Abstract
Background
Radiofrequency catheter ablation (RFA) has emerged as an important treatment strategy for AF. The potential cost-effectiveness of RFA for AF, relative to antiarrhythmic drug (AAD) therapy, has not been fully explored from a U.S. perspective.
Methods and Results
We constructed a Markov disease simulation model for a hypothetical cohort of drug- refractory paroxysmal AF patients managed either with RFA ± AAD or AAD alone. Costs and quality-adjusted life years (QALYs) were projected over 5 years. Model inputs were drawn from published clinical trial and registry data, from new registry and trial data analysis, and from data prospectively collected from AF patients managed with RFA at our institution. We assumed no benefit form ablation on stroke, heart failure or death, but did estimate changes in quality-adjusted life expectancy using data from several AF cohorts.
In the base case scenario, cumulative costs with the RFA and AAD strategies were $26,584 and $19,898, respectively. Over 5 years, quality adjusted life expectancy was 3.51 QALYs with RFA, versus 3.38 for the AAD group. The incremental cost-effectiveness ratio for RFA vs. AAD was thus $51,431/QALY. Model results were most sensitive to time horizon, the relative utility weights of successful ablation vs. unsuccessful drug therapy, and to the cost of an ablation procedure.
Conclusions
RFA ± AAD for symptomatic, drug-refractory paroxysmal AF appears to be reasonably cost-effective compared with AAD therapy alone from the perspective of the US health care system, based on improved quality of life and avoidance of future health care costs.
Keywords: atrial fibrillation, ablation, antiarrhythmia agents, cost-benefit analysis
Since its earliest reports little more than a decade ago1, 2 radiofrequency catheter ablation (RFA) for AF has undergone rapid evolution in its techniques and emerged as an important option in the management of AF patients.3 Several small randomized studies have established that ablation reduces AF recurrence more effectively than antiarrhythmic drugs (AADs) in patients who have failed previous AAD treatment,4 and yields superior improvements in symptoms and quality of life.5–7
As the population of patients with AF is large and growing,8 management decisions about AF are likely to have important implications for future population health and health care spending.9, 10 To date, information regarding the potential cost-effectiveness of catheter ablation for AF relative to medical therapy is limited. While a few previous studies have suggested that the up-front costs of catheter ablation may be partly – if not fully – offset by the avoidance of later AF-related resource utilization11–14 or adverse events such as stroke,15 none of these studies has directly integrated data on changes in quality of life related to maintenance of sinus rhythm.
To gain a better understanding of the benefits and costs of AF ablation relative to medical therapy, we developed a disease simulation Markov model for a hypothetical cohort of patients with paroxysmal AF who have failed previous treatment with one or more AADs. Drawing from a variety of sources, we estimated costs, quality-adjusted life expectancy, and cost-effectiveness of RFA ± AAD relative to continued AAD therapy alone over a 5-year time horizon.
METHODS
Modeling Strategy and Basic Assumptions
We developed a Markov disease-simulation model comparing RFA ± AAD with AAD therapy alone for patients with paroxysmal AF refractory to one or more AADs. This population was chosen because consensus guidelines have endorsed ablation in these patients;3, 16 ablation appears to potentially yield better results in paroxysmal compared with persistent AF patients;17–19 and because there is a larger body of randomized evidence available for paroxysmal AF patients (as opposed to persistent AF or mixed populations) from which to derive model parameters. The published literature suggests that the majority of patients currently referred for ablation are male, under age 60, and do not have advanced heart failure.4 We therefore modeled outcomes for a cohort of 60 year old men without severe structural heart disease.
The model has a 5-year time horizon, which we believe is long enough to capture most of the major changes in resource consumption and quality of life after an initial decision on AF management, and because this corresponds roughly with the longest follow-up of outcomes with either ablation or AAD therapy published in the literature.20, 21 The impact of time horizon was tested in sensitivity analysis. The cycle length was one month, and all analyses were based on the societal perspective. Future costs and quality-adjusted life years were discounted at 3% per year, and this rate was also varied in sensitivity analysis.22 We used TreeAge Pro 2005 software (TreeAge, Williamstown, MA) for all model programming and calculations.
For the sake of estimating cost-effectiveness over a medium-term time horizon, we assumed that patients assigned to drug therapy cannot “cross over” to ablation within the model. This construction more accurately estimates the true incremental value of ablation compared with drug therapy, as opposed to one that ultimately compared an up-front ablation strategy with “delayed” ablation after additional drug failure, assuming that the rate of drug failure would be significant.5, 6, 13
Model Structure
Figures 1–3 summarize the basic structure, health states, and possible transitions between health states used in the model. Two basic Markov processes were constructed: one for patients completing an uncomplicated ablation or having a reversible procedural complication, and another for the AAD therapy cohort (Figure 1). With the exception of a procedure-related stroke, ablation patients experiencing a non-fatal procedural complication incurred an immediate cost and short-term disutility, but after that followed the same path as patients without a procedural complication. On the other hand, we assumed that a procedure-related stroke would impact both quality adjusted life expectancy and costs in the long-term, and these effects were estimated from previous literature (states not shown in Figure 2).15
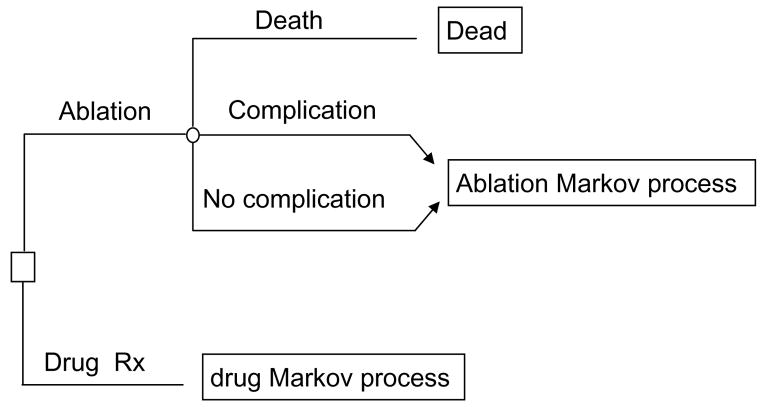
Figure 1.
Simplified structure of the decision analytic model. Following the initial decision (square box) to pursue ablation, patients face a finite probability (chance node represented by open circle) of procedural death, nonfatal complication, or no complication. All patients surviving ablation then enter the ablation Markov process (see subsequent figures).
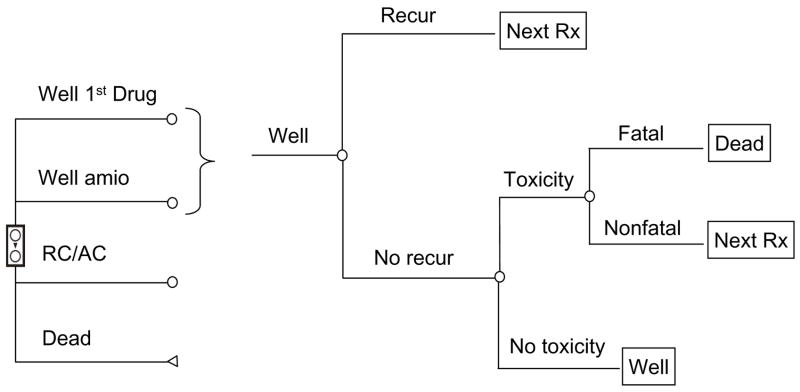
Figure 3.
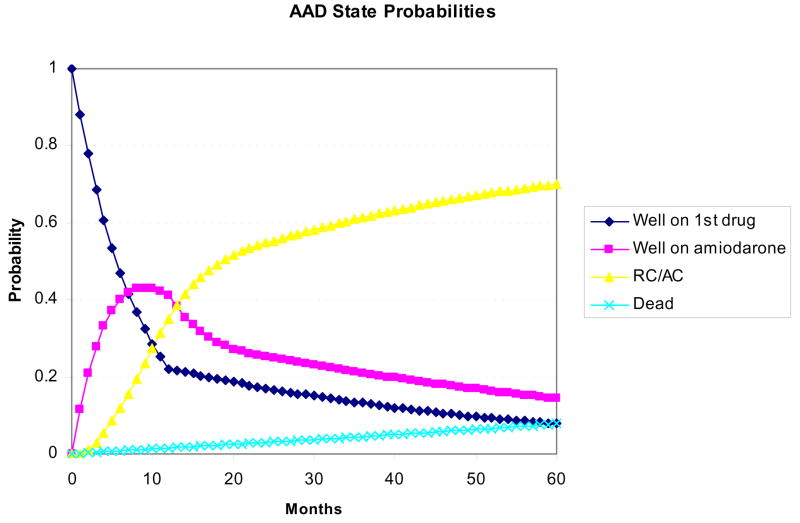
AAD Markov process. The three main treatment options in the AAD group are shown from top to bottom on the left. Within each well state, patients may experience recurrent symptomatic AF or drug toxicity, in which case they would progress to the next treatment (“next Rx”), or they could remain well. All failures of first line drug treatment would next try amiodarone, and all amiodarone failures would move on to rate control and anticoagulation (“RC/AC”).
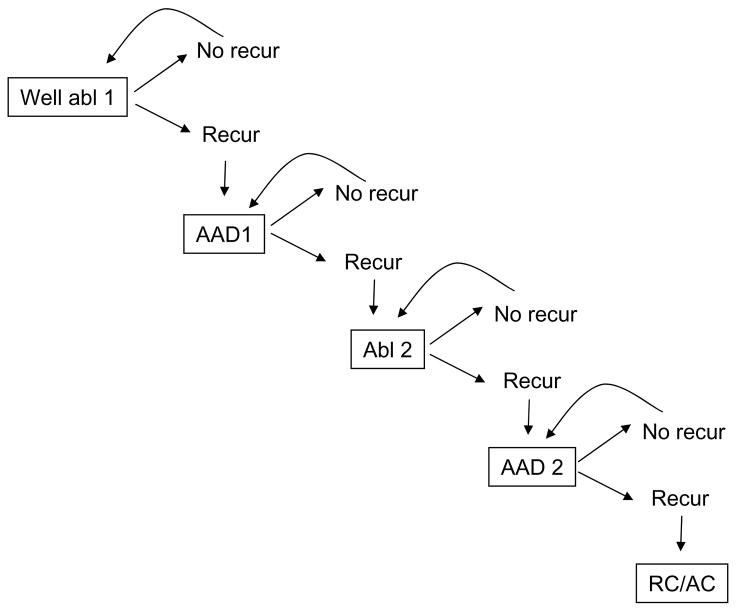
Figure 2.
Ablation Markov process. This simplified diagram demonstrates the major health states (boxes) and possible transitions between health states (arrows) in the ablation limb of the decision tree. In each state, patients face a finite risk of age-associated mortality (not shown). Otherwise, patients experiencing symptomatic recurrence of AF (or drug toxicity) progress to the next treatment, and patients remaining in sinus rhythm continue in the same state.
The ablation Markov process (Figure 2) assumes that patients will progress stepwise from one therapy to the next, based on whether or not they experience symptomatic AF recurrences on their current treatment. Thus, patients recurring after a first ablation restart guideline-recommended first-line AAD therapy with either sotalol or flecainide (moving from the ‘well abl 1’ to the ‘AAD 1’ health state),16 based on numerous reports that some patients respond to previously ineffective AADs following ablation. Patients with recurrent AF despite one ablation and the addition of first-line AAD therapy (or those experiencing drug toxicity) proceed to repeat ablation (moving from ‘AAD 1 to ‘Abl 2’). Those who recur despite a second ablation commence treatment with second-line AAD treatment (moving from ‘Abl 2 to AAD 2’), and patients failing second-line drug treatment cease further efforts at rhythm control and are treated with pharmacologic rate control. We made the conservative assumption that ablation patients would not be treated with amiodarone.
The AAD Markov process follows generally similar logic (Figure 3). Patients initially receive a first-line drug (sotalol or flecainide) and enter the “Well 1st drug” state. In the event of toxicity or therapeutic failure, they proceed to treatment with amiodarone (“well amio” state), and in the event of amiodarone failure, are treated with rate control (“RC/AC”). Amiodarone was chosen as the second-line agent for all patients in the drug “arm” based on its superiority over other drugs at maintaining sinus rhythm.23–25
All patients face a background rate of mortality based on their age and gender, and can therefore transition from any health state directly to death in either arm of the model (not shown in figures).26 Aside from small risks of procedural death or fatal drug toxicity (see Appendix), the model assumes that the risk of death is the same for all health states and interventions except following major stroke after an ablation. Further, because the potential for either drug therapy or ablation to reduce the long-term risk of stroke has not been proven, we assumed that the incidence of stroke would be the same for both therapies, and therefore did not explicitly consider stroke as an outcome in the model, except as a potential complication of ablation. In keeping with current recommendations,3 we also assumed that long-term anticoagulation practices, and related costs and complications, would be equivalent between groups.
Risks of both fatal and non-fatal antiarrhythmic drug toxicity were taken from the literature (see online Supplemental Material) and applied equally to ablation and non-ablation patients. In the ablation arm of the model, it was assumed that patients having drug toxicity after a single ablation would develop recurrent AF and undergo repeat ablation, while patients having drug toxicity after two ablations would then be managed with rate control.
Model Inputs
Inputs for the model were drawn from a variety of sources, including completed clinical trials, a large registry of new-onset AF patients, prospectively collected data from patients treated at our institution, and analysis of Medicare claims data. Risks of events were allowed to vary over time by incorporating probability tables and tunnel states into the model. The base case estimate, range of values used in sensitivity analysis, data sources and additional details are provided in the Appendix. Some general discussion regarding data sources and key inputs is provided here.
Event Probabilities
The risks of procedural complications following AF ablation were taken from published randomized and nonrandomized series and a large international survey on AF ablation outcomes.27 The likelihood of successful sinus rhythm maintenance for each of the strategies in both the ablation and drug “arms” of the model were taken as much as possible from recent randomized studies5, 28, 29 but were cross-referenced with more general sources.27
We assumed a single procedure efficacy rate of 60% for ablation, and calibrated the model to achieve a 25% rate of repeat ablation (with some patients achieving successful rhythm control on AAD therapy after one ablation) and a 10% overall failure rate with the ablation strategy. We assumed that 75% of patients treated with first-line drugs (and no ablation) would recur within one year, and that 65% of patients treated with amiodarone as second-line therapy would recur within one year. These recurrence rates with AAD therapy are slightly higher than in a recent AAD comparative efficacy trial24, but are consistent with recurrence rates in recent randomized studies comparing ablation with drug therapy in paroxysmal AF patients with previous drug failure.5, 28, 29
Costs
The costs of drug therapy for AF were primarily derived from the FRACTAL registry30 and the health economic sub-study of the AFFIRM trial.31 The cost of catheter ablation was estimated from our local cost-accounting system and recently published estimates from the US and Canada12, 15 were used in sensitivity analysis.
Quality of life adjustment
Although quality of life has been assessed in studies of many AF interventions,32 there is very little published information available regarding utilities in AF patients,33 particularly in AF ablation populations. To address this lack of data, we derived utilities for 3 separate populations of AF patients in order to estimate the likely changes that might be observed after successful ablative or drug therapy.
For drug treated patients, we used SF-12 questionnaire34 data from the FRACTAL registry35 and calculated utilities for these patients using the method proposed by Brazier.36 For ablation patients, we used a similar transformation of responses to the SF-36 questionnaire 37, 38 to estimate the change in utility experienced by a prospective cohort of patients undergoing catheter ablation at Beth Israel Deaconess Medical Center. Finally, we also calculated utilities using SF-36 data for patients enrolled in the Atrial Fibrillation vs. Antiarrhythmic Drugs (A4) trial5, 39 in order to estimate the comparative changes in utility for patients treated with drugs vs. ablation. Based on these analyses, the change in utility from baseline to successful sinus rhythm maintenance was set at 0.065 (see Appendix for additional details). In the model, utilities corresponding with each health state were summed over the amount of time spent in that health state in order to calculate quality-adjusted life years.
Secondary Analyses
One-way sensitivity analyses40 for all model inputs were performed and plotted in a “tornado” diagram. Two-way sensitivity analysis was performed on the utility of successful sinus rhythm maintenance after ablation and the utility of the rate control health state in order to better display the joint impact of these important variables on the model’s cost-effectiveness estimates.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
RESULTS
Base-case results
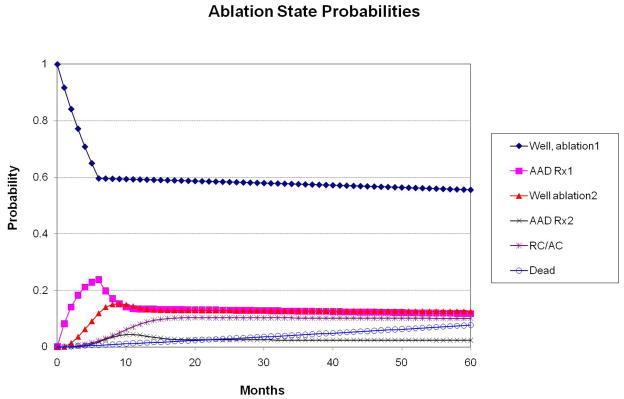
Figures 4A and 4B display the state probabilities for the ablation and AAD cohorts, respectively, plotted against time. Based on the model inputs, 60% of ablation patients remain well following a single procedure, ~10% eventually fail all therapies and progress to a rate control and anticoagulation strategy, and the remainder of ablation patients either achieve control of their AF with a second ablation and/or adjunctive AAD therapy, or die during follow-up. For the AAD cohort, the majority of patients fail first-line treatment over one year and transition to amiodarone. However, over time, roughly two-thirds of patients fail AAD treatment altogether and progress to a rate-control and anticoagulation strategy. Based on the low estimates of fatality from procedural complications or drug toxicity, projected all-cause mortality was equivalent between groups (7.7% ablation vs. 7.8% AAD).
Figure 4.
State probabilities over time for the ablation (panel A) and AAD (panel B) arms of the model. Based on the defined transition probabilities, roughly 60% of ablation patients remained well after a single procedure, 10% eventually failed all treatments, and the remainder achieved success with concomitant AAD therapy and/or a second ablation. In the AAD arm, roughly 50% of patients fail both first and second-line drug therapy within 2 years and are subsequently managed with rate control and anticoagulation only (“RC/AC”).
In the base case scenario, cumulative costs with the RFA and AAD strategies were $26,584 and $19,898, respectively. The initial difference in costs between strategies is roughly $10,000 but narrows over time as a larger proportion of AAD patients have symptomatic recurrences, leading to increased resource consumption and changes in therapy. Over 5 years, the RFA cohort lived 3.51 QALYs, versus 3.38 for the AAD group. The incremental cost-effectiveness ratio (iCER) for RFA vs. AAD was thus $51,431/QALY. Removing the age and gender related background mortality from the model increased costs in both groups to a similar extent, and modestly increased the incremental quality-adjusted life expectancy in favor of ablation (3.64 vs. 3.50 QALYs), slightly reducing the iCER to $47,333 per QALY.
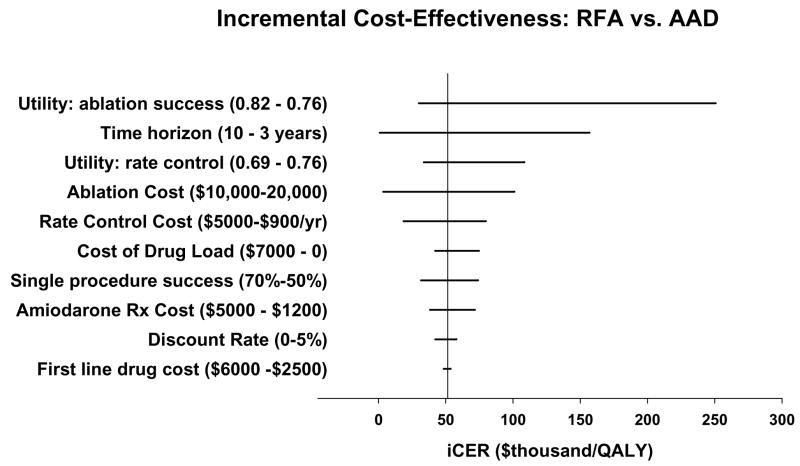
Sensitivity analysis
Figure 5 displays the impact of varying key input variables on the iCER. Within the plausible ranges for these variables (see also Appendix), the model was insensitive to changes to the discount rate, the probability or cost of procedural complications or drug toxicity, or assumptions about the efficacy or cost of first-line antiarrhythmic drug therapy. In no case did changes in these parameters increase the iCER above $60,000 per QALY.
Figure 5.
Tornado diagram displaying the results of key one-way sensitivity analyses on the incremental cost-effectiveness ratio (iCER) for ablation compared with AAD therapy. The base-case result is denoted by the vertical line, and the changes to the iCER by varying individual parameters within plausible limits (shown in parentheses) are shown in the horizontal bars.
The model was moderately sensitive to the cost of amiodarone therapy, the cost of drug loading, the cost of AF care under a rate control strategy, and the assumed rate of single procedure efficacy with ablation. Changes in these parameters did not increase the iCER to more than $80,000 per QALY.
The model results were most sensitive to the time horizon of the analysis, the cost of ablation, and to the relative utility weights of successful ablation vs. unsuccessful drug therapy. Predictably, higher assumed values for the cost of ablation increased the iCER, which was ~$100,000 per QALY at a single procedure ablation cost of $20,000. Holding all other parameters constant, a time horizon of 3 years resulted in incremental costs of ~$9900 and an unfavorable iCER of $157,000 per QALY, while a time horizon of 10 years resulted in near cost neutrality and larger incremental QALY gains favoring ablation, resulting in an iCER of <$1,000 per QALY.
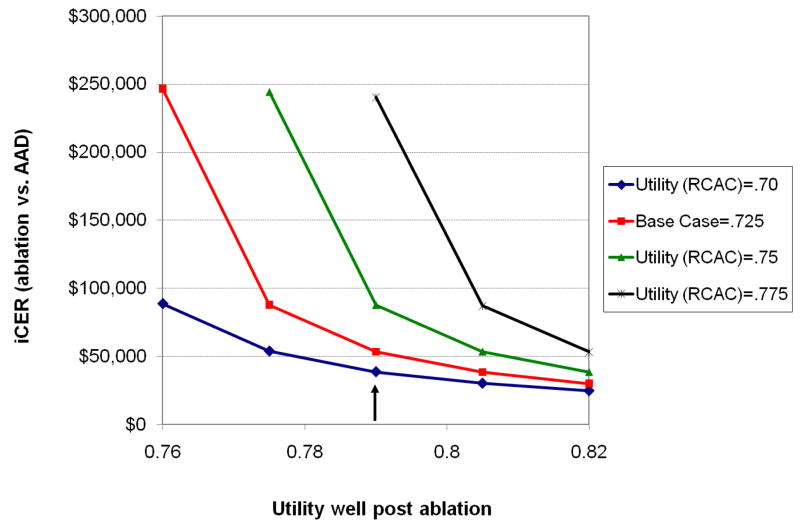
As shown in Figure 5, relatively small changes in the utility weights assigned to either the well states following ablation or the rate control state had a significant impact on the estimated iCER. The interplay between these variables is further highlighted in Figure 6. In general, the iCER for ablation vs. AAD therapy was <$100,000 as long as the difference between these utility scores was 0.04 or greater. Larger differences in utility resulted in quite favorable iCERs, while smaller differences yielded iCERs in the economically unattractive range.
Figure 6.
Two-way sensitivity analysis on utility values. Each line represents a different assumed utility score for the rate control and anticoagulation (“RCAC”) health state, ranging from.70 to.775, with the red line denoting the base case value. The effect of varying the utility for the well-states following ablation (from 0.76 to 0.82 on the x-axis) on the iCER (y-axis) are shown. The vertical arrow indicates the base case value for utility in a well state following ablation (0.79).
DISCUSSION
Multiple studies have demonstrated clinical benefits of catheter ablation for AF, including improved maintenance of sinus rhythm and improved quality of life, compared with AAD therapy. In the current study, we used a disease simulation model to project costs and quality-adjusted life expectancy for a population of 60 year old men with drug-refractory atrial fibrillation who were treated with either RF ablation (± adjunctive AADs) or continued medical therapy. Using assumptions based largely on the current medical literature, we found that ablation was reasonably cost-effective compared with AAD therapy alone from the perspective of the US health care system, even within a relatively short time horizon, and even without assuming that ablation reduces the risk of stroke, heart failure, or death. The modeled results were most sensitive to assumptions about the cost of ablation, and the difference in quality of life (utility) between successful and unsuccessful sinus rhythm maintenance.
Few previous studies have examined the potential cost-effectiveness of catheter ablation for AF. Chan et al.,15 also using a Markov model, projected the potential cost-effectiveness of “left atrial catheter ablation,” compared with amiodarone or rate control, based on hypothetical reductions in the risk of stroke following ablation. Potential improvements in quality adjusted life expectancy resulting from successful maintenance of sinus rhythm alone were not considered. Although a large non-randomized series from one center has suggested that AF ablation reduces morbidity and mortality relative to drug therapy,20 adequately powered randomized studies to test this hypothesis have not been completed. We constructed our model assuming no benefit for ablation on the risk of stroke; if such a benefit is eventually proven, it would only improve the cost-effectiveness of ablation.
Khaykin et al.12 performed a cost comparison of catheter ablation for AF and antiarrhythmic drug therapy based on health care utilization patterns from Canada and France using Canadian price weights, and concluded that costs would equalize over 3–8 years of follow-up (4.5–11 years with 3% discounting applied) due to higher long-term health care costs with AAD treatment. While we used a US rather than Canadian perspective, our results regarding the relative costs of ablation and drug therapy are qualitatively similar to this previous study. Longer term extrapolations of our model indicate cost neutrality after about 10 years, an estimate within the range of the previous authors’ sensitivity analyses. The longer time for this equalization of costs in our model may be due both to the higher up-front cost of ablation in the US, and to the fact that our model shunts the drug cohort toward less expensive rate controlling drugs over time. In contrast, Khaykin et al’s study12 assumed the usage of antiarrhythmic drugs would remain constant over long periods of time, an assumption we find unlikely.41
Most recently, an economic model of a randomized pilot study of AF ablation as first-line therapy for paroxysmal AF was performed,13 again using Canadian price weights. In this study, costs were nearly equal after 2 years, in large part because of a 49% rate of crossover to ablation in patients initially assigned to AAD therapy. We did not explicitly evaluate a delayed ablation strategy in our model, since we believe the pertinent health policy and reimbursement question is not the timing of ablation, but rather its incremental value over available rhythm control therapies.
The major difference between our study and prior economic assessments of AF ablation is the explicit incorporation of quality of life-adjustment as it pertains to sinus rhythm maintenance, which permits the expression of results in dollars per QALY, the recommended metric of cost-effectiveness analysis.22 The utility weights used in our analysis were derived from analysis of empirical SF-12 and SF-36 data from three separate AF populations (two treated with ablation), with highly concordant results (see Appendix). In these populations, mean utilities varied from ~0.72 to 0.80, which agree well with cross-sectional EQ-5D scores published by the Euro Heart Survey for AF.33
Our sensitivity analyses indicate that, until and unless morbidity and mortality benefits are proven, the cost-effectiveness of AF ablation relative to AAD therapy will remain highly contingent on the incremental gains in quality of life the procedure can provide, at least over a short to medium-term time horizon. This implies that AF ablation is unlikely to be cost-effective for patients who enjoy preserved baseline quality of life despite their AF, or for patients whose quality of life is not likely to substantially improve despite elimination of AF (e.g. patients with poor quality of life mainly due to other health problems). We believe these observations offer insights into patient selection for AF ablation that are congruent with clinical judgment and current consensus recommendations.3, 16
Our model may suggest additional insights into what factors may increase or decrease the likelihood of ablation being cost effective. Increased single procedure efficacy, reduced procedural cost, and/or increased symptomatic benefit would all improve the cost-effectiveness of ablation relative to AADs. On the other hand, a short time horizon, reduced costs for drug loading and follow-up care, better baseline quality of life, a smaller change in quality of life following ablation, and a greater probability of symptom reduction on AAD therapy would all tend to make ablation less attractive from a cost-effectiveness perspective.
We believe the assumptions used in our base case were conservative and, if anything, slightly biased against ablation. For example, longer time horizons would improve the cost-effectiveness of ablation, but we do not feel the current evidence base permits extrapolation of results beyond 5 years. Additional conservative assumptions in our model included not using amiodarone (known to have greater efficacy than alternative drugs) in the ablation arm of the model, and estimating single procedure efficacy with ablation at 60%, with a 25% rate of repeat ablation. Although centers of excellence have reported better results than these, we feel these estimates are probably representative of current general practice.18 Although non-randomized studies42–44 have suggested that AF ablation in patients with reduced LV systolic function can improve LV function, functional status, and quality of life, we did not incorporate improvements in heart failure into our model.
Our study has important limitations. First, the results of this model cannot be directly applied to other sub-sets of the AF population (e.g. newly detected, persistent, or long-standing persistent), as the baseline characteristics of those patients, the ablation methods required to achieve long-term success, and the outcomes with ablation and drug therapy are probably all different than those we estimated. We did not model all possible treatment strategies for patients with paroxysmal AF. In selected patients, pharmacologic rate control alone, or pacemaker implantation in conjunction with ablation of the AV junction may be reasonable alternatives. At present, however, there is very little data directly comparing these strategies with ablation from which to draw model inputs, and our model assumed that patients were seeking rhythm control strategies due to dissatisfaction with rate control alone.
As always with cost-effectiveness modeling studies, ours required simplifying assumptions and use of some uncertain parameters. One area of particular difficulty in this model was estimating when symptomatic AF recurrences would prompt a change in therapeutic strategy, as trials of AF interventions typically report all detected recurrences (or time to first recurrence) regardless of whether or not such recurrences would actually be considered a true therapeutic failure. For this reason, we attempted to define model parameters that mimic published results, e.g. in terms of repeat ablation, drug discontinuation, and concomitant use of AADs following ablation.
Finally, we recognize that not all technical approaches to catheter ablation for AF are the same, and that ablation techniques are rapidly evolving. This was one more reason that we chose to focus on ablation for paroxysmal AF, where the procedural focus remains more or less focused on electrical isolation of the pulmonary veins.3
Supplementary Material
Acknowledgments
Funding Sources. Dr. Reynolds was supported by grant K23 HL077171 from NIH.
Footnotes
Conflict of Interest Disclosures. Dr. Reynolds reports consulting fees from Biosense Webster, Inc and Sanofi-Aventis. Dr. Josesphson reports consulting fees from Biosense Webster, Inc.
References
- 1.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 2.Jais P, Haissaguerre M, Shah DC, Chouairi S, Gencel L, Hocini M, Clementy J. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation. 1997;95:572–576. doi: 10.1161/01.cir.95.3.572. [DOI] [PubMed] [Google Scholar]
- 3.Calkins H, Brugada J, Packer DL, Cappato R, Chen SA, Crijns HJG, Damiano RJ, Davies DW, Haines DE, Haissaguerre M, Iesaka Y, Jackman W, Jais P, Kottkamp H, Kuck KH, Lindsay BD, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Natale A, Pappone C, Prystowsky E, Raviele A, Ruskin JN, Shemin RJ. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. Heart Rhythm. 2007;4:816–861. doi: 10.1016/j.hrthm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Noheria A, Kumar A, Wylie JV, Jr, Josephson ME. Catheter ablation vs antiarrhythmic drug therapy for atrial fibrillation: a systematic review. Arch Intern Med. 2008;168:581–586. doi: 10.1001/archinte.168.6.581. [DOI] [PubMed] [Google Scholar]
- 5.Jais P, Cauchemez B, Macle L, Daoud E, Khairy P, Subbiah R, Hocini M, Extramiana F, Sacher F, Bordachar P, Klein G, Weerasooriya R, Clementy J, Haissaguerre M. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: The A4 Study. Circulation. 2008;118:2498–2505. doi: 10.1161/CIRCULATIONAHA.108.772582. [DOI] [PubMed] [Google Scholar]
- 6.Oral H, Pappone C, Chugh A, Good E, Bogun F, Pelosi F, Jr, Bates ER, Lehmann MH, Vicedomini G, Augello G, Agricola E, Sala S, Santinelli V, Morady F. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med. 2006;354:934–941. doi: 10.1056/NEJMoa050955. [DOI] [PubMed] [Google Scholar]
- 7.Wazni OM, Marrouche NF, Martin DO, Verma A, Bhargava M, Saliba W, Bash D, Schweikert R, Brachmann J, Gunther J, Gutleben K, Pisano E, Potenza D, Fanelli R, Raviele A, Themistoclakis S, Rossillo A, Bonso A, Natale A. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA. 2005;293:2634–2640. doi: 10.1001/jama.293.21.2634. [DOI] [PubMed] [Google Scholar]
- 8.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 9.Coyne KS, Paramore C, Grandy S, Mercader M, Reynolds MR, Zimetbaum P. Assessing the direct costs of treating nonvalvular atrial fibrillation in the United States. Value Health. 2006;9:348–356. doi: 10.1111/j.1524-4733.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- 10.Wattigney WA, Mensah GA, Croft JB. Increasing trends in hospitalization for atrial fibrillation in the United States, 1985 through 1999. Circulation. 2003;108:711–716. doi: 10.1161/01.CIR.0000083722.42033.0A. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg A, Menen M, Mickelsen S, MacIndoe C, Binder M, Nawman R, West G, Kusumoto FM. Atrial fibrillation ablation leads to long-term improvement of quality of life and reduced utilization of healthcare resources. J Interv Card Electrophysiol. 2003;8:59–64. doi: 10.1023/a:1022348216072. [DOI] [PubMed] [Google Scholar]
- 12.Khaykin Y, Morillo CA, Skanes AC, McCracken A, Humphries K, Kerr CR. Cost comparison of catheter ablation and medical therapy in atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:907–913. doi: 10.1111/j.1540-8167.2007.00902.x. [DOI] [PubMed] [Google Scholar]
- 13.Khaykin Y, Wang X, Natale A, Wazni OM, Skanes AC, Humphries KH, Kerr CR, Verma A, Morillo CA. Cost comparison of ablation versus antiarrhythmic drugs as first-line therapy for atrial fibrillation: an economic evaluation of the RAAFT pilot study. J Cardiovasc Electrophysiol. 2009;20:7–12. doi: 10.1111/j.1540-8167.2008.01303.x. [DOI] [PubMed] [Google Scholar]
- 14.Weerasooriya R, Jais P, Le Heuzey JY, Scavee C, Choi KJ, Macle L, Raybaud F, Hocini M, Shah DC, Lavergne T, Clementy J, Haissaguerre M. Cost analysis of catheter ablation for paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2003;26:292–294. doi: 10.1046/j.1460-9592.2003.00035.x. [DOI] [PubMed] [Google Scholar]
- 15.Chan PS, Vijan S, Morady F, Oral H. Cost-effectiveness of radiofrequency catheter ablation for atrial fibrillation. J Am Coll Cardiol. 2006;47:2513–2520. doi: 10.1016/j.jacc.2006.01.070. [DOI] [PubMed] [Google Scholar]
- 16.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey J-Y, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc J-J, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL Acc/Aha Task Force M; Esc Committee For Practice G. ACC/AHA/ESC 2006 Guidelines for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation) Circulation. 2006;114:e257–354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 17.Oral H, Knight BP, Tada H, Ozaydin M, Chugh A, Hassan S, Scharf C, Lai SW, Greenstein R, Pelosi F, Jr, Strickberger SA, Morady F. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation. 2002;105:1077–1081. doi: 10.1161/hc0902.104712. [DOI] [PubMed] [Google Scholar]
- 18.Cheema A, Vasamreddy CR, Dalal D, Marine JE, Dong J, Henrikson CA, Spragg D, Cheng A, Nazarian S, Sinha S, Halperin H, Berger R, Calkins H. Long-term single procedure efficacy of catheter ablation of atrial fibrillation. J Interv Card Electrophysiol. 2006;15:145–155. doi: 10.1007/s10840-006-9005-9. [DOI] [PubMed] [Google Scholar]
- 19.Neumann T, Vogt J, Schumacher B, Dorszewski A, Kuniss M, Neuser H, Kurzidim K, Berkowitsch A, Koller M, Heintze J, Scholz U, Wetzel U, Schneider MAE, Horstkotte D, Hamm CW, Pitschner H-F. Circumferential pulmonary vein isolation with the cryoballoon technique: results from a prospective 3-center study. J Am Coll Cardiol. 2008;52:273–278. doi: 10.1016/j.jacc.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 20.Pappone C, Rosanio S, Augello G, Gallus G, Vicedomini G, Mazzone P, Gulletta S, Gugliotta F, Pappone A, Santinelli V, Tortoriello V, Sala S, Zangrillo A, Crescenzi G, Benussi S, Alfieri O. Mortality, morbidity, and quality of life after circumferential pulmonary vein ablation for atrial fibrillation: outcomes from a controlled nonrandomized long-term study. J Am Coll Cardiol. 2003;42:185–197. doi: 10.1016/s0735-1097(03)00577-1. [DOI] [PubMed] [Google Scholar]
- 21.Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 22.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the panel on cost-effectiveness in health and medicine. JAMA. 1996;276:1253–1258. [PubMed] [Google Scholar]
- 23.Roy D, Talajic M, Dorian P, Connolly S, Eisenberg MJ, Green M, Kus T, Lambert J, Dubuc M, Gagne P, Nattel S, Thibault B. Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators. N Engl J Med. 2000;342:913–920. doi: 10.1056/NEJM200003303421302. [DOI] [PubMed] [Google Scholar]
- 24.Singh BN, Singh SN, Reda DJ, Tang XC, Lopez B, Harris CL, Fletcher RD, Sharma SC, Atwood JE, Jacobsen AK, Lewis HD, Raisch DW, Ezekowitz MD. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med. 2005;352:1861–1872. doi: 10.1056/NEJMoa041705. [DOI] [PubMed] [Google Scholar]
- 25.The AFFIRM Investigators. Maintenance of sinus rhythm in patients with atrial fibrillation: an AFFIRM substudy of the first antiarrhythmic drug. J Am Coll Cardiol. 2003;42:20–29. doi: 10.1016/s0735-1097(03)00559-x. [DOI] [PubMed] [Google Scholar]
- 26.National Center for Health Statistics. [Accessed September 1, 2006];United States Life Tables. 2003 Available at: http://www.cdc.gov/nchs/products/pubs/pubd/lftbls/life/1966.htm. [PubMed]
- 27.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Yl, Kalman J, Kim Y-H, Klein G, Packer D, Skanes A. World-wide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005;111:1100–1105. doi: 10.1161/01.CIR.0000157153.30978.67. [DOI] [PubMed] [Google Scholar]
- 28.Pappone C, Augello G, Sala S, Gugliotta F, Vicedomini G, Gulletta S, Paglino G, Mazzone P, Sora N, Greiss I, Santagostino A, LiVolsi L, Pappone N, Radinovic A, Manguso FSV. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation. J Am Coll Cardiol. 2006;48:2340–2347. doi: 10.1016/j.jacc.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 29.Stabile G, Bertaglia E, Senatore G, De Simone A, Zoppo F, Donnici G, Turco P, Pascotto P, Fazzari M, Vitale DF. Catheter ablation treatment in patients with drug-refractory atrail fibrillation: a prospective, multi-centre, randomized controlled study (Catheter Ablation for the Cure of Atrial Fibrillation Study) Eur Heart J. 2006;27:216–221. doi: 10.1093/eurheartj/ehi583. [DOI] [PubMed] [Google Scholar]
- 30.Reynolds MR, Essebag V, Zimetbaum P, Cohen DJ. Healthcare resource utilization and costs associated with recurrent episodes of atrial fibrillation: the FRACTAL registry. J Cardiovasc Electrohysiol. 2007;18:628–633. doi: 10.1111/j.1540-8167.2007.00819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marshall DA, Levy AR, Vidaillet H, Fenwick E, Slee A, Blackhouse G, Greene HL, Wyse G, Nichol G, O’Brien BJ. Cost-effectiveness of rhythm versus rate control in atrial fibrillation. Ann Intern Med. 2004;141:653–661. doi: 10.7326/0003-4819-141-9-200411020-00005. [DOI] [PubMed] [Google Scholar]
- 32.Reynolds MR, Ellis E, Zimetbaum P. Quality of life in atrial fibrillation: measurement tools and impact of interventions. J Cardiovasc Electrophys. 2008;19:762–768. doi: 10.1111/j.1540-8167.2007.01091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dagres N, Nieuwlaat R, Vardas PE, Andresen D, Levy S, Cobbe S, Kremastinos DT, Breithardt G, Cokkinos DV, Crijns HJ. Gender-related differences in presentation, treatment, and outcome of patients with atrial fibrillation in Europe: a report from the Euro Heart Survey on Atrial Fibrillation. J Am Coll Cardiol. 2007;49:572–577. doi: 10.1016/j.jacc.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 34.Ware J, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Reynolds MR, Lavelle T, Essebag V, Cohen DJ, Zimetbaum P. Influence of age, gender, and AF recurrence on quality of life outcomes in a population of new-onset AF patients: the FRACTAL registry. Am Heart J. 2006;152:1097–1103. doi: 10.1016/j.ahj.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care. 2004;42:851–859. doi: 10.1097/01.mlr.0000135827.18610.0d. [DOI] [PubMed] [Google Scholar]
- 37.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 38.Brazier J, Roberts J, Deverill M. The estimation of a preferenced based measure of health from the SF-36. Journal of Health Economics. 2002;21:271–292. doi: 10.1016/s0167-6296(01)00130-8. [DOI] [PubMed] [Google Scholar]
- 39.Reynolds MR, Cauchemez B, Macle L, Daoud EG, Jais P. Health state utilities improve with catheter ablation for AF more than with drug therapy: analysis from a randomized trial. Circulation. 2007;115:e574–575. (abstract) [Google Scholar]
- 40.Briggs A, Sculpher M, Buxton M. Uncertainty in the economic evaluation of health care technologies: the role of sensitivity analysis. Health Econ. 1994;3:95–104. doi: 10.1002/hec.4730030206. [DOI] [PubMed] [Google Scholar]
- 41.Nieuwlaat R, Prins MH, Le Heuzey J-Y, Vardas PE, Aliot E, Santini M, Cobbe SM, Widdershoven JWMG, Baur LH, Levy S, Crijns HJGM. Prognosis, disease progression, and treatment of atrial fibrillation patients during 1 year: follow-up of the Euro Heart Survey on atrial fibrillation. Eur Heart J. 2008;29:1181–1189. doi: 10.1093/eurheartj/ehn139. [DOI] [PubMed] [Google Scholar]
- 42.Chen MS, Marrouche NF, Khaykin Y, Gillinov AM, Wazni O, Martin DO, Rossillo A, Verma A, Cummings J, Erciyes D, Saad E, Bhargava M, Bash D, Schweikert R, Burkhardt D, Williams-Andrews M, Perez-Lugones A, Abdul-Karim A, Saliba W, Natale A. Pulmonary vein isolation for the treatment of atrial fibrillation in patients with impaired systolic function. J Am Coll Cardiol. 2004;43:1004–1009. doi: 10.1016/j.jacc.2003.09.056. [DOI] [PubMed] [Google Scholar]
- 43.Gentlesk PJ, Sauer WH, Gerstenfeld EP, Lin D, Dixit S, Zado E, Callans D, Marchlinski FE. Reversal of left ventricular dysfunction following ablation of atrial fibrillation. Journal of Cardiovascular Electrophysiology. 2007;18:9–14. doi: 10.1111/j.1540-8167.2006.00653.x. [DOI] [PubMed] [Google Scholar]
- 44.Hsu L-F, Jais P, Sanders P, Garrigue S, Hocini M, Sacher F, Takahashi Y, Rotter M, Pasquie J-L, Scavee C, Bordachar P, Clementy J, Haissaguerre M. Catheter ablation for atrial fibrillation in congestive heart failure. N Engl J Med. 2004;351:2373–2383. doi: 10.1056/NEJMoa041018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.