Abstract
Light-activated ion channels provide a precise and non-invasive optical means for controlling action potential firing, but the genes encoding these channels must first be delivered and expressed in target cells. Here we describe a method for bestowing light sensitivity onto endogenous ion channels that does not rely on exogenous gene expression. The method utilizes a synthetic photoisomerizable small molecule, or Photoswitchable Affinity Label (PAL), that specifically targets K+ channels. PALs contain a reactive electrophile, enabling covalent attachment of the photoswitch to naturally occurring nucleophiles in K+ channels. Ion flow through PAL-modified channels is turned on or off by photoisomerizing PAL with different wavelengths of light. We show that PAL treatment confers light sensitivity onto endogenous K+ channels in isolated neurons and intact neural structures, allowing rapid optical regulation of excitability without genetic modification.
INTRODUCTION
Eukaryotic cells possess ion channels that are directly activated by voltage or ligands, but not by light. Consequently, electrical or chemical stimuli are often used to elicit physiological responses in excitable cells. However, light stimulation has several advantages over electrodes or chemical perfusion devices. Light is non-invasive and can be projected on tissue with great temporal and spatial precision. It can be focused on subcellular structures, single cells, or projected diffusely to regulate the activity of many cells simultaneously1–5. But how can light be used to manipulate the activity of “blind” cells that have no natural photoresponsive proteins?
One popular approach to manipulate the activity of excitable cells with light has been to use a “caged” neurotransmitter (for example glutamate) that is liberated from a photolabile protecting group (the cage) upon exposure to light1,5. Photorelease of caged glutamate accurately mimics the kinetics of synaptic transmission and has been used to map neuronal circuits6–9. However, glutamate uncaging is ill-suited for inducing sustained activity because prolonged uncaging can lead to the accumulation of desensitized receptors and local depletion of the caged neurotransmitter4. Photorelease is irreversible and diffusion of the liberated neurotransmitter can result in unintended activation of receptors on untargeted cells.
To circumvent the limitations associated with using a freely diffusible light sensitive compound, several types of light-activated proteins have been used to control neuronal activity. A light-activated K+ channel (SPARK), consisting of a photoswitchable ligand attached to a genetically engineered Shaker K+ channel, allows reversible suppression of action potential firing10. LiGluR, a light-activated glutamate receptor, containing a different photoswitchable ligand attached to a genetically engineered iGluR611, reversibly depolarizes cells and promotes neuronal firing12. Finally, channelrhodopsin-2 (ChR2) and halorhodopsin (NpHR), which use the natural photoswitch retinal, allow light to trigger or inhibit action potential firing13–19. Each of these proteins can impart light sensitivity on neuronal firing, but only if their gene is first introduced into the cell of interest and the protein expressed in sufficient abundance on the plasma membrane. However, exogenous expression of proteins can be non-uniform and slow, requiring days to weeks, and is not currently practical in some organisms. Genes encoding light-activated proteins can also be introduced transgenically into organisms12,19–22 but this may perturb the development and function of cells expressing the genes.
Here we describe a new method, based on a Photoswitchable Affinity Label (PAL), to confer light sensitivity to proteins without requiring genetic engineering and exogenous gene expression. Consequently, the PAL approach can be used to photosensitize endogenous proteins and control their activity in freshly obtained, genetically unadulterated cells or tissues. The PAL molecules described here specifically target voltage-gated K+ channels and act as covalently tethered channel blockers. The tether is photoisomerizable and can be shortened or elongated by exposure to different wavelengths of light, allowing or disallowing the blocking moiety to reach the pore. When applied to neurons, PAL enables control of endogenous K+ channels with light, resulting in optical control of electrical excitability without genetic modification.
RESULTS
The PAL approach
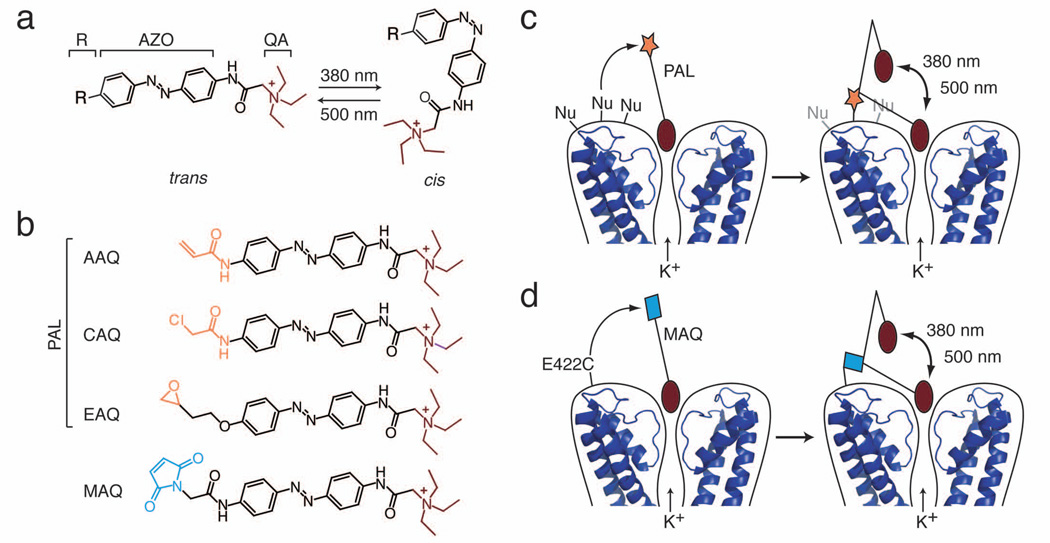
PALs are derivatives of the photoisomerizable molecule azobenzene (AZO; Fig. 1a). Connected to one end of AZO is a protein-binding ligand, in this case a quaternary ammonium group (QA), which binds to the pore of K+ channels and blocks ion conduction. On the other end is an electrophilic group (R) that covalently tethers the photoswitch to the channel. We have designed PALs with three different electrophilic groups, acrylamide (AAQ), chloroacetamide (CAQ) or epoxide (EAQ) (Fig. 1b; see supplementary methods for synthesis) to enable attachment to nucleophilic amino acid side chains. Binding of the QA to the K+ channel pore promotes attachment of these PALs if the channel possesses a nucleophile at ~20 Å from the QA binding site, matching the length of the PAL molecule. Hence, the covalent attachment of PALs to channels is promoted by ligand binding, as in classical affinity labeling23. After the photoswitch is tethered, the QA can reach the pore and block ion conduction only when the AZO is in its elongated trans form, but not in its bent cis form (Fig. 1c). Thus, channels are unblocked by exposure to 360–400 nm light, which photoisomerizes the AZO from trans to cis. The reverse cis to trans conversion, which restores channel block, occurs slowly in the dark (τ=~5 minutes) and is accelerated by long wavelength light (450–560 nm)10,24.
Figure 1. The PAL approach for imparting light sensitivity onto native ion channels.
(a) PAL molecules consist of a photoisomerizable azobenzene group (AZO) flanked by a quaternary ammonium (QA; burgundy) and a covalent attachment group (R). Exposure to 380 nm light isomerizes the AZO to its shorter cis form whereas exposure to 500 nm light favors the trans configuration.
(b) PAL molecules contain a promiscuous reactive group (orange): acrylamide (Acryl-AZO-QA or AAQ), chloroacetamide (CAQ), or epoxide (EAQ). In contrast, MAQ contains a maleimide (blue) designed to react with an engineered cysteine.
(c) After QA binds to the channel pore, PAL reacts via its promiscuous reactive group with an endogenous nucleophile (Nu) on native K+ channels. Once tethered, the photoswitch allows control of ionic current using light. In 500 nm light, the photoswitch is extended, blocking ion conduction. In 380 nm light, AZO isomerizes to its cis form, retracting the QA and allowing ion conduction. Molecular coordinates from KcsA (PDB ID: 2A9H) were drawn using MacPyMol (DeLano, W.L. The PyMOL Molecular Graphics System (2002); http://www.pymol.org).
(d) To generate the SPARK channel, the maleimide group of MAQ attaches to a cysteine (E422C) genetically engineered in a Shaker K+ channel.
PAL-modified channels differ in several ways from the previously described SPARK channel10. SPARK is based on a Shaker K+ channel engineered to contain an extracellular cysteine that serves as the covalent attachment site for its photoswitch (Fig. 1d). Additional mutations were introduced in the channel to shift voltage-dependent activation and minimize inactivation. The SPARK photoswitch is maleimide-AZO-QA (MAQ, Fig. 1b), which covalently attaches to the channel via a maleimide group often considered selective for cysteines. Thus, to impart light sensitivity using SPARK, two components must be added, the channel gene and the MAQ photoswitch. In contrast, the PAL photoswitch acts on endogenous K+ channels that have no introduced cysteine and no mutations to modify gating. Hence PAL is a one-component system for conferring light sensitivity.
PAL imparts light sensitivity to K+ channels
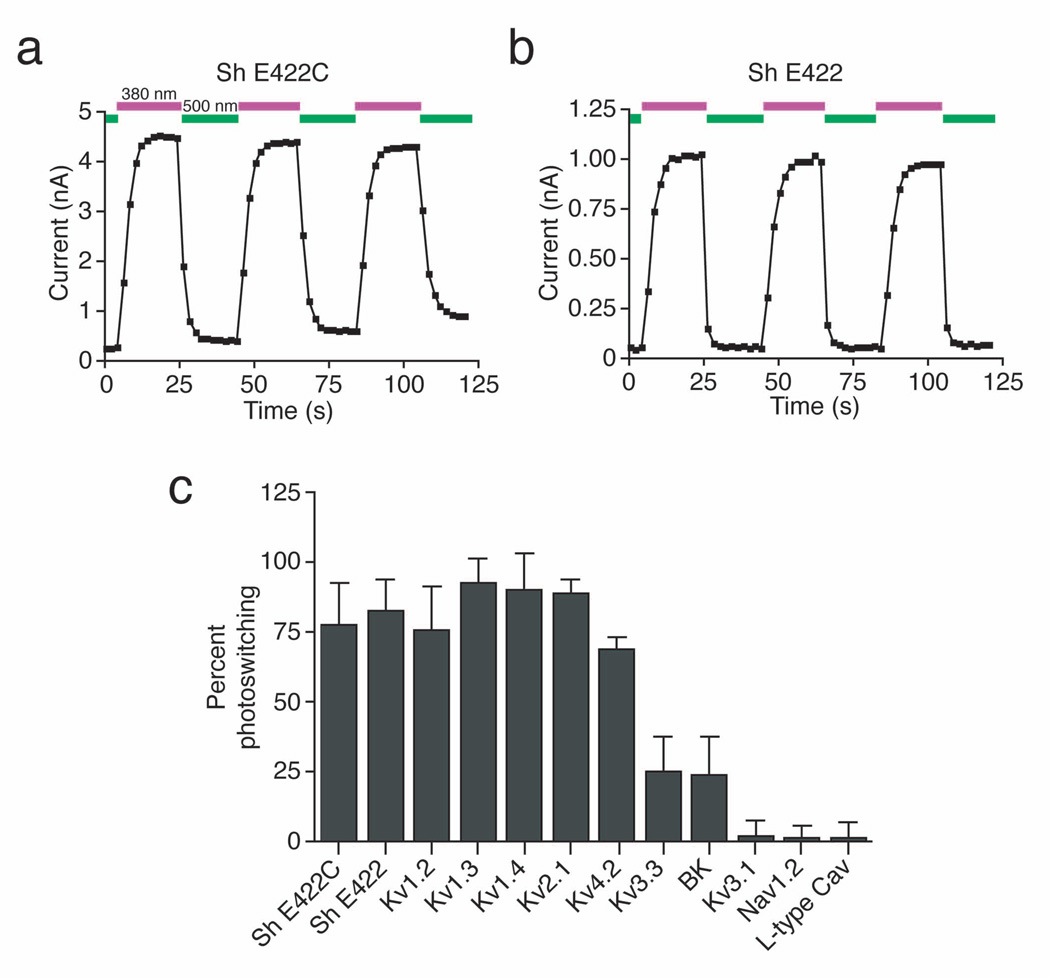
We assessed the feasibility of the PAL approach using whole-cell patch clamp recording in cells heterologously expressing Shaker K+ channels. First, we engineered an appropriately positioned nucleophilic site in Shaker (E422C) to maximize the probability of attachment of the PAL photoswitch to the channel. Addition of AAQ to Shaker E422C photosensitized this channel, enabling regulation of ion flow with light (Fig. 2a). Importantly, AAQ also imparted light sensitivity to a Shaker channel lacking the cysteine substitution (E422; Fig. 2b) and indeed, conferred light sensitivity on a Shaker channel devoid of extracellular cysteines (data not shown). The fraction of current that could be photoregulated after AAQ treatment (% photoswitching) was similar for E422C and E422 Shaker channels (77 ± 15% and 83 ± 11% respectively, n = 4 cells; Fig. 2c). CAQ and EAQ also photosensitized E422 Shaker (data not shown). Hence, PAL molecules can find a covalent attachment site at an appropriate distance from the channel pore, such that light-elicited changes in photoswitch length allow or disallow block by the QA group. PAL-modified channels could be photoswitched repeatedly with little decrement in the fraction of current regulated and no apparent photobleaching of the photoswitch (Fig. 2a and b). Thus, PAL molecules can be used to endow persistent light sensitivity to a wild-type Shaker channel, allowing rapid and reversible control of channel function.
Figure 2. Photocontrol of K+ channels expressed in HEK293 cells.
(a) AAQ photosensitizes Shaker channels that contain an engineered nucleophilic attachment site (Sh E422C). Voltage-gated K+ currents were elicited by pulsing from −70 to +30 mV for 250 ms. 500 nm light (green) blocks current through the channels whereas 380 nm light (violet) unblocks the channels.
(b) AAQ photosensitizes wild-type Shaker channels (Sh E422). Pulse protocols as in (a).
(c) Percent photoswitching for different channels treated with AAQ (400 µM, 15 min). We defined percent photoswitching as the difference between the steady-state current in 380 and 500 nm light, divided by the current in 380 nm light. The extent of photoswitching is similar for Shaker with or without the engineered cysteine (n = 4 for each). AAQ treatment also strongly photosensitizes Kv1.2, 1.3, 1.4, 2.1, and 4.2. Currents through Kv3.3 and BK are only modestly affected by light after AAQ treatment. Kv3.1 is insensitive to AAQ as are voltage-gated Na+ channels expressed in HEK293T cells and L-type Ca2+ channels endogenous to GH3 cells (n = 4–7 cells for each channel type). Current were elicited by stepping from −70 to +30 mV (K+ channels), −80 to 0 mV (Na+ channels), and −40 to +20 mV (Ca2+ channels).
If PAL molecules can impart light sensitivity on wild-type Shaker, perhaps they can also act on other QA-sensitive K+ channels. We expressed various channels in HEK293 cells and quantified photosensitivity after AAQ treatment (Fig. 2c). Several K+ channels became light sensitive, including Kv1.2, Kv1.3, Kv1.4, Kv2.1 and Kv4.2 (% photoswitching for Kv1.2, 76 ± 16%; Kv1.3, 93 ± 9%; Kv1.4, 90 ± 13%; Kv2.1, 89 ± 5%; Kv4.2, 69 ± 4%; n = 4 cells each; Fig. 2c). Light sensitivity was less pronounced for Kv3.3 (25 ± 13% photoswitching, n = 5 cells) and BK (24 ± 13% photoswitching, n = 4 cells). In contrast, Kv3.1 exhibited no detectable photosensitivity after AAQ treatment (2 ± 6% photoswitching, n = 6 cells). Moreover, neither voltage-gated Na+ channels (Nav1.2) nor voltage-gated Ca2+ channels (L-type) became light sensitive after AAQ treatment (1 ± 5% and −2 ± 5% photoswitching respectively, n = 4–7 cells).
PAL photosensitizes K+ channels in cultured neurons
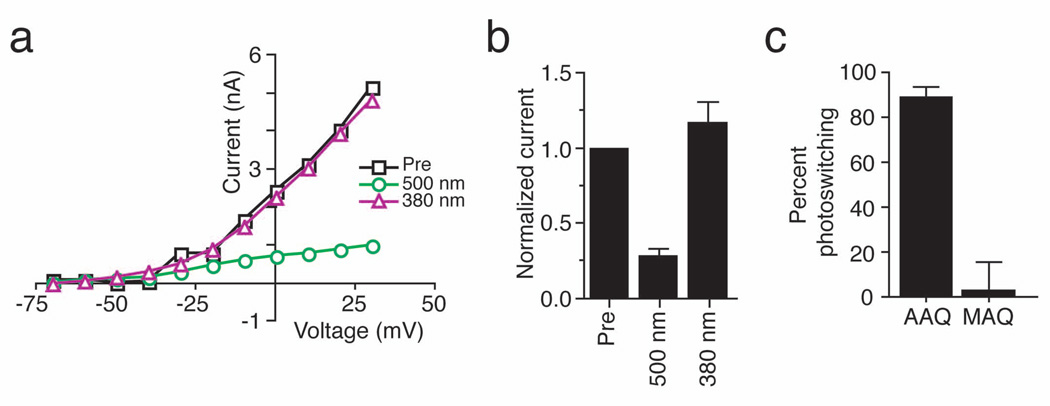
We next tested whether PAL molecules could photosensitize endogenous K+ channels in neurons. We recorded voltage-gated outward K+ currents from cultured hippocampal neurons before and after AAQ application. Steady-state I–V curves indicate that AAQ application and exposure to 500 nm light blocked a large fraction of voltage-gated K+ currents (Fig. 3a and b; average current blocked 72 ± 9%, n = 5). Photoisomerization of AAQ to its cis configuration with 380 nm light completely relieved K+ channel block such that the I–V curve overlapped with that before AAQ treatment (Fig. 3a and b; average current recovered under 380 nm, 117 ± 29%, n = 5). Voltage-gated K+ currents were photoswitched to a similar extent in neurons treated with CAQ (data not shown). In contrast, MAQ failed to impart light sensitivity on endogenous neuronal K+ channels (Fig. 3c; 88 ± 5% and 2 ± 13% photoswitching for AAQ and MAQ respectively, n = 6 each).
Figure 3. Photocontrol of native K+ current in cultured hippocampal neurons.
(a) Steady-state I–V curves from a voltage-clamped hippocampal pyramidal neuron before (Pre; black squares) and after a 15 min application of 300 µM AAQ. Photoisomerization of AAQ to the trans state with 500 nm light (green circles) reduces voltage-gated K+ current whereas illumination with 380 nm light (violet triangles) restores current to levels similar to those measured prior to AAQ treatment.
(b) AAQ-treated channels are completely unblocked by 380 nm light. Voltage-gated currents (elicited by stepping from −70mV to +30mV) measured after AAQ treatment were normalized to those measured prior to AAQ application (n = 5).
(c) Percent photoswitching for K+ current in hippocampal neurons treated with AAQ (200 µM) or MAQ (250 µM) (n = 6 for each). Light has no effect on the steady-state I–V curve from a non-transfected neuron treated with MAQ. MAQ only imparts light-sensitivity on neurons transfected with the Shaker E422C channel10.
In darkness, the PAL photoswitch will relax to its trans configuration, blocking PAL-modified K+ channels and potentially causing depolarization of the membrane potential. To prevent tonic blockade of K+ channels, 380 nm light can be used to keep PAL-modified channels unblocked (Fig. 3a and b). However, because the spontaneous cis to trans isomerization of the photoswitch occurs over many minutes in the dark 10, flashes of 380 nm light (1 second per minute) were sufficient to maintain 94 ± 4% of the K+ current unblocked in AAQ-treated neurons (n = 10). Thus, the state of PAL-modified K+ channels can be set with the appropriate illumination conditions, preventing tonic K+ channel blockade.
Despite having a reactive electrophile and causing K+ channel blockade in darkness and 500 nm light, PAL photoswitches did not have obvious deleterious effects on cultured hippocampal neurons. Neurons remained active with a normal resting membrane potential (vehicle, −54 ± 13 mV, n = 10; AAQ, −54 ± 13 mV, n = 23; p > 0.1 unpaired t-test) and normal membrane resistance (vehicle, 77 ± 20 MΩ, n = 10; AAQ, 113 ± 65 MΩ, n = 6; p > 0.1 unpaired t-test) for several hours after PAL treatment. Further, there was no significant difference in cell death between neurons treated for 15 minutes with vehicle alone (5 ± 3%), AAQ (9 ± 3%) or CAQ (8 ± 3%; n = 4–8 fields each, p > 0.05; One-way ANOVA; Supplementary Fig. 1). EAQ appeared more toxic and was not used further in our studies (25 ± 11% cell death). Similar results were obtained after treatment for 60 minutes, 4-times longer than needed to impart light sensitivity (Supplementary Fig. 1). We also tested neurons up to 6 days after a 15 minutes treatment with AAQ and found no significant toxicity (3 ± 1% cell death) compared to treatment with vehicle alone (4 ± 1%). Additional experiments showed that AAQ injection into the vitreous humor of the rat eye successfully imparted light sensitivity onto retinal ganglion cells (RGCs), but had no effect on either the histology of the retina or on the rod- and cone-driven light response, as measured in electroretinogram recordings (Borges and Kramer, unpublished observations). Hence, at least for our treatment conditions, AAQ does not appear toxic to neurons in vitro or in vivo.
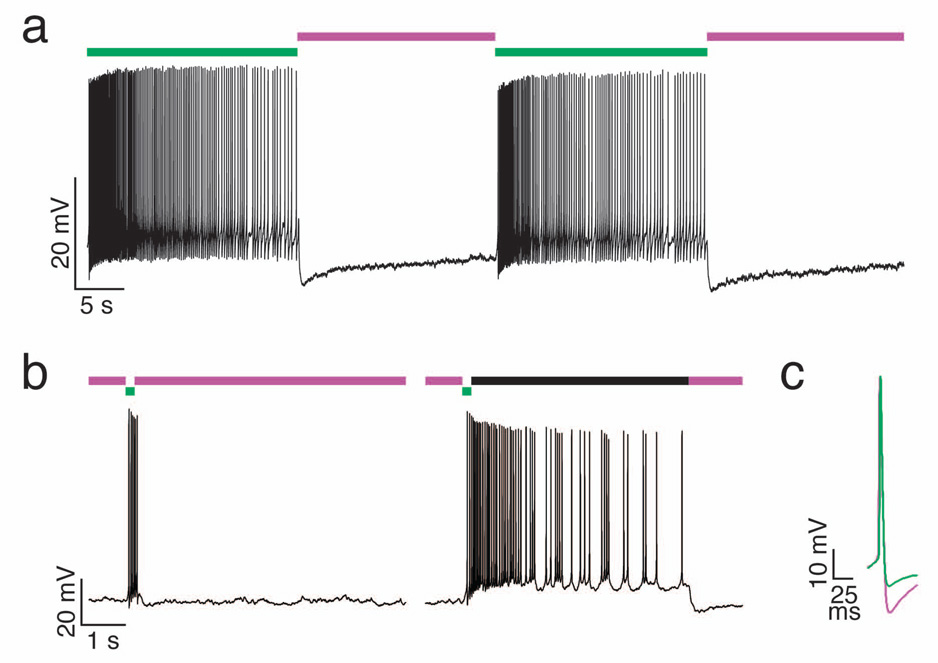
Like other light-activated ion channels, optical control of PAL-modified K+ channels can alter the frequency of action potential firing in treated neurons. To allow reliable measurement of photoregulation of firing frequency, we induced continuous firing by injecting depolarizing current in AAQ-treated neurons exposed to 500 nm light. Subsequent photoisomerization of AAQ with 380 nm light, which unblocks K+ channels, rapidly suppressed action potential firing (Fig. 4a). High-frequency firing resumed upon continuous illumination with 500 nm light. In addition, flashes of 500 nm light followed by darkness resulted in sustained firing that long outlived the light stimulus (Fig. 4b), reflecting the stability of the photoswitch in its trans configuration. Hence, light flashes of either wavelength are sufficient to toggle on and off channel blockade and neuronal excitability, eliminating the need for continuous illumination. This feature differentiates PAL-mediated control from regulation by glutamate uncaging and ChR2, which generally affects neurons only during the illumination period. The persistent nature of PAL photoisomers after brief illumination minimizes potential photodamage both to the photoswitch and to target cells by reducing the total amount of light required for optical control. Photocontrol of PAL-modified channels with continuous or intermittent light occurred rapidly, resulting in modulation of neuronal firing within hundreds of milliseconds after the onset of illumination, with the exact delay varying with light intensity. We also found that the action potential afterhyperpolarization was more pronounced in 380 nm than 500 nm light, consistent with K+ channel regulation (Fig. 4c).
Figure 4. Photocontrol of neuronal firing.
(a) Current clamp recording from a hippocampal pyramidal neuron treated with AAQ. Depolarizing current (90 pA) was injected to induce continuous action potential firing in 500 nm light (green). Illumination with 380 nm light (violet) rapidly suppresses action potential firing. High frequency firing resumes upon illumination with 500 nm light.
(b) Current clamp recording from a hippocampal pyramidal neuron treated with AAQ. Depolarizing current (15 pA) was injected to bring the cell close to threshold. Left: a 200 ms flash of 500 nm light (green) induces a transient burst of action potential that is terminated rapidly upon illumination with 380 nm light (violet). Right: a persistent burst of action potential that outlast the light stimulus can be generated when the cell is illuminated briefly with 500 nm light (green) followed by darkness (black bar). Firing ceases once the neuron is illuminated with 380 nm light (violet).
(c) Block of K+ channels with AAQ in 500 nm light (green) decreases the action potential afterhyperpolarization compared to that under 380 nm light (violet). Action potentials were induced with 500 ms depolarizing current injection (120 pA in 380 nm light, 100 pA in 500 nm light).
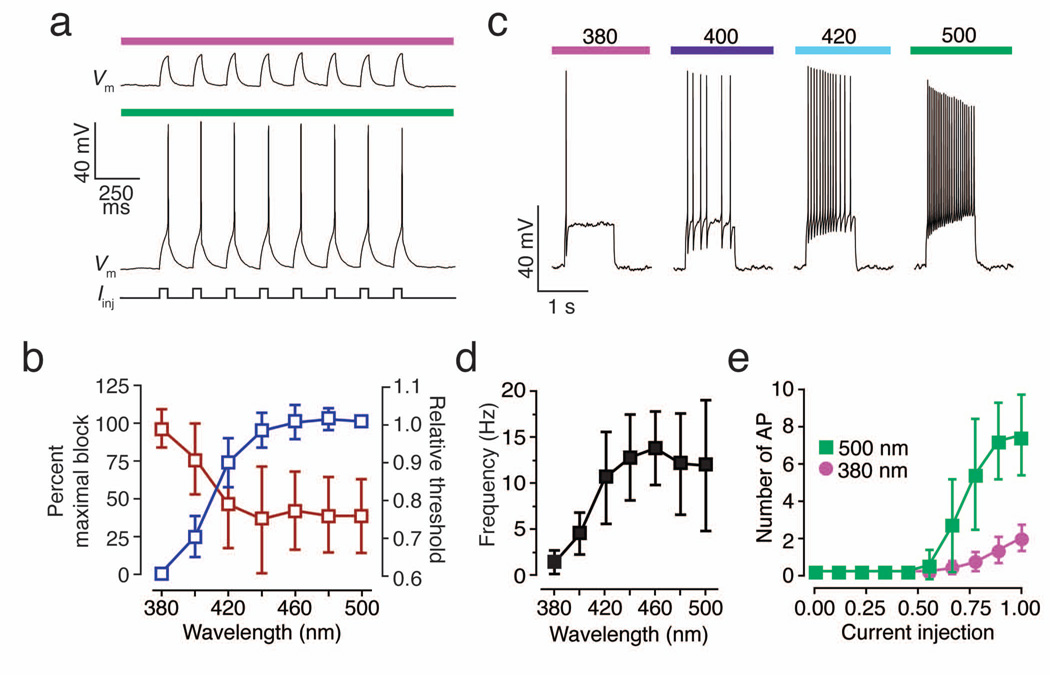
Unlike other light-activated channels, PAL targets native ion channels that control cellular excitability, thereby altering the threshold for action potential firing. Current injections that failed to elicit action potential firing under 380 nm light reliably induced firing when a neuron was illuminated with 500 nm light (Fig. 5a). The relative proportion of cis and trans photoisomers of azobenzene-containing photoswitches is dependent on wavelength10,24. This photoequilibrium can be exploited to adjust the extent of K+ channel block in a graded manner to fine-tune cellular excitability. Thus, the amount of depolarizing current required to induce action potential firing decreased as the number of blocked K+ channels was increased with longer wavelengths (Fig. 5b). Photoregulation of voltage-gated K+ channels also allows control of spike frequency adaptation. Neurons responding to depolarizing current injection with a single spike in 380 nm light fired repetitively when illuminated with visible light (400–500 nm; Fig. 5c) and the frequency of firing was graded depending on the wavelength of illumination (Fig. 5d). Photoregulation of AAQ-modified channels thus modulates firing threshold (i.e. rheobase) as well as the number of action potentials fired in response to a given current injection (Fig. 5e).
Figure 5. Modulation of neuronal excitability with light.
(a) PAL imparts photocontrol over the current threshold for eliciting action potentials in hippocampal neurons. Current pulses (250 pA, 50 ms; 5 Hz) that fail to induce spikes in 380 nm light, reliably elicit spikes in 500 nm light.
(b) Control of voltage-gated K+ channel blockade and action potential threshold by different wavelengths of light. Graded block of voltage-gated K+ current with increasing wavelengths of light (blue, n = 5 cells) decreases the current injection threshold for eliciting spikes (red, n = 6 cells).
(c) PAL imparts photocontrol over spike frequency adaptation. Current injection (300 pA, 1s) triggers an adapting response consisting of a single spike when K+ channels are unblocked with 380 nm light. K+ channel blockade with longer wavelength light (indicated above colored bars) gradually decreases spike frequency adaptation, allowing the same current injection to elicit repetitive, high frequency firing.
(d) Relationship between illumination wavelength and average spike frequency during depolarizing pulses (n = 9 cells).
(e) Blockade of PAL-modified channels using 500 nm light decreases firing threshold and increases the number of action potentials fired in response to a given current injection (n = 6 cells. Currents were normalized to the maximal amount of current injected in each cell. Current injections lasted 500 ms and ranged from 15 to 360 pA).
Targeted photosensitivity with optical imprinting
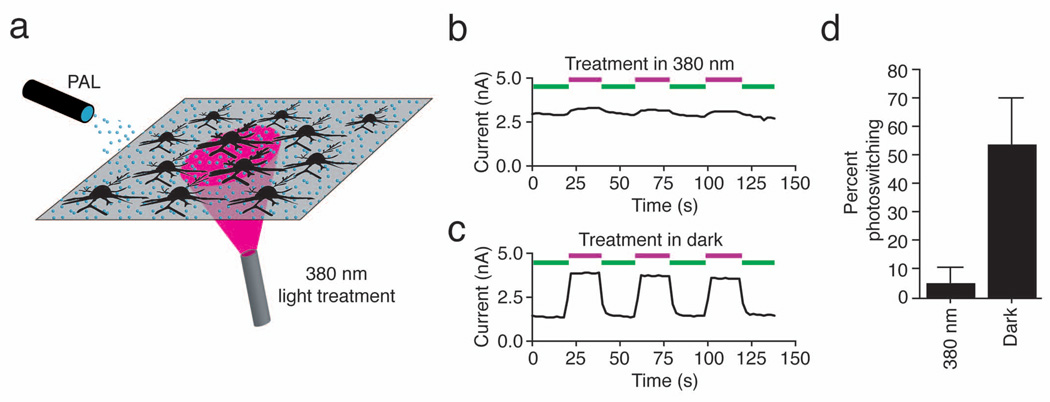
Channel modification by AAQ is dependent on the presence of a nucleophile at the correct distance from the QA binding site. We have shown that covalent attachment occurs when cells are treated in the dark with AAQ in its extended trans form. However, the reactive moiety of PAL will encounter different amino acids when the photoswitch is in its shorter cis configuration. To test whether AAQ can attach to K+ channels in its cis form, we applied it while illuminating a subpopulation of neurons with 380 nm light (Fig. 6a). The remaining neurons were kept in darkness and thus were exposed to AAQ in its trans form. Neurons illuminated with 380 nm light during PAL treatment showed little photosensitivity, whereas those kept in darkness exhibited light-regulated K+ currents (Fig. 6b, c and d; treatment in 380 nm light, 5 ± 5% photoswitching, n = 10; treatment in darkness, 54 ± 16% photoswitching, n = 9). The magnitude of the unblocked K+ current in the two groups of cells was not significantly different (3,515 ± 1022pA, n = 9 and 4,355 ± 1466 pA respectively, n = 10; p > 0.1, Student’s unpaired t-test). Hence, photosensitization of K+ channels by PAL is itself sensitive to light. This feature can be exploited to pre-program specific cells to become light sensitive by employing selective illumination during PAL treatment.
Figure 6. Local illumination during PAL treatment imprints photosensitivity onto specific neurons.
(a) Illustration of experimental setup. A hippocampal neuronal culture was uniformly exposed to 300 µM AAQ. The microscope objective was used to illuminate a subpopulation of neurons with 380 nm light with the remaining neurons were kept in darkness. After treatment, AAQ was replaced with extracellular solution and whole-cell recordings were obtained.
(b) Photosensitization of voltage-gated outward current is prevented in a neuron locally exposed to 380 nm light during AAQ treatment. Currents were elicited by stepping from −70 mV to +30 mV for 250 ms every 2 sec.
(c) Photosensitization of voltage-gated outward current is robust in a neuron kept in darkness during AAQ treatment. Recordings in panels b and c were from neurons on the same coverslip.
(d) Exposure to 380 nm light during AAQ treatment prevents photosensitization of K+ channels (n = 9 and 10 neurons for reaction in the dark and under 380 nm light respectively).
PAL-mediated optical control of activity in neural tissue
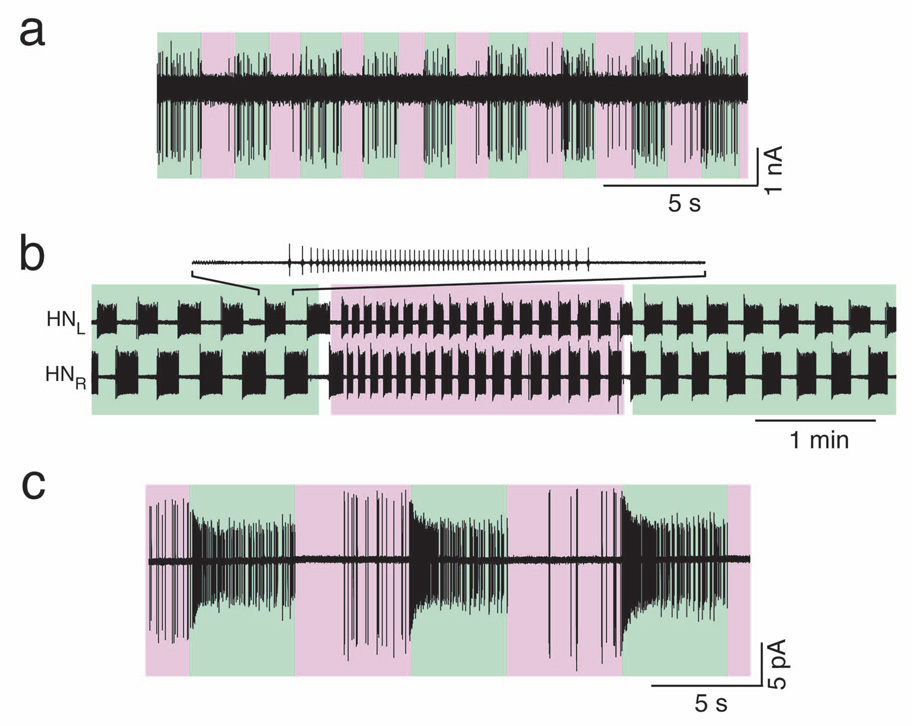
So far, we have shown that PAL molecules enable optical control of cells in culture. For the approach to be effective in tissue, the only additional requirement is that the photoswitch and light reach the cells of interest. We evaluated PAL-mediated optical control of neuronal firing in freshly obtained rat cerebellar slices. After pretreatment with AAQ, we recorded from cerebellar basket cells using the loose-patch configuration. Recordings were obtained in the presence of AMPA, NMDA, and GABAA receptor antagonists, leaving the basket cells synaptically isolated from the rest of the circuit. Blocking AAQ-modified K+ channels with 500 nm light promoted the firing of basket cells while unblocking channels with 360 nm light decreased their firing (Fig. 7a; Photoregulation was observed in 6/6 basket cells in AAQ-treated slices and 0/8 basket cells in untreated slices). These results demonstrate that the penetration of AAQ and the delivery of light are not significantly impeded in brain tissue.
Figure 7. Photocontrol of action potential firing in intact circuits.
(a) Recording from a cerebellar basket cell. Unblocking AAQ-modified K+ channels with 360 nm light (violet) prevents basket cell firing while blocking AAQ-modified K+ channels with 500 nm light (green) depolarizes basket cells, resulting in action potential firing.
(b) Simultaneous loose-patch recordings of bursting activity from the left and right heart interneurons (HNL and HNR) in the leech heart beat CPG. Blocking AAQ-modified K+ channels with 500 nm light (green) increased the bursting cycle period. Unblocking AAQ-modified channels with 380 nm light (violet) decreased the period.
(c) Loose-patch recording of PAL-mediated photoresponses in a rat RGC from a flat-mounted retina. Blocking AAQ-modified K+ channels with 500 nm light (green) promotes high-frequency firing; 380 nm light (violet) inhibits firing.
We next tested AAQ on the medicinal leech Hirudo medicinalis, a system in which the introduction of foreign genes required for other types of light-activated proteins is not widely used. Specifically, we obtained extracellular recordings from the heart central pattern generator (CPG) interneurons (HN cells). HN cells control the contraction of the heart by bursting in alternation and imposing this rhythm on heart motor neurons. The neuropeptide FMRFamide decreases the burst period of HN cells, possibly by modulating voltage-gated K+ currents25. By allowing specific and reversible photoregulation of K+ channels, the PAL approach provides a means to assess the contribution of K+ channels in the bursting pattern of HN cells. We found that unblocking AAQ-modified K+ channels with 380 nm light decreased burst period of HN cells while 500 nm light extended the period (Fig. 7b, n = 4 pairs). This is consistent with modeling studies that predict changes in HN cell burst pattern upon modulation of K+ channels26. The ability to photoregulate neurons in the leech heartbeat CPG demonstrates that the PAL approach is a powerful method to control K+ channels and electrical activity in an intact neural circuit without genetic modification.
The installation of light sensitivity on neurons may enable the artificial input of information downstream from sites of damage or degeneration. For example, the loss of visual function caused by degeneration of rods and cones could be alleviated by treatments that impart light sensitivity on downstream neurons that are normally light insensitive. We tested whether PAL treatment could impart light sensitivity on RGCs, which relay information from the retina to the brain. Loose-patch recordings obtained from RGCs after AAQ treatment showed that their firing increased in 500 nm light and decreased in 380 nm light (Fig. 7c), owing to the block and unblock of AAQ-modified K+ channels. Robust photocontrol of firing was observed in 15/23 RGCs in AAQ-treated retina and 0/10 RGCs in non-AAQ-treated retina. The retina contains a small fraction of RGCs (~2.5%) that express melanopsin and are intrinsically photosensitive27, but it is unlikely that these cells account for the AAQ results reported here.
DISCUSSION
Here we describe a new set of chemical tools for the optical control of endogenous K+ channels and neuronal excitability based on photoswitchable affinity labels (PALs). Traditional affinity labels consist of a ligand that covalently attaches to its target protein via a reactive group. Affinity labels have been used to identify amino acids involved in ligand binding as well as to map distances between selected residues and a ligand binding site in a variety of proteins. Affinity labels can cause permanent activation or inhibition of protein function since covalent attachment results in persistent occupancy of the ligand binding site23. We have expanded on the affinity labeling concept by including a photoisomerizable azobenzene linker between the ligand and the reactive group, enabling photoregulation of ligand occupancy and protein function. By using the pore-blocking QA as the ligand, we have designed PALs selective for K+ channels. The inclusion of a promiscuous reactive group resulted in covalent attachment and photoregulation of several types of K+ channels, but not Na+ and Ca2+ channels. The modular nature of PALs enables easy modification of each functional group in the molecule, yielding a combinatorial toolkit for optical regulation of endogenous proteins. For instance, the reactive group could be altered to enable selective attachment to particular amino acids or protein sequences and the photoisomerizable moiety modified to alter the spectral sensitivity or photoswitching kinetics. Finally, inclusion of the appropriate ligand may target PAL molecules to particular types of ion channels and receptors, allowing optical control of a variety of physiological functions.
Genetically encoded light-activated proteins, such as ChR2, LiGluR, SPARK, and NpHR, have emerged as potent tools for mapping microcircuitry because their expression can be restricted to subpopulations of neurons. In contrast, PAL molecules target intrinsic cellular proteins and thus impart photosensitivity on all treated cells, as long as they express the photoswitch target. Widespread photosensitivity may facilitate functional analysis of processes that involve the coordinated firing of multiple cells. However, if regulation of particular cells is desired, there are three ways to restrict PAL-mediated photocontrol. First, PAL can be applied locally so that only a restricted cell or group of cells becomes photosensitized. Second, local illumination can be used during treatment to optically imprint PAL and thus photosensitize particular cells. Third, after PAL treatment, light of the appropriate wavelength can be projected locally to regulate excitability in individual cells or groups of cells. The key asset of the PAL approach is that light sensitivity can be installed onto freshly obtained tissue, unadulterated by exogenous gene expression and possible developmental consequences of ectopic protein expression.
ChR2 and LiGluR are effective tools for driving precise spike trains in neurons12–14,16,17 because they encode non-specific cation channels that directly lead to membrane depolarization. In contrast, the molecular targets of PAL are voltage-gated K+ channels, which act as a “brake” rather than a direct trigger of activity. Thus, although light flashes can induce action potential firing in PAL-treated neurons, the temporal fidelity is lower than with other light-activated ion channels that directly depolarize the membrane. Instead, the strength of PAL lies in its persistent and effective control over excitability, which enables light to modulate neuronal firing reversibly. Moreover, because photoisomerization can be controlled bi-directionally, the level of cellular excitability can be titrated by controlling the equilibrium between cis and trans photoisomers using different wavelengths of light. Although the isomerization of azobenzene-containing photoswitches occurs over a broad range of wavelengths (cis: 360–400nm; trans: 450–560nm), wavelengths longer than 560 nm do not affect the photoswitch10,24. Thus, it may be possible to combine PAL-mediated optical control with imaging of activity-dependent fluorescent dyes to generate an all-optical system for controlling and recording neuronal excitability.
The distinct mechanism of action of PAL explains why it is particularly effective in controlling the activity of neurons that are spontaneously active; their voltage-gated K+ channels are continually opening and therefore continuously subject to optical regulation with PAL. But in theory, PAL should allow modulation of excitability even in quiescent neurons when they are receiving depolarizing input. More complex features of excitability, such as action potential firing threshold and spike frequency adaptation, can also be rendered light sensitive using PAL. In addition, AAQ treatment allows optical neuromodulation of pacemaker activity in the leech heartbeat CPG, mimicking the actions of a neuropeptide. Voltage-gated K+ channels participate in the control of excitability in a large number of cell types and thus play a crucial role in the regulation of neuronal communication, endocrine and exocrine secretion, cardiac signaling and vascular contraction. By enabling photocontrol of endogenous K+ channels, the PAL approach provides a powerful means to control these physiological functions with light.
Because it targets endogenous proteins the PAL approach is applicable to systems in which introduction of foreign genes is impractical or difficult. Indeed, it is conceivable that modified PAL molecules might ultimately be useful for imparting optical control of neurons in humans, without requiring gene therapy. A particularly relevant tissue for PAL-mediated optical regulation is the retina, the sole part of the nervous system that is exposed to light in vivo. Our data show that AAQ confers light-sensitivity onto RGCs that are not intrinsically light sensitive, raising the possibility that PAL treatment, along with an appropriate optical system, may be used as an alternative to multielectrode-based retinal prosthetic devices28 to restore visual function in retinas with damaged or degenerated rod and cone photoreceptors.
METHODS
Cell culture, plasmids and transfection
HEK293 cells were grown in DMEM containing 5% fetal bovine serum (FBS). For HEK293T cells, 500 µg/ml G-418 was also included. For electrophysiology, cells were plated at 12 × 103 cells/cm2 on poly-L-lysine coated coverslips and transfected using the calcium phosphate method29. The Shaker H4 used here contained the Δ6–46 deletion to minimize fast inactivation30. Kv4.2 was co-transfected with KChIP3, at a 4:1 ratio31. Recordings were performed 24–48 hours after transfection for K+ channels and 96 hours for Nav1.2. L-type Ca2+ channels currents were recorded from GH3 cells grown in F-12K containing 15% horse serum and 2.5% FBS, 48 hours after plating. Hippocampal neurons were prepared from neonatal rats according to standard procedures32, plated at 50 × 103 cells/cm2 on poly-L-lysine coated coverslips and grown in MEM containing 5% FBS, 20 mM glucose, B27 (Invitrogen), glutamine and Mito+ Serum Extender (BD Biosciences). Recordings were performed 14–25 days after plating. Animal care and experimental protocols were approved by the UC Berkeley Animal Care and Use Committee.
PAL attachment
Cells were incubated at 37°C in the dark for 15 minutes with 200–300 µM (hippocampal neurons) or 400 µM (HEK293, HEK293T and GH3 cells) AAQ diluted in bath solution. Cerebellar slices were treated at room temperature in the dark with 200 µM AAQ for 8 minutes. Retinas and leech ganglia were treated with 150 µM and 400 µM AAQ respectively.
Illumination for photoswitching was provided using a xenon lamp (175 W) with narrow band pass filters (380BP10 and 500BP5). Light output was measured using a handheld Newport meter (840-C model). At the back of the objective, light output was 0.3 mW/cm2 for the 380 nm light and 2.5 mW/cm2 the 500 nm light. When measured through a 40X objective and normalized to the focal area at the specimen plane, light output was 0.5 mW/mm2 and 3.5 mW/mm2 for the 380 nm and 500 nm light respectively. For some experiments, illumination was provided with a monochromator (TILL Photonics, Polychrome V).
Data analysis
Percent photoswitching is defined as the difference between current in 380 and 500 nm divided by current in 380 nm light. All data reported in the text or shown in bar graphs are averages ± standard deviation.
Electrophysiological recordings from cultured cells
Recordings were made in the whole-cell patch clamp configuration using pipettes with 3–5 MΩ resistance. To elicit voltage-gated K+ currents from neurons and HEK293 cells, holding potential was set to −70 mV and stepped to +30mV for 250 ms. To elicit voltage-gated Na+ currents, HEK293T cells expressing Nav1.2 were held at −80 mV and stepped to 0 mV for 250 ms. L-type Ca2+ currents were recorded in GH3 cells and elicited by stepping to +20 mV for 200 ms from a holding potential of −40mV. Bath and intracellular solutions compositions are provided in the supplementary methods.
Tissue preparation and recordings
Parasagittal cerebellar slices (300 µm) were prepared from P14–P20 rats using standard techniques approved by the UCLA Animal Care Committee. After sectioning, slices were incubated for 30 minutes at 35 °C in aCSF (composition in supplementary methods) then brought to room temperature for PAL treatment and recording. Loose-patch recordings were made in the presence of 6,7-dinitroquinoxaline-2,3-[1H,4H]-dione, gabazine and [RS]-3-[2-carboxypiperazin-4-yl]-propyl-1-phosphonic acid using pipettes with 3–5 MΩ resistance. The depth of recorded basket cells was approximately 25–30 µm. Retinas were dissected from P50–P60 rats under continuous flow of oxygenated saline solution (composition in supplementary methods). Dissected retinas were treated at 37°C with 20 U/ml cysteine-activated papain for 8–20 minutes then washed with saline containing 10 mg/ml BSA and 10 mg/ml trypsin inhibitor followed by PAL treatment and recording using the loose-patch configuration. Dissections and recordings from leech ganglia were conducted as described previously33. Extracellular signals from heart interneurons (HNs) were obtained using the loose-patch configuration.
Supplementary Material
Supplementary Figure 1 Neuronal survival after PAL treatment
Supplementary Methods
ACKNOWLEDGEMENTS
We thank E. Isacoff, J. Chambers, S.-Y. Choi and S. Jackman for helpful comments, J. Flannery and K. Greenberg for help with RGC experiments, D. Johnston, (University of Texas at Austin) B. Rothberg (University of Texas Health Science Center at San Antonio), B. Rudy (New York University), W. Catterall (University of Washington) and J. Trimmer (University of California Davis) for providing plasmids. This work was supported by the Howard Hughes Medical Institute (KB), the National Science Foundation (IOB-BSC to WBK), Microsoft Research Labs (WBK) and the National Institutes of Health (GM057027 to DT, MH43396 to WBK, and EY16249 to RHK). DAW, QG and WBK thank A. Blankenship and M. Feller for the loan of their xenon lamp.
Footnotes
Supplementary information is available on the Nature Methods website.
REFERENCES
- 1.Callaway EM, Yuste R. Stimulating neurons with light. Curr. Opin. Neurobiol. 2002;12:587–592. doi: 10.1016/s0959-4388(02)00364-1. [DOI] [PubMed] [Google Scholar]
- 2.Kramer RH, Chambers JJ, Trauner D. Photochemical tools for remote control of ion channels in excitable cells. Nat. Chem. Biol. 2005;1:360–365. doi: 10.1038/nchembio750. [DOI] [PubMed] [Google Scholar]
- 3.Miesenbock G, Kevrekidis IG. Optical imaging and control of genetically designated neurons in functioning circuits. Annu. Rev. Neurosci. 2005;28:533–563. doi: 10.1146/annurev.neuro.28.051804.101610. [DOI] [PubMed] [Google Scholar]
- 4.Zhang F, Wang LP, Boyden ES, Deisseroth K. Channelrhodopsin-2 and optical control of excitable cells. Nat. Methods. 2006;3:785–792. doi: 10.1038/nmeth936. [DOI] [PubMed] [Google Scholar]
- 5.Ellis-Davies GC. Caged compounds: photorelease technology for control of cellular chemistry and physiology. Nat. Methods. 2007;4:619–628. doi: 10.1038/nmeth1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalva MB, Katz LC. Rearrangements of synaptic connections in visual cortex revealed by laser photostimulation. Science. 1994;265:255–258. doi: 10.1126/science.7912852. [DOI] [PubMed] [Google Scholar]
- 7.Callaway EM, Katz LC. Photostimulation using caged glutamate reveals functional circuitry in living brain slices. Proc. Natl. Acad. Sci. USA. 1993;90:7661–7665. doi: 10.1073/pnas.90.16.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shepherd GM, Pologruto TA, Svoboda K. Circuit analysis of experience-dependent plasticity in the developing rat barrel cortex. Neuron. 2003;38:277–289. doi: 10.1016/s0896-6273(03)00152-1. [DOI] [PubMed] [Google Scholar]
- 9.Nikolenko V, Poskanzer KE, Yuste R. Two-photon photostimulation and imaging of neural circuits. Nat. Methods. 2007;4:943–950. doi: 10.1038/nmeth1105. [DOI] [PubMed] [Google Scholar]
- 10.Banghart M, Borges K, Isacoff E, Trauner D, Kramer RH. Light-activated ion channels for remote control of neuronal firing. Nat. Neurosci. 2004;7:1381–1386. doi: 10.1038/nn1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volgraf M, et al. Allosteric control of an ionotropic glutamate receptor with an optical switch. Nat. Chem. Biol. 2006;2:47–52. doi: 10.1038/nchembio756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szobota S, et al. Remote control of neuronal activity with a light-gated glutamate receptor. Neuron. 2007;54:535–545. doi: 10.1016/j.neuron.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Zhang F, et al. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- 14.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 15.Bi A, et al. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron. 2006;50:23–33. doi: 10.1016/j.neuron.2006.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, et al. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc. Natl. Acad. Sci. USA. 2005;102:17816–17821. doi: 10.1073/pnas.0509030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han X, Boyden ES. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS ONE. 2007;2:e299. doi: 10.1371/journal.pone.0000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat. Neurosci. 2007 doi: 10.1038/nn1891. [DOI] [PubMed] [Google Scholar]
- 19.Arenkiel BR, et al. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron. 2007;54:205–218. doi: 10.1016/j.neuron.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagel G, et al. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr. Biol. 2005;15:2279–2284. doi: 10.1016/j.cub.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, et al. High-speed mapping of synaptic connectivity using photostimulation in Channelrhodopsin-2 transgenic mice. Proc. Natl. Acad. Sci. USA. 2007;104:8143–8148. doi: 10.1073/pnas.0700384104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schroll C, et al. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr. Biol. 2006;16:1741–1747. doi: 10.1016/j.cub.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 23.Wold F. Affinity labeling--an overview. Methods Enzymol. 1977;46:3–14. doi: 10.1016/s0076-6879(77)46005-1. [DOI] [PubMed] [Google Scholar]
- 24.Gorostiza P, et al. Mechanisms of photoswitch conjugation and light activation of an ionotropic glutamate receptor. Proc. Natl. Acad. Sci. USA. 2007;104:10865–10870. doi: 10.1073/pnas.0701274104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nadim F, Calabrese RL. A slow outward current activated by FMRFamide in heart interneurons of the medicinal leech. J. Neurosci. 1997;17:4461–4472. doi: 10.1523/JNEUROSCI.17-11-04461.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill AA, Lu J, Masino MA, Olsen OH, Calabrese RL. A model of a segmental oscillator in the leech heartbeat neuronal network. J. Comput. Neurosci. 2001;10:281–302. doi: 10.1023/a:1011216131638. [DOI] [PubMed] [Google Scholar]
- 27.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lakhanpal RR, et al. Advances in the development of visual prostheses. Curr. Opin. Ophthalmol. 2003;14:122–127. doi: 10.1097/00055735-200306000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Xia Z, Dudek H, Miranti CK, Greenberg ME. Calcium influx via the NMDA receptor induces immediate early gene transcription by a MAP kinase/ERK-dependent mechanism. J. Neurosci. 1996;16:5425–5436. doi: 10.1523/JNEUROSCI.16-17-05425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoshi T, Zagotta WN, Aldrich RW. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 1990;250:533–538. doi: 10.1126/science.2122519. [DOI] [PubMed] [Google Scholar]
- 31.An WF. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–556. doi: 10.1038/35000592. [DOI] [PubMed] [Google Scholar]
- 32.Higgins D, Banker GA. Primary dissociated cell cultures. In: Banker GA, Goslin KA, editors. Culturing Nerve Cells. Cambridge, Massachusetts: MIT Press; 1998. [Google Scholar]
- 33.Masino MA, Calabrese RL. Phase relationships between segmentally organized oscillators in the leech heartbeat pattern generating network. J. Neurophysiol. 2002;87:1572–1585. doi: 10.1152/jn.00336.2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Neuronal survival after PAL treatment
Supplementary Methods