Abstract
Eg5 is a plus end directed kinesin related motor protein (KRP) previously shown to be involved in the assembly and maintenance of the mitotic spindle. KRPs are molecular motors capable of generating forces upon MTs in dividing cells and driving structural rearrangements necessary in the developing spindle. In vitro experiments demonstrate that loss of Eg5 results in cell cycle arrest and defective centrosome separation resulting in the development of monopolar spindles. Here we describe mice with a genetrap insertion in Eg5. Heterozygous mutant mice appear phenotypically normal. In contrast, embryos homozygous for the Eg5 null allele recovered at embryonic days 2.5–3.5 display signs of a proliferation defect as reduced cell numbers and failure of compaction and progression to the blastocyst stage was observed. These data, in conjunction with previous in vitro data, suggest that loss of Eg5 results in abnormal spindle structure, cell cycle arrest and thereby reduced cell proliferation of early cleavage pre-implantation embryos. These observations further support the conclusion that Eg5 is essential for cell division early in mouse development, and that maternal contribution may sustain the embryo through the maternal to zygotic transition at which point supplies of functional Eg5 are exhausted, preventing further cell cleavage.
Introduction
The mitotic spindle is a highly organized and dynamic microtubule (MT) array that plays a pivotal role in mitosis and allows for the equal segregation of genetic material between two daughter nuclei. Spindle assembly requires the nucleation and cross-linking of cytoplasmic MTs into polar MT bundles radiating outward from the duplicated centrosomes, with minus ends focused at duplicated centrosome and plus ends radiating outward. Arrangement of MTs leads to separation and migration of centrosomes to opposing poles of the spindle and the formation of an antiparallel MT lattice between them. A number of MT motors have been shown to localize to the spindle and MT matrices, suggesting a role for the motors in producing the sliding forces that allow for centrosome migration and chromosomal segregation.
Cytoplasmic dynein and other motors from the kinesin-like protein family have been shown to generate MT sliding forces capable of driving centrosome separation and chromosome segregation, maintaining the forces that hold the mitotic spindle together, and controlling MT dynamics within the spindle. Originally identified in Xenopus laevis [1], Eg5 is a plus-end directed kinesin related protein (KRP) that associates with MTs of the mitotic spindle. A highly conserved N-terminal motor domain places Eg5 in the kinesin-5 family of KRPs, which includes cut7 from Schizosaccharomyces pombe [2], Kip1p and Cin8p from Saccharomyces cerevisiae [3, 4], KLP61F from Drosophila melanogaster [5], XIEg5 from Xenopus laveis [6], and HsEg5 from humans [7]. Eg5 functions as an anti-parallel homotetrameric structure, having two motor domains at opposing ends of a central stalk [6, 8], and is capable of binding and generating forces upon MTs of the mitotic spindle that can directly contribute to spindle assembly and elongation [9, 10]. Current studies demonstrate that Eg5 and related Kinesin-5 family members carry out similar functions and that mutations in this family of genes cause failure of centrosome separation, spindle assembly, and maintenance of a bipolar spindle [2, 3, 5–7, 11]. Additionally, Eg5 inhibition by immuno-depletion, RNA interference, and small molecule inhibitors, such as monastrol, results in mitotic arrest and monopolar spindles [3, 6, 7, 12–16].
In this work, we disrupted Eg5 to investigate its role in cell division and mammalian development in vivo. We show that mice lacking functional Eg5 are not viable and die early in embryogenesis at pre-implantation stages. Homozygous embryos arrested after 2.5 days post-coitum (dpc) showed abnormalities in early cellular proliferation of pre-implantation embryos. Given that loss of Eg5 in vitro results in monopolar spindles and cell cycle arrest, our data suggests that loss of Eg5 in vivo results in abnormal spindle structure, cell cycle arrest and thereby reduced cell proliferation of early cleavage pre-implantation embryos.
Results
Generation of Eg5 KO mice
To characterize the role of Eg5 in mouse development, mice lacking Eg5 were generated from a genetrapped embryonic stem cell line (ES cell, XE579, available from Baygenomics Genetrap Consortium) containing an insertion in the genomic locus of Eg5. Multiple highly chimeric mice (>70%) were generated upon injection of the genetrapped ES cells into recipient albino B6 (C57BL/6J-Tyrc-2J)) blastocysts. Chimeric males were crossed with WT 129Sv/Ev females and offspring were genotyped by PCR analysis and/or Southern blotting to identify germline transmission of the Eg5 KO allele. No phenotypic differences were observed between Eg5+/+ and Eg5+/− offspring.
To generate Eg5−/− mice, Eg5+/− males and females were intercrossed and 249 offspring were genotyped (Table 1). Out of 249 offspring, no viable Eg5−/− mice were detected. The ratio of heterozygous mice to wild-type mice was 1.8 to 1. These observations suggest that loss of endogenous Eg5 through genetrap targeting of Eg5 results in embryonic lethality.
Table 1. Genotyping and detection of genetrapped allele in mouse embryos.
No Eg5−/− embryos were detected by nested PCR genotyping between E7.5-P21. One partially resorbed embryo was detected at E 6.5.
Table 1. Genotypes of offspring from heterozygous matings
| Age | Number of Mice |
||||
|---|---|---|---|---|---|
| Wildtype | Heterozygous | Homozygous | * Other | Total | |
| P21 | 90 | 159 | 0 | 0 | 249 |
| E6.5–11.5 | 35 | 58 | 1 | 9 | 103 |
| E3.5 | 26 | 73 | 8 | 7 | 114 |
| E2.5 | 19 | 58 | 16 | 5 | 98 |
| E1.5 | 9 | 20 | 8 | 5 | 42 |
Embryos lost or genotype not determined
Disruption of the Eg5 locus in mice
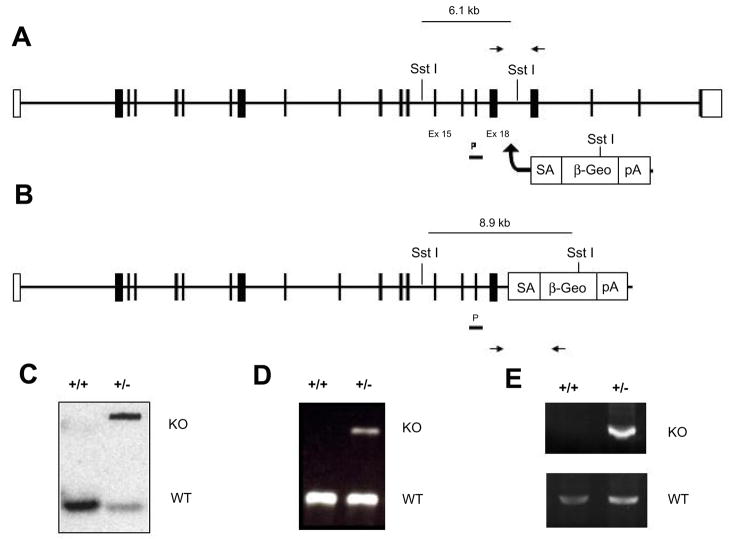
The genomic locus of Eg5 is located on mouse chromosome 19 with a non-expressed pseudogene located on mouse chromosome 6. BLAST analysis between Eg5 mRNA transcript sequence (EMBL:AJ223293) and bacterial artificial chromosome sequence (EMBL:AC137605) containing the genomic sequence, revealed that the gene contains 21 exons. Insertion of the genetrap vector within intron 18 results in deletion of a portion of the tail domain of Eg5 and generates a fusion transcript between Eg5 exon 18 and the β-galactosidase/neomycin gene (βGeo) contained within the genetrap vector (Figure 1). To confirm the transcription of truncated mRNA, RT-PCR was used to detect mutant mRNA which splices from exon 18 to the βGeo cassette using the cryptic splice site found in the En2 intronic sequence contained in the genetrap vector.
Figure 1. Generation and detection of Eg5 genetrapped KO allele.
Genetrapped ES cells with a genetrapping vector integrated in intronic sequence between exons 18 and 19 of the endogenous Eg5 locus (A). The genetrap vector consists of a portion of the engrailed 2 intronic sequence containing splice acceptor sequence, a Lac-Z/neomycin (βGeo) cassette, and a poly-adenylated tail. (B) Transcription of the genetrapped allele results in a truncated LacZ tagged fusion protein. Genotyping of genetrapped mice was performed by Southern and PCR analysis. (C) Integration of the genetrap vector in intron 18 introduces an alternate SstI restriction site detectable by southern analysis using a probe to exon 17. (D) PCR analysis detected both wildtype (225 bp) and genetrapped (327 bp) alleles in founder mice and offspring. (E) RT-PCR was used to detect both endogenous Eg5 and mutant Eg5-LacZ fusion transcripts.
The insertion is predicted to lead to a C-terminal truncation, resulting in deletion of 129 amino acids of the tail domain of Eg5. This deletion eliminates a portion of the tail domain which has been shown to be required for Eg5 localization and function in the spindle. Within the deleted tail region lies the BimC box, which in the human homologue has been shown to contain a cdc2 phosphorylation site (T937) involved in regulation of Eg5 activity[7, 17]. Eg5 normally functions as a bipolar homotetramer [18] in the mitotic spindle where it binds two antiparallel MTs emanating from opposing spindle poles and, with its plus end directed motor activity, is predicted to push these poles apart [9]. The truncation would additionally eliminate the Eg5 tail domain potentially required for formation of functional Eg5 homotetramers. Mutations in both the tail domain and cdc2 phosphorylation site introduced in vitro have been shown to independently inhibit endogenous Eg5 activity, and thereby prevent normal spindle formation and stability[17]. This suggests that a C-terminal truncation resulting from genetrap insertion, would result in a functionally inactive mutant form of Eg5 in mice.
Phenotype of Eg5−/− mice in early embryogenesis
To determine when the loss of Eg5 induces embryonic lethality, timed matings of heterozygous mice were used to generate embryos for analysis at different stages of development. From E7.5 to E11.5, 89 embryos were collected of which 80 appeared morphologically and developmentally normal. The remaining 9 embryos were resorbed and DNA could not be recovered for genotyping. PCR genotyping did not reveal any Eg5−/− embryos beyond E 6.5 (Table 1). Analysis of embryos collected at E6.5 revealed one embryo that was morphologically smaller and partially resorbed. Genotyping of this embryo identified the first Eg5−/− embryo, suggesting that embryonic lethality occurs much earlier in development.
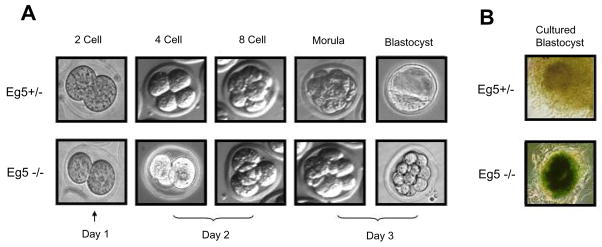
Analysis of embryos collected at E3.5 revealed a broad array of embryonic stages, ranging from the 4 cell stage to normal blastocyst stage embryos. PCR genotyping of these embryos suggest a proliferation defect due to loss of Eg5, as most pre-blastocyst stage embryos (<16 cell stage) were Eg5−/−. Embryos appear to have arrested prematurely as most E3.5 Eg5−/− embryos displayed fewer cell numbers, 8–16 with some a few as 4 blastomeres, and most had not undergone compaction (Figure 2A). Additionally, Eg5−/− numbers (7.5%) were still lower than the expected Mendelian ratios, suggesting that the effects of loss of Eg5 manifest earlier in development. Further analysis of embryos collected at E2.5 revealed an increase in the number of Eg5−/− embryos (16.8%), with a significant number of homozygous embryos displaying early proliferation defects as most ranged from 4–8 cell stages, compared to most wild-type embryos containing 8 to 16 cells. Embryos collected at E1.5 genotyped at near Mendelian ratios, and Eg5+/+, Eg5+/− and Eg5−/− embryos were morphologically indistinguishable (Table 1).
Figure 2. Loss of Eg5 results in early embryonic lethality due to a proliferation defect.
(A) Genotyping of E3.5 embryos revealed that Eg5−/− embryo numbers were reduced and appear to arrest at the 8 cell stage, most of which fail to undergo compaction. No Eg5−/− blastocyst stage embryos were detected at this stage of development. At E2.5 Eg5−/− embryos approached near Mendelian ratios and these embryos were observed to reach the 4–8 cell stage. The presence of homozygous embryos at E2.5 and reduced numbers at E3.5, in addition to failure to undergo compaction and progression to the blastocyst stage suggest a proliferation defect at the 4–8 cell stage resulting in the embryonic lethality associated with the loss of Eg5. (B) Cells of the inner cell mass stain positive for β-galactosidase in heterozygous embryos, as observed by LacZ staining of cultured blastocysts.
Lac-Z staining showed that Eg5 expression was broadly detected throughout heterozygous embryos collected at E10.5 (data not shown). Staining of cultured blastocysts, revealed broad staining of the inner cell mass (ICM) (Figure 2B). These observations demonstrate that genetrapped ES cells do contribute to the developing embryo and produce translatable messenger RNAs. Additionally, this embryonic lethality was rescued by transgenic mice expressing an Eg5 transgene (data not shown) removing any potential secondary effects due to position effect, additional genetraps, and/or mutations in these genetrapped ES cells.
Discussion
Here we report that murine Eg5 is essential for early developing embryos, as the loss of this MT motor causes early embryonic lethality in pre-implantation embryos. Genotype analysis of offspring from heterozygous intercrossing of mice containing a genetrapped allele of Eg5 yielded no homozygous deficient animals. Eg5-null embryos were collected from E1.5–3.5 with one resorbed embryo recovered at E6.5. Previous in vitro data demonstrating the necessity of Eg5 in assembly and maintenance of the mitotic spindle leads us to suggest that loss of Eg5 in the early cleavage embryo also prevents proper spindle assembly leading to mitotic arrest and a proliferation defect in the developing embryos. Our data have shown that Eg5−/− embryos collected at E2.5 show early signs of a cellular proliferation defect, as these embryos appear to arrest at the 4–8 cell stage. Twenty-four hours later a decrease in the number of Eg5−/− embryos is observed and these homozygous embryos have arrested at the 8 cell stage and fail to undergo compaction and progression to the blastocyst stage.
The key question regarding these observations lies in how embryos lacking a functional Eg5 allele are capable of undergoing early cell cleavages and surviving to E2.5 without Eg5 present for spindle assembly. One potential explanation that would allow an early cleavage embryo to undergo 3 to 4 cell divisions and then arrest lies in previous evidence linking maternal contribution of mRNAs and proteins to the developing embryo. An enrichment of Eg5 mRNA and protein levels is seen in Xenopus and murine oocytes. A burst of maternal mRNA and protein production is observed during the latter stages of oogenesis, and an additional surge is seen in oocyte maturation, when Eg5 mRNAs are poly-adenylated and translated [19–22]. Fully-grown mouse oocytes contain about 200 times more RNA and about 3000 times more ribosomes than somatic cells. Additionally, fully grown mouse oocytes contain about 50 to 60 times the protein levels found in mammalian liver cells [23]. Presumably these maternal products are required for early cell cycle progression and development, as transcription appears to be effectively repressed from meiotic maturation to early development and fertilization [24, 25]. Following fertilization, maternal protein levels decrease as the developing embryo proceeds through the early cleavage stages and maternal proteins are diluted amongst each new daughter cell. Additionally, maternal transcripts are degraded in preparation for zygotic genome activation (ZGA) [19].
Following fertilization, several key events occur throughout embryonic development, with one of the earliest described events being the maternal to zygotic transition (MZT) in which the developmental program is switched from maternally derived transcripts and proteins to zygotic-derived products. The key marker of this transition is the activation of the embryonic genome or zygotic genome activation. ZGA occurs globally in the mouse by the 2-cell stage and current data suggests that it is rather promiscuous, thereby establishing the unique profile of gene expression required for further embryonic development [26]. Parallel to ZGA, destruction of maternal mRNAs has also been observed at this stage of embryo development [19], and while most maternal mRNAs have been degraded by the 2–4 cell stage, this may not hold true for maternal proteins. The activities of several maternally derived enzymes remain constant in developing embryos through the 8–16 cell stage [27, 28], with one particular enzyme, glucose phosphate isomerase, still present at the late blastula stage [27].
Based on these observations, it remains quite plausible that early embryonic translation of maternal mRNA transcripts may allow for sufficient pools of Eg5 molecules to exist so that early rounds of mitosis may proceed through to the 4–8 cell stage and potentially even to the 16 cell stage. Upon degradation of these maternal transcripts, additional cellular divisions would necessitate ZGA and production of embryonic sources of Eg5. Thus, in our genetrapped mice, homozygous null embryos would not produce functional Eg5 monomers upon ZGA thereby triggering mitotic arrest due to loss of Eg5 function. As in vitro experiments have shown that loss of Eg5 results in monopolar spindles and mitotic arrest, proliferation defects observed in Eg5 null pre-implantation embryos can most likely be attributed to mitotic arrest due to spindle defects associated with the loss of this crucial component of spindle assembly and maintenance.
As a member of the kinesin-5 superfamily, Eg5 is nearly universally required for proper spindle development and recent targeting for the development of new chemotherapeutic treatments raises significant interest in its basic function during cell division. A further understanding of its role in early embryonic development and basic cell biology is therefore crucial for targeting its inhibition in the clinic. The study of mitotic spindle development and mitotic arrest in Eg5 null embryos will therefore be the subject of future investigations.
Materials and Methods
Generation of Eg5KO mice
Eg5 genetrapped embryonic stem cells (Baygenomics ES cell line XE579) were used to generate chimeric mice. Chimeric mice were produced by injection of genetrapped ES cells into albino B6 (C57BL/6J-Tyrc-2J)) blastocysts and germline transmission was established by backcrossing F1 offspring to wild-type 129S6/SvEvTac mice to obtain progeny. The genetrap allele was confirmed by Southern analysis. Southern blots containing SstI digested genomic DNA were probed with a 209 bp fragment of exon 17 generated by PCR amplification from wildtype genomic 129 DNA using the primers forward 5′-AAATTCTTGCTGAATCTGATGGA-3′ and reverse 5′-CTCTTGCTTAGGCAGGATGC-3′. Mouse lines were maintained by backcrossing to 129SvEv wild-type mice.
Genotyping of Eg5KO mice
PCR genotyping was performed on tails prepared by overnight digestion at 55 °C in lysis buffer (50 mM Tris-Cl [pH 8.0], 50 mM EDTA [pH 8.0], 100 mM NaCl, 0.5% SDS, 1 mg/ml proteinase K), phenol-chloroform extracted, and ethanol precipitated. DNA pellets were resuspended in 100 μl 1× Tris-EDTA and 2.5 μl of genomic DNA was used for PCR genotyping. Yolk sacs from E8.5–11.5 embryos were lysed at 95 °C in 100 μl of 50 mM NaOH for 30 min. Lysed yolk sacs were neutralized with 20 μl of 0.5 M Tris-Cl [pH 8.0] and 2.5 μl of genomic DNA was used for PCR genotyping. For E6.5–8.5 embryos, whole embryos were lysed at 95 °C in 100 μl of 50 mM NaOH for 30 min. Genomic DNA was genotyped by PCR amplification using the following primers:
Wildtype allele: Fwd 5′-GGCTTAGTGTCCGCAGTAGC-3′ and Rev 5′-GTGGCGCACACCTTTAATTT-3′ (225 bp PCR product); KO allele: Fwd 5′-GGCTTAGTGTCCGCAGTAGC-3′ and Rev 5′-AGAGGGACCTGGCTCCTATG-3′ (372 bp PCR product).
Genotyping of pre-implantation embryos was performed on whole embryos lysed at 95 °C in 20 μl of 50 mM NaOH for 30 min. Lysed embryos were neutralized with 4 μl of 0.5 M Tris-Cl [pH 8.0] and 5 μl of genomic DNA was genotyped by nested PCR amplification using the following primers:
Wildtype allele: Fwd 5′-GGCTTAGTGTCCGCAGTAGC-3′ and Rev 5′-GTGGCGCACACCTTTAATTT-3′; KO allele: Fwd 5′-GGCTTAGTGTCCGCAGTAGC-3′ and Rev 5′-GAGGGACCTGGCTCCTATG-3′ and nested primers - wildtype allele: Fwd 5′-AGGACCAGCACCACTTTCC-3′ and Rev 5′-GGAGGCAGTGAGTTTGAAGC-3′ and KO allele: Fwd 5′-ATGAACTTGCCCATCTCGAC-3′ and Rev 5′-AGACCTTGGGACCACCTCAT-3′
Culturing of pre-implantation embryos
Male and female mice were caged together overnight and plugs were checked the following morning. Fertilization was assumed to occur at midnight and embryos were staged accordingly (noon on the first day is termed E0.5). Blastocysts were flushed from the uteri at E3.5 and transferred to 24 well tissue culture plates containing blastocyst culturing media (Knockout Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Carlsbad, CA) containing 20% FBS, 2 mM L-glutamine, 0.1 mM 2-mercaptoethanol and 2% Penicillin/Streptomycin). Hatched embryos attached to the tissue culture dish and the ICM continued to proliferate, forming large cellular masses.
β-galactosidase staining of cultured pre-implantation embryos
Embryos collected at E3.5 were cultured for 3 days, fixed for 20 min in 4% paraformaldehyde, and stained in lacZ stain (10X stock – 5mM K4Fe(CN)6 ferrocyanide crystalline, K3Fe(CN)6 ferricyanide trihydrate, 2mM MgCl2, 0.02% sodium deoxcholate) containing 5 mM EGTA and 1 mg/ml X-Gal (5-bromo-4-chloro-3-indolyl-beta-D-galactopyranoside) overnight at 37 °C. E10.5 embryos were stained similarly with a longer fixation of embryos for 2hrs in 4% paraformaldehyde prior to staining.
Generation of mouse embryonic fibroblasts
Mouse embryonic fibroblasts (MEFs) were derived from E13.5 wild-type and Eg5+/− embryos. After removal of the head, liver, and gastrointestinal tract, each embryo was washed with phosphate-buffered saline and minced, and the tissue was placed in a 15-ml conical tube. After centrifugation, tissue pellets were resuspended in 1 ml of trypsin solution (0.25% trypsin, 0.005% EDTA) and the tissue was digested at 37 °C for 10 min. After pipetting several times, embryos were further digested with 1 ml of trypsin at 37 °C for 10 min. Trypsin was inactivated with DMEM (Invitrogen, Carlsbad, CA) containing 10% FBS, 2 mM L-glutamine, and 2% penicillin/streptomycin. Following pipetting several times, single cell suspensions were plated on 100mm tissue culture dishes and incubated at 37 °C for 2 to 3 days until cells reached confluency. These cultures were designated P0.
Transcript Analysis
Total RNA was extracted from tissues and MEFs using RNA STAT-60 (Tel-Test, Friendswood, TX). 1μg DNase free RNA was used for reverse transcription (RT) of cDNA using Superscript First-Strand Synthesis System and random hexamer RT primers (Invitrogen, Carlsbad, CA). Endogenous Eg5 transcripts were amplified using the following primers: Eg5 Ex17F: 5′-AAATTCTTGCTGAATCTGATGGA-3′ and Eg5 Ex20R: 5′-CCTCTGCTGGAAGAGCAATC-3′. Genetrapped fusion transcripts were amplified using a forward primer to the endogenous Eg5 locus and a reverse primer located within the βGeo cassette of the genetrap vector. Eg5 Ex17F1: 5′-AAATTCTTGCTGAATCTGATGGA-3′ and LacZ Rev: 5′-AAATTCAGACGGCAAACGAC-3′.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Le Guellec R, Paris J, Couturier A, Roghi C, Philippe M. Cloning by differential screening of a Xenopus cDNA that encodes a kinesin-related protein. Mol Cell Biol. 1991;11:3395–3398. doi: 10.1128/mcb.11.6.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hagan I, Yanagida M. Novel potential mitotic motor protein encoded by the fission yeast cut7+ gene. Nature. 1990;347:563. doi: 10.1038/347563a0. [DOI] [PubMed] [Google Scholar]
- 3.Hoyt MA, He L, Loo KK, Saunders WS. Two Saccharomyces cerevisiae kinesin-related gene products required for mitotic spindle assembly. J Cell Biol. 1992;118:109–120. doi: 10.1083/jcb.118.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roof DM, Meluh PB, Rose MD. Kinesin-related proteins required for assembly of the mitotic spindle. J Cell Biol. 1992;118:95–108. doi: 10.1083/jcb.118.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heck MM, Pereira A, Pesavento P, Yannoni Y, Spradling AC, Goldstein LS. The kinesin-like protein KLP61F is essential for mitosis in Drosophila. J Cell Biol. 1993;123:665–679. doi: 10.1083/jcb.123.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sawin KE, LeGuellec K, Philippe M, Mitchison TJ. Mitotic spindle organization by a plus-end-directed microtubule motor. Nature. 1992;359:540. doi: 10.1038/359540a0. [DOI] [PubMed] [Google Scholar]
- 7.Blangy A, Lane HA, d’Herin P, Harper M, Kress M, Nigg EA. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell. 1995;83:1159–1169. doi: 10.1016/0092-8674(95)90142-6. [DOI] [PubMed] [Google Scholar]
- 8.Kashina AS. A bipolar kinesin. Nature. 1996;379:270. doi: 10.1038/379270a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kapitein LC, Peterman EJG, Kwok BH, Kim JH, Kapoor TM, Schmidt CF. The bipolar mitotic kinesin Eg5 moves on both microtubules that it crosslinks. Nature. 2005;435:114. doi: 10.1038/nature03503. [DOI] [PubMed] [Google Scholar]
- 10.Kashina AS, Rogers GC, Scholey JM. The bimC family of kinesins: essential bipolar mitotic motors driving centrosome separation. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1997;1357:257. doi: 10.1016/s0167-4889(97)00037-2. [DOI] [PubMed] [Google Scholar]
- 11.Enos AP, Morris NR. Mutation of a gene that encodes a kinesin-like protein blocks nuclear division in A. nidulans. Cell. 1990;60:1019. doi: 10.1016/0092-8674(90)90350-n. [DOI] [PubMed] [Google Scholar]
- 12.Kapoor TM, Mayer TU, Coughlin ML, Mitchison TJ. Probing Spindle Assembly Mechanisms with Monastrol, a Small Molecule Inhibitor of the Mitotic Kinesin, Eg5. J Cell Biol. 2000;150:975–988. doi: 10.1083/jcb.150.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayer TU, Kapoor TM, Haggarty SJ, King RW, Schreiber SL, Mitchison TJ. Small Molecule Inhibitor of Mitotic Spindle Bipolarity Identified in a Phenotype-Based Screen. Science. 1999;286:971–974. doi: 10.1126/science.286.5441.971. [DOI] [PubMed] [Google Scholar]
- 14.Weil D, Garcon L, Harper M, Dumenil D, Dautry F, Kress M. Targeting the kinesin Eg5 to monitor siRNA transfection in mammalian cells. Biotechniques. 2002;33:1244–1248. doi: 10.2144/02336st01. [DOI] [PubMed] [Google Scholar]
- 15.Stout JR, Rizk RS, Kline SL, Walczak CE. Deciphering protein function during mitosis in PtK cells using RNAi. BMC Cell Biol. 2006;7:26. doi: 10.1186/1471-2121-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu C, Zhao J, Bibikova M, Leverson JD, Bossy-Wetzel E, Fan JB, Abraham RT, Jiang W. Functional Analysis of Human Microtubule-based Motor Proteins, the Kinesins and Dyneins, in Mitosis/Cytokinesis Using RNA Interference. Mol Biol Cell. 2005;16:3187–3199. doi: 10.1091/mbc.E05-02-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawin KE, Mitchison TJ. Mutations in the Kinesin-Like Protein Eg5 Disrupting Localization to the Mitotic Spindle. PNAS. 1995;92:4289–4293. doi: 10.1073/pnas.92.10.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kashlna AS, Baskin RJ, Cole DG, Wedaman KP, Saxton WM, Scholey JM. A bipolar kinesin. Nature. 1996;379:270. doi: 10.1038/379270a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winston N, Bourgain-Guglielmetti F, Ciemerych MA, Kubiak JZ, Senamaud-Beaufort C, Carrington M, Brechot C, Sobczak-Thepot J. Early Development of Mouse Embryos Null Mutant for the Cyclin A2 Gene Occurs in the Absence of Maternally Derived Cyclin A2 Gene Products. Developmental Biology. 2000;223:139. doi: 10.1006/dbio.2000.9721. [DOI] [PubMed] [Google Scholar]
- 20.Zeng F, Schultz RM. Gene Expression in Mouse Oocytes and Preimplantation Embryos: Use of Suppression Subtractive Hybridization to Identify Oocyte- and Embryo-Specific Genes. Biol Reprod. 2003;68:31–39. doi: 10.1095/biolreprod.102.007674. [DOI] [PubMed] [Google Scholar]
- 21.Houliston E, Le Guellec R, Kress M, Philippe M, Le Guellec K. The Kinesin-Related Protein Eg5 Associates with both Interphase and Spindle Microtubules during Xenopus Early Development. Developmental Biology. 1994;164:147. doi: 10.1006/dbio.1994.1187. [DOI] [PubMed] [Google Scholar]
- 22.Ferhat L, Cook C, Chauviere M, Harper M, Kress M, Lyons GE, Baas PW. Expression of the Mitotic Motor Protein Eg5 in Postmitotic Neurons: Implications for Neuronal Development. J Neurosci. 1998;18:7822–7835. doi: 10.1523/JNEUROSCI.18-19-07822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knobil E, Neill JD. The Physiology of reproduction. Vol. 1988;1:102–117. [Google Scholar]
- 24.Bolton VN, Oades PJ, Johnson MH. The relationship between cleavage, DNA replication, and gene expression in the mouse 2-cell embryo. J Embryol Exp Morphol. 1984;79:139–163. [PubMed] [Google Scholar]
- 25.Wasserman P. The Physiology of Reproduction. Raven; New York: 1988. [Google Scholar]
- 26.Ma J, Svoboda P, Schultz RM, Stein P. Regulation of Zygotic Gene Activation in the Preimplantation Mouse Embryo: Global Activation and Repression of Gene Expression. Biol Reprod. 2001;64:1713–1721. doi: 10.1095/biolreprod64.6.1713. [DOI] [PubMed] [Google Scholar]
- 27.Johnson MH. The molecular and cellular basis of preimplantation mouse development. Biol Rev Camb Philos Soc. 1981;56:463–498. doi: 10.1111/j.1469-185x.1981.tb00356.x. [DOI] [PubMed] [Google Scholar]
- 28.Magnuson T, Epstein CJ. Genetic control of very early mammalian development. Biol Rev Camb Philos Soc. 1981;56:369–408. doi: 10.1111/j.1469-185x.1981.tb00354.x. [DOI] [PubMed] [Google Scholar]