Abstract
The major inhibitory neurotransmitter in the brain, γ-aminobutyric acid (GABA), has only partial efficacy at certain subtypes of GABAA receptors. To characterize these minor receptor populations in rat and mouse brains, we used autoradiographic imaging of t-butylbicyclophosphoro[35S]thionate ([35S]TBPS) binding to GABAA receptors in brain sections and compared the displacing capacities of 10 mM GABA and 1 mM 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol (THIP), a competitive GABA-site agonist. Brains from GABAA receptor α1, α4, δ, and α4 + δ subunit knockout (KO) mouse lines were used to understand the contribution of these particular receptor subunits to “GABA-insensitive” (GIS) [35S]TBPS binding. THIP displaced more [35S]TBPS binding than GABA in several brain regions, indicating that THIP also inhibited GIS-binding. In these regions, GABA prevented the effect of THIP on GIS-binding. GIS-binding was increased in the cerebellar granule cell layer of δ KO and α4 + δ KO mice, being only slightly diminished in that of α1 KO mice. In the thalamus and some other forebrain regions of wild-type mice, a significant amount of GIS-binding was detected. This GIS-binding was higher in α4 KO mice. However, it was fully abolished in α1 KO mice, indicating that the α1 subunit was obligatory for the GIS-binding in the forebrain.
Our results suggest that native GABAA receptors in brain sections showing reduced displacing capacity of [35S]TBPS binding by GABA (partial agonism) minimally require the assembly of α1 and β subunits in the forebrain and of α6 and β subunits in the cerebellar granule cell layer. These receptors may function as extrasynaptic GABAA receptors.
Keywords: GABAA receptor, [35S]TBPS autoradiography, THIP, Gaboxadol, Partial agonism, Thalamus, Cerebellum
1. Introduction
γ-Aminobutyric acid (GABA) A-type receptors are pentameric ligand-gated anion channels mediating inhibitory currents in the adult mammalian central nervous system. They are composed from 16 receptor subunits (α1-6, β1-3, γ 1-3, δ, ε, θ and π) with different brain regional gene expression profiles (Wisden et al., 1992), forming the basis for GABAA receptor diversity. Several receptor subunit combinations (subtypes) can be distinguished by their affinities for various drugs (Olsen et al., 1990; Korpi and Lüddens 1993; Sieghart 1995; Mitchell et al., 2008). Receptor subtypes may also differ in their synaptic versus extrasynaptic location, conductance and opening time (Brickley, et al., 1999; Birnir and Korpi, 2007; Mody, 2008).
t-Butylbicyclophosphorothionate (TBPS) is a picrotoxin-type GABAA receptor blocker, which is thought to bind inside the GABAA receptor ion channel. This view is suggested by experiments carried out using site-directed mutagenesis and cysteine accessibility methods (Xu et al., 1995; Bali and Akabas, 2007). Dissociation of [35S]TBPS correlates well with the function of the ion channel measured by anion flux (Im and Blakeman, 1991). In general, [35S]TBPS dissociates from its binding sites when agonists are applied and this effect can be inhibited by antagonists (Squires et al. 1983; Ticku and Ramanjaneylu, 1984; Lüddens and Korpi, 1995). It is likely that [35S]TBPS is dissociated from its channel binding sites due to agonist binding-mediated allosteric effects on the channel structure, but the effect of receptor desensitization for the dissociation, especially during long incubations, cannot be fully excluded. The [35S]TBPS binding assay has been used as a simple biochemical assay of GABAA receptor function, even though it is an indirect method and the detailed binding mechanisms are still unknown.
Previous experiments with [35S]TBPS have revealed a proportion of GABAA receptors displaying atypical allosteric coupling between the agonist and channel sites: GABA fails to induce full displacement of [35S]TBPS, leaving the so-called GABA-insensitive [35S]TBPS binding (GIS-binding) (Sinkkonen et al., 2001b). This binding component is detectable in brain sections by autoradiography. GIS-binding is enriched in the cerebellar granule cell layer and thalamic nuclei, two brain regions in which GABAA receptor subunits δ, α6 and α4 are enriched (Wisden et al., 1992; Pirker et al., 2000). Experiments with recombinant receptors have revealed that many GABAA receptor subtypes may contribute to GIS-binding (Sinkkonen et al., 2001a). Interestingly, GABAA receptors containing α6 subunit express more GIS-binding than α1 subunit-containing receptors, and mice lacking α6 have reduced GIS-binding (Sinkkonen et al., 2001a). Mice heterozygous for γ2 subunit deletion display increased GIS-binding, suggesting a role for dimeric αβ receptors (Sinkkonen et al., 2004a). In line with this observation, transgenic mice ectopically expressing extrasynaptic dimeric α6β GABAA receptors in the hippocampus have increased GIS-binding and increased tonic inhibition in that brain region (Wisden et al., 2002; Sinkkonen et al., 2004b). Additionally, these α6 transgenic mice have increased amount of GABAA receptors in the hippocampus, in which the competitive GABA-site agonist 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridine-3-ol (THIP, gaboxadol) is stronger than GABA (Saarelainen et al., 2008). GABA actually inhibits the THIP effect in [35S]TBPS binding autoradiography of this mouse model and in electrophysiology of recombinant α6β3(δ) receptors. Thus, GABA is a partial agonist at the novel extrasynaptic receptors of this mouse line. δ KO mice do not have deficient forebrain GIS-binding (Sinkkonen et al., 2001a). The obvious hypothesis for the present study was that the α4 KO mice would have dramatically reduced levels of GIS-binding, similarly to the cerebellum of α6 KO mice.
In this study, we have characterized the capacities of high concentrations of GABA and THIP to displace [35S]TBPS binding in a number of animal models using ligand autoradiography method. First, we studied the brain regional distribution of displacing capacities using rat brain sections. Then, we used mouse lines deficient in α1, α4, δ, or α4 and δ subunits, to see which of the subunits was responsible for the GIS-binding in the forebrain.
2. Methods
2.1. Animals
Male Wistar rats (n=9, Harlan Netherlands B.V, Netherlands) were decapitated at four months of age and whole brains were carefully dissected out, rinsed in ice-cold saline and frozen on dry ice. Frozen brains were wrapped in plastic and stored at −80 °C until used.
Four different GABAA receptor subunit knockout mouse lines were used along with respective wild-type controls. The α1 subunit knockout mice (α1 KO; n=5) and corresponding littermate wild-type controls (WT2; n=6) were of a mixed C57BL/6J, FVB, and Strain 129S1/X1 genetic background and were created by interbreeding heterozygous α1 KO mice (Vicini et al., 2001). The α4 subunit knockouts (α4 KO; n=3 for [35S]TBPS autoradiography, n=6 for [3H]Ro 15-4513 autoradiography), δ subunit knockouts (δ KO; n=3), combined α4 and δ subunit knockouts (α4+δ KO; n=5) and their wild-type controls (WT1; n=4 for [35S]TBPS autoradiography, n=6 for [3H]Ro 15-4513 autoradiography) were of a mixed C57BL6/J and Strain 129S1/X1 genetic background. They were created by first interbreeding α4 KO (Chandra et al., 2006) and δ KO (Mihalek et al., 1999) and then interbreeding the double heterozygous offspring. Mice were decapitated at the age of 2-3 months, and whole brains were carefully dissected out, rinsed in ice-cold saline and frozen on dry ice. The frozen brains were wrapped in plastic and stored at −80 °C until used.
All animals were group housed, given standard rodent chow and tap water ad libitum, and maintained on a 12-h alternating light/dark schedule, at the temperature of 20-22 °C. All procedures were approved by the Institutional Animal Use and Care committees of the University of Pittsburgh, the University of Helsinki, and the Southern Finland provincial government.
2.2. Ligand autoradiography
[35S]TBPS autoradiography to examine brain regional distribution of GIS-binding was carried out as described in detail (Sinkkonen et al., 2001b). Frozen brains were cut to 14-μm frontal or horizontal sections using a Leica CM 3050 S cryostat. Sections were thaw-mounted to gelatin-coated object glasses and stored at -80 °C until autoradiography. Each object glass contained sections from all mouse groups to be compared, with the cutting order being balanced for the groups.
Sections were preincubated in an ice-water bath for 15 min in 50 mM Tris-HCl (pH 7.4) supplemented with 120 mM NaCl to remove traces of endogenous GABA, thus minimizing its effect on [35S]TBPS binding kinetics. Basal [35S]TBPS binding was performed in fresh incubation buffer of the above composition supplemented with 2 nM [35S]TBPS (700-900 CPM/μl final incubation solution; PerkinElmer Life and Analytical Sciences, Boston, USA) at 20-22 °C for 90 min. Displacement of [35S]TBPS binding was studied in the presence of various concentrations of GABA, THIP or GABA + THIP. After preliminary experiments with a range of saturating millimolar GABA (1-70 mM; Sigma Chemical Company, St. Louis, USA) and THIP (1-10 mM; THIP-HCl, H. Lundbeck A/S, Copenhagen, Denmark) concentrations, optimal concentrations for revealing interations between GABA and THIP were found to be 10 mM GABA and 1 mM THIP, as reversal of THIP inhibition needed about 10-fold higher concentration of GABA. In the TBPS binding assay, THIP acts like a full agonist (Rabe et al., 2000). The nonspecific binding was determined in the presence of 100 μM picrotoxinin (Sigma). After incubation, to focus on the GIS binding, the sections were subjected to a thorough washing step (three times 30 min in ice-cold, NaCl-free 10 mM Tris-HCl, pH 7.4). Sections were then dipped in ice-cold distilled water (0-4 °C) and dried under a fan at 20-22 °C. Then, the sections were exposed to Biomax MR films (Eastman Kodak, Rochester, USA) with plastic 14C-microscale standards (GE Healthcare, Little Chalfont, Buckinghamshire, UK) for two days (basal binding conditions) to three weeks (other binding conditions).
The autoradiographic assay of [3H]Ro 15-4513 binding was carried out as previously described (Mäkelä et al., 1997). Briefly, mouse brain sections were preincubated in an ice-water bath for 15 min in 50 mM Tris-HCl (pH 7.4) supplemented with 120 mM NaCl. Final incubation in the preincubation buffer was performed in the dark with 15 nM [3H]Ro 15-4513 (PerkinElmer) at 0-4 °C for 60 min. The binding was also studied in the presence of 1 and 10 μM diazepam (Orion Pharma, Espoo, Finland; dissolved in dimethylsuphoxide) to reveal the α6 and α4 subunit-dependent diazepam-insensitive binding. Nonspecific binding was determined in the presence of 10 μM flumazenil (Hoffman-La Roche, Basel, Switzerland). It was at the background level (not shown). After incubation, the sections were washed in ice-cold incubation buffer (0-4 °C) twice for 60 s. Sections were then dipped into ice-cold distilled water, air-dried at room temperature, and exposed with plastic 3H-microscale standards to Biomax MR films for 5 weeks.
Films of rat brain sections and [3H]Ro 15-4513 experiment were quantified using MCID M5 image analysis devices and programs (Imaging Research Inc., St. Catharines, Ontario, Canada). Those of [35S]TBPS experiments on mouse sections were first scanned using an EPSON Expression 1680 Pro scanner and EPSON Scan v. 1.11e program and then analysed with Scion Image analysis program (Scion Corporation, Frederick, Maryland, USA). The microscale standards were used as the reference and the resulting binding values were converted to radioactivity levels estimated for grey matter areas (nCi/g for 35S and nCi/mg for 3H). Images from representative films were produced by scanning the films using the same scanner and scanning program and Corel Paint Shop Pro Photo XI (version 11.20, Corel Corporation, Ottawa, Ontario, Canada) and Corel Draw X3.
2.3. Statistics
Specific [35S]TBPS binding values were determined by subtracting the nonspecific binding values from the corresponding total binding values. Binding densities for each brain area were averaged from measurements from three to nine animals. Paired or standard t-test and one-way analysis of variance and Bonferroni post-hoc test were used when determining statistical significance of the differences between groups (Prism program, version 5.00, GraphPad Software, San Diego, CA).
3. Results
3.1. Brain regional distribution of [35S]TBPS displacing capacities of 10 mM GABA and 1 mM THIP
Basal [35S]TBPS binding was seen throughout the rat brain (Fig. 1). Picrotoxinin at 100 μM displaced almost all the [35S]TBPS binding, leaving a negligible level of nonspecific binding. A saturating GABA concentration, 10 mM, inhibited the binding almost to the background levels in most brain areas. Significant binding was left in certain areas, such as the thalamus and granule cell layer of cerebellum, revealing the GIS-binding as shown previously (Sinkkonen et al., 2001b). When THIP was applied at 1 mM, a concentration which has been shown to inhibit [35S]TBPS binding more effectively than 10 mM GABA in regions with GIS-binding and less effectively elsewhere (Saarelainen et al., 2008), a different binding pattern was revealed. THIP inhibited the binding to a lesser degree in most brain areas compared to GABA, although its effect was greater in a few regions (Table 1). In the external layer of the cerebral cortex, the ventroposterolateral thalamus and granule cell layer of the cerebellum there was less [35S]TBPS binding in the presence of THIP than GIS-binding (Table 1).
Fig. 1.

The effects of GABA and THIP on picrotoxinin-sensitive [35S]TBPS binding in serial frontal sections of rat brain. BrS, brain stem; G, gelatinosus nucleus of thalamus; Gr, granule cell layer of cerebellum; nRT, reticular nucleus of thalamus; Th, thalamus; VL, ventrolateral thalamus; VPL, ventroposterolateral thalamus; SN, substantia nigra. Representative images were scanned and processed using identical scaling for brightness and contrast; the basal images were scanned from films after 2-day exposure and the other images from films after 21-day exposure.
Table 1.
[35S]TBPS binding and its modulation by GABA and THIP in frontal sections of the rat brain as revealed by quantitative autoradiography.
| Brain area | Basal binding (nCi/mg) |
GABA 10mM (% of basal) |
THIP 1mM (% of basal) |
|---|---|---|---|
| Olfactory bulb | 295±36 | 3.1±0.6 | 3.4±0.7 |
| Cerebral cortex, external layer | 543±66 | 1.3±0.2 | 1.1±0.2b |
| Cerebral cortex, internal layer | 1026±73 | 0.9±0.1 | 1.2±0.2a |
| Anterior cortical amygdaloid nucleus | 1840±261 | 1.1±0.2 | 2.0±0.2c |
| Medial amygdaloid nucleus | 1535±198 | 1.0±0.2 | 2.3±0.4b |
| Anterior amygdaloid area | 2049±219 | 1.1±0.2 | 2.0±0.3b |
| Caudate-putamen anterior | 630±42 | 0.9±0.1 | 1.5±0.2b |
| Caudate-putamen posterior | 523±23 | 1.1±0.1 | 1.7±0.1b |
| Globus pallidus | 1704±158 | 0.7±0.1 | 2.6±0.2c |
| Globus pallidus-ventral pallidum | 1641±164 | 0.8±0.1 | 2.5±0.2c |
| Globus pallidus-basal nucleus of Meynert | 1395±94 | 0.8±0.1 | 2.8±0.2c |
| Nucleus vertical limb of diagonal band | 1787±147 | 2.1±0.2 | 2.1±0.2 |
| Nucleus horizontal limb of diagonal band | 1819±150 | 1.6±0.1 | 2.5±0.3a |
| Lateral preoptic area | 1527±167 | 1.2±0.1 | 1.6±0.3 |
| Lateral hypothalamus | 1375±166 | 0.7±0.1 | 1.6±0.3b |
| CA3 area of hippocampus | 510±59 | 1.5±0.2 | 1.4±0.2 |
| CA4 area of hippocampus | 541±40 | 1.3±0.2 | 2.3±0.3c |
| Reticular nucleus of thalamus | 82±9 | 4.3±2.6 | 20±6b |
| Gelatinosus nucleus of thalamus | 1334±125 | 3.0±0.4 | 2.6±0.2 |
| Ventrolateral thalamic nucleus | 1049±105 | 3.4±0.6 | 2.6±0.3 |
| Ventroposterolateral thalamic nucleus | 907±88 | 4.2±0.5 | 3.0±0.5b |
| Medial geniculate nucleus | 1030±29 | 2.6±0.2 | 2.8±0.2 |
| Substantia nigra, reticular part | 1487±115 | 0.8±0.2 | 1.8±0.1b |
| Paranigral nucleus | 1028±123 | 1.0±0.2 | 3.0±0.7b |
| Oculomotor nucleus | 1198±138 | 1.3±0.2 | 3.4±0.4c |
| Nucleus lateral lemniscus dorsal/ventral | 1452±190 | 1.4±0.2 | 2.6±0.4b |
| Superficial grey layer of superior colliculus | 1642±121 | 1.4±0.2 | 3.0±0.3b |
| Inferior colliculus | 1701±119 | 1.2±0.1 | 2.4±0.2c |
| Olivary pretectal nucleus | 1279±131 | 2.4±0.2 | 2.9±0.3a |
| Anterior pretectal area | 1808±80 | 1.5±0.2 | 3.1±0.3b |
| Central gray | 1076±93 | 1.0±0.1 | 2.1±0.3a |
| Brain stem | 493±71 | 1.6±0.1 | 9.2±1.9b |
| Granule cell layer of cerebellum | 281±26 | 7.1±0.6 | 4.6±0.4b |
The values (mean ± SEM, n = 9) of basal binding are shown as nCi/mg, others are per cent values of the corresponding basal [35S]TBPS binding.
p < 0.05 for the significance of difference between the proportions of remaining [35S]TBPS binding after application of THIP compared to GABA (paired t-test).
p < 0.01 for the significance of difference between the proportions of remaining [35S]TBPS binding after application of THIP compared to GABA (paired t-test).
p < 0.001 for the significance of difference between the proportions of remaining [35S]TBPS binding after application of THIP compared to GABA (paired t-test).
3.2. GABA-insensitive, THIP-sensitive [35S]TBPS binding in different knockout mouse lines
Recently, Saarelainen et al. (2008) demonstrated that transgenic mice over-expressing the GABAA receptor α6 subunit gene ectopically in forebrain had increased GIS-binding in the hippocampus, and that THIP decreased [35S]TBPS binding more efficiently than GABA. They also found that GABA competitively inhibited the effect of THIP on the binding in a concentration-dependent manner (i.e., high concentration of GABA reversed the THIP-induced inhibition of the GIS-binding). Recombinant dimeric α6β3 receptors expressed in HEK 293 cells possessed similar properties. In the present study, we wanted to explore which receptor subtype might be responsible for the GIS-binding detected in the native brain (Fig. 1). Since this binding is enriched in the thalamus and cerebellar granule cell layer, we concentrated on these brain regions. We used knockout mouse lines lacking the genes encoding for α1, α4, δ and α4 + δ subunits of the GABAA receptor (α1 KO, α4 KO, δ KO and α4 + δ KO, respectively) with respective wild-type controls, and performed [35S]TBPS autoradiography with them.
High basal [35S]TBPS binding was seen in all mouse lines (Fig. 2a, Fig. 3a). There were certain differences in the basal [35S]TBPS binding between the wild-type and knockout mice (Table 2). In the thalamus, α4 and α4 + δ KO mice had significantly increased basal binding when compared to the wild-type mice, and in the δ KO mice the trend was similar (p = 0.10). In contrast, α1 KO mice had significantly decreased [35S]TBPS binding in the thalamus as compared to the wild-type mice. In the granule cell layer of the cerebellum, δ KO and α4 + δ KO mice had increased basal binding, where as α4 KO and α1 KO mice had unaltered binding.
Fig. 2.
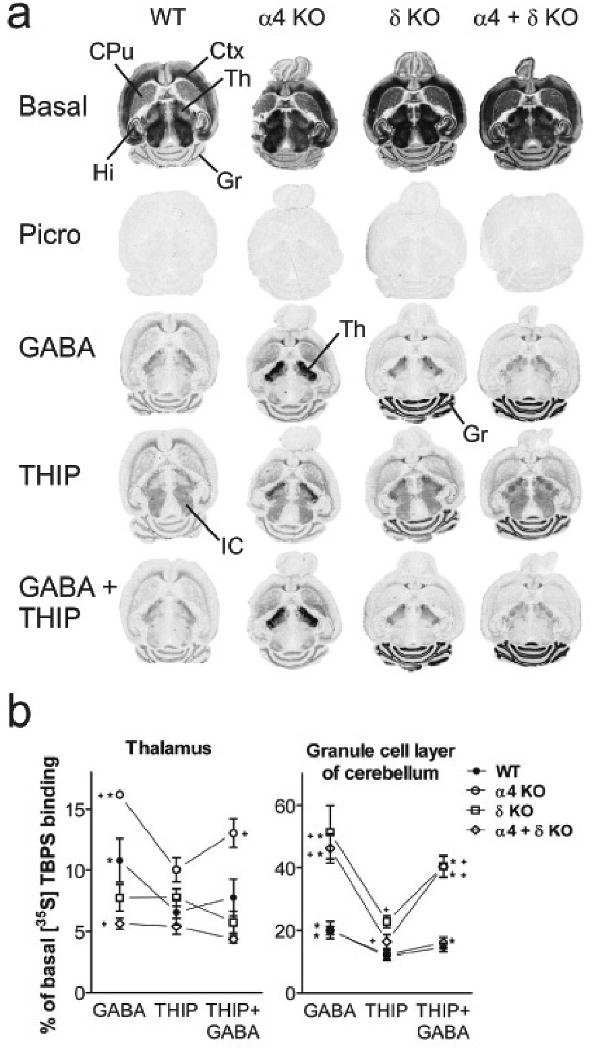
[35S]TBPS binding and its modulation by GABA, THIP or both in horizontal sections of wild-type, α4 KO, δ KO and α4+δ KO mouse brains. a, Representative images were scanned and processed using identical scaling for brightness and contrast; the basal images were scanned from films after 2-day exposure and the other images from films after 21-day exposure. Ctx, cortex; CPu, caudate-putamen; Gr, granule cell layer of cerebellum; Hi, hippocampus; IC, inferior colliculus; Th, thalamus. b, Quantitative autoradiography results of the effects of 10 mM GABA, 1 mM THIP or both on [35S]TBPS binding in the thalamus and granule cell layer of cerebellum of different mouse lines. Data are means ± SEM (n = 3 for α4 KO, δ KO, n = 4 for wild type and n = 5 for α4+δ KO mice), expressed as percent of the corresponding basal [35S]TBPS binding value. *p < 0.05 for the significance of the difference between GABA and THIP or between THIP and THIP + GABA (paired t-test). + p < 0.05 for the significance of the difference from the corresponding wild-type value (Bonferroni t-test).
Fig. 3.
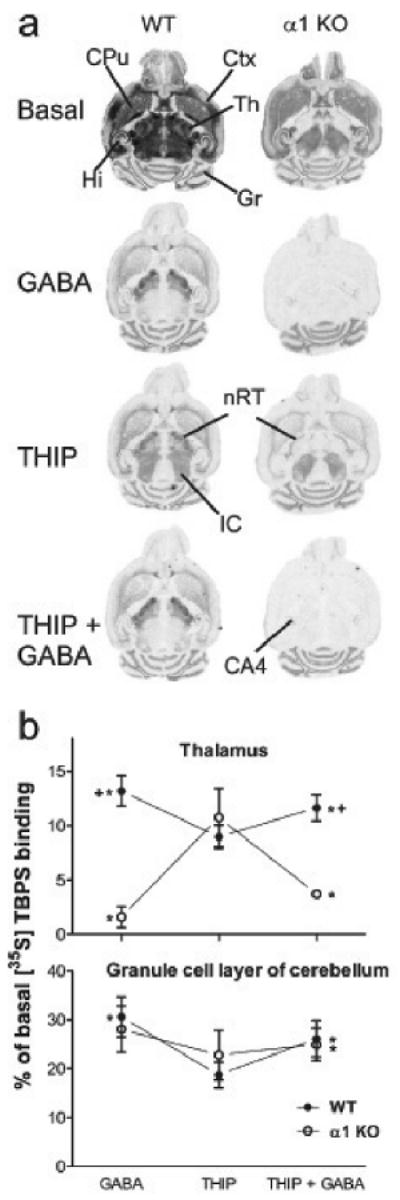
Modulation of [35S]TBPS binding by GABA and THIP in α1 KO mice. a, Representative images of [35S]TBPS binding and its modulation by 10 mM GABA, 1 mM THIP or both on wild-type and α1 KO mouse horizontal brain sections. CA4, CA4 area of the hippocampus; Ctx, cortex; CPu, caudate-putamen; Gr, granule cell layer of the cerebellum; Hi, hippocampus; nRT, reticular nucleus of the thalamus; Th, thalamus; IC, inferior colliculus. All the binding was abolished in the presence of 100 μM picrotoxinin as in Figs 1 and 2 (not shown). The representative images were scanned and processed using identical scaling for brightness and contrast, but the basal binding image was taken from films exposed only for 2 days rather than 21 days. b, Quantitative proportions of the [35S]TBPS binding in the presence of 10 mM GABA, 1 mM THIP or both in the thalamus and granule cell layer of cerebellum. Data are means ± SEM (n = 5 for α1 KO and n = 6 for wild-type mice) and expressed as percent of the corresponding basal [35S]TBPS binding value. *p < 0.05 for the significance of the difference between GABA and THIP or between THIP and THIP + GABA (paired t-test). + p < 0.05 for the significance of the difference from the corresponding wild-type value (Bonferroni t-test).
Table 2.
Basal [35S]TBPS binding in the thalamus and cerebellar granule cell layer in horizontal brain sections of GABAA receptor subunit knockout mice and their wild-type controls.
| Mouse line | Thalamus | Granule cell layer |
|---|---|---|
| WT1 | 635±93 | 322±55 |
| δ KO | 923±113 | 654±38b |
| α4 KO | 975±51a | 364±37 |
| α4+ δ KO | 959±60a | 677±46b |
| WT2 | 618±64 | 220±34 |
| α1 KO | 70±4c | 226±31 |
Values are means ± SEM in nCi/g (n=4 for wild-type WT1, n=3 for δ KO and α4 KO, n=5 for α4 + δ KO and α1 KO, n=6 for wild-type WT2).
p < 0.05 for the significance of the difference from the corresponding value of the wild-type control (t-test).
p < 0.01 for the significance of the difference from the corresponding value of the wild-type control (t-test).
p < 0.001 for the significance of the difference from the corresponding value of the wild-type control (t-test).
The wild-type mice displayed the same kind of GIS-binding profile as described earlier (Sinkkonen et al., 2001b). In wild-type mice, similar to rats (Fig. 1), 1 mM THIP inhibited significantly more [35S]TBPS binding when compared to 10 mM GABA in the granule cell layer of the cerebellum and in the thalamus (Figs 2-3). Significantly less binding remained after application of THIP when compared to co-application of GABA and THIP (Figs 2b, 3b), which suggests that GABA acts as a partial agonist when compared to THIP. Also in the α4, δ and α4 + δ KO mice, THIP inhibited significantly more binding in the cerebellar granule cell layer than GABA (Fig. 2b), the reduced displacing capacity of GABA being the clearest in the δ subunit-deficient mice. The same was true for the thalamus of α4 KO mice, but not for that of the δ subunit-deficient mice, in which GABA and THIP exhibited similar inhibitions. Since the basal [35S]TBPS was increased in these mice (Table 2), the amount of GIS-binding was increased in all of them, with the binding relative to the basal binding being unchanged or even slightly reduced as compared to wild-type mice.
To see how γ2 subunit-dependent binding sites were altered in the α4 KO brain, we performed labelling of the benzodiazepine sites with [3H]Ro 15-4513 (Mäkelä et al., 1997). We found out that thalamic labelling was increased in α4 KO mice (30.8 ± 1.2 nCi/mg vs. 23.8 ± 0.5 nCi/mg, p < 0.001, t-test, mean ± SEM, n=6; Fig. 4). The α4 subunit-dependent diazepam-insensitive binding was almost absent (1.8 ± 0.1 nCi/mg vs. 0.7 ± 0.1 nCi/mg, p < 0.001, t-test, mean ± SEM, n=6).
Fig. 4.

[3H]Ro 15-4513 binding and its modulation by 1 and 10 μM diazepam (DZ) in α4 KO and wild-type brain sections. Ctx, cerebral cortex; Gr, granule cell layer of the cerebellum; Hi, hippocampus; OB, olfactory bulb; Th, thalamus.
Since the α1 KO mouse line derived from a different genetic background than the other KO mouse lines, wild-type littermate controls (WT 2) of the α1 KO mice were used. In α1 KO mice, the [35S]TBPS binding profile in the thalamus differed from the other mouse lines. The basal binding was strongly reduced and the GIS-binding was at background level (Fig. 3). This indicates that α1 subunit is essential for the GIS-binding in almost all the forebrain areas. In addition, GABA was significantly more effective than THIP (Fig. 3), and co-application of GABA and THIP inhibited the binding more than THIP alone (Fig. 3). In the cerebellar granule cell layer, the α1 KO mice did not differ from their wild-type controls, except for not revealing any statistically significant differences between the inhibitions by GABA, THIP and GABA + THIP.
There are two brain areas that merit a special note in the α1 KO brains, namely the reticular nucleus of thalamus and the CA4 region of hippocampus (Fig. 3a). The reticular nucleus of thalamus has a very low basal [35S]TBPS binding (Table 1), but retains a high level of binding in the presence of 1 mM THIP (24 ± 4.5 % of basal, mean ± SEM, n=5) and becomes therefore clearly visible in the α1 KO brains. The low THIP sensitivity of this area might be due to the presence of α3- and non-α1 subunit-containing GABAA receptors (Ebert et al., 1994; Mozrzymas et al., 2007). The native α3 receptors obviously do not contribute to GIS-binding. The stratum oriens of the CA4 region retains some binding in the presence of 10 mM GABA (6.4 ± 1.5 % of basal, mean ± SEM, n=5), perhaps suggesting the presence of some unknown GABAA subtypes or e.g., “ectopic” α6 subunit expression. GIS-binding was present in the CA4 area of all genotypes.
4. Discussion
In this study we have demonstrated GABA-insensitive [35S]TBPS binding to GABAA receptors in several brain regions of the rat and mouse: the cerebellar granule cell layer, various thalamic nuclei, cerebral cortex, caudate-putamen, colliculi and the CA4 area of the hippocampus. We also deciphered the effects of inactivating the genes encoding for α1, α4 and δ subunits of the GABAA receptor. The knockout mouse lines helped to understand the native GABAA receptor subtypes where GABA is weaker than THIP and may act like a partial agonist.
Previous electrophysiological findings on recombinant receptors (Storustovu and Ebert, 2006) and [35S]TBPS autoradiography on brain regional distributions of GIS binding (Sinkkonen et al., 2001b) suggested that the reduced displacing capacity of GABA would be caused by GABAA receptors containing a δ subunit, two β subunits and two α subunits, either α4 (forebrain) or α6 (cerebellum) (see Introduction). The correlation between α4 subunit expression and GIS-binding in the forebrain (Sinkkonen et al., 2001b) and high THIP sensitivity and efficacy of the α4 subunit-containing GABAA receptors (Krogsgaard-Larsen et al., 2004) would have made them a likely population of receptors responsible for GIS-binding. Therefore, the present experiments with α4 KO and δ KO mouse brains were surprising, as the forebrain GIS-binding was not abolished: although its proportion of the basal binding was either reduced (α4 + δ KO), unaltered (δ KO) or even increased (α4 KO), it was clearly present in all these mouse lines, especially if we take into consideration the increase in the basal [35S]TBPS binding in these mouse models (Table 2).
Preliminary data suggest that various compensations among the remaining GABAA receptor subunits take place in the thalamus of α4 KO mice [decreased δ subunit expression and transport to the cell membrane (Peng et al., 2007; Zhang et al., 2007), increased amounts of α1 and α2 subunits (Peng et al., 2007), with a subtle increase in γ2 subunit on the plasma membrane (Peng et al., 2007)], which suggest that other subunits than α4 are responsible for reduced displacing capacity of GABA and inhibition of THIP effects by GABA in the forebrain. We found here a 29% increase in thalamic γ2 subunit-dependent benzodiazepine-site labelling in α4 KO mice (Fig. 4), indicating normal availability of γ2 subunits. Based on these results, the native α4 subunit-containing receptors cannot have the similar GABA pharmacology as the structurally related α6 subunit-containing receptors in the cerebellum, although these receptor subtypes share many other selective pharmacological features, such as insensitivity to benzodiazepine agonists and sensitivity to furosemide antagonism (Korpi et al., 1995; Wafford et al., 1996).
Against expectations, the GIS-binding the thalamic nuclei and elsewhere in the forebrain was abolished when the gene encoding for α1 subunit was inactivated (Fig. 3). In recombinant GABAA receptors, α1βγ2 receptors produce hardly any GIS-binding. In contrast, α1β receptors lacking γ2 subunit display significant GIS-binding (Sinkkonen et al., 2004a). Hence, a simple explanation for forebrain GIS-binding is the existence of dimeric α1β GABAA receptors in the native brain. This is supported by strongly increased and widespread forebrain GIS-binding in the γ2 subunit knockout heterozygous mice (Sinkkonen et al., 2004a). As shown earlier (Sinkkonen et al., 2001a), the δ KO mice displayed increased GIS-binding in the granule cell layer of the cerebellum, indicating that δ subunit is not compulsory for GIS-binding. Deleting the GABAA receptor α1 subunit did not affect the cerebellar GIS-binding, which strengthens the importance of the α6 subunit in GIS-binding in the granule neurons. Furthermore, the increased α1 subunit might also explain the increased thalamic GIS-binding in the α4 KO mice (see above).
Compensatory changes occurring in subunit knockout mouse models may complicate the interpretation of our binding data. In the thalamus of α1 KO mice, the GABAA receptor α4 subunit does not associate with gephyrin (Kralic, et al., 2006), a protein essential for synaptic clustering of GABAA receptors (Essrich, et al., 1998) even in the presence of the γ2 subunit and gephyrin. This suggests that the α4 subunit does not replace the missing synaptic α1 subunits. Immunoreactivity for the α3 and α4 subunits was increased (Kralic, et al., 2006). Our knowledge of receptor assembly is incomplete, which leaves us speculating on the precise subunit compositions and subcellular distributions of GABAA receptors with specific binding properties. Thus, the precise subunit composition of GABAA receptors formed in the thalamus of α1 KO mice is not known. Homomeric β3 GABAA receptors are known to produce [35S]TBPS binding that is not displaced by GABA (Slany et al., 1995), and this is probably a consequence of lack of α/β subunit interfaces required for binding of GABA (see, Smith and Olsen, 1995). Homomeric β3 receptors do not bind GABA (Davies et al., 1997; Slany et al., 1995). [35S]TBPS binding to β3 receptors is strongly inhibited by allosteric agonists, such as barbiturates, neurosteroids and propofol (Davies et al., 1997; Slany et al., 1995), similarly to GIS-binding to native receptors in the cerebellum and thalamus (Sinkkonen et al., 2001b). However, the role of β3 subunits in producing GIS-binding has been excluded by unaltered GIS-binding in the β3−/− mouse brains (Sinkkonen et al., 2001a). Finally, more generally, it is possible that the agonist and channel binding sites are not normally coupled in GABAA receptors where two β subunits are situated as neighbours, as suggested previously (Sinkkonen et al., 2004a). This hypothesis remains to be tested e.g. in recombinant GABAA receptors with known concatenated subunit configurations (see e.g., Kaur et al., 2009).
On the other hand, we think that the present results demonstrate brain regional differences in the efficacy of allosteric coupling between GABA and channel sites in specific receptor populations rather than in the amounts of receptors being unable to bind GABA, since GABA has to bind to the receptor in order to produce inhibition of the THIP effect that we observed. Although we cannot fully rule out the role of receptor desensitisation as a functional correlate to displacement of [35S]TBPS binding by agonists, Saarelainen et al. (2008) found in recombinant receptors expressing α6β3±δ subunits that THIP-induced currents were greater than GABA-induced currents and that simultaneous application of THIP and GABA produced currents that approached in amplitude those induced by high GABA alone, which indicated differences in the agonist efficacy. Recombinant α1β and α6β receptors lacking γ2 subunit display significant GIS-binding (Sinkkonen et al., 2004a), indicating a deficient coupling between GABA and ion channel sites. The γ2+/− mice have a lack of γ2 subunits and an increased assembly of αβ-like receptors with a consequential increase in GIS-binding (Sinkkonen et al., 2004a; Lorez et al., 2000). Now, an opposite situation can be speculated for the α1 KO mice as the neurons are lacking the most abundant α subunit and there would be a surplus of γ2 subunits. If the formation of dimeric αβ receptors is normally due to the shortage of γ2, the surplus of γ2 could switch the assembly kinetics of GABAA receptors towards negligible formation of dimeric αβ receptors and all other α subunits would be assembled in pentamers with γ2 subunits. Our data speak for the formation of GIS-binding in GABAA receptors assembled from only α1 or α6 and β subunits, being deficient of γ2 subunits.
One limitation in using the present ligand autoradiography procedure as the method for describing the functional state of GABAA receptors is that [35S]TBPS used to label GABAA receptors may penetrate the cell membrane and reach also to the intracellular space. It is unclear whether the radioactivity seen in the autoradiographs represents functional GABAA receptors or internalized receptors or their early assembly products or degrading receptors with intact ion channels. The in vitro imaging of the maximal agonist efficacy at displacing [35S]TBPS binding relies on millimolar concentrations of agonists, while the assumed extrasynaptic receptor pool normally faces much lower levels of “spillover” GABA (about 0.1-0.4 μM; Richerson and Wu, 2003; Tossman et al., 1986). The experiments with transgenic mice over-expressing α6 subunits in the hippocampal CA1 region indicate that the dimeric α6β receptors are functional in situ and in behaving animals (Sinkkonen et al., 2004b; Saarelainen et al., 2008). These transgenic mice show increased hippocampal GIS-binding and are exceptionally sensitive to THIP both in vitro and in vivo, indicating that new GABAA receptors are pharmacologically active. In line with our studies, the α1 KO mice have reduced sedative/hypnotic effects of THIP (Blednov et al., 2003). However, the α4 KO mice that have plenty of forebrain THIP-sensitive GIS-binding (the present study), are insensitive to ataxic, sedative and analgesic effects of THIP (Chandra et al., 2006). Also in the δ KO mice, hypnotic (Boehm et al., 2006) and electroencephalographic effects (Winsky-Sommerer et al., 2007) of THIP are reduced. Further studies are needed to assess the behavioural pharmacological significance of GIS-binding, which may well be different for the cerebellar and forebrain binding sites. For example, it would important to study the THIP sensitivity of GIS-binding in GABAA receptor β3 subunit-deficient mice, which are insensitive to hypnotic effects of THIP (Ugarte et al., 2000). The interest in the mechanisms of action of THIP is increasing as the drug may be clinically significant (see, Wafford and Ebert, 2006) and as it may be a useful tool in the pharmacological distinction between the synaptic γ2 subunit-containing GABAA receptors mediating fast phasic currents and the extrasynaptic often δ subunit-containing receptors mediating tonic “background” conductance (Farrant and Nusser, 2005).
In conclusion, we suggest that in the thalamus and other forebrain regions there are extrasynaptic α1β receptors (with and without δ subunits) that are responsible for THIP-sensitive GIS-binding. The α6 subunit-containing receptors mediate similar pharmacology with partial GABA agonism in the granule cell layer of the cerebellum. Based on these findings, there is a need for further study on minor GABAA receptor populations containing the most prevalent α1 subunit.
Acknowledgments
The authors wish to thank Aira Säisä and Okko Savonius for technical assistance in autoradiography and Bjarke Ebert (H. Lundbeck A/S, Copenhagen, Denmark) for the generous donation of THIP. This work was supported by the Academy of Finland, the Sigrid Juselius foundation, and the United States National Institutes of Health grants AA10422, AA13004, and DE14184.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bali M, Akabas MH. The location of a closed channel gate in the GABAA receptor channel. J Gen Physiol. 2007;129:145–159. doi: 10.1085/jgp.200609639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnir B, Korpi ER. The impact of sub-cellular location and intracellular neuronal proteins on properties of GABAA receptors. Curr Pharm Des. 2007;13:3169–3177. doi: 10.2174/138161207782341330. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Jung S, Alva H, Wallace D, Rosahl T, Whiting PJ, Harris RA. Deletion of the α1 or β2 subunit of GABAA receptors reduces actions of alcohol and other drugs. J Pharmacol Exp Ther. 2003;304:30–36. doi: 10.1124/jpet.102.042960. [DOI] [PubMed] [Google Scholar]
- Boehm SL, 2nd, Homanics GE, Blednov YA, Harris RA. δ-subunit containing GABAA receptor knockout mice are less sensitive to the actions of 4,5,6,7-tetrahydroisoxazolo-[5,4-c];pyridin-3-ol. Eur J Pharmacol. 2006;541:158–162. doi: 10.1016/j.ejphar.2006.02.054. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Single-channel properties of synaptic and extrasynaptic GABAA receptors suggest differential targeting of receptor subtypes. J Neurosci. 1999;19:2960–2973. doi: 10.1523/JNEUROSCI.19-08-02960.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra D, Jia F, Liang J, Suryanarayanan A, Werner D, Spigelman I, Houser CR, Olsen RW, Harrison NL, Homanics GE. GABAA receptor α4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc Nat Acad Sci USA. 2006;103:15230–15235. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PA, Kirkness EF, Hales TG. Modulation by general anaesthetics of rat GABAA receptors comprised of α1β3 and β3 subunits expressed in human embryonic kidney 293 cells. Br J Pharmacol. 1997;120:899–909. doi: 10.1038/sj.bjp.0700987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert B, Wafford KA, Whiting PJ, Krogsgaard-Larsen P, Kemp JA. Molecular pharmacology of γ-aminobutyric acid type A receptor agonists and partial agonists in oocytes injected with different α, β, and γ receptor subunit combinations. Mol Pharmacol. 1994;46:957–963. [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the γ2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: Phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Im WB, Blakeman DP. Correlation between γ-aminobutyric acidA receptor ligand-induced changes in t-butylbicyclophosphoro[35S]thionate binding and 36Cl- uptake in rat cerebrocortical membranes. Mol Pharmacol. 1991;39:394–398. [PubMed] [Google Scholar]
- Kaur KH, Baur R, Sigel E. Unanticipated structural and functional properties of δ-subunit-containing GABAA receptors. J Biol Chem. 2009;284:7889–96. doi: 10.1074/jbc.M806484200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpi ER, Lüddens H. Regional γ-aminobutyric acid sensitivity of t-butylbicyclophosphoro[35S]thionate binding depends on γ-aminobutyric acidA receptor α subunit. Mol Pharmacol. 1993;44:87–92. [PubMed] [Google Scholar]
- Kralic JE, O'Buckley TK, Khisti RT, Hodge CW, Homanics GE, Morrow AL. GABAA receptor α-1 subunit deletion alters receptor subtype assembly, pharmacological and behavioral responses to benzodiazepines and zolpidem. Neuropharmacology. 2002;43:685–694. doi: 10.1016/s0028-3908(02)00174-0. [DOI] [PubMed] [Google Scholar]
- Kralic JE, Sidler C, Parpan F, Homanics GE, Morrow AL, Fritschy JM. Compensatory alteration of inhibitory synaptic circuits in cerebellum and thalamus of γ-aminobutyric acid type A receptor α1 subunit knockout mice. J Comp Neurol. 2006;495:408–421. doi: 10.1002/cne.20866. [DOI] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P, Frolund B, Liljefors T, Ebert B. GABAA agonists and partial agonists: THIP (gaboxadol) as a non-opioid analgesic and a novel type of hypnotic. Biochem Pharmacol. 2004;68:1573–1580. doi: 10.1016/j.bcp.2004.06.040. [DOI] [PubMed] [Google Scholar]
- Lorez M, Benke D, Lüscher B, Möhler H, Benson JA. Single-channel properties of neuronal GABAA receptors0 from mice lacking the γ2 subunit. J Physiol. 2000;527:11–31. doi: 10.1111/j.1469-7793.2000.t01-1-00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüddens H, Korpi ER. GABA antagonists differentiate between recombinant GABAA/benzodiazepine receptor subtypes. J Neurosci. 1995;15:6957–6962. doi: 10.1523/JNEUROSCI.15-10-06957.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä R, Uusi-Oukari M, Homanics GE, Quinlan JJ, Firestone LL, Wisden W, Korpi ER. Cerebellar γ-aminobutyric acid type A receptors: Pharmacological subtypes revealed by mutant mouse lines. Mol Pharmacol. 1997;52:380–388. doi: 10.1124/mol.52.3.380. [DOI] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman L, Li Z, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor δ subunit knockout mice. Proc Nat Acad Sci USA. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell EA, Herd MB, Gunn BG, Lambert JJ, Belelli D. Neurosteroid modulation of GABAA receptors: molecular determinants and significance in health and disease. Neurochem Int. 2008;52:588–95. doi: 10.1016/j.neuint.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Mody I. Extrasynaptic GABAA receptors in the crosshairs of hormones and ethanol. Neurochem Int. 2008;52:60–4. doi: 10.1016/j.neuint.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozrzymas JW, Barberis A, Vicini S. GABAergic currents in RT and VB thalamic nuclei follow kinetic pattern of α3- and α1-subunit-containing GABAA receptors. Eur J Neurosci. 2007;26:657–665. doi: 10.1111/j.1460-9568.2007.05693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, McCabe RT, Wamsley JK. GABAA receptor subtypes: Autoradiographic comparison of GABA, benzodiazepine, and convulsant binding sites in the rat central nervous system. J Chem Neuroanat. 1990;3:59–76. [PubMed] [Google Scholar]
- Peng Z, Chandra D, Homanics GE, Olsen RW, Houser CR. 2007 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience, 2007; 2007. Multiple changes in GABAA receptor subunit localization in the thalamus of an α4 subunit-deficient mouse. Program No 350.1/F5. Online. [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: Immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Rabe H, Picard R, Uusi-Oukari M, Hevers W, Lüddens H, Korpi ER. Coupling between agonist and chloride ionophore sites of the GABAA receptor: Agonist/antagonist efficacy of 4-PIOL. Eur J Pharmacol. 2000;409:233–242. doi: 10.1016/s0014-2999(00)00838-4. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wu Y. Dynamic equilibrium of neurotransmitter transporters: Not just for reuptake anymore. J Neurophys. 2003;90:1363–1374. doi: 10.1152/jn.00317.2003. [DOI] [PubMed] [Google Scholar]
- Saarelainen KS, Ranna M, Rabe H, Sinkkonen ST, Moykkynen T, Uusi-Oukari M, Linden AM, Lüddens H, Korpi ER. Enhanced behavioral sensitivity to the competitive GABA agonist, gaboxadol, in transgenic mice over-expressing hippocampal extrasynaptic α6β GABAA receptors. J Neurochem. 2008;105:338–350. doi: 10.1111/j.1471-4159.2007.05136.x. [DOI] [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of γ-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- Sinkkonen ST, Mihalek RM, Homanics GE, Lüddens H, Korpi ER. Altered atypical coupling of γ-aminobutyrate type A receptor agonist and convulsant binding sites in subunit-deficient mouse lines. Mol Brain Res. 2001a;86:179–183. doi: 10.1016/s0169-328x(00)00273-4. [DOI] [PubMed] [Google Scholar]
- Sinkkonen ST, Uusi-Oukari M, Tupala E, Sarkioja T, Tiihonen J, Panula P, Lüddens H, Korpi ER. Characterization of γ-aminobutyrate type A receptors with atypical coupling between agonist and convulsant binding sites in discrete brain regions. Mol Brain Res. 2001b;86:168–178. doi: 10.1016/s0169-328x(00)00275-8. [DOI] [PubMed] [Google Scholar]
- Sinkkonen ST, Luscher B, Lüddens H, Korpi ER. Autoradiographic imaging of altered synaptic αβγ2 and extrasynaptic αβ GABAA receptors in a genetic mouse model of anxiety. Neurochem Int. 2004a;44:539–547. doi: 10.1016/j.neuint.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Sinkkonen ST, Vekovischeva OY, Moykkynen T, Ogris W, Sieghart W, Wisden W, Korpi ER. Behavioural correlates of an altered balance between synaptic and extrasynaptic GABAAergic inhibition in a mouse model. Eur J Neurosci. 2004b;20:2168–2178. doi: 10.1111/j.1460-9568.2004.03684.x. [DOI] [PubMed] [Google Scholar]
- Slany A, Zezula J, Tretter V, Sieghart W. Rat β3 subunits expressed in human embryonic kidney 293 cells form high affinity [35S]t-butylbicyclophosphorothionate binding sites modulated by several allosteric ligands of γ-aminobutyric acid type A receptors. Mol Pharmacol. 1995;48:385–391. [PubMed] [Google Scholar]
- Smith GB, Olsen RW. Functional domains of GABAA receptors. Trends Pharmacol Sci. 1995;16:162–168. doi: 10.1016/s0165-6147(00)89009-4. [DOI] [PubMed] [Google Scholar]
- Squires RF, Casida JE, Richardson M, Saederup E. [35S]t-butylbicyclophosphorothionate binds with high affinity to brain-specific sites coupled to γ-aminobutyric acid-A and ion recognition sites. Mol Pharmacol. 1983;23:326–336. [PubMed] [Google Scholar]
- Storustovu SI, Ebert B. Pharmacological characterization of agonists at δ-containing GABAA receptors: Functional selectivity for extrasynaptic receptors is dependent on the absence of γ2. J Pharmacol Exp Ther. 2006;316:1351–1359. doi: 10.1124/jpet.105.092403. [DOI] [PubMed] [Google Scholar]
- Ticku MK, Ramanjaneyulu R. Differential interactions of GABA agonists, depressant and convulsant drugs with [35S]-t-butylbicyclophosphorothionate binding sites in cortex and cerebellum. Pharmacol Biochem Behav. 1984;21:151–158. doi: 10.1016/0091-3057(84)90145-x. [DOI] [PubMed] [Google Scholar]
- Tossman U, Jonsson G, Ungerstedt U. Regional distribution and extracellular levels of amino acids in rat central nervous system. Acta Physiol Scand. 1986;127:533–545. doi: 10.1111/j.1748-1716.1986.tb07938.x. [DOI] [PubMed] [Google Scholar]
- Ugarte SD, Homanics GE, Firestone LL, Hammond DL. Sensory thresholds and the antinociceptive effects of GABA receptor agonists in mice lacking the β3 subunit of the GABAA receptor. Neuroscience. 2000;95:795–806. doi: 10.1016/s0306-4522(99)00481-9. [DOI] [PubMed] [Google Scholar]
- Vicini S, Ferguson C, Prybylowski K, Kralic J, Morrow AL, Homanics GE. GABAA receptor α1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. J Neurosci. 2001;21:3009–3016. doi: 10.1523/JNEUROSCI.21-09-03009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafford KA, Ebert B. Gaboxadol--a new awakening in sleep. Curr Opin Pharmacol. 2006;6:30–36. doi: 10.1016/j.coph.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Thompson SA, Thomas D, Sikela J, Wilcox AS, Whiting PJ. Functional characterization of human γ-aminobutyric acidA receptors containing the α4 subunit. Mol Pharmacol. 1996;50:670–678. [PubMed] [Google Scholar]
- Winsky-Sommerer R, Vyazovskiy VV, Homanics GE, Tobler I. The EEG effects of THIP (gaboxadol) on sleep and waking are mediated by the GABAA δ-subunit-containing receptors. Eur J Neurosci. 2007;25:1893–1899. doi: 10.1111/j.1460-9568.2007.05455.x. [DOI] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W, Cope D, Klausberger T, Hauer B, Sinkkonen ST, Tretter V, Lujan R, Jones A, Korpi ER, Mody I, Sieghart W, Somogyi P. Ectopic expression of the GABAA receptor α6 subunit in hippocampal pyramidal neurons produces extrasynaptic receptors and an increased tonic inhibition. Neuropharmacology. 2002;43:530–549. doi: 10.1016/s0028-3908(02)00151-x. [DOI] [PubMed] [Google Scholar]
- Xu M, Covey DF, Akabas MH. Interaction of picrotoxin with GABAA receptor channel-lining residues probed in cysteine mutants. Biophys J. 1995;69:1858–1867. doi: 10.1016/S0006-3495(95)80056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Chandra D, Homanics GE, Houser CR. 2007 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience, 2007; 2007. Ultrastructural localization of the δ subunit of the GABAA receptor in the mouse thalamus and its alterations in α4 subunit-deficient mice. Program No 350.2/F6. Online. [Google Scholar]


