Abstract
Small-vessel vasculitis (SVV) is a chronic autoinflammatory condition linked to antineutrophil cytoplasm autoantibodies (ANCAs). Here we show that chromatin fibers, so-called neutrophil extracellular traps (NETs), are released by ANCA-stimulated neutrophils and contain the targeted autoantigens proteinase-3 (PR3) and myeloperoxidase (MPO). Deposition of NETs in inflamed kidneys and circulating MPO-DNA complexes suggest that NET formation triggers vasculitis and promotes the autoimmune response against neutrophil components in individuals with SVV.
SVV is a relapsing-remitting autoinflammatory disorder leading to necrotic inflammation of small-sized blood vessels and capillaries1. ANCAs directed against granule proteins of neutrophils, namely against PR3 in Wegener's granulomatosis and MPO in microscopic polyangiitis, are implicated in the pathogenesis of SVV2. In vitro studies have demonstrated an activating effect of ANCAs on cytokine-primed neutrophils3, which was further corroborated by animal models of these diseases4,5. However, the basic mechanism that induces the life-threatening exacerbations of vasculitis and the sustained autoimmune response against neutrophil components remains elusive.
A unique type of cell death of neutrophil granulocytes has recently been discovered that is characterized by the active release of chromatin fibers, so-called NETs, that trap and kill invading microbes extracellularly6. However, this glutinous DNA web can also stick to the endothelium and cause tissue damage during sepsis7, similar to neutrophil-induced inflammation of capillaries in SVV. The stimuli leading to NETs can be of varying nature but are always dependent on the respiratory burst of neutrophils8.
As ANCA can activate the respiratory burst by binding to PR3 or MPO on the neutrophil surface3, we examined whether ANCA-mediated activation of neutrophils induces NET formation. We primed isolated neutrophils with tumor necrosis factor-α and incubated them with purified IgG from individuals with SVV or healthy donors as performed previously3. We observed robust NET formation (as determined by immunofluorescence6,8; Supplementary Methods online) in neutrophils incubated with ANCA-IgG (Fig. 1a and Supplementary Table 1 online) but not in those incubated with control IgG, in which most nuclei retained the typical lobulated structure (Fig. 1b). After 180 min, we found that 23% of neutrophils incubated with ANCA-IgG produced NETs, compared to 11% of control IgG–treated neutrophils (Fig. 1c). Incubation with phorbol 12-myristate 13-acetate (PMA), known as a strong inducer of NETs, triggered NET production in 38% of all neutrophils (Fig. 1c). We also induced NETs with a PR3-specific mouse monoclonal antibody (Supplementary Fig. 1 online), supporting the hypothesis that PR3-specific autoantibodies within the ANCA-IgG fraction trigger NET formation. ANCA-induced cell death of neutrophils was previously regarded as a dysregulated form of apoptosis9, but the link to NETs had not been noticed. The morphological changes of neutrophil nuclei clearly indicated to us that ANCA-induced NETs were of nuclear rather than of mitochondrial origin, as recently described for activated eosinophils10.
Figure 1.
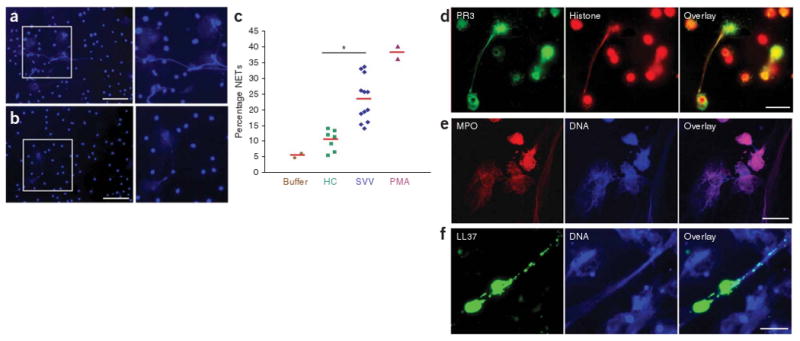
ANCA-induced formation of NETs containing autoantigens PR3 and MPO. (a,b) Fluorescence imaging of isolated tumor necrosis factor-α–primed neutrophils incubated with ANCA IgG (a) and control IgG (b) and stained for DNA. Scale bars, 50 μm. (c) Quantification of NET formation by fluorescence microscopy analysis. The percentage of cells with enlarged nuclei producing extracellular DNA fibers after 180 min incubation with buffer control, IgG from healthy controls (HC; n = 7), IgG from individuals with SVV (n = 12), or PMA as positive control is shown. Red horizontal bars indicate average percentage of each group. *P < 0.05. (d–f) Immunofluorescence analysis of autoantigens and immunostimulatory LL37 on PMA-induced NETs. (d) Immunofluorescence analysis of NETs stained with histone-specific antibody (red) and PR3-specific antibody (green). (e) Immunofluorescence analysis of NETs stained with MPO-specific antibody (red) and DNA stained with Hoechst dye (blue). (f) Immunofluorescence analysis of NET-forming neutrophils stained with LL37-specific antibody (green) and DNA stained with Hoechst dye (blue). Scale bars, 5 μm.
Next, we asked whether the targeted autoantigens, PR3 and MPO, are present on NETs. Immunofluorescence analysis of PMA-induced NETs revealed that both PR3 and MPO localized with the extracellular chromatin fibers (Fig. 1d,e). Notably, using an ELISA approach, we observed that PR3 bound DNA-coated wells, but not albumin-coated wells (Supplementary Fig. 2 online), indicating that not only MPO11 but also PR3 may directly interact with the DNA in NETs. Sera from people with SVV showed strong reactivity with NETs, showing that the epitopes targeted in vivo were still accessible on NETs (Supplementary Fig. 3 online). Moreover, we found that an antimicrobial peptide made by neutrophils, called LL37, was enriched on parts of the chromatin fibers of NETs (Fig. 1f). As shown recently, LL37 is key in converting self-DNA into an activator of plasmacytoid dendritic cells (pDCs) in psoriasis, an autoimmune disease of the skin12. Hence, extracellular NETs in conjunction with LL37 could have a pathogenic role in SVV.
Seeking in vivo evidence of NET formation, we analyzed a panel of kidney needle biopsies from subjects with SVV and acute deterioration of kidney function (n = 15) by in situ immunofluorescence microscopy. Indeed, we found the typical components of NETs, DNA in combination with histone and neutrophil granule proteins, located in close proximity to neutrophil infiltrates in affected glomeruli and in the interstitium (Fig. 2a–d and Supplementary Fig. 4 online). NETs were prominent in specimens with strong neutrophil infiltration (9 of 15 biopsies; Supplementary Table 2 online), suggesting that NET formation occurs predominantly during active disease. In situ NETs were decorated with the autoantigens MPO and PR3 (Fig. 2b,c) and were partially coated with the immunostimulatory peptide LL37 (Fig. 2d). DNA tangles of NETs contribute to the acute tissue damage of small blood vessels during sepsis7. Likewise, ANCA-induced NET formation in glomerular capillaries may be detrimental to the entire glomerulum, which is in agreement with the focal nature of rapid progressive glomerulonephritis in subjects with SVV. Moreover, the persistence of NETs in the tissue could be prolonged by actin, an inhibitor of DNase released by NET-forming neutrophils, or by as yet undetermined genetic defects of DNAse function in subjects with SVV.
Figure 2.
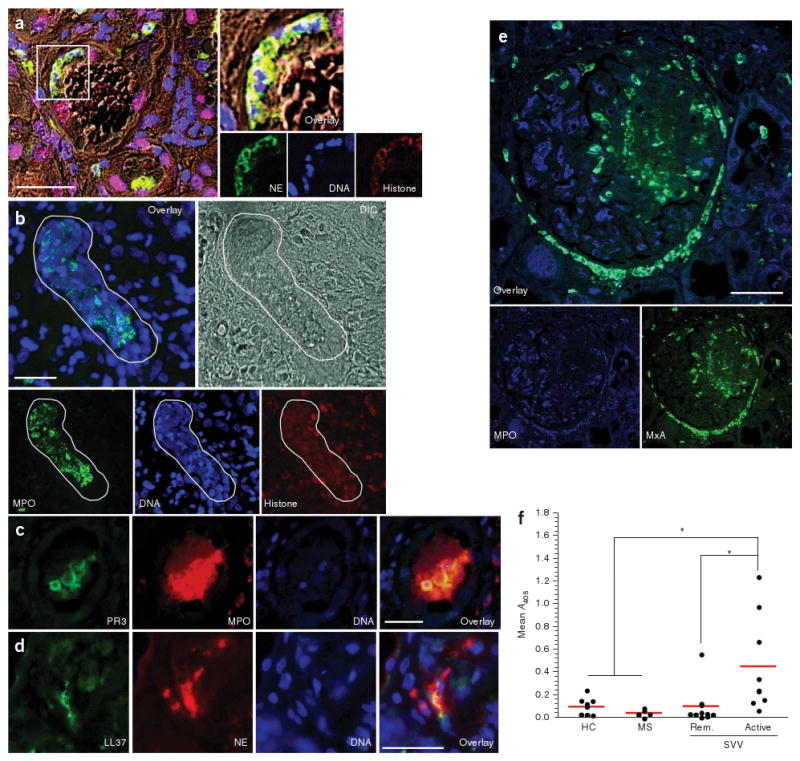
In vivo evidence for NET formation in individuals with SVV. (a–d) In situ immunofluorescence analysis of paraffin-embedded tissue sections of kidney needle biopsies from individuals with SVV glomerulonephritis. Tissue structure was visualized by differential interference contrast (DIC) microscopy. NETs were identified by colocalization of DNA, histone and neutrophil granule markers. (a) Colocalization of DNA (blue), histone (red) and neutrophil elastase (green) indicates intraglomerular NET formation. Blow up of boxed area shows NET deposition inside glomerular capsule. (b) Confocal immunofluorescence analysis showing large areas of colocalized DNA in blue, histone in red and MPO in green (defined by white line), indicating deposition of NETs that show autoantigen in the inflamed kidney of individuals with SVV. (c) Colocalization of MPO (red), PR3 (green) and DNA (blue), showing NET-autoantigen deposits inside the glomerulus. (d) Immunostaining of LL37 in kidney tissue of humans with SVV shows extracellularly located LL37 (green), DNA (blue) and NE (red), indicating NETs that are coated with LL37 and granular components. (e) MxA expression in close proximity to intra- and periglomerular neutrophil infiltrates. A glomerulus with extensive segmental necrosis and with pronounced extracellular MPO (green) deposition is shown. Numerous MxA (blue)-positive cells are scattered around MPO-positive neutrophil infiltrates and extracellular MPO deposits in the necrotic area. Tubular epithelia are partially MxA positive. Scale bars, 50 μm (a) and 25 μm (b-e). (f) Quantification of MPO-DNA complexes in the serum samples. The mean optical density as measured by capture ELISA using serum samples from healthy donors (n = 9), individuals with multiple sclerosis (MS; n = 5) and individuals with SVV in remission (Rem.; n = 10) and with active disease (n = 9) is shown; *P < 0.05. This study was approved by the local ethical committee of the Ludwig Maximilian University in Munich.
Autoinflammatory conditions such as psoriasis can be driven by pDCs, which produce large amounts of interferon-α (IFN-α) in the presence of DNA and LL37 (ref. 12). As these cells reside in close proximity to glomeruli and tubuli of the kidney and increase in number under pathological conditions13, we searched for functional signs of local IFN-α production, which results in the upregulation of cytosolic myxovirus resistance protein A (MxA) in surrounding tissue cells14. Indeed, we observed increased expression of MxA in six of ten biopsies in close proximity to intraglomerular neutrophil infiltrates, strongly suggesting that pDC activation and local production of IFN-α occur in active SVV lesions (Fig. 2e, Supplementary Fig. 5 and Supplementary Table 2 online). This was further supported by significantly (P < 0.01) increased concentrations of INF-α measured in serum samples from individuals with active SVV, but not in individuals with inactive SVV or in healthy controls (Supplementary Fig. 6 online).
Increased levels of circulating nucleosomes, which have previously been observed in ANCA-associated SVV15, may be a further hint for NET formation. Chromatin in NETs is most likely degraded by extracellular DNases to soluble nucleosomes, and these fragments should therefore appear in the plasma during active disease. Using MPO-specific capture and subsequent DNA-specific detection antibodies, we identified circulating MPO-DNA complexes, especially in individuals with active SVV, that were absent in control sera from healthy individuals or subjects with multiple sclerosis (Fig. 2f). We obtained similar results when we measured nucleosome-associated MPO activity (Supplementary Fig. 7 online). This indicated that circulating nucleosomes are, at least in part, derived from NET-forming (netting) neutrophils.
In summary, we show that NETs occur in an autoinflammatory disorder in the absence of microbial infection. These fibrous DNA deposits, containing the targeted autoantigens PR3 and MPO, are suited to activate pDCs and autoreactive B cells in a Toll-like receptor 9–dependent manner12,16. ANCAs may perpetuate a vicious circle of NET production that maintains the delivery of antigen-chromatin complexes to the immune system. The propensity of neutrophils to form NETs in individuals with SVV may be further enhanced by bacterial infections with Staphylococcus aureus, which is known to strongly induce NETs8 and which seems to be linked to relapses during SVV2. Further studies are required to address whether suppression of NET formation, for instance with reactive oxygen species scavengers8, can ameliorate vasculitis and abrogate chronic autoimmunity in individuals suffering from SVV.
Supplementary Material
Acknowledgments
We would like to thank W. Samtleben for providing serum samples, R. Bittner for technical assistance and E. Csernok and A. Kniepert for help with serum samples. Moreover, we are grateful to M. Sixt and E. Meinl for helpful suggestions and H. Wekerle for his constant interest and support of this work. We also thank M. Monestier (Temple University) for providing us with histone-specific monoclonal antibody and G. Koch (University of Freiburg) for the kind gift of the antibody specific for MxA. This work was funded by the Deutsche Forschungsgemeinschaft (SFB 571). It was further supported by the US National Institutes of Health (AI053194) and by fellowships from the Susan G. Komen for the Cure Foundation and the Alexander von Humboldt Foundation.
Footnotes
Note: Supplementary information is available on the Nature Medicine website.
Author Contributions: K.K. performed most of the experiments. M.K. performed IFN-α ELISA and contributed to data analysis and discussion. U.S., W.B. and W.L.G. provided patient material and clinical data. Z.W. provided critical input on data analysis. H.-J.G. provided kidney biopsies and clinical data. H.-J.G. and V.B. performed immunostainings and analysis of histology. K.K. and D.E.J. designed and supervised the study, analyzed data and wrote the manuscript. D.E.J. secured the funding. All authors discussed results and commented on the manuscript.
References
- 1.Jennette JC, Falk RJ. N Engl J Med. 1997;337:1512–1523. doi: 10.1056/NEJM199711203372106. [DOI] [PubMed] [Google Scholar]
- 2.Kallenberg CGM, Heeringa P, Stegeman CA. Nat Clin Pract Rheumatol. 2006;2:661–670. doi: 10.1038/ncprheum0355. [DOI] [PubMed] [Google Scholar]
- 3.Falk RJ, Terrell RS, Charles LA, Jennette JC. Proc Natl Acad Sci USA. 1990;87:4115–4119. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfister H, et al. Blood. 2004;104:1411–1418. doi: 10.1182/blood-2004-01-0267. [DOI] [PubMed] [Google Scholar]
- 5.Xiao H, et al. J Clin Invest. 2002;110:955–963. doi: 10.1172/JCI15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brinkmann V, et al. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 7.Clark SR, et al. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs TA, et al. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harper L, Ren Y, Savill J, Adu D, Savage COS. Am J Pathol. 2000;157:211–220. doi: 10.1016/S0002-9440(10)64532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yousefi S, et al. Nat Med. 2008;14:949–953. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 11.Murao S, Stevens FJ, Ito A, Huberman E. Proc Natl Acad Sci USA. 1988;85:1232–1236. doi: 10.1073/pnas.85.4.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lande R, et al. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 13.Woltman AM, et al. Kidney Int. 2007;71:1001–1008. doi: 10.1038/sj.ki.5002187. [DOI] [PubMed] [Google Scholar]
- 14.Wenzel J, et al. J Pathol. 2005;205:435–442. doi: 10.1002/path.1721. [DOI] [PubMed] [Google Scholar]
- 15.Holdenrieder S, et al. Ann NY Acad Sci. 2006;1075:318–327. doi: 10.1196/annals.1368.043. [DOI] [PubMed] [Google Scholar]
- 16.Leadbetter EA, et al. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


