Abstract
Purpose
To examine distributed patterns of language processing in healthy controls and patients with epilepsy using magnetoencephalography (MEG), and to evaluate the concordance between laterality of distributed MEG sources and language laterality as determined by the intracarotid amobarbitol procedure (IAP).
Methods
MEG was performed in ten healthy controls using an anatomically-constrained, noise-normalized distributed source solution (dSPM). Distributed source modeling of language was then applied to eight patients with intractable epilepsy. Average source strengths within temporoparietal and frontal lobe regions of interest (ROIs) were calculated and the laterality of activity within ROIs during discrete time windows was compared to results from the IAP.
Results
In healthy controls, dSPM revealed activity in visual cortex bilaterally from ~80-120ms in response to novel words and sensory control stimuli (i.e., false fonts). Activity then spread to fusiform cortex ~160-200ms, and was dominated by left hemisphere activity in response to novel words. From ~240-450ms, novel words produced activity that was left-lateralized in frontal and temporal lobe regions, including anterior and inferior temporal, temporal pole, and pars opercularis, as well as bilaterally in posterior superior temporal cortex. Analysis of patient data with dSPM demonstrated that from 350-450ms, laterality of temporoparietal sources agreed with the IAP 75% of the time, whereas laterality of frontal MEG sources agreed with the IAP in all eight patients.
Discussion
Our results reveal that dSPM can unveil the timing and spatial extent of language processes in patients with epilepsy and may enhance knowledge of language lateralization and localization for use in preoperative planning.
Keywords: MEG, language, dynamic statistical parametric mapping, epilepsy
INTRODUCTION
Magnetoencepholography (MEG), when combined with high-resolution MRI, is gaining widespread acceptance as a presurgical language mapping tool in the evaluation of patients with epilepsy (Papanicolaou et al., 1999, Bowyer et al., 2005a, Breier et al., 2005, Billingsley-Marshall et al., 2007, Lee et al., 2006, Papanicolaou et al., 2004). To date, most studies have focused on late activity (>200ms) to auditory or visual word stimuli that are presumed to reflect sustained N400 effects elicited during lexical-semantic processing (Halgren et al., 2002, Salmelin, 2007). The vast majority of these studies have used an equivalent current dipole (ECD) or other focal source solutions to lateralize N400 effects and have demonstrated a strong concordance between MEG language lateralization and the intracarotid amobarbitol procedure (IAP) (Bowyer et al., 2005b, Papanicolaou et al., 1999, Breier et al., 2000, Billingsley-Marshall et al., 2007, Maestu et al., 2002, Papanicolaou et al., 2004, Hirata et al., 2004). When focal source solutions are employed, N400 effects are reliably localized within left or right perisylvian cortex, centered on the superior and middle temporal gyri (Billingsley-Marshall et al., 2007), supramarginal gyrus, or within the superior temporal sulcus (Lee et al., 2006). Although each region is undoubtedly critical to language, it is well known that language processing is distributed in nature, simultaneously and sequentially recruiting multiple areas within temporal, frontal, and parietal cortex (Geschwind, 1970, Marinkovic, 2004). Therefore, ECD models, which assume that the magnetic field is generated by a focal source, will underestimate the regions involved in complex cognitive functions such as language.
To overcome limitations of focal source models, several distributed source solutions have been proposed (Dale et al., 2000, Bowyer et al., 2005a, Hamalainen and Ilmoniemi, 1994). In particular, Dale et al. have developed a distributed source solution that produces noise-normalized dynamic statistical parametric maps (dSPMs) of cortical activity similar to those generated for fMRI or PET (Dale et al., 2000), and may offer an advantage for studying localization of language processes. This noise-normalized dSPM method has been successfully used to unveil the cortical dynamics of language processing in healthy controls by comparing event-related fields (ERFs) evoked by novel versus repeating word stimuli (Halgren et al., 2002, Dhond et al., 2001, Dale et al., 2000, Dhond et al., 2003, Marinkovic et al., 2003). Despite its appeal, dSPM of language has yet to be applied to patients with epilepsy and compared to results obtained from the IAP.
In this study, we examine spatiotemporal patterns of language processing in healthy controls and patients with epilepsy using dSPM and a novel word-reading task designed to isolate language-specific processes. In patients with epilepsy, we compare language laterality results obtained from dSPM sources within temporoparietal and frontal lobe ROIs to language laterality results obtained from the IAP. We hypothesized that language-related activity would spread along a posterior to anterior gradient, becoming increasingly left-lateralized in healthy controls within temporoparietal and frontal lobe ROIs. We further hypothesized that laterality would be greatest from ~350-450ms (i.e., capturing peak N400 responses), and that laterality of N400 responses in these regions would show strong concordance with the IAP. If this method of source modeling shows the hypothesized pattern, it could enhance preoperative planning by increasing knowledge of language lateralization and localization in patients with epilepsy.
MATERIALS AND METHODS
Participants
Participants in this investigation were ten right-handed, healthy controls and eight patients with intractable epilepsy (seven right-handed, one left-handed) between the ages of 21-54. The study was approved by the Institutional Review Boards at the University of California, San Diego (UCSD) and New York University (NYU) and each subject's consent was obtained in accordance with the ethical standards promulgated in the Declaration of Helsinki. The control group consisted of seven males and three females with no known history of neurological disorder, loss of consciousness, or serious medical or psychiatric condition. All control data were collected at UCSD. The patients included four females and four males who were recruited from the UCSD and NYU Epilepsy Clinics and were undergoing presurgical evaluation at the time of enrollment in the study. IAP results were available for all eight patients and indicated left hemisphere language dominance in six patients, right language dominance in one, and bilateral language dominance in one patient. Patient characteristics, neuropsychological data, and IAP results are presented for all patients in Table 1.
Semantic Judgment Task
All participants completed the same semantic judgment task in which they were instructed to respond by lifting their finger in response to low-frequency target items (i.e., animals; see supplemental Figure). Task stimuli were presented visually as white letters on a black background in 12-point, Arial font. Stimuli consisted of 400 novel words that were presented only once, 400 “old” words (20 repetitions of 10 words), 400 consonant letter strings, 400 false font stimuli, and 40 target words. All real word stimuli were 4-8 letter nouns, with a written lexical frequency of 3-80 per 10 million (Francis and Kucera, 1982). The false font stimuli were comprised of alphabet-like characters that were matched in size and number of strokes to a real letter in the English alphabet; however, they did not resemble actual letters. In addition, false fonts were each matched to a novel word in the number of characters to visually control for the length of the stimulus. Therefore, they were designed to control for visual stimulus features, but not lexical, syntactic, or semantic content. The experimental task was organized into two separate lists, each consisting of 20 blocks, with approximately 42 stimuli per block. Each of the repeating words was presented once per block in approximately the same order, resulting in 20-30 seconds (~42 intervening stimuli) between presentations of a given repeated word. Novel words, letter strings, and false fonts were presented in random order, with approximately 10 of each per block. Data were collected using a rapid stimulus onset asynchrony (SOA; 600ms) and a very large number of trials per condition in order to obtain MEG data with a high signal-to-noise ratio (SNR) in a short time frame (~20 minutes), thereby maximizing the clinical utility of the task. Although this presentation rate is much faster than is customary, it is still much slower than typical word reading speed. The sequence of stimulus conditions was balanced to ensure that each condition was preceded by every other condition with equal likelihood. The task was programmed using Presentation software (Neurobehavioral Systems, Inc).
MEG recording
Prior to the MEG session, subjects were screened for MEG artifacts resulting from dental work or excessive eye-blinking. Magnetic fields at UCSD were recorded by an Elekta-Neuromag whole-head MEG system (Helsinki, Finland) with 204 planar dc-SQUID gradiometers and 102 magnetometers in a magnetically-shielded room (IMEDCO-AG, Switzerland) at the UCSD Radiology Imaging Laboratory. Magnetic fields at NYU were recorded by a whole-head Magnes 3600 WH MEG system with 248 radial magnetometers (4D Neuroimaging, San Diego) in a magnetically-shielded room. At both centers, pairs of EOG electrodes were used to detect eye blinks and movements. The translation between the MEG coordinate systems and each participant's structural MRI was made using three head position coils placed on the scalp and fiducial landmarks (Hamalainen, 1993). Signals were recorded continuously with 1000 Hz sampling rate and minimal on-line filtering (.1-200 Hz). Data were then low-pass filtered off-line at 20 Hz (transition band = 4 Hz) and downsampled by a factor of four before separate averages were created for each subject. Single trials were rejected based on amplitude criteria supplemented by visual inspection or any cases where responses were contaminated with eyeblinks (>280 microvolts in the EOG electrode). All off-line averaging at NYU and UCSD was performed using the same filtering, downsampling, and artifact rejection criteria.
Procedure
MRI scanning and image processing
All imaging for UCSD participants was performed at the UCSD Radiology Imaging Laboratory on a General Electric 1.5T EXCITE HD scanner with an 8-channel phased-array head coil. Image acquisitions included a conventional 3-plane localizer, GE calibration scan, and two T1-weighted volume acquisition pulse sequences (TE = 3.8ms, TR=10.7ms, TI = 1000 ms, flip angle= 8 deg, FOV=25.6 cm, matrix=256 × 192, slice thickness=1.0mm). MRI data for all NYU participants was acquired using a 3T Siemens Allegra head-only MRI scanner (TE = 3.25, TR = 2530, TI = 1100 ms, flip angle = 7 deg, FOV = 25.6 cm, matrix = 256× 256, slice thickness = 1.3 mm). Acquisition parameters on both scanners were optimized for increased gray/white matter image contrast. The image files in DICOM format were transferred to a Linux workstation for morphometric analysis. The two T1-weighted images were rigid body registered to each other and reoriented into a common space, roughly similar to alignment based on the AC-PC line. Images were corrected for non-linear warping caused by non-uniform fields created by the gradient coils. Image intensities were normalized and made uniform with the FreeSurfer (3.0.5) software package (http://surfer.nmr.mgh.harvard.edu).
Cortical Surface Reconstruction
Geometric representations of the cortical surface were constructed from the T1-weighted structural volumetric images using procedures described previously (Dale et al., 1999, Fischl et al., 1999a, Fischl et al., 2002). First, segmentation of cortical white matter was performed and the estimated border between gray and white matter was tessellated, providing a topographically correct representation of the surface. This representation of the folded cortical surface was used to derive the locations and orientations of the dipoles used in the analysis of the MEG data. Next, the folded surface tessellation was “inflated” in order to unfold cortical activation patterns. For intersubject averaging, the reconstructed surface for each subject was morphed into an average spherical representation, optimally aligning sulcal and gyral features across subjects while minimizing metric distortion (Fischl et al., 1999b). This surface-based registration procedure results in a substantial reduction in anatomical and functional variability across subjects relative to the Talairach normalization approach (Fischl et al., 1999b).
Inverse Solution: Spatiotemporal Analysis
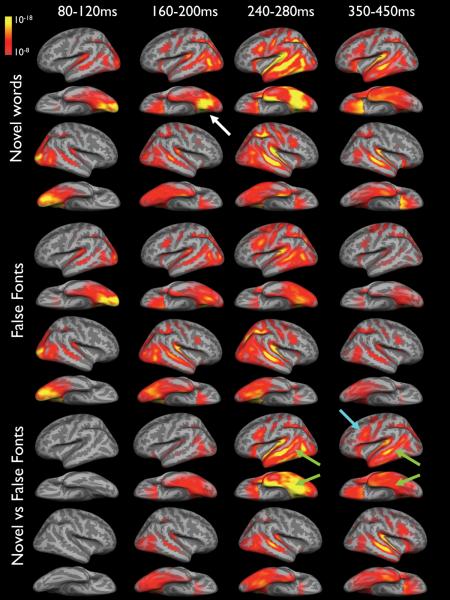
To estimate the time courses of cortical activity using a distributed source solution, a noise-normalized, anatomically constrained linear estimation approach was applied to gradiometer data at UCSD and magnetometer data at NYU (Dale et al., 2000). This method is based on the observation that the main cortical generators of MEG and EEG signals are localized in the gray matter. Once the exact shape of the cortical surface is known, this information can be used to reduce the MEG solution space. Furthermore, normalization procedures are used that take into account the noise sensitivity at each spatial location, allowing for statistical parametric maps. First, the cortical surface was subsampled to about 2500 dipole locations per hemisphere (Dale et al., 2000). Second, the forward solution at each location was calculated using a boundary element model. Third, dipole power was estimated at each cortical location every 4 ms and divided by the predicted noise power obtained from a weighted average of all conditions for each individual. The square roots of these values were then averaged on the cortical surface across individuals after aligning their sulcal-gyral patterns (Dale et al., 2000, Dhond et al., 2001). Using this procedure, spatiotemporal activity estimates were computed from 0-1000 ms following stimulus presentation. This method generates statistical maps that are F-distributed and represent the mean activity for the entire group of participants throughout the time course. Figure 1 demonstrates estimated cortical activity produced by novel words, false fonts, and the comparison of these conditions (i.e., novel words vs false fonts) at selected time intervals for the healthy controls. The comparison condition was designed to provide the best estimate of language processing because it minimizes the contribution of activity associated with non-language related functions (e.g., sensory and attentional processing) that would presumably also be evoked by false fonts. Selected time intervals are those that were of theoretical interest based on previous MEG (Marinkovic et al., 2003, Dhond et al., 2001) and intracranial (Halgren et al., 1994b, Halgren et al., 2006) studies of language processing.
Figure 1.
Average dynamic statistical parametric maps of cortical responses to novel words (top), false fonts (middle), and the novel words vs false fonts (bottom) for 10 healthy controls. Activity is seen bilaterally in occipital cortex ~80-120ms. By ~160ms, activity to novel words peaks in the left ventral occipital-temporal area (white arrow). By ~240ms, activity is observed within the left superior, middle, and inferior temporal lobe, in addition to left prefrontal cortex and is greater for novel words relative to false fonts. Activity is also seen within the right temporal lobe by ~240ms in both conditions. By ~350 ms, activity to novel words remains left lateralized in temporal cortex, but is bilateral in multiple frontal regions. The bottom panel demonstrates the absolute power differences in the mean waveforms. As can be seen, there is an absence of activity in this subtraction condition from ~80-120ms. This initial response was followed by a left-lateralized pattern of activity after ~240ms in left temporal (green arrows) and prefrontal (blue arrow) believed to reflect lexical, syntactic, and semantic processing. In each condition, significance thresholds are set at a minimum of p < 10-8 (full red), with p< 10-18 indicating peak activity (full yellow). These values represent significance levels associated with the noise-normalized dipole strength and can be conceptualized as estimates of the signal-to-noise at each vertex.
Laterality Index
In order to compare language lateralization results from the dSPM approach to those obtained from the IAP, activity sources associated with novel words vs false fonts were modeled and average spatiotemporal activity estimates of the difference waveform were calculated across the cortical surface for each of the epilepsy patients. Laterality indices were then derived within temporoparietal and frontal lobe ROIs for two different time windows (240-280ms and 350-450ms) according to the following formula: [(left - right) / (left + right)] x 100. These time windows were selected based on evidence that initial lexical access and early semantic processing emerges within posterior language cortex ~240-280ms, whereas N400 responses within posterior and anterior language regions peak between 350-450ms following exposure to a verbal stimulus (Bowyer et al., 2005b, Fujimaki et al., 2009). In each case, values greater than 10 were arbitrarily used as a cut-off to indicate left language dominance, values less than -10 were used to indicate right language dominance, and values between -10 and 10 to indicate bilateral language representation.
RESULTS
Healthy control data
In response to both novel words and false fonts, the earliest activity is seen bilaterally in occipital cortex ~80-120ms, representing early visual processing (see Figure 1). By ~160ms, activity to novel words peaked in the ventral occipital-temporal area and was strongly left lateralized. By ~240ms, activity had spread to more anterior regions including the left superior, middle, and inferior temporal lobe, in addition to left prefrontal cortex and was noticeably stronger to novel words relative to false fonts. Activity was also seen within the right temporal lobe by ~240ms in both conditions. By ~350 ms, activity to novel words remained left lateralized in temporal cortex, but appears more prominent in multiple frontal regions as well. After ~80-120ms, activity to false fonts was much less robust than novel words and was not strongly lateralized. Figure 1 (bottom panel) shows the comparison of novel words vs false fonts, demonstrating the absolute power differences in the mean waveforms. As can be seen, there is limited activity in this subtraction condition from ~80-120ms, suggesting that the novel words and false fonts were well-matched in their visual attributes. This initial response was followed by a strong left-lateralized pattern of activity after ~240ms that was sustained and more distributed at later latencies.
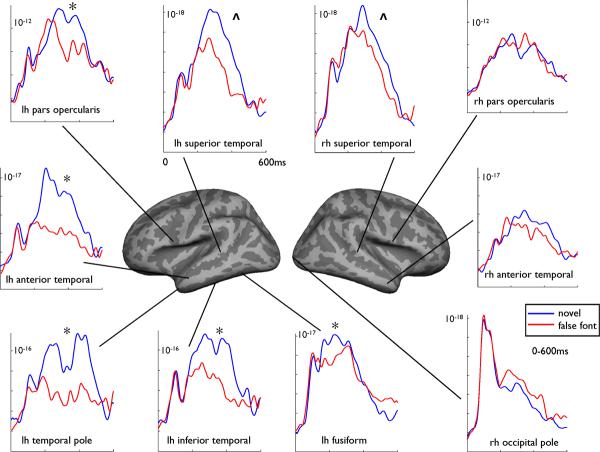
To explore statistical differences between novel words and false fonts at the group level, condition (2) by hemisphere (2) repeated measures analysis of variance (RM ANOVAs) were performed on the average activity for each time window within regions of interest (ROIs). ROIs were determined a priori based on previous research from MEG (Dhond et al., 2001, Marinkovic et al., 2003) and intracranial (Halgren et al., 2006) studies of language processing. Figure 2 portrays the estimated time courses extracted from selected ROIs for the left and right hemispheres for the group. As expected, no significant difference emerged between novel words and false fonts within the occipital pole from 80-120ms [F(1,9) = 1.2, p >.05)]. RM ANOVA revealed a significant condition by hemisphere interaction in the 160-200ms time frame within the ventral occipito-temporal cortex, demonstrating greater responses to novel words relative to false fonts in the left hemisphere only [F(1,9) = 7.8, p <.05)]. Within the 350-450ms time window, condition by hemisphere interactions revealed novel > false font effects in the left, but not right, hemisphere within several temporal lobe regions, including the inferior (ventral) temporal cortex [F(1,9) = 7.8, p <.05)], anterior temporal cortex [F(1,9) = 8.0, p <.05)], and temporal pole [F(1,9) = 7.4, p <.05)]. Within the frontal lobe, condition by hemisphere interactions revealed novel > false font effects in left pars opercularis [F(1,9) = 9.2, p <.05)] and left orbitofrontal cortex [F(1,9) = 6.2, p <.05)]. Main effects of condition were seen in posterior superior temporal cortex, revealing novel > false font responses regardless of hemisphere [F(1,9) = 5.4, p <.05)].
Figure 2.
Noise-normalized dipole strengths from 0-600ms for novel words (blue) and false fonts (red) within selected regions of interest for the healthy control group. Significance (*) denotes regions producing a significant condition x hemisphere interaction. Main effects of condition are indicated by (“ ^ ”).
Patient Data
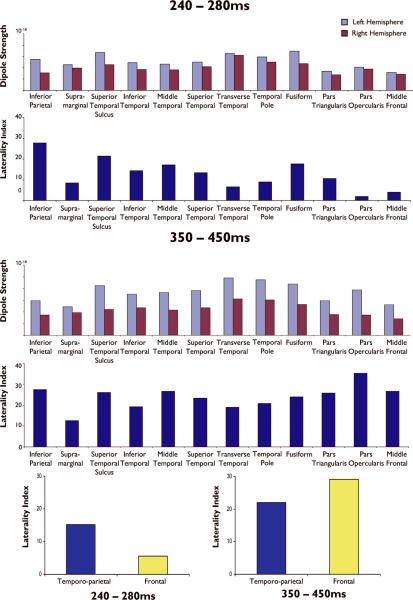
Figure 3 shows the average noise-normalized dipole strength and laterality indices for the patient group for the 240-280 ms and 350-450ms time windows. During the 240-280ms time window, sources within the inferior parietal lobule, superior temporal sulcus, and fusiform gyrus showed the strongest laterality effect. Within the 350-450ms time window, laterality was strongest in pars opercularis relative to the other ROIs. However, most temporal and frontal lobe regions showed stronger average source strength and laterality effects during this later time window when compared to their laterality estimates from 240-280ms (see Figure 3; bottom panel). Consistent with our control data, average source strength and laterality during the 350-450ms time window appeared to capture the peak N400 effect within both posterior and anterior language regions. Therefore, this time window was selected as optimal for comparing our individual patient MEG data to results from the IAP.
Figure 3.
Noise-normalized dipole strengths and laterality indices for each temporal, parietal, and frontal lobe subregion averaged across the patients for the 240-280ms and 350-450ms time windows. Positive values for the laterality indices reflect left > right activity.
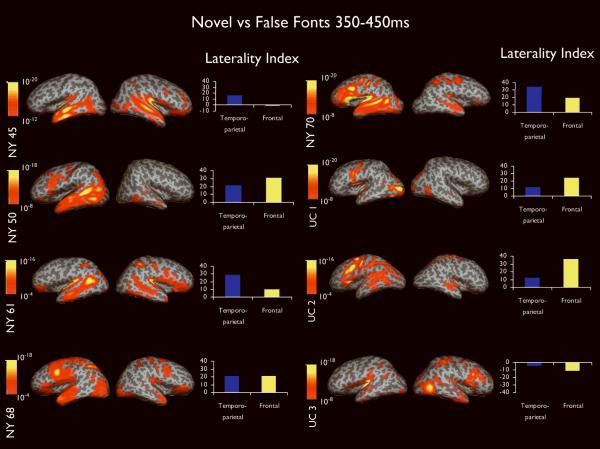
Figure 4 portrays dSPMs of novel words versus false font condition averaged across 350-450ms for each patient, as well as the laterality index for temporoparietal and frontal lobe ROIs. As can be seen, activity appears lateralized in most patients and widely distributed among temporal, parietal, and frontal lobe regions implicated in lexical-semantic processing. Six of eight patients showed evidence of left language dominance on the IAP and across temporoparietal and frontal lobe ROIs (see supplemental Table). In all six of these patients (NY50, NY61, NY68, NY70, UC1, and UC2), the IAP and MEG agreed. In addition, NY68 and NY70 completed the same semantic judgment task during intracranial recordings. Clear focal N400 responses were recorded with subdural electrodes placed on the left cortex in or near Wernicke's and Broca's areas in both patients, providing further validation of the MEG sources. In the patient for whom the IAP suggested right language dominance (UC3), frontal lobe MEG sources agreed, whereas temporoparietal sources suggested bilateral language. In the one patient for whom the IAP suggested bilateral language (NY45), frontal MEG sources agreed, but temporoparietal sources suggested left language representation. In no case did the IAP and MEG produce completely discordant results.
Figure 4.
Dynamic statistical parametric maps and laterality indices calculated from 350-450ms for each of the eight patients. Activity is displayed on the inflated surface of each hemisphere. Sulci and gyri are shown in dark and light gray, respectively. Color bars range from moderate (orange) to high (yellow) activity and are relative to each patient's baseline. Values on the color bars represent significance values associated with the noise-normalized dipole strength at each vertex on the cortical surface. Laterality indices represent the difference between the hemispheres where positive values reflect greater left hemisphere activity and negative values greater right hemisphere activity.
DISCUSSION
The goals of this study were to (1) demonstrate the spatiotemporal pattern of language processing using distributed source modeling (i.e., dSPM) and a task designed to isolate language, and (2) evaluate the concordance between the laterality of temporoparietal and frontal lobe MEG sources and language lateralization as determined by the IAP in patients with epilepsy. In support of our initial hypothesis, dSPM revealed a left-lateralized, distributed pattern of cortical activity that evolved over time, peaking ~350-450ms within multiple perisylvian regions. In healthy controls, bilateral responses emerged in occipital cortex ~100ms, which is consistent with early visual processing in retinotopic areas (Hagler et al., 2008). This activity proceeds anteriorly along the ventral visual stream, becoming left-lateralized ~160ms in occipito-temporal cortex in response to novel words—a finding that is consistent with studies implicating this region in processing orthographic information or visual word forms (Allison et al., 1999). Activity then became distributed and bilateral ~240ms, but remained left-lateralized for novel words in most temporal and frontal lobe regions, including anterior and inferior temporal, temporal pole, and pars opercularis. All of these regions have previously been implicated in lexical-semantic processing and described as intracranial generators of the N400 (Halgren et al., 1994a, Halgren et al., 1994b). Surprisingly, posterior superior temporal cortex (i.e., Wernicke's area) was not among the most lateralized regions in healthy controls, but rather produced strong source amplitudes within both hemispheres from ~240-450ms (see Figure 2). The strength of the sources within these regions is reflected by the fact that most language-related ECDs are localized within superior temporal cortex (Papanicolaou et al., 2004, Halgren et al., 2002, Salmelin et al., 1996). As a result, simultaneously active, but weaker sources generated from other essential language cortex can be overshadowed when focal source solutions are employed. This may be especially important for left anterior and ventral temporal areas that are described as strong local intracranial generators of the N400 associated with word recognition (Halgren et al., 2006). These regions are not only obscured by MEG when focal source solutions are applied, but they are frequently undetected by fMRI due to susceptibility artifacts (Devlin et al., 2000).
DSPM was then applied to eight patients with long-standing refractory epilepsy who are representative of the surgical cases seen in our clinics. Consistent with our second hypothesis, laterality effects in patients were strongest from 350-450ms—the time window likely capturing peak N400 responses. Temporoparietal MEG sources within this time window agreed with the IAP 75% of the time, whereas frontal MEG sources agreed in all eight cases. Very high concordance between frontal MEG sources and the IAP has been reported by Bowyer et al. using a current source density technique (MR-FOCUSS) during a similar time interval (Bowyer et al., 2005b). In their study, this late time interval was superior for eliciting activity within Broca's area, whereas earlier time intervals (~230-290) were superior for eliciting activity within Wernicke's area and associated temporoparietal cortex. Our results suggest that although initial lexical access within temporoparietal regions may begin ~240 ms, peak amplitudes and laterality of sources within frontal and temporal perisylvian regions are stronger from 350-450ms. These latter sources likely reflect a combination of language skills, including sustained lexical access, semantic processing, and syntactic analysis (Fujimaki et al., 2009, Salmelin, 2007). Although stronger concordance between frontal lobe MEG sources and the IAP was not anticipated, it should be noted that the IAPs at both institutions are heavily weighted toward the evaluation of expressive rather than receptive language cortex. Therefore, although prefrontal MEG sources, especially within Broca's area may show somewhat stronger concordance with the IAP, temporal MEG sources may be better predictors of postoperative outcome following language-dominant temporal lobe resections.
Results obtained from dSPM reinforce and expand upon ECD solutions by demonstrating the simultaneous recruitment of temporal, parietal, and frontal lobe regions involved in language processing. Knowledge of simultaneous, distributed activity could assist with tailoring resections in some patients with temporal and extratemporal epilepsy, and could identify patients with hemispheric dissociation in temporal and frontal language cortex (Kamada et al., 2007). It is also possible that the degree of activity within anterior and basal temporal cortex, as observed in the dSPMs of many patients, could help to explain why some patients show language decline following anterior temporal lobectomy (ATL), whereas others do not (Hermann et al., 1999). In particular, cortical stimulation studies have revealed language areas in the basal temporal cortex of the dominant temporal lobe in 40-100% of patients with TLE (Luders et al., 1986, Luders et al., 1991), and there is some evidence of improved neuropsychological outcome when basal temporal language cortex is spared (Mikuni et al., 2006). A systematic evaluation of whether identification of basal temporal sources adds to the prediction of postsurgical language outcome is needed to establish the relevance of these dSPM sources.
Taken together, our data suggest that distributed source modeling of peak N400 responses using a visual, semantic decision task may enhance language lateralization and localization in preoperative evaluations. Importantly, the dSPM method employed in this study normalizes for noise sensitivity at each location, providing statistical maps that estimate the reliability of observed signals at each location and time point and does not require prior channel selection (Dale et al., 2000). Despite the potential clinical value of our study, there are several additional points that should be addressed. First, the spatial resolution of MEG is limited relative to fMRI or PET (Dale and Halgren, 2001, Dale et al., 2000). Therefore, the localization accuracy of our findings would be greatly enhanced with convergent data from fMRI. Previous studies that have compared language localization results between fMRI and MEG using ECD models have found poor agreement (Billingsley-Marshall et al., 2007). Perhaps greater agreement would be obtained across modalities using distributed source solutions that produce statistical maps comparable to the ones produced for fMRI (Dale et al., 2000). Second, it is important to note that our results do not contradict current ECD methods for determining language lateralization.
Such models have shown strong concordance with the IAP in large, independent cohorts of epilepsy patients (Papanicolaou et al., 2004, Lee et al., 2006). Rather, we propose that dSPM may enhance existing methods by providing additional insight into the distribution of temporoparietal and frontal language cortex. These data may be especially important for identifying dissociations in expressive and receptive language cortex that may not be captured by focal source solutions or the IAP.
Supplementary Material
ACKNOWLEDGMENTS
The project described was supported by Grant Number K23NS056091 and NS18741 from the National Institute of Neurological Disorders and Stroke. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institute of Health. We also greatly acknowledge support from GE Healthcare. Thank you to Saeed Afaneh for assistance with the figures included in this manuscript and Mazyar Ahmadi for assistance with task development.
Footnotes
Conflict of Interest: We confirm that we have read the journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. Eric Halgren has equity interest in CorTechs Labs, Inc, and also serves on its Board of Directors.
Anders M. Dale is a founder and holds equity in CorTechs Labs, Inc and also serves on the Scientific Advisory Board. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies.
The remaining authors have no conflict of interest to disclose.
REFERENCES
- ALLISON T, PUCE A, SPENCER DD, MCCARTHY G. Electrophysiological studies of human face perception. I: Potentials generated in occipitotemporal cortex by face and non-face stimuli. Cereb Cortex. 1999;9:415–30. doi: 10.1093/cercor/9.5.415. [DOI] [PubMed] [Google Scholar]
- BILLINGSLEY-MARSHALL RL, CLEAR T, MENCL WE, SIMOS PG, SWANK PR, MEN D, SARKARI S, CASTILLO EM, PAPANICOLAOU AC. A comparison of functional MRI and magnetoencephalography for receptive language mapping. J Neurosci Methods. 2007;161:306–13. doi: 10.1016/j.jneumeth.2006.10.020. [DOI] [PubMed] [Google Scholar]
- BOWYER SM, FLEMING T, GREENWALD ML, MORAN JE, MASON KM, WEILAND BJ, SMITH BJ, BARKLEY GL, TEPLEY N. Magnetoencephalographic localization of the basal temporal language area. Epilepsy Behav. 2005a;6:229–34. doi: 10.1016/j.yebeh.2004.12.003. [DOI] [PubMed] [Google Scholar]
- BOWYER SM, MORAN JE, WEILAND BJ, MASON KM, GREENWALD ML, SMITH BJ, BARKLEY GL, TEPLEY N. Language laterality determined by MEG mapping with MR-FOCUSS. Epilepsy Behav. 2005b;6:235–41. doi: 10.1016/j.yebeh.2004.12.002. [DOI] [PubMed] [Google Scholar]
- BREIER JI, CASTILLO EM, SIMOS PG, BILLINGSLEY-MARSHALL RL, PATARAIA E, SARKARI S, WHELESS JW, PAPANICOLAOU AC. Atypical language representation in patients with chronic seizure disorder and achievement deficits with magnetoencephalography. Epilepsia. 2005;46:540–8. doi: 10.1111/j.0013-9580.2005.48904.x. [DOI] [PubMed] [Google Scholar]
- BREIER JI, SIMOS PG, ZOURIDAKIS G, PAPANICOLAOU AC. Lateralization of activity associated with language function using magnetoencephalography: a reliability study. J Clin Neurophysiol. 2000;17:503–10. doi: 10.1097/00004691-200009000-00010. [DOI] [PubMed] [Google Scholar]
- DALE AM, FISCHL B, SERENO MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- DALE AM, HALGREN E. Spatiotemporal mapping of brain activity by integration of multiple imaging modalities. Curr Opin Neurobiol. 2001;11:202–8. doi: 10.1016/s0959-4388(00)00197-5. [DOI] [PubMed] [Google Scholar]
- DALE AM, LIU AK, FISCHL BR, BUCKNER RL, BELLIVEAU JW, LEWINE JD, HALGREN E. Dynamic statistical parametric mapping: combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron. 2000;26:55–67. doi: 10.1016/s0896-6273(00)81138-1. [DOI] [PubMed] [Google Scholar]
- DEVLIN JT, RUSSELL RP, DAVIS MH, PRICE CJ, WILSON J, MOSS HE, MATTHEWS PM, TYLER LK. Susceptibility-induced loss of signal: comparing PET and fMRI on a semantic task. Neuroimage. 2000;11:589–600. doi: 10.1006/nimg.2000.0595. [DOI] [PubMed] [Google Scholar]
- DHOND RP, BUCKNER RL, DALE AM, MARINKOVIC K, HALGREN E. Spatiotemporal maps of brain activity underlying word generation and their modification during repetition priming. J Neurosci. 2001;21:3564–71. doi: 10.1523/JNEUROSCI.21-10-03564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DHOND RP, MARINKOVIC K, DALE AM, WITZEL T, HALGREN E. Spatiotemporal maps of past-tense verb inflection. Neuroimage. 2003;19:91–100. doi: 10.1016/s1053-8119(03)00047-8. [DOI] [PubMed] [Google Scholar]
- FISCHL B, SALAT DH, BUSA E, ALBERT M, DIETERICH M, HASELGROVE C, VAN DER KOUWE A, KILLIANY R, KENNEDY D, KLAVENESS S, MONTILLO A, MAKRIS N, ROSEN B, DALE AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- FISCHL B, SERENO MI, DALE AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999a;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- FISCHL B, SERENO MI, TOOTELL RB, DALE AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999b;8:272–84. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANCIS WN, KUCERA H. Frequency analysis of English usage: Lexicon and grammar. Houghton Mifflin; Boston: 1982. [Google Scholar]
- FUJIMAKI N, HAYAKAWA T, IHARA A, WEI Q, MUNETSUNA S, TERAZONO Y, MATANI A, MURATA T. Early neural activation for lexico-semantic access in the left anterior temporal area analyzed by an fMRI-assisted MEG multidipole method. Neuroimage. 2009;44:1093–102. doi: 10.1016/j.neuroimage.2008.10.021. [DOI] [PubMed] [Google Scholar]
- GESCHWIND N. The organization of language and the brain. Science. 1970;170:940–4. doi: 10.1126/science.170.3961.940. [DOI] [PubMed] [Google Scholar]
- HAGLER DJ, JR., HALGREN E, MARTINEZ A, HUANG M, HILLYARD SA, DALE AM. Source estimates for MEG/EEG visual evoked responses constrained by multiple, retinotopically-mapped stimulus locations. Hum Brain Mapp. 2008 doi: 10.1002/hbm.20597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALGREN E, BAUDENA P, HEIT G, CLARKE JM, MARINKOVIC K, CHAUVEL P, CLARKE M. Spatio-temporal stages in face and word processing. 2. Depth-recorded potentials in the human frontal and Rolandic cortices. J Physiol Paris. 1994a;88:51–80. doi: 10.1016/0928-4257(94)90093-0. [DOI] [PubMed] [Google Scholar]
- HALGREN E, BAUDENA P, HEIT G, CLARKE JM, MARINKOVIC K, CLARKE M. Spatio-temporal stages in face and word processing. I. Depth-recorded potentials in the human occipital, temporal and parietal lobes [corrected] J Physiol Paris. 1994b;88:1–50. doi: 10.1016/0928-4257(94)90092-2. [DOI] [PubMed] [Google Scholar]
- HALGREN E, DHOND RP, CHRISTENSEN N, VAN PETTEN C, MARINKOVIC K, LEWINE JD, DALE AM. N400-like magnetoencephalography responses modulated by semantic context, word frequency, and lexical class in sentences. Neuroimage. 2002;17:1101–16. doi: 10.1006/nimg.2002.1268. [DOI] [PubMed] [Google Scholar]
- HALGREN E, WANG C, SCHOMER DL, KNAKE S, MARINKOVIC K, WU J, ULBERT I. Processing stages underlying word recognition in the anteroventral temporal lobe. Neuroimage. 2006;30:1401–13. doi: 10.1016/j.neuroimage.2005.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMALAINEN M, HARI R, ILMONIEMI RJ, KNUUTILA J, LOUNASMAA OV. Magnetoencephalography--theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev Mod Phys. 1993;65:413–497. [Google Scholar]
- HAMALAINEN MS, ILMONIEMI RJ. Interpreting magnetic fields of the brain: minimum norm estimates. Med Biol Eng Comput. 1994;32:35–42. doi: 10.1007/BF02512476. [DOI] [PubMed] [Google Scholar]
- HERMANN BP, PERRINE K, CHELUNE GJ, BARR W, LORING DW, STRAUSS E, TRENERRY MR, WESTERVELD M. Visual confrontation naming following left anterior temporal lobectomy: a comparison of surgical approaches. Neuropsychology. 1999;13:3–9. doi: 10.1037//0894-4105.13.1.3. [DOI] [PubMed] [Google Scholar]
- HIRATA M, KATO A, TANIGUCHI M, SAITOH Y, NINOMIYA H, IHARA A, KISHIMA H, OSHINO S, BABA T, YORIFUJI S, YOSHIMINE T. Determination of language dominance with synthetic aperture magnetometry: comparison with the Wada test. Neuroimage. 2004;23:46–53. doi: 10.1016/j.neuroimage.2004.05.009. [DOI] [PubMed] [Google Scholar]
- KAMADA K, SAWAMURA Y, TAKEUCHI F, KURIKI S, KAWAI K, MORITA A, TODO T. Expressive and receptive language areas determined by a non-invasive reliable method using functional magnetic resonance imaging and magnetoencephalography. Neurosurgery. 2007;60:296–305. doi: 10.1227/01.NEU.0000249262.03451.0E. discussion 305-6. [DOI] [PubMed] [Google Scholar]
- LEE D, SAWRIE SM, SIMOS PG, KILLEN J, KNOWLTON RC. Reliability of language mapping with magnetic source imaging in epilepsy surgery candidates. Epilepsy Behav. 2006;8:742–9. doi: 10.1016/j.yebeh.2006.02.012. [DOI] [PubMed] [Google Scholar]
- LUDERS H, LESSER RP, HAHN J, DINNER DS, MORRIS H, RESOR S, HARRISON M. Basal temporal language area demonstrated by electrical stimulation. Neurology. 1986;36:505–10. doi: 10.1212/wnl.36.4.505. [DOI] [PubMed] [Google Scholar]
- LUDERS H, LESSER RP, HAHN J, DINNER DS, MORRIS HH, WYLLIE E, GODOY J. Basal temporal language area. Brain. 1991;114(Pt 2):743–54. doi: 10.1093/brain/114.2.743. [DOI] [PubMed] [Google Scholar]
- MAESTU F, ORTIZ T, FERNANDEZ A, AMO C, MARTIN P, FERNANDEZ S, SOLA RG. Spanish language mapping using MEG: a validation study. Neuroimage. 2002;17:1579–86. doi: 10.1006/nimg.2002.1235. [DOI] [PubMed] [Google Scholar]
- MARINKOVIC K. Spatiotemporal dynamics of word processing in the human cortex. Neuroscientist. 2004;10:142–52. doi: 10.1177/1073858403261018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARINKOVIC K, DHOND RP, DALE AM, GLESSNER M, CARR V, HALGREN E. Spatiotemporal dynamics of modality-specific and supramodal word processing. Neuron. 2003;38:487–97. doi: 10.1016/s0896-6273(03)00197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIKUNI N, MIYAMOTO S, IKEDA A, SATOW T, TAKI J, TAKAHASHI J, OHIGASHI Y, HASHIMOTO N. Subtemporal hippocampectomy preserving the basal temporal language area for intractable mesial temporal lobe epilepsy: preliminary results. Epilepsia. 2006;47:1347–53. doi: 10.1111/j.1528-1167.2006.00610.x. [DOI] [PubMed] [Google Scholar]
- PAPANICOLAOU AC, SIMOS PG, BREIER JI, ZOURIDAKIS G, WILLMORE LJ, WHELESS JW, CONSTANTINOU JE, MAGGIO WW, GORMLEY WB. Magnetoencephalographic mapping of the language-specific cortex. J Neurosurg. 1999;90:85–93. doi: 10.3171/jns.1999.90.1.0085. [DOI] [PubMed] [Google Scholar]
- PAPANICOLAOU AC, SIMOS PG, CASTILLO EM, BREIER JI, SARKARI S, PATARAIA E, BILLINGSLEY RL, BUCHANAN S, WHELESS J, MAGGIO V, MAGGIO WW. Magnetocephalography: a noninvasive alternative to the Wada procedure. J Neurosurg. 2004;100:867–76. doi: 10.3171/jns.2004.100.5.0867. [DOI] [PubMed] [Google Scholar]
- SALMELIN R. Clinical neurophysiology of language: the MEG approach. Clin Neurophysiol. 2007;118:237–54. doi: 10.1016/j.clinph.2006.07.316. [DOI] [PubMed] [Google Scholar]
- SALMELIN R, SERVICE E, KIESILA P, UUTELA K, SALONEN O. Impaired visual word processing in dyslexia revealed with magnetoencephalography. Ann Neurol. 1996;40:157–62. doi: 10.1002/ana.410400206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.