.3±2.9 | 0.054 | FTD > PA | |
| 26 Weeks | 25.5±4.1 | 23.1±4.3 | 7.6±3.1 | 21.2±2.7 | 0.037 | FTD > PA | |
| Change | -2.2±4.4 | 3.6±3.6 | -2.1±2.3 | 0.0±2.4 | 0.539 | ||
| TFLS | |||||||
| Baseline | 35.2±3.4 | 36.5±3.1 | 42.4±4.2 | 37.1±2.1 | 0.425 | ||
| 26 Weeks | 30.5±4.4 | 31.6±4.0 | 40.3±4.5 | 32.8±2.6 | 0.370 | ||
| Change | -4.4±1.5 | -4.9±1.8 | -2.1±2.4 | -4.3±1.1 | 0.650 | ||
| UPDRS | |||||||
| Baseline | 9.2±2.1 | 3.3±1.1 | 7.2±2.4 | 6.8±1.2 | 0.090 | ||
| 26 Weeks | 8.6±2.8 | 2.4±0.8 | 13.6±4.1 | 7.5±1.7 | 0.046 | PA > SD | |
| Change | -0.6±1.2 | -0.8±1.3 | 6.4±2.1 | 0.7±1.0 | 0.017 | PA > SD |
Mean ± SEM values shown. Bold values indicate statistical significance, ANOVA with Tukey post-hoc.
Overall ANOVA.
p < 0.05 Tukey post hoc test.
EXIT25
There were no baseline differences in EXIT25 score between groups. The SD group declined over 26 weeks, by 4.67±1.8 points (p =0.004, 95%CI: 1.5, 7.8; Table 3). Variable declines were observed in the FTD and PA groups.
MMSE
There were no baseline differences in MMSE (F[2,34]=1.085, p=0.35). We fitted a model that included terms for linear change in MMSE over time, diagnostic group and time by group interaction. In all subjects combined MMSE declined 2.21±0.7 points over 26 weeks (p=0.002; 95% CI= -3.59, -0.84).
Functional/Motor Scales
CDR-SOB
There were baseline differences in CDR-SOB between the subject groups (F[2,32]=7.388, p=0.002; Table 3). The FTD group had higher (more impaired) baseline CDR-SOB scores than the PA group (p = 0.002) and there was a non-significant trend towards a higher CDR-SOB in the FTD group than the SD group (p=0.071). In all subjects combined, the overall change in CDR-SOB was 0.39 ±0.33 points (p=0.24, 95% CI= -0.25, 1.03; Table 3) over the course of the study.
TFLS
There were no baseline differences in TFLS score between the three groups (p=0.475; Table 3). The FTD and SD groups declined over time on the TFLS at a similar rate (Figure 1D). The FTD group declined by -4.4±1.5 points (p=0.022, 95%CI: -8.59, -0.8) and the SD group declined by -4.9±1.8 points (p=0.018 95%CI: -8.8, -1.0) over 26 weeks. The PA subjects demonstrated variable change of -2.1±2.4 points (p=0.452, 95%CI: -3.6, 7.9) over 6 months.
UPDRS
There were no differences in baseline UPDRS score between groups (Table 3). The PA group's UPDRS score increased by 6.4±2.1 points (p=0.003; 95%CI = 2.05, 10.38), whereas the other two groups remained relatively stable on the UPDRS over 26 weeks (Figure 1E).
Discussion
We found that most FTLD subjects were able to tolerate 26 weeks of open-label memantine treatment. A transient improvement in total NPI score was observed over the first 16 weeks of treatment which was most apparent in the FTD group. Variable declines on most of the other secondary outcome measures were observed in each FTLD clinical subtype. The SD and FTD groups showed the greatest declines on the cognitive and functional measures but remained stable on the UPDRS, whereas the PA group remained relatively stable on the cognitive and functional measures, but declined on the UPDRS. Since this was an open-label study, it is not possible to know whether any of the changes on the outcome measures were related to memantine treatment, per se. Moreover, without a placebo group, a rigorous analysis of AE frequency was not possible. Nonetheless, the longitudinal data acquired as part of the study may prove useful in planning future FTLD clinical trials.
An unexpected finding of the study was a transient decrease (improvement) in NPI score that was maximal at week 16, but increased back to baseline by week 26 most prominently in the FTD group. At baseline, the FTD group had the highest NPI scores, and also appeared to display the largest magnitude change on the NPI. Since the NPI reflects caregiver reports of behavior and includes the degree of distress caused by these behaviors, it is highly likely that some of the observed changes were related to non-treatment related aspects of study participation such as more frequent clinic visits and placebo effect. In addition, as six of the subjects had been on antipsychotic medication prior to study enrollment, it is possible that some of the improvement in NPI could have been due to a reduction in side effects from these medications since only two of the subjects required antipsychotic medication treatment after the baseline visit. A smaller, 26 week, open-label study of memantine in FTD and SD failed to demonstrate any improvements on the NPI, however the reported analysis was limited to comparing the first and last observations, and thus may have missed the transient change we observed.23 A number of placebo-controlled trials of memantine for AD 24, 25, as well as one small case series in FTD 11, have demonstrated improvements in NPI score that were similar in magnitude and time course to that we observed. With these results in mind, our findings suggest that placebo-controlled efficacy studies of memantine for FTLD are warranted.
We compared longitudinal changes in behavioral, cognitive and functional scales in all three subtypes of FTLD that were measured over a relatively short time course typical of a clinical trial. For the cognitive and functional measures, the FTD and SD groups followed a similar pattern of decline, whereas the PA group remained relatively stable over the 26 weeks of follow up. In contrast, the PA group displayed a large decline on the UPDRS-motor scale, and a modest decline on the TFLS over the same time period. The striking increase in parkinsonism that we observed is consistent with the frequent association of the clinical PA syndrome with tau-related neuropathology at autopsy, often in the form of Corticobasal Degeneration or Progressive Supranuclear Palsy, syndromes often associated with atypical parkinsonism. 26-28
Although this was an open-label treatment study that by definition precludes any comment about the efficacy of memantine for the symptomatic treatment of FTLD, our data provide additional rationale for conducting a randomized placebo-controlled trial of memantine in FTLD. Furthermore, these data suggest that focusing on the FTD and SD syndromes in such a trial may allow for greater power to detect an effect of memantine on the behavioral and functional decline caused by FTLD. Finally, the variable declines observed on many of the outcome measures used for this study suggest that development of more FTLD-sensitive cognitive and functional outcome measures and better operational criteria for subject inclusion and exclusion will be necessary for future large randomized controlled clinical trials in FTLD. 29, 30
Acknowledgments
Supported by: Forest Research Institute, K23NS408855, The John Douglas French Foundation, P50AG-03-006-01, P01AG019724, The L. Hillblom Foundation and the State of CA.
Footnotes
National Clinical Trial identification number: NCT00187525
John Neuhaus, PhD and Adam Boxer, MD, PhD conducted the statistical analysis.
Disclosures: ALB received research support from Forest, Elan, Wyeth and Myriad and has been a consultant for Genentech. AML is a member of speakers bureaus for Forest, Pfizer, and Schwarz and a consultant for Pfizer. BLM has been a consultant and/or speaker for Novartis, Elan, Genentech and Pfizer and received research support from Forest. The remaining authors have no financial conflict. Forest Research Institute (FRI) was not involved collection, management, analysis or interpretation of the data. FRI was not involved in preparation or approval of the manuscript, although it was given an opportunity to review the manuscript before submission. No changes were made based on this review.
References
- 1.Boxer AL, Miller BL. Clinical features of frontotemporal dementia. Alzheimer Dis Assoc Disord. 2005;19 1:S3–6. doi: 10.1097/01.wad.0000183086.99691.91. [DOI] [PubMed] [Google Scholar]
- 2.Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 3.Huey ED, Putnam KT, Grafman J. A systematic review of neurotransmitter deficits and treatments in frontotemporal dementia. Neurology. 2006;66(1):17–22. doi: 10.1212/01.wnl.0000191304.55196.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pijnenburg YA, Sampson EL, Harvey RJ, Fox NC, Rossor MN. Vulnerability to neuroleptic side effects in frontotemporal lobar degeneration. Int J Geriatr Psychiatry. 2003;18(1):67–72. doi: 10.1002/gps.774. [DOI] [PubMed] [Google Scholar]
- 5.Moretti R, Torre P, Antonello RM, Cattaruzza T, Cazzato G, Bava A. Rivastigmine in frontotemporal dementia: an open-label study. Drugs Aging. 2004;21(14):931–937. doi: 10.2165/00002512-200421140-00003. [DOI] [PubMed] [Google Scholar]
- 6.Kertesz A, Morlog D, Light M, Blair M, Davidson W, Jesso S, et al. Galantamine in frontotemporal dementia and primary progressive aphasia. Dement Geriatr Cogn Disord. 2008;25(2):178–185. doi: 10.1159/000113034. [DOI] [PubMed] [Google Scholar]
- 7.Mendez MF, Shapira JS, McMurtray A, Licht E. Preliminary findings: behavioral worsening on donepezil in patients with frontotemporal dementia. Am J Geriatr Psychiatry. 2007;15(1):84–87. doi: 10.1097/01.JGP.0000231744.69631.33. [DOI] [PubMed] [Google Scholar]
- 8.Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat Rev Drug Discov. 2006;5(2):160–170. doi: 10.1038/nrd1958. [DOI] [PubMed] [Google Scholar]
- 9.Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ. Memantine in moderate-to-severe Alzheimer's disease. N Engl J Med. 2003;348(14):1333–1341. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 10.Tariot PN, Farlow MR, Grossberg GT, Graham SM, McDonald S, Gergel I. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. Jama. 2004;291(3):317–324. doi: 10.1001/jama.291.3.317. [DOI] [PubMed] [Google Scholar]
- 11.Swanberg MM. Memantine for behavioral disturbances in frontotemporal dementia: a case series. Alzheimer Dis Assoc Disord. 2007;21(2):164–166. doi: 10.1097/WAD.0b013e318047df5d. [DOI] [PubMed] [Google Scholar]
- 12.Amadoro G, Ciotti MT, Costanzi M, Cestari V, Calissano P, Canu N. NMDA receptor mediates tau-induced neurotoxicity by calpain and ERK/MAPK activation. Proc Natl Acad Sci U S A. 2006;103(8):2892–2897. doi: 10.1073/pnas.0511065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”;. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 14.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 15.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141(11):1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 16.Royall DR, Mahurin RK, Gray KF. Bedside assessment of executive cognitive impairment: the executive interview. J Am Geriatr Soc. 1992;40(12):1221–1226. doi: 10.1111/j.1532-5415.1992.tb03646.x. [DOI] [PubMed] [Google Scholar]
- 17.Kertesz A, Davidson W, Fox H. Frontal Behavioral Inventory: Diagnostic Criteria for Frontal Lobe Dementia. Can J Neurol Sci. 1997;24:29–36. doi: 10.1017/s0317167100021053. [DOI] [PubMed] [Google Scholar]
- 18.Yesavage JA, Brink TL, Rolse TL, Lum O, Huang V, Adey M, et al. Development and validity of a Geriatric Depression Scale: A preliminary report. J Psychiatric Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 19.Cummings JL, Mega M, Gray K, Thompson-Rosenberg J, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 20.Cullum CM, Saine K, Chan LD, Martin-Cook K, Gray KF, Weiner MF. Performance-Based instrument to assess functional capacity in dementia: The Texas Functional Living Scale. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14(2):103–108. [PubMed] [Google Scholar]
- 21.Fahn S, Elton RL . Committee motUD. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Calne D, Goldstein M, editors. Recent Developments in Parkinson's Disease. Florham Park NJ: Macmillan Health-care Information; 1987. pp. 153–163. [Google Scholar]
- 22.McCulloch CE, Searle SR. Generalized, Linear and Mixed Models. New York: John Wiley and Sons; 2001. [Google Scholar]
- 23.Diehl-Schmid J, Forstl H, Perneczky R, Pohl C, Kurz A. A 6-month, open-label study of memantine in patients with frontotemporal dementia. Int J Geriatr Psychiatry. 2008 doi: 10.1002/gps.1973. [DOI] [PubMed] [Google Scholar]
- 24.Gauthier S, Loft H, Cummings J. Improvement in behavioural symptoms in patients with moderate to severe Alzheimer's disease by memantine: a pooled data analysis. Int J Geriatr Psychiatry. 2007 doi: 10.1002/gps.1949. [DOI] [PubMed] [Google Scholar]
- 25.Cummings JL, Schneider E, Tariot PN, Graham SM. Behavioral effects of memantine in Alzheimer disease patients receiving donepezil treatment. Neurology. 2006;67(1):57–63. doi: 10.1212/01.wnl.0000223333.42368.f1. [DOI] [PubMed] [Google Scholar]
- 26.Hodges JR, Davies RR, Xuereb JH, Casey B, Broe M, Bak TH, et al. Clinicopathological correlates in frontotemporal dementia. Ann Neurol. 2004;56(3):399–406. doi: 10.1002/ana.20203. [DOI] [PubMed] [Google Scholar]
- 27.Josephs KA, Petersen RC, Knopman DS, Boeve BF, Whitwell JL, Duffy JR, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology. 2006;66(1):41–48. doi: 10.1212/01.wnl.0000191307.69661.c3. [DOI] [PubMed] [Google Scholar]
- 28.Boxer AL, Geschwind MD, Belfor N, Gorno-Tempini ML, Schauer GF, Miller BL, et al. Patterns of brain atrophy that differentiate corticobasal degeneration syndrome from progressive supranuclear palsy. Arch Neurol. 2006;63(1):81–86. doi: 10.1001/archneur.63.1.81. [DOI] [PubMed] [Google Scholar]
- 29.Freedman M. Frontotemporal dementia: recommendations for therapeutic studies, designs, and approaches. Can J Neurol Sci. 2007;34 1:S118–124. doi: 10.1017/s0317167100005680. [DOI] [PubMed] [Google Scholar]
- 30.Knopman DS, Boeve B, Caselli RJ, Graff-Radford NR, Kramer JH, Mendez MF, et al. Longitudinal Tracking of FTLD: Toward Developing Clinical Trial Methodology. Alzheimer Dis Assoc Disord. 2007;21(4):S58–S63. doi: 10.1097/WAD.0b013e31815bf69d. [DOI] [PubMed] [Google Scholar]