Abstract
Objective
Lower life expectancy in men is generally attributed to higher likelihood of risky behavior and because men develop chronic conditions earlier. If sex-related differences in survival are independent of pre-infection chronic health and health behavior, it would suggest that survival differences may occur due to sex differences in quality of care and biologic response to infection and these differences may contribute to sex differences in life expectancy. We assessed if sex-related survival difference following community-acquired pneumonia (CAP) is due to differences in clinical characteristics, quality of care, or immune response.
Design, setting, and subjects
Prospective observational cohort of 2183 subjects with CAP.
Measurements and main results
Mean age was 64.9 years. Compared to women, men were more likely to smoke and had more comorbidity. At emergency department presentation, men had different biomarker patterns, as evidenced by higher inflammation (tumor necrosis factor (TNF), interleukin (IL)-6, and IL-10) and fibrinolysis (D-dimer), and lower coagulation biomarkers (antithrombin-III and Factor IX) (P<0.05). Small differences in favor of men were seen in care quality, including antibiotic timing and compliance with American Thoracic Society guidelines. Men had lower survival at 30, 90, 365 days. The higher one-year mortality was not attenuated when adjusted for differences in demographics, smoking, resuscitation, insurance, and vaccination status, comorbidity, hospital characteristics, and illness severity (unadjusted hazard ratio (HR)=1.35, p=0.003 and adjusted HR=1.29, p=0.004). HR were no longer statistically significant when additionally adjusted for differences in ED concentrations of TNF, IL-6, IL-10, D-dimer, antithrombin-III, and Factor IX (adjusted HR=1.27, p=0.17). Patterns of biomarkers observed in men were associated with worse survival over one year.
Conclusions
Lower survival among men following CAP was not explained by differences in chronic diseases, health behaviors, and quality of care. Patterns of inflammatory, coagulation, and fibrinolysis biomarkers among men may explain reduced short and long-term survival.
Keywords: sex, disparities, sepsis, pneumonia, inflammation mediators, quality of health care
Women live longer than men. The prevailing assumption is that this observation is largely because men are more likely to engage in risky behavior, such as smoking, and develop chronic life-threatening diseases, such as cardiovascular disease and cancer, earlier (1). A further possibility could be differences in susceptibility to, and outcome from, acute infection. Despite advances in antibiotics and modern intensive care, life-threatening bacterial infections such as community-acquired pneumonia (CAP), remain one of the most common reasons for hospitalization and death in western societies, especially among older individuals (2-4). Several studies suggest that men are more susceptible to infections (1) and once an infection occurs, they are more likely to die (2, 5-7). However, some report the opposite effect (8-11). Clarifying whether susceptibility and response to acute infections independently contribute to sex disparities in longevity would have important implications for efforts designed to mitigate these disparities.
Sex differences in the outcomes of infection can occur due to differences in clinical characteristics, including socio-demographics, chronic health, health behaviors, differences in quality of care, or differences in the immune response to infection. If sex inequalities in survival are merely due to differences in pre-infection clinical characteristics, then differences in susceptibility and response per se would not contribute to differences in life expectancy. On the other hand, differences in survival that persist after adjustment for these risk factors could potentially implicate differences in the biologic response, or in the quality of care provided, as risk factors not just for survival from infection but for overall life expectancy. However, no large study has simultaneously examined the role of all of these factors. Previous population-based studies using administrative databases had limited information regarding clinical characteristics (2, 7, 9, 10, 12). Cohort studies generally record more clinical data, but published studies have several limitations, such as including patients with heterogeneous sources of infections (8, 10, 11, 13, 14), being limited to those patients who were sufficiently ill to require admission to an intensive care unit (14-16), being conducted at a single center (11), or only examining short-term outcomes (5, 8, 17). Sex-related differences in immune response to infection have been mostly studied in young, proestrous animals and in young, healthy human volunteers (18). These studies suggest that females have a proinflammatory whereas males have an anti-inflammatory immune profile (18). However, these results may not be generalizable to older adults with multiple chronic health conditions, who are at highest risk of developing infections. Indeed a small, single-center study of older subjects with sepsis found TNF concentrations were lower and IL-10 concentrations were higher among women, contrary to results in animals (5).
We therefore analyzed data from a large prospective cohort study of older adults with community-acquired pneumonia (CAP) to determine if differences in clinical characteristics alone explain sex-related difference in survival. We chose CAP because by studying a single infectious process we avoided the confounding effect of combining different sources of infection, the distribution of which varies by sex (2). We examined sex-related differences in both short- and long-term survival, and we compared quality of care and patterns of immune response, testing whether any identified differences explained differences in survival.
MATERIAL AND METHODS
Subjects and design
We analyzed data from Genetic and Inflammatory Markers of Sepsis (GenIMS), a prospective multicenter observational cohort study. Subjects were enrolled in emergency departments (ED) of 28 academic and community hospitals in Pennsylvania, Connecticut, Michigan, and Tennessee from December 2001 to November 2003. Eligible patients were ≥18 years old and had a clinical and radiological diagnosis of CAP using criteria by Fine et al (19). Exclusion criteria included transfer from another hospital, discharge from an acute care hospital within the previous ten days, diagnosis of pneumonia within the previous 30 days, chronic dependency on mechanical ventilation, cystic fibrosis, active pulmonary tuberculosis, admission for palliative care, prior enrollment in the study, incarceration, and pregnancy. Institutional Review Boards of all participating hospitals approved the study and written informed consent was obtained from all subjects.
Of the 2320 subjects enrolled, we excluded 137 subjects because the clinical team ruled out CAP during the first three days of hospitalization. We restricted our analyses to the remaining 2183 subjects.
Clinical characteristics and outcome
We prospectively collected detailed baseline and sequential clinical information using structured subject or proxy interviews, bedside assessment by study nurses, and from medical records. We classified subjects as insured if they had commercial health insurance, Medicare, or Medicaid coverage. We assessed co-morbidity using the Charlson co-morbidity score (20). We assessed severity of illness on presentation using the Acute Physiology and Chronic Health Evaluation III (APACHE III) score and the Pneumonia Severity Index (19, 21). We also analyzed the acute physiology component of the APACHE III score separately, excluding points for age and chronic health conditions, to quantify physiological derangements. We also analyzed the PSI score without points attributed to age because this score assigns fewer age points to women than men. We defined severe sepsis as pneumonia with acute organ dysfunction following the 2001 International Consensus Criteria (22). We defined acute organ dysfunction as a new Sequential Organ Failure Assessment (SOFA) score of ≥ 3 in any of six organ systems (23). Based on Medicare quality monitoring system criteria (24) and the American Thoracic Society (ATS) guidelines (25), we compared quality of hospital care for CAP by assessing timing of initial antibiotic therapy and whether antibiotics were compliant with ATS guidelines. We also compared measures of therapeutic intensity, by noting whether patients were admitted to hospital and to intensive care units (ICUs), and for subjects admitted to an ICU, utilization of various treatments. We ascertained one-year survival and cause of death by National Death index (NDI) search and using NDI-coded causes of death (26). The reliability of NDI for epidemiological studies has been previously validated (27).
Laboratory procedures
We assessed differences in the immune response to infection by comparing circulating concentrations of biomarkers at ED presentation and during the first week of hospital course within inflammatory (TNF, IL-6, IL-10), coagulation (Factor IX, thrombin-antithrombin complexe (TAT), antithrombin III), and fibrinolysis (plasminogen activator inhibitor (PAI)-1, and D-dimer) systems. Our choice of markers was based on two considerations: prior evidence of association between the biomarker and outcome (28, 29), and logistics, such as stability of the marker, ease of assay, and costs. Blood was collected at enrollment and, for admitted patients, daily for the first week and weekly thereafter until hospital discharge or day 28. We did not obtain samples in ED from subjects presenting after 11 PM or on weekends or holidays for logistic reasons. Details of sample processing have been described previously (30). We analyzed inflammatory biomarkers using an automated chemiluminescent immunoassay analyzer (IMMULITE, Diagnostic Products Corp., Los Angeles, CA). Coagulation and fibrinolysis biomarkers were analyzed in a randomly selected subset by a commercial laboratory (Essoterix, Agoura Hills CA, USA). Thus, we analyzed inflammatory biomarkers in 1429 and coagulation and fibrinolysis biomarkers in 734 subjects.
Statistical analyses
We conducted univariate comparisons using Chi-squared tests, t-tests, or their nonparametric equivalents, as appropriate. We conducted multivariable analyses using generalized linear models for severity of illness, logistic regression model for risk of severe sepsis, mixed models for biomarkers, generalized estimating equations (GEE) models for quality of care, and Cox proportional hazards models for survival analyses. We selected variables from demographic, health behavior, and chronic health domains for multivariable analyses if they were significant in the univariate analysis (p<0.1). For quality of care models, we also adjusted for initial severity of illness. For survival analyses, we calculated hazard ratios for serial models, initially constructing a model without covariates and then adjusting for demographics, health behavior, and chronic health domains. We used mixed models to compare biomarkers to account for correlation of repeated measures over time and Tobit models to account for truncated data (31). We used GEE models where hospital characteristics were treated as a cluster level covariate to compare quality of care because care quality for subjects treated within the same hospital may be correlated. For the logistic regression and Cox proportional hazards models, we conducted additional analyses to assess whether the association between sex and outcomes were affected by hospital characteristics by incorporating these factors as fixed effects. We compared survival over different time intervals following occurrence of CAP using a Gray’s model (32) over one-year and at earlier time points at 30 and 90-days. To assess if menopausal status influenced differences in survival, we compared survival among men and women stratified by age.
We used two approaches to assess if sex-differences in inflammatory, coagulation, and fibrinolysis biomarkers explained differences in survival. First, we examined change in hazard ratios in survival models incorporating variables from demographics, health behavior, and chronic health domains and then additionally adjusting for inflammatory, coagulation, and fibrinolysis biomarkers that differed between men and women. Second, we examined sex differences in ED concentrations of the biomarkers that differed between men and women, adjusted by survival status. ED concentrations of biomarkers were associated with survival over different time points and a Gray’s model was used to identify the time point at which the biomarker was no longer associated with differences in outcome.
Analyses were performed using SAS 9.0 (SAS Institute, Cary, NC, USA) assuming statistical significance at p<0.05. We estimated that a sample size of 1800 men and women would be required to show a 5% absolute difference in one-year mortality (β=0.8 and α=0.05).
RESULTS
Pre-hospitalization characteristics
Of the 2183 subjects, 1136 were men and 1047 were women (Table 1). The mean age of both sexes in the cohort was 64.9 years. Approximately half of all subjects were recruited from EDs of large (≥250 beds) teaching hospitals. Smoking was more prevalent among men compared to women (70.9% vs. 58.3%, p<0.0001). Men were more likely to have at least one chronic health condition, based on their Charlson score (70% vs. 65.5%, p=0.03). In particular, cardiac disease, AIDS, and neoplastic disease were more prevalent among men. Approximately half the subjects who reported vaccination status had received a pneumococcal or influenza vaccine. Men were more likely to have received an influenza vaccine, but no differences were seen in pneumococcal vaccination between men and women. Most subjects had health insurance, though men were less likely to have health insurance (92.7% vs. 95.8%, p=0.002). Only 5.6% of subjects had a ‘do not resuscitate’ order prior to hospitalization; again, this was less frequent among men (4.7% vs. 6.7%, p=0.04). Men were less likely to have received antibiotics prior to presentation to the ED. On average, symptoms were present for slightly longer in men (5.1 vs. 4.7 days for men and women). Following evaluation in ED, 288 (13.2%) subjects were discharged home and 1895 (86.8%) required hospital admission. An equal proportion of men and women were hospitalized (86.7% vs. 86.9%, p=0.89).
Table 1.
Pre-hospitalization characteristics*
| Variable | All subjects N=2183 |
Men n=1136 |
Women n=1047 |
P value |
|---|---|---|---|---|
| Demographics | ||||
| Age, mean (sd) | 64.9 (18.0) | 64.9 (18.0) | 64.9 (18.1) | 0.94 |
| Race, white, n (%) | 1738 (79.6) | 919 (80.9) | 819 (78.2) | 0.12 |
| Health insurance, n (%) | 2056 (94.1) | 1053 (92.7) | 1003 (95.8) | 0.002 |
| Health behaviors | ||||
| Ever smoked, n (%) | 1415 (64.8) | 805 (70.9) | 610 (58.3) | <0.0001 |
| Years smoked, (mean, sd) | 31.1 (16.4) | 31.8 (16.2) | 30.1 (16.6) | 0.06 |
| Charlson comorbidity score>0, n (%) | 1481 (67.8) | 795 (70.0) | 686 (65.5) | 0.02 |
| COPD**, n (%) | 513 (23.6) | 270 (23.8) | 243 (23.3) | 0.76 |
| Cardiac disease, n (%) | 513 (26.5) | 331 (32.1) | 182 (20.2) | <0.0001 |
| Renal disease, n (%) | 51 (2.3) | 29 (2.6) | 22 (2.1) | 0.48 |
| Cirrhosis, n (%) | 5 (0.7) | 3 (0.8) | 2 (0.6) | 0.72 |
| Diabetes , n (%) | 414 (19.0) | 222 (19.5) | 192 (18.3) | 0.47 |
| Neoplastic disease, n (%) | 132 (7.2) | 85 (8.7) | 47 (5.5) | 0.007 |
| AIDS, n (%) | 37 (1.7) | 26 (2.3) | 11 (1.1) | 0.02 |
| ‘Do not resuscitate’ order prior to admission, n (%) |
123 (5.6) | 53 (4.7) | 70 (6.7) | 0.04 |
| Vaccination status | ||||
| Influenza vaccine in preceding eight months, n (%) |
807 (47.8) | 451 (50.3) | 356 (45.0) | 0.02 |
| Pneumococcal vaccine, n (%) | 764 (45.7) | 404 (45.7) | 360 (45.7) | 0.99 |
| Symptoms and therapy prior to hospitalization | ||||
| Duration (median, mean, IQR) | 3, 4.9 (1-5) | 3, 5.1 (1-6) | 3, 4.7, (1-6) | 0.02 |
| Received antibiotics within seven days, n (%) |
370 (17.0) | 164 (14.5) | 206 (19.7) | 0.0010 |
| Hospital characteristics | ||||
| Teaching hospital n (%) | 1200 (55.1) | 574 (54.8) | 626 (55.3) | 0.84 |
| Bed size n (%) | ||||
| <100 beds | 55 (2.5) | 23 (2) | 3.2 (3.1) | 0.28 |
| 100-249 beds | 952 (43.7) | 511 (53.7) | 441 (42.1) | |
| 250-499 | 841 (38.6) | 432 (38.1) | 409 (39.1) | |
| >500 | 332 (15.2) | 165 (15.8) | 167 (14.7) | |
Data was missing for fewer than 5% of cohort for most variables
COPD — chronic obstructive pulmonary disease
Severity of illness and severe sepsis
At presentation to ED, men had greater illness severity, reflected by higher mean physiology components of the APACHE III score (38.8 vs. 37.2, p = 0.02). These differences were small but remained significant when adjusted for age, race, Charlson score, and smoking, vaccination, insurance, and resuscitation status (p=0.02). Men had higher PSI scores compared to women (100.3 vs. 86.5, p<0.0001) and this difference persisted when PSI scores were compared without points for age (35.3 vs. 31.6, p=0.004).
Severe sepsis occurred in 588 (31%) subjects. Of these, approximately half had severe sepsis on the first day of hospitalization, and 85% cases occurred by the fourth day after presentation. Men had higher risk of severe sepsis compared to women (29.2% vs. 24.5%, unadjusted odds ratio (OR)=1.3, 95% CI=1.1-1.5, p=0.01) (Table 2). The OR remained unchanged, but did not remain statistically significant, when adjusted for age, race, Charlson score, and smoking, vaccination, insurance, and resuscitation status (adjusted OR=1.2, 95% CI=0.99-1.5, p=0.07). The incidence of cardiac, respiratory, neurologic, coagulation, and liver dysfunction was similar, but men were more likely to have renal dysfunction compared to women (Table 2). The median length of hospital stay was similar among men and women (5 days in each case, p=0.84).
Table 2.
Risk of severe sepsis and organ dysfunction
| Variable | Men | Women | P value |
|---|---|---|---|
| Risk of severe sepsis, n (%) | 332 (29.2) | 256 (24.5) | 0.01 |
| Organ dysfunction | |||
| Respiratory | 178 (15.7) | 156 (14.9) | 0.61 |
| Cardiac | 44 (3.9) | 31 (3.0) | 0.24 |
| Renal | 193 (17.0) | 138 (13.2) | 0.01 |
| Liver | 8 (0.7) | 4 (0.4) | 0.3 |
| Neurologic | 64 (5.6) | 50 (4.8) | 0.36 |
| Coagulation | 18 (1.6) | 7 (0.7) | 0.04 |
Immune response
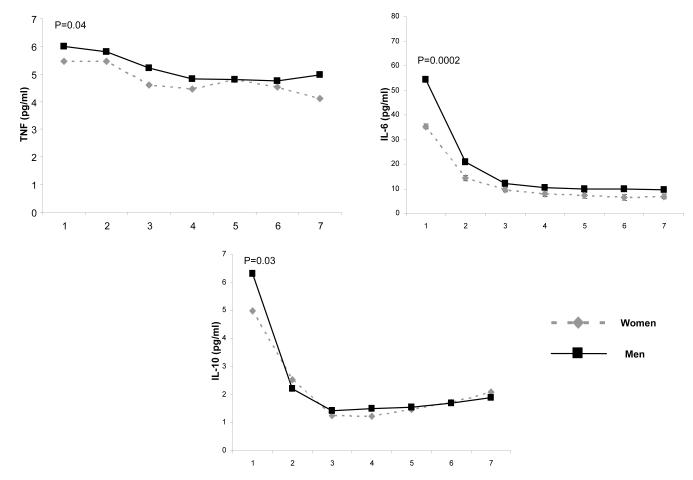
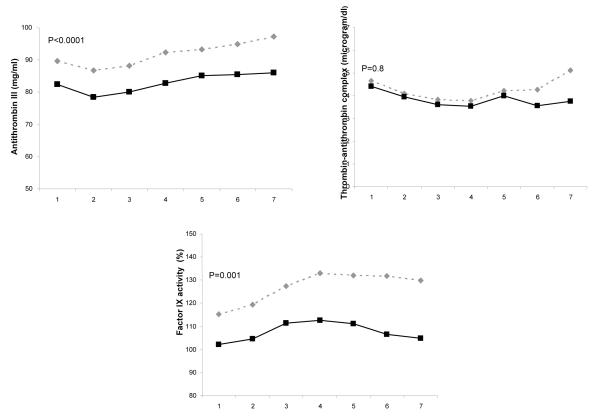
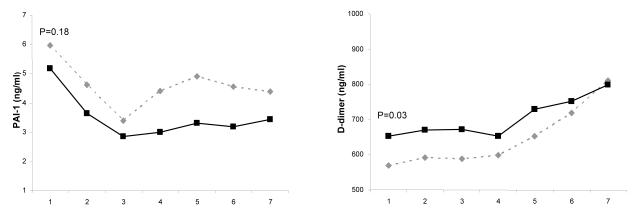
On presentation to ED, inflammatory biomarkers, including TNF, IL-6, and IL-10, were higher among men compared to women (Figure 1). Coagulation biomarkers, such as antithrombin-III and Factor IX, were lower among men, but no differences were seen in TAT complexes. Fibrinolysis biomarkers, such as D-dimer concentrations, were higher among men, but PAI-1 concentrations were similar for both groups. On ED presentation, men had lower platelet count compared to women. The median (interquartile range) platelet count, expressed as 103 cells/mm3, for men and women were 224 (169-285) and 264.5 (205-339) (p=0.005). These differences remained significant when adjusted for age, race, Charlson score, insurance and smoking status (Figure 1).
Figure 1.
Comparison of host response to infection over the initial seven days after presentation to the emergency department for inflammatory, coagulation, and fibrinolysis markers in 1427, 734, and 734 men and women, respectively. Panel A shows inflammatory biomarkers (TNF, IL-6, and IL-10). Data were missing in five subjects for TNF, three subjects for IL-6, and nine subjects for IL-10. Panel B shows coagulation biomarkers (Factor VII, antithrombin III, and thrombin-antithrombin (TAT) complexes). Data were missing in two subjects for factor VII and four subjects for TAT complexes. Panel C shows biomarkers of fibrinolysis (plasminogen activator (PAI)-1 and D-dimer). Data were missing in one subject for PAI-1 and two subjects for D-dimer. P values are shown for differences in day 1 concentration for each biomarker and adjusted for age, race, Charlson score, insurance and smoking status.
Quality and intensity of care
Compared to women, men were more likely to receive antibiotics within four and eight hours after presentation to the ED, but results were statistically significant in the univariate analyses only at eight hours (Table 3). This association remained significant when adjusted for age, race, Charlson score, insurance status, physiology components of the APACHE III score, and hospital characteristics (teaching status and bed size) (p=0.01). Similar trends were seen when analyses were stratified by PSI scores (Table 3). No sex differences were found in compliance of antibiotic therapy with ATS guidelines.
Table 3.
Indices of quality and intensity of care
| Variable | All subjects n=2183 |
Men n=1136 |
Women n=1047 |
P value |
|---|---|---|---|---|
| Quality of care | ||||
| Antibiotics adequate as per American Thoracic Society guidelines, n (%) |
1698 (77.8) | 892 (78.5) | 806 (77.0) | 0.39 |
| Received antibiotics within 4 hours, n (%) |
||||
| All subjects | 1580 (72.4) | 840 (73.9) | 740 (70.7) | 0.09 |
| PSI I-III | 755 (69.8) | 355 (72.3) | 400 (67.7) | 0.10 |
| PSI IV, V | 825 (74.9) | 485 (75.2) | 340 (74.6) | 0.81 |
| Received antibiotics within 8 hours, n (%) |
||||
| All subjects | 1982 (90.8) | 1052 (92.6) | 930 (88.9) | 0.002 |
| PSI I-III | 963 (89) | 446 (90.8) | 517 (87.5) | 0.08 |
| PSI IV, V | 1019 (92.6) | 606 (94) | 413 (90.6) | 0.04 |
| Intensity of care | ||||
| Admitted to hospital from emergency department, n (%) |
1895 (86.8) | 985 (86.7) | 910 (87) | 0.89 |
| Code status was changed to ‘do not resuscitate’ n (%) |
76 (3.7%) | 37 (3.4%) | 39 (3.9%) | 0.54 |
| Made ‘comfort measures only’, n (% ) |
57 (2.6%) | 31 (2.7%) | 26 (2.5%) | 0.72 |
| Admitted to ICU from emergency department, n (%) |
74 (3.4%) | 50 (4.4%) | 24 (2.3%) | 0.007 |
| Admitted to ICU during hospital stay, n (%) |
303 (13.9%) | 168 (14.8%) | 135 (12.9%) | 0.20 |
| Interventions during ICU stay | ||||
| Pulmonary artery catheter at any stage, n (%) |
23 (7.8%) | 14 (8.3%) | 9 (6.7%) | 0.59 |
| Mechanically ventilated at any stage, n (%) |
123 (40.6%) | 70 (41.7%) | 53 (39.3%) | 0.67 |
| Made ‘comfort measures only’, n (%) |
26 (8.9%) | 15 (8.9%) | 11 (8.2%) | 0.81 |
With one exception, no sex-related differences in the intensity of hospital care were observed. Equivalent proportions of men and women were admitted to hospital after evaluation in ED (Table 3). Men were almost twice as likely to be initially admitted to the ICU after evaluation in ED (4.4% vs. 2.2%, p=0.007 for men and women), perhaps due to the observed higher illness severity among men. However, no differences were seen in ICU utilization during hospital stay. Once admitted to the ICU, no differences were seen in the use of pulmonary artery catheters or mechanical ventilation. Use of comfort measures or ‘do not resuscitate’ orders were also similar for both groups.
Survival
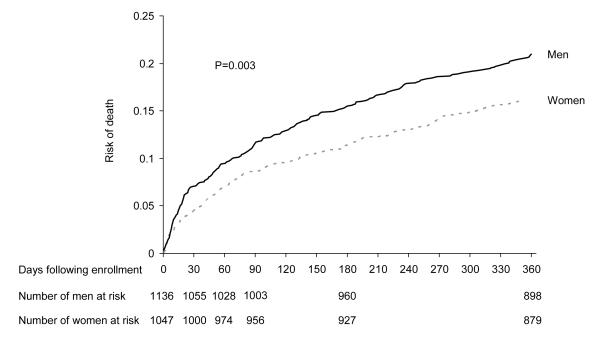
Men had higher risk of death at earlier time points, such as 30-days (7% vs. 4.5%, p=0.01) and 90-days (11.4% vs. 8.6%, p=0.02), and at one year (21% vs. 16%, p=0.002). The HRs were similar over ten consecutive time intervals over one year (data not shown). Men had higher risk of death compared to women over one year (hazards ratio (HR) = 1.35, 95% CI = 1.11-1.65, p=0.003) (Figure 2). The HR remained unchanged and the association was significant when adjusted for age, race, smoking, resuscitation, insurance, and vaccination status, Charlson comorbidity index, and physiology components of the APACHE III score (adjusted HR=1.29, 95% CI = 1.05-1.59, p=0.004). Hospital site or hospital characteristics were not significant predictors in any model and the addition of these variables to the models did not change the risk estimates (data not shown).
Figure 2.
Failure plots showing higher risk of death for men compared to women over one year following hospitalization for community acquired pneumonia. P=0.003 using log-rank test.
We analyzed risk of death stratified by severe sepsis occurrence, age (younger and older than 50 years), smoking status, and Charlson score (Table 4). For each analysis, the stratified ORs of death at one year were similar to those seen in the full analyses, suggesting that severe sepsis occurrence, age, smoking status, and chronic health conditions did not influence the association between sex and risk of death.
Table 4.
Odds of death (men vs. women) at one year stratified by severe sepsis occurrence, age, smoking status, and chronic health conditions*
| Variable | Odds ratio | 95% CI | P value |
|---|---|---|---|
| All subjects (n=2183) | 1.39 | 1.12 – 1.73 | 0.003 |
| Severe sepsis | |||
| Wit h severe sepsis (n=588) | 1.16 | 0.82 – 1.62 | 0.41 |
| Without severe sepsis (n=1658) | 1.42 | 1.13 – 1.79 | 0.003 |
| Age | |||
| ≤50 years (n=525) | 1.05 | 0.5 – 2.4 | 0.91 |
| >50 years (n=1658) | 1.42 | 1.13 – 1.79 | 0.003 |
| Smoking | |||
| Never smoked (n=768) | 1.2 | 0.83-1.73 | 0.35 |
| Ever smoked (n=1415) | 1.5 | 1.13-2 | 0.004 |
| Chronic health conditions | |||
| Charlson score = 0 or no chronic health condition (n=702) |
1.64 | 0.96 – 2.8 | 0.07 |
| Charlson score > 0 or at least one chronic health condition (n=1481) |
1.29 | 1.01 – 1.64 | 0.04 |
p value for interaction between sex and each of these variables for one-year mortality was not significant
Causes of death were different between men and women (p=0.005) (Table 5). Men were more likely to die due to cardiovascular disease (32.2% vs. 24.4%) and cancer (26.8% vs. 17.1%) and women were more likely to die due to chronic lower respiratory disease (20.7% vs. 11%).
Table 5.
Causes of death*
| Causes of death | Men (n, %) | Women (n, %) | P value |
|---|---|---|---|
| Cardiovascular | 73 (32.2) | 40 (24.4) | 0.005 |
| Cancer | 61 (26.8) | 28 (17.1) | |
| Infection | 34 (15) | 25 (15.2) | |
| Chronic lower respiratory disease |
25 (11) | 34 (20.7) | |
| Renal failure | 14 (6.2) | 10 (6.1) | |
| Others | 20 (8.8) | 27 (16.5) |
Causes of death were missing in 15 subjects
Immune response and sex-differences in survival
For biomarkers that differed between men and women in the ED, patterns of circulating concentrations of these biomarkers were associated with survival at different time points. Lower concentrations of factor IX were associated with lower survival over 30 days; higher concentrations of IL-6 and IL-10 were associated with lower survival over 180 days; and higher TNF and D-dimer and lower antithrombin-III were associated with lower survival over one year (P<0.05, data not shown). Compared to the HR (men vs. women) estimated in the unadjusted model and the model adjusted for demographics, health behaviors, comorbidities, and illness severity, the HR in the model additionally adjusted for biomarkers that differed between men and women were no longer statistically significant (adjusted HR=1.27, 95% CI=0.9-1.8, p=0.17). Table 6 shows ED concentrations among survivors and non-survivors adjusted by sex. In this adjusted analysis, the magnitude of difference was similar between men and women in the groups who did and did not survive. However, the patterns of TNF, IL-6, IL-10, D-dimer, antithrombin-III, and Factor IX observed among men were associated with worse survival.
Table 6.
Selected inflammatory, coagulation, and fibrinolysis biomarkers concentration in emergency department among men and women, adjusted by survivor status*
| Marker | Non-Survivors |
Survivors |
||
|---|---|---|---|---|
| Men | Women | Men | Women | |
| Survival at 30 days | ||||
| Factor IX (%) | 103.85 | 117.21 | 103.44 | 116.74 |
| Survival at 180 days | ||||
| Interleukin-6 (pg/ml) | 74.94 | 54.05 | 40.44 | 28.78 |
| Interleukin-10 (pg/ml) | 8.33 | 7.17 | 5.15 | 4.43 |
| Survival at one year | ||||
| TNF (pg/ml) | 6.55 | 5.98 | 5.31 | 4.85 |
| Antithrombin-III (mg/ml) | 81.45 | 87.35 | 84.77 | 90.92 |
| D-dimer (ng/ml) | 888.91 | 837.14 | 502.7 | 473.42 |
Using a Gray’s model, we showed that concentrations of different biomarkers were associated with survival over different time periods. ED concentrations of factor IX were associated with survival over 30-days, IL-6 and IL-10 with survival over 180-days, and TNF, antithrombin-III and D-dimer with survival over one year. Table shows concentrations of these biomarkers among survivors and non-survivors at the corresponding time-points, stratified by sex.
DISCUSSION
In a large, multicenter cohort of older adults, we found that men with CAP were less likely to survive after an infection compared to women. This difference was not explained by differences in demographics, health behavior, chronic health conditions, and quality of care. Biologic response to CAP was different among men and women, with higher pro (TNF, IL-6) and anti- (IL-10) inflammatory biomarkers, decreased circulating concentrations of coagulation factors, including antithrombin III and Factor IX, and increased D-dimer, suggesting greater fibrinolysis among men. Although the magnitude of differences in biomarker concentrations were similar between survivors and non-survivors among men and women, the patterns of inflammatory, coagulation, and fibrinolysis markers among men were more likely to be associated with worse outcomes in our analyses and prior studies (28, 30). To our knowledge, this study is the largest study comparing biologic response to infection between men and women. Our results suggest that immune response to infection is an important target for interventions to reduce sex disparities in the outcome of infection.
Prior studies suggest potential reasons for lower life expectancy of men include higher prevalence of risk taking behavior and higher burden of chronic diseases. In our study men were more likely to smoke and had higher burden of chronic health conditions. Yet, the higher hazard ratio for men was not attenuated and remained statistically significant when adjusted for these risk factors. Men presenting with CAP of an equivalent illness severity to women were also more likely to die, as the survival difference was not explained by adjustment for physiological derangements on presentation. Furthermore, sex-related differences in survival following CAP occurred at earlier time points, during the acute illness, and these differences persisted over one year, when subjects appeared to have recovered from the acute illness. Prior studies showing increased susceptibility to infection for men (1) and our results demonstrating worse long-term survival following an acute infection have important implications for the understanding of sex-related differences in life expectancy.
A key finding of our study is that the inflammatory, coagulation, and fibrinolysis responses to infection on presentation to the ED differ between men and women and independent of age, chronic health, and smoking status. There are several possible explanations for the differences observed. First, sex hormones have been suggested to play a role, but are less likely to be important because most women in our study were post menopausal and the pattern of immune response in our study is different from those reported in proestrous animals. However, a recent study by May et al. (33) suggested that estradiol concentrations are higher in older women compared to men during critical illness. We did not measure levels of sex hormones in our study. If sex hormones do play a role in older adults, we speculate that they may modify immune response when subjects are exposed to infection and prior to hospitalization. Second, the pattern of immune response may reflect the higher severity of illness we observed among men. Third, there is an increasingly recognized interaction between acute infection and chronic disease (34), implying differences in immune response could occur because men had more chronic health conditions. Fourth, microbiologic factors, such as qualitative or quantitative differences in infection burden due to smoking, may lead to higher inflammatory and lower coagulation biomarkers. Finally, cellular mosaics in women due to differential inactivation of the paternal and maternal X-chromosomes in different cells and genes related to inflammation present on them, such as interleukin-1 receptor-associated kinases (IRAK)-1, may influence immune response (18, 35, 36).
In addition to examining differences in survival and immune response, we also examined differences in severity of illness and quality of care. We showed small differences in the severity of illness between men and women at presentation to the ED. Men presented approximately half a day later after the onset of symptoms and were less likely to receive antibiotics prior to presenting to the ED. Whether these differences reflect patient preferences or occurred because lower proportion of men had health insurance, and if these differences account for higher severity of illness on presentation to the ED is less clear. We also showed a small difference in quality of care. Men were more likely to receive antibiotics within eight hours despite adjusting for hospital characteristics and severity of illness. While we could not assess if antibiotic therapy was delayed due to differences in clinical presentation of CAP, if anything, this difference would favor a better outcome for men.
Our study had several strengths. First, our finding of lower male survival was independent of hospital characteristics and observed in 28 academic and community hospitals in four geographic regions of the US. Second, we adjusted for several factors that could confound this association, including risk factors in the demographic, chronic health, and health behavior domains. Furthermore, the higher risk of death persisted when we adjusted for differences in the initial severity of illness between men and women. We showed that the higher risk of death for men was similar in subjects with and without severe sepsis and those with and without chronic health conditions. Although we cannot exclude residual confounding due to sub-clinical disease, these risk factors are likely to be more prevalent among women (37). Third, by considering subjects with CAP alone, we avoided confounding associated with different types of infection. Our results differ from prior cohort studies that recruited subjects following surgery and trauma (8, 10, 11). Compared to our cohort, these subjects have different characteristics, including differences in age, health behaviors, and chronic health conditions. These studies often did not adjust for differences in illness severity. Furthermore, they examined outcomes of hospital- in contrast to community-acquired infection, with different etiologic agents and predisposing factors.
Our study has limitations. First, we cannot ascribe cause-effect relationship to specific biomarkers and mediators upstream in the immune response could explain these differences. Second, we measured a broad panel of biomarkers but several other biomarkers could play an important role in explaining sex differences in outcomes of CAP. Finally, our results cannot be generalized to non-infectious conditions or other infections since sex differences could manifest differently with different infections.
In summary, we were unable to explain the higher male mortality from CAP on the basis of differences in clinical characteristics or quality of care. We observed different patterns of activation of the inflammatory, coagulation and fibrinolysis systems between men and women and these patterns were associated with short and long-term survival differences between men and women.
Acknowledgments
Support: GenIMS was funded by NIGMS R01 GM61992 with additional support from GlaxoSmithKline for enrollment and clinical data collection, Diagnostic Products Corporation for the cytokine assays.
REFERENCES
- 1.Klein SL. The effects of hormones on sex differences in infection: from genes to behavior. Neurosci Biobehav Rev. 2000;24:627–638. doi: 10.1016/s0149-7634(00)00027-0. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Merrill CT, Elixhauser A. Hospitalization in the United States. Agency for Healthcare Research and Quality; Rockville, MD: 2002. HCUP Fact book no 6. AHRQ Publication No. 05-0056. [Google Scholar]
- 4.National Center for Health Statistics [Accessed on 1st February 2008]; www.cdc.gov/nchs.
- 5.Schroder J, Kahlke V, Staubach KH, et al. Gender differences in human sepsis. Arch Surg. 1998;133:1200–1205. doi: 10.1001/archsurg.133.11.1200. [DOI] [PubMed] [Google Scholar]
- 6.Raine R, Goldfrad C, Rowan K, et al. Influence of patient gender on admission to intensive care. J Epidemiol Community Health. 2002;56:418–423. doi: 10.1136/jech.56.6.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaplan V, Angus DC, Griffin MF, et al. Hospitalized community-acquired pneumonia in the elderly: age- and sex-related patterns of care and outcome in the United States. Am J Respir Crit Care Med. 2002;65:766–772. doi: 10.1164/ajrccm.165.6.2103038. [DOI] [PubMed] [Google Scholar]
- 8.Crabtree TD, Pelletier SJ, Gleason TG, et al. Gender-dependent differences in outcome after the treatment of infection in hospitalized patients. JAMA. 1999;282:2143–2148. doi: 10.1001/jama.282.22.2143. [DOI] [PubMed] [Google Scholar]
- 9.Dombrovskiy VY, Martin AA, Sunderram J, et al. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 10.Eachempati SR, Hydo L, Barie PS. Gender-based differences in outcome in patients with sepsis. Arch Surg. 1999;134:1342–1347. doi: 10.1001/archsurg.134.12.1342. [DOI] [PubMed] [Google Scholar]
- 11.Croce MA, Fabian TC, Malhotra AK, et al. Does gender difference influence outcome? J Trauma. 2002;53:889–894. doi: 10.1097/00005373-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 13.Mokart D, Leone M, Sannini A, et al. Predictive perioperative factors for developing severe sepsis after major surgery. Br J Anaesth. 2005;95:776–781. doi: 10.1093/bja/aei257. [DOI] [PubMed] [Google Scholar]
- 14.Frink M, Pape HC, van Griensven M, et al. Influence of sex and age on mods and cytokines after multiple injuries. Shock. 2007;27:151–156. doi: 10.1097/01.shk.0000239767.64786.de. [DOI] [PubMed] [Google Scholar]
- 15.Offner PJ, Moore EE, Biffl WL. Male gender is a risk factor for major infections after surgery. Arch Surg. 1999;134:935–938. doi: 10.1001/archsurg.134.9.935. [DOI] [PubMed] [Google Scholar]
- 16.Rello J, Ollendorf DA, Oster G, et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122:2115–2121. doi: 10.1378/chest.122.6.2115. [DOI] [PubMed] [Google Scholar]
- 17.O’Keefe GE, Hunt JL, Purdue GF. An evaluation of risk factors for mortality after burn trauma and the identification of gender-dependent differences in outcomes. J Am Coll Surg. 2001;192:153–160. doi: 10.1016/s1072-7515(00)00785-7. [DOI] [PubMed] [Google Scholar]
- 18.Sperry JL, Minei JP. Gender dimorphism following injury: making the connection from bench to bedside. J Leukoc Biol. 2008 March;83(3):499–506. doi: 10.1189/jlb.0607360. [DOI] [PubMed] [Google Scholar]
- 19.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community acquired pneumonia. N Engl J Med. 1997;336:243–50. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 22.Levy MM, Fink M, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 23.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 24.Medicare Quality Monitoring System [Accessed 1st February 2008]; http://www.cms.hhs.gov/QualityInitiativesGenInfo/15_MQMS.asp.
- 25.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–72. S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Death Index [Accessed 1st Frebruary 2008]; http://www cdc gov/nchs/ndi htm.
- 27.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140:1016–1019. doi: 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]
- 28.Kinasewitz GT, Yan SB, Basson B, et al. Universal changes in biomarkers of coagulation and inflammation occur in patients with severe sepsis, regardless of causative micro-organism. Crit Care. 2004;8:R82–R90. doi: 10.1186/cc2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinsky MR, Vincent JL, Deviere J, et al. Serum cytokine levels in human septic shock. Relation to multiple-system organ failure and mortality. Chest. 1993;03:565–575. doi: 10.1378/chest.103.2.565. [DOI] [PubMed] [Google Scholar]
- 30.Kellum JA, Kong L, Fink MP, et al. Understanding the Inflammatory Cytokine Response in Pneumonia and Sepsis: Results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167:1655–1663. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Epstein MP, Lin X, Boehnke M. A tobit variance-component method for linkage analysis of censored trait data. Am J Hum Genet. 2003;72:611–620. doi: 10.1086/367924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasal J, Jovanovic Z, Clermont G, et al. Comparison of Cox and Gray’s survival models in severe sepsis. Crit Care Med. 2004;32:700–707. doi: 10.1097/01.ccm.0000114819.37569.4b. [DOI] [PubMed] [Google Scholar]
- 33.May AK, Dossett LA, Norris PR, et al. Estradiol is associated with mortality in critically ill trauma and surgical patients. Crit Care Med. 2008;36:62–68. doi: 10.1097/01.CCM.0000292015.16171.6D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reade MC, Milbrandt EB, Angus DC. The impact of chronic disease on response to infection: more than just reduced physiological reserve? In: Vincent JL, editor. Yearbook of Intensive Care and Emergency Medicine. Springer-Verlag; Berlin: 2007. pp. 197–207. [Google Scholar]
- 35.Migeon BR. The role of X inactivation and cellular mosaicism in women’s health and sex-specific diseases. JAMA. 2006;295:1428–1433. doi: 10.1001/jama.295.12.1428. [DOI] [PubMed] [Google Scholar]
- 36.Arcaroli J, Silva E, Maloney JP, et al. Variant IRAK-1 Haplotype Is Associated with Increased Nuclear Factor-{kappa}B Activation and Worse Outcomes in Sepsis. Am J Respir Crit Care Med. 2006;173:1335–1341. doi: 10.1164/rccm.200603-341OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abe Y, Rundek T, Sciacca RR, et al. Ultrasound Assessment of Subclinical Cardiovascular Disease in a Community-Based Multiethnic Population and Comparison to the Framingham Score. Am J Cardiol. 2006;98:1374–1378. doi: 10.1016/j.amjcard.2006.06.034. [DOI] [PubMed] [Google Scholar]