Abstract
XPB and XPD subunits of TFIIH are central genome caretakers involved in nucleotide excision repair (NER), although their respective role within this DNA repair pathway remains difficult to delineate. To obtain insight into the function of XPB and XPD, we studied cell lines expressing XPB or XPD ATPase-deficient complexes. We show the involvement of XPB, but not XPD, in the accumulation of TFIIH to sites of DNA damage. Recruitment of TFIIH occurs independently of the helicase activity of XPB, but requires two recently identified motifs, a R-E-D residue loop and a Thumb-like domain. Furthermore, we show that these motifs are specifically involved in the DNA-induced stimulation of the ATPase activity of XPB. Together, our data demonstrate that the recruitment of TFIIH to sites of damage is an active process, under the control of the ATPase motifs of XPB and suggest that this subunit functions as an ATP-driven hook to stabilize the binding of the TFIIH to damaged DNA.
Keywords: DNA repair, helicase, TFIIH, XPB, XPD
Introduction
DNA and RNA helicases are a ubiquitous, yet diverse, group of enzymes present in viruses, prokaryotes and eukaryotes (Delagoutte and von Hippel, 2003). They convert chemical energy of nucleoside triphosphate hydrolysis to the mechanical energy necessary to transiently separate the strands of duplex nucleic acids (Tuteja and Tuteja, 2004). By this mean, they provide the single-stranded DNA or RNA intermediates necessary for replication, transcription, recombination or repair. Furthermore, it has been shown that helicases can also effectively displace bound proteins from DNA or RNA (von Hippel, 2004). There are several known human diseases caused by defective helicases (Ellis, 1997). Among these disorders, the cancer-prone Xeroderma pigmentosum (XP), alone or in combination with the Cockayne syndrome (CS), and the Trichothiodystrophy (TTD) are noteworthy as they entail mutations in the XPB and XPD superfamily 2 helicases. Both of these helicases are part of the same TFIIH complex. TFIIH is composed of a seven-subunit core (XPB, XPD, p62, p52, p44, p34 and p8/TTD-A) associated with the CAK subcomplex (Cdk7, cyclin H, and MAT1) (Giglia-Mari et al, 2004; Ranish et al, 2004). TFIIH functions in both transcription initiations of mRNA and rRNA (Iben et al, 2002), as well as in nucleotide excision repair (NER) (Schaeffer et al, 1993).
XPB and XPD patients are photosensitive and display a 1000-fold increase in melanoma risk because of defects in the NER function of TFIIH (Lehmann, 2003). NER removes a broad spectrum of DNA lesions including UV-induced pyrimidine dimers and bulky, helix-distorting adducts caused by toxic chemicals such as the anticancer drug cisplatin (Sancar, 1996). In mammalian cells, the proteins necessary for the incision reaction include XPC-HR23b, TFIIH, XPA, RPA and the nucleases XPG and ERCC1-XPF (Araujo et al, 2000). The removal of lesions requires their recognition by the repair factor XPC-HR23b and the subsequent opening of the DNA duplex by TFIIH. The single-stranded structure is then stabilized by XPA and RPA, and the margins of the resulting DNA bubble are recognized by XPG and ERCC1-XPF, thereby generating 3′ and 5′ incisions relative to the damage, respectively (O'Donnovan et al, 1994; Sijbers et al, 1996).
As XPB and XPD helicases are both integral parts of TFIIH, their individual molecular roles in NER remain difficult to delineate. As XPB and XPD are helicases with opposite polarities, it was originally suggested that they could cooperate to open DNA on the 5′ and 3′ sides of a lesion, respectively (Schaeffer et al, 1994). Indeed, mutation of the ATPase activity of either XPB or XPD results in the inability to remove DNA lesions (Sung et al, 1988; Guzder et al, 1994). Refining these proposals, recent data bring into question the direct role of the helicase activity of XPB in NER and transcription, and suggest that only the ATPase activity is required (Lin et al, 2005; Coin et al, 2007; Richards et al, 2008). Supporting the prime role of the ATPase activity of XPB in TFIIH functions, we recently showed that this activity was regulated by the p52 subunit of TFIIH (Coin et al, 2007) and by the damage recognition factor XPC (Bernardes de Jesus et al, 2008). Contrary to XPB, the helicase activity of XPD, which is regulated by the p44 subunit of TFIIH (Coin et al, 1998), is required for efficient opening of the DNA around the damage, but is dispensable for transcription (Tirode et al, 1999; Coin et al, 2007).
To further our understanding of the mechanistic details of XPB and XPD function, we analysed the behaviour of ATPase-deficient TFIIH complexes in vivo. We found that a TFIIH complex deficient in the ATPase of XPB was not recruited to sites of DNA damage, whereas a complex deficient in the ATPase of XPD did. More surprisingly, we discovered that the recruitment of TFIIH to these sites does not require the helicase activity of XPB but depends on two motifs, a R-E-D residue loop and a positively charged flexible Thumb (ThM) motif that were identified in a homologue of XPB from the thermophilic organism Archaeoglobus fulgidus (Fan et al, 2006). We analysed the molecular details of R-E-D and ThM impact on XPB activities and found that they were required to stimulate ATP hydrolysis in the presence of DNA. We propose a mechanism in which XPB functions as an ATP-dependent hook that uses the ATPase, R-E-D and ThM motifs to anchor TFIIH to the sites of DNA damage during DNA repair.
Results
The ATPase activity of XPB anchors TFIIH to the sites of DNA damage in vivo
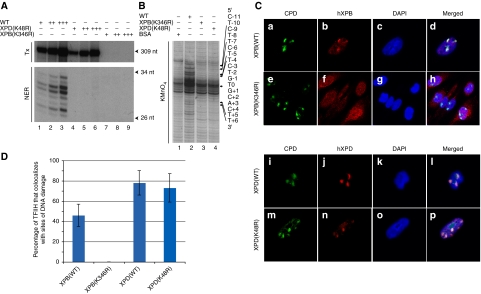
To functionally discriminate between the ATPase activities of XPB and XPD, we produced recombinant TFIIH/XPD(K48R) and TFIIH/XPB(K346R) in baculovirus-infected cells (Tirode et al, 1999) and tested them in DNA repair and transcription assays. These complexes are mutated in the ATPase Walker A motif of XPD and XPB, respectively. When incubated in the presence of recombinant TBP, TFIIA, TFIIB, TFIIE and TFIIF transcription factors in addition to purified RNA polymerase II and a linearized DNA template containing the adenovirus major late promoter (Tirode et al, 1999), TFIIH/XPD(K48R) supported transcription, contrary to TFIIH/XPB(K346R), which was totally inactive (Figure 1A, upper panel). To test the repair capacity of the different TFIIH complexes, we used a reconstituted dual incision assay composed of the recombinant XPC-HR23b, XPA, RPA, XPG, ERCC1-XPF factors and a closed-circular plasmid (Pt-DNA) containing a single 1,3-intra-strand d(GpTpG) cisplatin-DNA crosslink as a template (Araujo et al, 2000). None of the mutated complexes was able to excise the damaged oligonucleotide (Figure 1A, lower panel). In a permanganate footprinting assay that measures the opening of the DNA around the lesion (Tapias et al, 2004), addition of TFIIH(WT) induced an increased sensitivity of nucleotides at positions T+5, T+6, T–4, T–5, and, to a lesser extent, T–7 and T–10 (Figure 1B, lane 2), indicative of DNA opening. In contrast, neither TFIIH/XPB(K346R) nor TFIIH/XPD(K48R) were able to open damaged DNA (compare lanes 3–4 with lane 2).
Figure 1.
The ATPase activity of XPB is required to anchor TFIIH to damaged chromatin. (A) A measure of 25, 50 and 100 ng of TFIIH(WT), TFIIH/XPD(K48R) or TFIIH/XPB(K346R) was tested either in a reconstituted transcription assay (Tx, upper panel) or in a dual incision assay (NER, lower panel) as described (Coin et al, 2004). The sizes of the incision and transcription products are indicated. (B) TFIIH(WT), TFIIH/XPD(K48R) or TFIIH/XPB(K346R) (100 ng) were incubated with a radio-labelled linear DNA fragment from the Pt-DNA plasmid and 40 ng of XPC-HR23b, 25 ng of XPA, 50 ng of RPA and 150 ng of XPG in a KMnO4 footprinting assay. Lane 1; Pt-DNA with BSA only. Residues are numbered with the central thymine of the crosslinked GTG sequence designated T0. Arrows indicate KMnO4 sensitive sites. Adducted strand residues to the 3′ and 5′ of T0 are denoted by positive and negative integers (+N, –N), respectively. (C) Stably transfected CHO-27-1 expressing a GFP-tagged version of the human WT or K346R XPB proteins (upper panel) and stably transfected CHO-UV5 cells expressing an HA-tagged version of the human WT or K48R XPD proteins (bottom panel) (Winkler et al, 2000) were UV irradiated at 100 J/m2 through the 3 μm pore filter and fixed 30 min later. Immunofluorescent labelling was performed using a rabbit polyclonal anti-GFP (panels b and f), a rat monoclonal anti-HA (panels j and n) or a mouse monoclonal anti-CPD (panels a, e, i, m). Nuclei were counterstained with DAPI (panels c, g, k, o), and slides were merged (panels d, h, l, p). (D) Quantitative analysis of the recruitment of TFIIH to sites of DNA damage in transfected cells. Values represent averages±s.d. (n=100 sites of DNA damage) from three independent experiments.
To analyse the behaviour of ATP-deficient TFIIH complexes in vivo, we used a stably transfected Chinese hamster ovary (CHO)-UV5 cell line expressing an HA-tagged version of the human XPD(K48R) protein (Winkler et al, 2000). Using the CHO-27-1 cells mutated in the hamster homologue of XPB (Ma et al, 1994), we also generated a stably transfected cell line expressing a C-terminally GFP-tagged version of the human XPB WT or K346R protein. The functionality of an XPB–GFP fusion construct was established earlier (Hoogstraten et al, 2002). We used immunofluorescent labelling after local UV irradiation of stably transfected cells (Volker et al, 2001) to assess the nuclear distribution pattern of XPB and XPD. Immunostaining with antibodies against cyclobutane pyrimidine dimers (CPDs) showed that UV damages were located in discrete local spots in the nucleus (Figure 1C, panels a, e, i, m). Both, human wild-type XPB and XPD proteins colocalized with CPD spots, indicating that TFIIH was efficiently recruited to the damaged sites in these cells (panels a–d and i–l). Surprisingly, although signals of XPD(K48R) colocalized with CPD spots in CHO-UV5 cells (panels m–p), signals of XPB(K346R) showed an homogenous distribution pattern through the nucleus (panels e–h), indicating that TFIIH/XPB(K346R) complex was not recruited to the damaged sites (Figure 1D). These data suggest that the accumulation of TFIIH to sites of DNA damage takes place in the absence of an active XPD protein but requires functional XPB.
New motifs in XPB required for the activity of TFIIH in NER
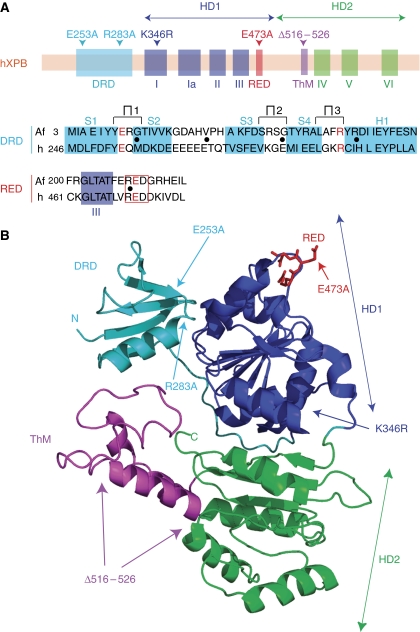
By introducing mutations in some of the seven canonical helicase motifs of XPB, we demonstrated recently that its helicase activity was not required for TFIIH repair function (Coin et al, 2007). Recently, three additional motifs were identified in a homologue of XPB from the thermophilic organism Archaeoglobus fulgidus (Fan et al, 2006). To determine whether these newly identified motifs have a function in the activities of the human TFIIH complex in transcription and repair, we designed four mutants (E253A, E253A/R283A, E473A and Δ516–526) depicted in Figure 2. We introduced an E253A substitution located at the end of the first β-strand that was combined, when indicated, with an R283A mutation located at the beginning of the α-helix of a putative damage recognition domain (DRD). We also designed an E473A substitution in the R-E-D residue loop to change the local negative charge of the motif, and we deleted the positively charged ThM domain from amino acid 516 to 526 (Δ516–526) (Figure 2).
Figure 2.
XPB conserved sequences, motifs and structural architecture. (A) Upper panel shows the location of the human XPB structural domains: the four classical helicase motifs (I, Ia, II and III) of the first helicase module (HD1) are indicated in blue. The three helicase motifs (IV, V and VI) of the second helicase module (HD2) are indicated in green. The putative damage recognition (DRD), R-E-D and Thumb (ThM) domains identified in an homologue of XPB from the thermophilic organism Archaeoglobus fulgidus (Fan et al, 2006) are indicated, respectively, in light blue, red and purple. The mutations E253A, R283A, K346R, E473A and Δ516–526 are annotated. Lower panel shows the sequence conservation of the DRD and R-E-D motifs between human (h) and Archaeoglobus fulgidus (Af) XPB proteins. The β-strands (S1–4) and the α-helix (H1) are indicated. The conserved helicase motif III indicated by a blue square is located close to the R-E-D motif indicated by a red opened square. The three hairpin loops potentially involved in DNA binding are indicated (∏1, ∏2, ∏3). Residues mutated in this study are marked in red. (B) View of the ribbon representation of AfXPB. The HD1 is indicated in blue, HD2 in green. The putative DRD, R-E-D and ThM domains are indicated in light blue, red and purple, respectively. The positions of the new mutations are indicated.
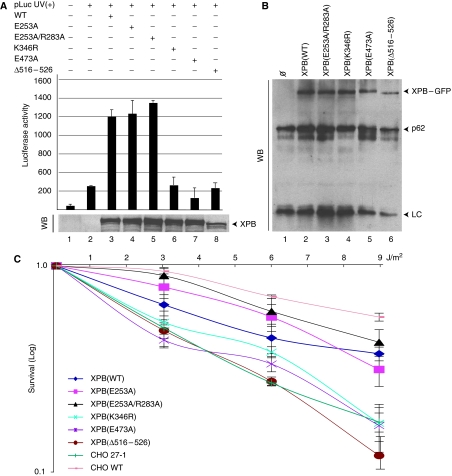
To investigate the importance of the DRD, R-E-D and ThM motifs of XPB in the repair function of TFIIH, we first performed a host-cell reactivation assay (Carreau et al, 1995). A UV damaged reporter construct, carrying a luciferase gene (pLuc) was transiently transfected into CHO-27-1 cells, together with vectors coding for β-galactosidase and for human XPB proteins. Transfection of either XPB(E253A) or XPB(E253A/R283A) restored luciferase expression that reached the level observed with XPB(WT) (Figure 3A, lanes 1–5). In marked contrast, XPB(E473A) and XPB(Δ516–526) were not able to restore luciferase expression (lanes 7–8), a defect also observed with XPB(K346R) (lane 6). The various XPB were expressed at a similar level with the exception of XPB(Δ516–526) whose expression was slightly reduced compared with the wild type (Figure 3A).
Figure 3.
Analysis of the function of the new motifs in NER in vivo. (A) CHO-27-1 cells were transfected with UV-irradiated pLuc plasmid expressing the luciferase gene (lanes 2–8) in combination with vector expressing either XPB(WT) (lane 3), XPB(E253A) (lane 4), XPB(E253A/R283A) (lane 5), XPB(K346R) (lane 6), XPB(E473A) (lane 7) or XPB(Δ516–526) (lane 8). The luciferase activity in cell lysates (48 h post-transfection), normalized with the internal β-galactosidase standard, assesses repair complementation. Results are expressed as relative luciferase activity. Values represent averages±s.d. from three independent experiments. A measure of 50 μg of total extract were resolved by SDS–PAGE and western blotted (WB) with a mouse anti-human XPB antibody (Coin et al, 2004). Note that XPB(Δ516–526) migrates slightly lower than the others because of the deletion. (B) TFIIH from 100 μg of extracts prepared from untransfected CHO-27-1 (lane 1) or CHO-27-1 stably expressing XPB (lanes 2–6) was immunoprecipitated with a polyclonal antibody against the p62 subunit of TFIIH and resolved by SDS–PAGE, followed by Western blotting with a mouse anti-human XPB and a mouse anti-p62 antibody. LC; light chain of the antibody. (C) Quantitative UV-survival analysis of transfected CHO-27-1 cell lines. Results are expressed as a ratio between the cells that survived after irradiation and the cells that survived without treatment. Values represent averages±s.d. from three independent experiments.
Next, we carried out a UV-survival assay and for that purpose we established CHO-27-1 cells stably expressing the new XPB–GFP mutant proteins. Immunoprecipitations using a rabbit polyclonal antibody, recognizing the hamster homologue of the core TFIIH subunit p62, demonstrated that the various XPB were efficiently incorporated into the hamster TFIIH complex (Figure 3B). The stably transfected CHO-27-1 cells were UV irradiated at different doses (3, 6 and 9 J/m2) and their survival was measured. Expression of XPB(WT), XPB(E253A) and XPB(E253A/R283A) induced a substantial rescue of the UV survival of the CHO-27-1 cells compared with nontransfected control (Figure 3C). On the other hand, the UV-survival curve of XPB(E473A) and XPB(Δ516–526) transfected cells fell into the range of both the nontransfected parental CHO-27-1 cells and those transfected with the NER-deficient XPB(K346R) control. These data indicate that the R-E-D and ThM domains of XPB are crucial for the repair activity of TFIIH, while the putative DRD is dispensable.
R-E-D and ThM motifs are needed for an optimal ATPase activity of XPB
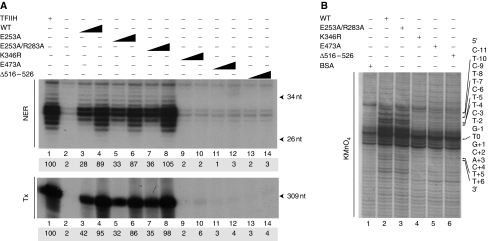
To decipher the molecular details of the repair defect generated by the E473A and Δ516–526 mutations, we produced recombinant TFIIH complexes in baculovirus-infected cells containing the corresponding mutated XPB subunits (Tirode et al, 1999). Western blot analysis of the recombinant TFIIH/XPB(E253A), XPB(E253A/R283A), XPB(E473A) and XPB(Δ516–526) complexes revealed a similar subunit composition compared with the TFIIH(WT) complex (data not shown). When tested in either a dual incision or in a transcription assays, TFIIH/XPB(E253A) and XPB(E253A/R283A) were as active as TFIIH(WT) in excising damaged DNA (Figure 4A, upper panel, compare lanes 5–8 with lanes 3–4) or synthesizing RNA (lower panel). In contrast, TFIIH/XPB(E473A) and XPB(Δ516–526) were inactive in repairing damaged DNA and in synthesizing RNA (Figure 4A, compare lanes 11–12 and 13–14 with lanes 3–4), similarly to TFIIH/XPB(K346R) (lanes 9–10).
Figure 4.
Mutations in R-E-D and ThM motifs impair the ATPase activity of XPB. (A) A measure of 25 and 75 ng of TFIIH(WT), TFIIH/XPB(E253A), XPB(E253A/R283A), XPB(K346R), XPB(E473A) or XPB(Δ516–526) was tested in a dual incision assay (NER, upper panel) or in a reconstituted transcription assay (Tx, lower panel) as described (Coin et al, 2004). Lane 1 contains highly purified Hela TFIIH (Giglia-Mari et al, 2004). Lane 2 contains all the factors except TFIIH. The sizes of the incision or transcription products are indicated. The transcription and repair signals were quantified using Genetool (Syngene). (B) A measure of 100 ng of the various TFIIH complexes were tested in a KMnO4 footprint assay (see Figure 1B). Lane 1; Pt-DNA with BSA only. Residues are numbered with the central thymine of the crosslinked GTG sequence designated T0. Arrows indicate KMnO4 sensitive sites. Adducted strand residues to the 3′ and 5′ of T0 are denoted by positive and negative integers (+N, –N).
In a permanganate footprint assay, TFIIH/XPB(E473A) and XPB(Δ516–526) were unable to open the damaged DNA (Figure 4B, lanes 5–6), compared with TFIIH(WT) or XPB(E253A/R283A) (lanes 2–3). Altogether, the above data drew our attention to the critical role of both the R-E-D and ThM motifs of XPB in damaged DNA opening.
R-E-D and ThM motifs are needed for the anchoring of TFIIH to the sites of DNA damage
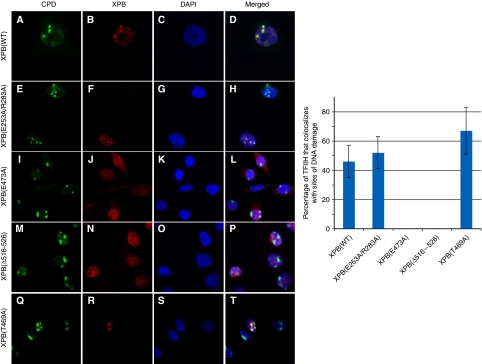
We next measured the recruitment of the TFIIH complexes carrying mutations in the newly identified motifs to sites of DNA damage in vivo. XPB(E253A/R283A) colocalized with CPD spots (Figure 5, panels e–h), indicating that the corresponding TFIIH complexes translocated to the sites of DNA photolesions. XPB(E473A) and XPB(Δ516–526), however, displayed a homogeneous distribution pattern through the nucleus (panels i–l and m–p), which parallels the pattern observed in Figure 1C with XPB(K346R). This homogenous distribution contrasted with the local accumulation to the damaged sites of a TFIIH/XPB(T469A) complex (panels q–t) containing a mutation that has been shown to impede the helicase activity of XPB but not the NER function of TFIIH (Coin et al, 2007). We conclude from these data that the R-E-D and ThM motifs are required, together with the Walker A motif, for the recruitment of the TFIIH complex to sites of DNA damage.
Figure 5.
Recruitment of TFIIH to local sites of DNA damage. (Left panel) CHO-27-1 cells were stably transfected with pEGFP plasmids expressing various forms of GFP-tagged XPB proteins. These cells were irradiated with UV light (100 J/m2) through the 3-μm pore filter and fixed 30 min later. Immunofluorescent labelling was performed using either a mouse monoclonal anti-CPD (panels A, E, I, M, Q) or a rabbit polyclonal anti-GFP (panels B, F, J, N, R). Nuclei were counterstained with DAPI (panels C, G, K, O, S), and slides were merged (panels D, H, L, P, T). (Right panel) Quantitative analysis of the recruitment of TFIIH to sites of DNA damage in transfected cells. Values represent averages±s.d. (n=100 sites of DNA damage) from three independent experiments.
R-E-D and ThM motifs stimulate the DNA-dependent TFIIH ATPase activity
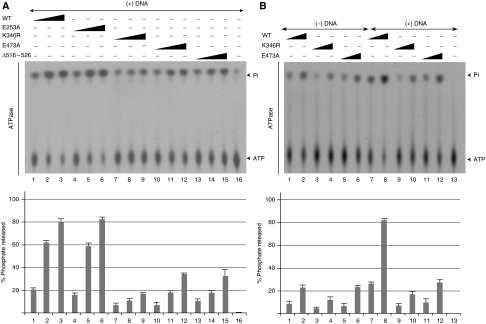
Data above show common biochemical and biological defects for TFIIH complexes mutated either in the ATPase, R-E-D or ThM motifs of XPB and suggest a link between the newly identified motifs of XPB and its ATPase activity. To assess the contributions of these motifs on the hydrolysis of ATP by XPB, we performed ATPase assay. Our data show that the TFIIH/XPB(E473A) and TFIIH/XPB(Δ516–526) displayed about 40% of residual ATPase activity compared with TFIIH(WT) (Figure 6A, compare lanes 1–3 with 10–12 and 13–15). TFIIH/XPB(E253A) or TFIIH/XPB(E253A/R283A) exhibited the same activity as TFIIH(WT) (lanes 1–6 and data not shown). TFIIH/XPB(K346R) showed only 20% residual ATPase activity (corresponding to that of XPD (Coin et al, 2006)), compared with TFIIH(WT) (compare lanes 1–3 with 7–9).
Figure 6.
Mutations in the R-E-D motif impair DNA-dependant TFIIH ATPase activity. (A) 50, 100, and 150 ng of TFIIH(WT), TFIIH/XPB(E253A), XPB(K346R), XPB(E473A) or XPB(Δ516–526) were tested in an ATPase assay in the presence of 200 ng of double-strand circular DNA (Coin et al, 2007). The average percentage±s.d. of phosphate released (Pi/(ATP+Pi)) from three independent experiments is represented in the graph. (B) 50 and 150 ng of TFIIH(WT), TFIIH/XPB(K346R) or TFIIH/XPB(E473A) were tested in an ATPase assay without (lanes 1–6) or with (lanes 7–12) 200 ng of double-strand circular DNA. The average percentage±s.d. of phosphate released (Pi/(ATP+Pi)) from three independent experiments is represented in the graph.
XPB and XPD are DNA-dependent ATPases (Roy et al, 1994). The ATPase activity of TFIIH(WT), low in the absence of DNA, is stimulated by double-stranded DNA (Figure 6B, compare lanes 1–2 with 7–8). In contrast, TFIIH/XPB(E473A) showed almost no DNA-induced ATPase stimulation (Figure 6B, compare lanes 5–6 with 11–12). More importantly, in the absence of DNA, TFIIH/XPB(WT) and XPB(E473A) displayed similar specific ATPase activities (Figure 6B, compare lanes 1–2 and 5–6) that were slightly higher than the TFIIH/XPB(K346R) activity (compare lanes 1–2, 5–6 and 3–4). Similar observations were obtained with TFIIH/XPB(Δ516–526) (Supplementary data 1). Altogether, these data indicate that the R-E-D and ThM motifs do not affect the basal intrinsic ATPase activity of XPB but are required for the stimulation of this activity by DNA.
Discussion
To efficiently protect the genome, cells need to detect all types of DNA structural alterations embedded in billions of normal base pairs. The identification of the various proteins that execute NER was done through extensive studies of human cells deficient in this repair pathway (Maillard et al, 2007). Both in vivo and in vitro experiments identified XPC as the first factor that binds the damaged DNA (Sugasawa et al, 1998; Volker et al, 2001; Riedl et al, 2003). TFIIH is recruited to the lesion immediately after XPC (Yokoi et al, 2000; Riedl et al, 2003), presumably through direct protein–protein interaction (Bernardes de Jesus et al, 2008). The role of TFIIH is devoted to the opening of the DNA around the damaged site, but the individual function of its helicase subunits in this step remains difficult to delineate.
Earlier studies from our laboratory have shown that mutations in the helicase motifs III (T469A) or VI (Q638A), which impaired the helicase activity of the XPB subunit, did not inhibit the NER activity of TFIIH (Coin et al, 2007), thus raising the question of the role of XPB in NER. Here, we showed that TFIIH containing mutation in the motif III of XPB is recruited to the DNA repair sites after UV irradiation. However, a mutation in the helicase motif Ia, which abolishes the ATPase activity of XPB, thwarts the accumulation of TFIIH to these sites. This implies that the recruitment of TFIIH to sites of damage is an active process that requires ATP hydrolysis. In contrast, the ATPase activity of XPD, the second helicase of TFIIH, is not required to recruit TFIIH to the damage sites, although it is needed for DNA repair.
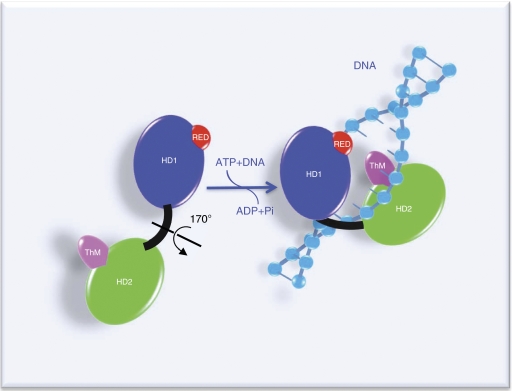
In addition to the aforementioned ATPase motif, we found that two additional motifs, the R-E-D and ThM motifs, are implicated in the recruitment of TFIIH to sites of DNA damage. These two domains, highly conserved in human XPB, were identified in an homologue of XPB from the thermophilic organism Archaeoglobus fulgidus and were suggested to be involved in TFIIH functions (Fan et al, 2006). Mutations in the R-E-D and ThM motifs mimicked the biochemical and biological defects obtained with a mutation in the ATPase motif. This suggests that the ATPase, R-E-D and ThM motifs work together to ensure a correct recruitment of TFIIH to the damaged sites before the opening and dual incision steps take place during NER. How the R-E-D and ThM motifs participate to the anchoring of TFIIH? The ThM domain has not been found in other helicases, including XPD (Bienstock et al, 2002; Fan et al, 2008; Liu et al, 2008; Wolski et al, 2008), but a similar helical protrusion has been observed in DNA polymerases (Doublie et al, 1998) and in Sulfolobus solfataricus SWI2/SNF2 ATPase Rad54 (Durr et al, 2005), in which it is expected to grip double-stranded DNA from the minor groove. The structure of XPB suggests that the energy furnished by the ATP hydrolysis is used to induce a flip of 170° of the HD2 domain after the binding of XPB to DNA (Fan et al, 2006) (Figure 7). The R-E-D (present in HD1) and the ThM (present in HD2) are then in close vicinity and are used to stabilize TFIIH on the DNA by introducing a wedge (the E473 residue) in the double-stranded DNA, gripped by the ThM motif. To obtain experimental evidence for this model, we compared the ATPase activities of the WT and mutated complexes with or without DNA. Indeed, like most SF1 and SF2 members, DNA stimulates the ATPase activity of TFIIH (Roy et al, 1994). In the presence of DNA, mutations in the R-E-D and ThM motifs induces 60% inhibition of the ATPase activity compare with TFIIH(WT). In the absence of DNA, the three ATPase activities are strictly identical and are slightly higher than the ATPase activity of the TFIIH complex mutated in the ATP-binding site of XPB. These data further support the model of the conformation change proposed above, as it demonstrates that R-E-D and ThM are used to stabilize the binding of XPB to DNA. Furthermore, the fact that these mutations inhibit both, TFIIH transcription and repair activities, suggests a common mode of recruitment of TFIIH to the promoters and to the damage sites.
Figure 7.
Proposed structure-based mechanism for binding of XPB to DNA. In this model, adapted from Fan et al (2006), XPB is in an opened conformation in the absence of DNA. When XPB binds to DNA, the rotation (170°) of the second helicase domain (HD2) together with the ThM domain, facilitated by HD1-mediated ATP hydrolysis, forms the closed and stable XPB–DNA complex.
The recruitment of TFIIH through the action of the ATPase activity of XPB may also induce a reorganization of the protein–DNA complexes in transcription and repair that will allow new protein–protein or protein–DNA contacts. Indeed, using photocrosslink experiments, we have shown that addition of ATP in NER induced a re-positioning of XPC on the damaged DNA, which dependent on TFIIH (Tapias et al, 2004). After the recruitment of TFIIH to the damaged DNA through the energy furnished by the ATPase activity of XPB, the DNA would be opened by XPD, which has a processive and robust helicase activity stimulated by the p44 subunit of the core TFIIH (Coin et al, 1998). Here, a mutation in the ATPase activity of XPD still allowed TFIIH to bind the damaged sites in vivo but was unable to open the DNA around the lesion. Altogether, our data brings a new conceptual view of the roles of XPB and XPD in NER by revealing their different molecular functions within this genome caretaking event.
Materials and methods
Cell lines
CHO-27-1 is a CHO mutant cell line belonging to the third rodent complementation group (the hamster ERCC3 gene is the homologue of the human XPB gene) (Hall et al, 2005). CHO-UV5 belongs to the second rodent complementation group (the hamster ERCC2 is the homologue of the human XPD gene) (Winkler et al, 2000).
Construction of the plasmids
Baculovirus allowing the expression of mutated XPB were constructed in the FLAG tag pSK278 vector (BD Biosciences). XPB was inserted at the BamHI/EcoRI site, in fusion with the FLAG tag at its 5′ side. The mutants were obtained by site-directed mutagenesis (Quickchange, Stratagene). The resulting vectors were recombined with baculovirus DNA (BaculoGold DNA, PharMingen) in Spodoptera frugiperda 9 (Sf9) cells. In vivo experiments were carried out with the pEGFP-N1 plasmid (Clontech) containing the XPB cDNA inserted in frame with the green fluorescent protein tag (Hoogstraten et al, 2002).
Stable cell lines
CHO-27-1 cells (106) were transfected with 2 μg of pEGFP-N1/XPB plasmid in 10 cm Petri dishes using lipofectamine (Invitrogen). Forty hours after transfection, the fluorescent cells were sorted on the FACS DIVa (BD; Becton, Dickinson and Company). The cells with the highest level of fluorescence (about 5% of total cells) were maintained in the selective medium with G418 (Geniticin, 800 μg/ml), expanded and analysed for XPB expression.
Damaged DNA substrates
Covalently closed circular Pt-DNA containing a single 1,3-intra-strand d(GpTpG) cisplatin–DNA crosslink was prepared as described (Frit et al, 2002).
Dual incision assay
Dual incision assay was carried out in 25 μl of Repair buffer (45 mM Hepes-KOH (pH 7.8), 5 mM MgCl2, 1 mM DTT, 0.3 mM EDTA, 10% glycerol, 2.5 μg BSA, 50 mM KCl) supplemented with 2 mM ATP. Each reaction contained 5 ng of XPG, 15 ng of XPF/ERCC1, 10 ng of XPC-HR23b, 50 ng of RPA and 25 ng of XPA. After pre-incubation 10 min at 30°C, 30 ng of Pt-DNA was added and reaction was continued for 90 min at 30°C. The excised fragment was detected on a 14% urea-acrylamide after annealing with 9 ng of the complementary oligonucleotide and addition of four radiolabelled dCMPα-P32 (3000 μCi/mmol) residues by Sequenase V2.1 (USB).
KMnO4 footprint assay
The damaged strand probe was obtained on Age1/Ase1 digestion of the Pt-DNA and radiolabelling at the 3′end in a Klenow reaction, the Pt adduct is located at 156 bp from the labelled end. The resulting fragment was purified by the ‘crush and soak' method after migration in a 5% nondenaturating PAGE. Reactions (75 μl) were carried out in 20 μl of Repair buffer (+2 mM ATP) containing the labelled cisplatinated probe (40 fmol) and 40 ng of XPC-HR23b, 25 ng of XPA, 50 ng of RPA and 150 ng of XPG. After incubation at 30 °C for 15 min, 3 μl of 120 mM KMnO4 was added, and oxidation was allowed to proceed for 3 min at room temperature before reduction by adding 6 μl of 14.6 M β-mercaptoethanol for 5 min on ice. After organic extraction and ethanol precipitation, dried pellets were resuspended in 100 μl of a solution containing 1 M piperidine, 1 mM EDTA and 1 mM EGTA and incubated at 90°C for 25 min. Samples were next ethanol precipitated, and final pellets were recovered in 10 μl of loading buffer and analysed in 8% urea PAGE.
ATPase assay
Protein fractions were incubated for 2 h at 30°C in the presence of 1 μCi [γ-32P]ATP (7000 Ci/mmol, ICN Pharmaceuticals) in a 20 μl reaction volume in 20 mM Tris–HCl pH 7.9, 4 mM MgCl2, 1 mM DTT, 50 μg/ml BSA and when indicated 200 ng of double-strand DNA (pcDNA3+). Reactions were stopped by addition of EDTA (50 mM) and SDS (1% (w/w)). The reactions were then diluted five-fold, spotted onto polyethylenimine (PEI) TLC plates (Merck), run in 0.5 M LiCl/1 M formic acid and autoradiographed.
Local UV irradiation and immunofluorescence
The cells were rinsed with PBS and covered with an isopore polycarbonate filter with pores of 3 μm diameter (Millipore, Badford, MA). Cells were then exposed to UV irradiation with a Philips TUV lamp (predominantly 254 nm) at a dose of 100 J/m2 (Volker et al, 2001). Subsequently, the filter was removed, the medium was added back to the cells, and they were returned to culture conditions for 30 min. Then, cells were fixed in 2% formaldehyde for 15 min at room temperature and permeabilized with PBS/0.5% Triton X-100 for 5 min. After washing with PBS-Tween (0.05%), the slides were incubated for 1 h with the indicated antibodies. After extensive washing with PBS-Tween, they were incubated for 1 h with Cy3-conjugated donkey anti-rabbit IgG, goat anti-mouse Alexa 488 IgG or goat anti-rat Alexa 488 IgG (Jackson Laboratories) diluted 1:400 in PBS-Tween/0.5% Foetal Calf Serum. The slides were counterstained for DNA with DAPI prepared in Vectashield mounting medium (Vector lab). All images were collected using a Leica Confocal TCS 4D microscope equipped with both UV laser and an Argon/Kripton laser, and standard filters to allow collection of the data at 488 and 568 nm. The software TCSTK was used for three-colour reconstructions, and figures were generated using the PLCHTK software.
Host-cell reactivation assay
The pGL3 vector expressing Photinus pyralis (firefly) luciferase was purchased from Promega and the pCH110 vector expressing the β-galactosidase from Invitrogen. The pGL3 vector was UV irradiated (254 nm, 1000 J/m2) at a concentration of 1 mg/ml in 10 mM Tris–HCl (pH 8.0) and 1 mM EDTA. CHO-27-1 cells were transfected in a six-well plate at a confluence of 95% using Lipofectamine Plus (Invitrogen). Each transfection mixture contained 500 ng of pGL3 (UV+/−), 100 ng of pCH110 (nonirradiated) and 10 ng of the various pcDNAXPB plasmids. After 4 h of incubation, the transfection reagents were replaced by medium. Cells were lysed after 24 h to measure luciferase activity on a microtiter plate luminometer (Dynex). All results (mean values of at least five measurements) were normalized by calculating the ratios between luciferase and galactosidase activities.
UV-survival assay
Cells (103) were plated per 6 cm petri dishes, cultured overnight and UV irradiated at 254 nm at various doses (0.5 J/m2/s). After 14 days, the cells are stained by trypan blue and counted.
Antibodies
Mouse monoclonal antibodies towards TFIIH subunits were used as described (Coin et al, 2007). Primary antibodies (the final dilutions are indicated in parentheses) used in fluorescent labelling were purified rabbit anti-GFP (Torrey Pines Biolabs, Inc) (1:1000), rat monoclonal anti-HA 3F10 (Roche) (1:1000) and mouse IgG monoclonal anti-CPD (TDM2) (1:2000) (MBL international corp.).
Supplementary Material
Figure 1S
Supplementary data 1
Review Process File
Acknowledgments
We are grateful to A Larnicol for her excellent technical expertise and to R Velez-Cruz for his critical reading and to A Poterszman for fruitful discussion. We are grateful to J Hoeijmakers and W Vermeulen for the CHO-UV5 cells. This study was supported by funds from the Ligue Contre le Cancer (Equipe Labellisée), from the French National Research Agency (ANR-08-GENOPAT-042) and from the Institut National du Cancer (INCA-2008-041). VO and BBJ are supported by the French ‘Association pour la Recherche contre le Cancer' (ARC). AZ is supported by the French ‘Ligue contre le Cancer'. Work in the JME and FC laboratory is supported by a European Research Council advanced grant.
Footnotes
The authors declare that they have no conflict of interest.
References
- Araujo SJ, Tirode F, Coin F, Pospiech H, Syvaoja JE, Stucki M, Hubscher U, Egly JM, Wood RD (2000) Nucleotide excision repair of DNA with recombinant human proteins: definition of the minimal set of factors, active forms of TFIIH, and modulation by CAK. Genes Dev 14: 349–359 [PMC free article] [PubMed] [Google Scholar]
- Bernardes de Jesus BM, Bjoras M, Coin F, Egly JM (2008) Dissection of the molecular defects caused by pathogenic mutations in the DNA repair factor XPC. Mol Cell Biol 28: 7225–7235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienstock RJ, Skorvaga M, Mandavilli BS, Van Houten B (2002) Structural and functional characterization of the human DNA repair helicase XPD by comparative molecular modeling and site-directed mutagenesis of the bacterial repair protein UvrB. J Biol Chem 27: 27. [DOI] [PubMed] [Google Scholar]
- Carreau M, Eveno E, Quilliet X, Chevalier-Lagente O, Benoit A, Tanganelli B, Stefanini M, Vermeulen W, Hoeijmakers JH, Sarasin A, Mezzina M (1995) Development of a new easy complementation assay for DNA repair deficient human syndromes using cloned repair genes. Carcinogenesis 16: 1003–1009 [DOI] [PubMed] [Google Scholar]
- Coin F, Auriol J, Tapias A, Clivio P, Vermeulen W, Egly JM (2004) Phosphorylation of XPB helicase regulates TFIIH nucleotide excision repair activity. EMBO J 23: 4835–4846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coin F, De Santis LP, Nardo T, Zlobinskaya O, Stefanini M, Egly JM (2006) p8/TTD-A as a Repair-Specific TFIIH Subunit. Mol Cell 21: 215–226 [DOI] [PubMed] [Google Scholar]
- Coin F, Marinoni JC, Rodolfo C, Fribourg S, Pedrini AM, Egly JM (1998) Mutations in the XPD helicase gene result in XP and TTD phenotypes, preventing interaction between XPD and the p44 subunit of TFIIH. Nat Genet 20: 184–188 [DOI] [PubMed] [Google Scholar]
- Coin F, Oksenych V, Egly JM (2007) Distinct roles for the XPB/p52 and XPD/p44 subcomplexes of TFIIH in damaged DNA opening during nucleotide excision repair. Mol Cell 26: 245–256 [DOI] [PubMed] [Google Scholar]
- Delagoutte E, von Hippel PH (2003) Helicase mechanisms and the coupling of helicases within macromolecular machines. Part II: Integration of helicases into cellular processes. Q Rev Biophys 36: 1–69 [DOI] [PubMed] [Google Scholar]
- Doublie S, Tabor S, Long AM, Richardson CC, Ellenberger T (1998) Crystal structure of a bacteriophage T7 DNA replication complex at 2.2 A resolution. Nature 391: 251–258 [DOI] [PubMed] [Google Scholar]
- Durr H, Korner C, Muller M, Hickmann V, Hopfner KP (2005) X-ray structures of the Sulfolobus solfataricus SWI2/SNF2 ATPase core and its complex with DNA. Cell 121: 363–373 [DOI] [PubMed] [Google Scholar]
- Ellis NA (1997) DNA helicases in inherited human disorders. Curr Opin Genet Dev 7: 354–363 [DOI] [PubMed] [Google Scholar]
- Fan L, Arvai AS, Cooper PK, Iwai S, Hanaoka F, Tainer JA (2006) Conserved XPB core structure and motifs for DNA unwinding: implications for pathway selection of transcription or excision repair. Mol Cell 22: 27–37 [DOI] [PubMed] [Google Scholar]
- Fan L, Fuss JO, Cheng QJ, Arvai AS, Hammel M, Roberts VA, Cooper PK, Tainer JA (2008) XPD helicase structures and activities: insights into the cancer and aging phenotypes from XPD mutations. Cell 133: 789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frit P, Kwon K, Coin F, Auriol J, Dubaele S, Salles B, Egly JM (2002) Transcriptional activators stimulate DNA repair. Mol Cell 10: 1391–1401 [DOI] [PubMed] [Google Scholar]
- Giglia-Mari G, Coin F, Ranish JA, Hoogstraten D, Theil A, Wijgers N, Jaspers NG, Raams A, Argentini M, van der Spek PJ, Botta E, Stefanini M, Egly JM, Aebersold R, Hoeijmakers JH, Vermeulen W (2004) A new, tenth subunit of TFIIH is responsible for the DNA repair syndrome trichothiodystrophy group A. Nat Genet 36: 714–719 [DOI] [PubMed] [Google Scholar]
- Guzder SN, Sung P, Bailly V, Prakash L, Prakash S (1994) RAD25 is a DNA helicase required for DNA repair and RNA polymerase II transcription. Nature 369: 578–581 [DOI] [PubMed] [Google Scholar]
- Hall H, Gursky J, Nicodemou A, Rybanska I, Kimlickova E, Pirsel M (2005) Characterization of ERCC3 mutations in the Chinese hamster ovary 27-1, UV24 and MMC-2 cell lines. Mutat Res 593: 177–186 [DOI] [PubMed] [Google Scholar]
- Hoogstraten D, Nigg AL, Heath H, Mullenders LH, van Driel R, Hoeijmakers JH, Vermeulen W, Houtsmuller AB (2002) Rapid switching of TFIIH between RNA polymerase I and II transcription and DNA repair in vivo. Mol Cell 10: 1163–1174 [DOI] [PubMed] [Google Scholar]
- Iben S, Tschochner H, Bier M, Hoogstraten D, Hozak P, Egly JM, Grummt I (2002) TFIIH plays an essential role in RNA polymerase I transcription. Cell 109: 297–306 [DOI] [PubMed] [Google Scholar]
- Lehmann AR (2003) DNA repair-deficient diseases, Xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. Biochimie 85: 1101–1111 [DOI] [PubMed] [Google Scholar]
- Lin YC, Choi WS, Gralla JD (2005) TFIIH XPB mutants suggest a unified bacterial-like mechanism for promoter opening but not escape. Nat Struct Mol Biol 12: 603–607 [DOI] [PubMed] [Google Scholar]
- Liu H, Rudolf J, Johnson KA, McMahon SA, Oke M, Carter L, McRobbie AM, Brown SE, Naismith JH, White MF (2008) Structure of the DNA repair helicase XPD. Cell 133: 801–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Westbroek A, Jochemsen AG, Weeda G, Bosch A, Bootsma D, Hoeijmakers JH, van der Eb AJ (1994) Mutational analysis of ERCC3, which is involved in DNA repair and transcription initiation: identification of domains essential for the DNA repair function. Mol Cell Biol 14: 4126–4134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard O, Camenisch U, Clement FC, Blagoev KB, Naegeli H (2007) DNA repair triggered by sensors of helical dynamics. Trends Biochem Sci 32: 494–499 [DOI] [PubMed] [Google Scholar]
- O'Donnovan A, Davies AA, Moggs JG, West SC, Wood RD (1994) XPG endonuclease makes the 3′ incision in human DNA nucleotide excision repair. Nature 371: 432–435 [DOI] [PubMed] [Google Scholar]
- Ranish JA, Hahn S, Lu Y, Yi EC, Li XJ, Eng J, Aebersold R (2004) Identification of TFB5, a new component of general transcription and DNA repair factor IIH. Nat Genet 36: 707–713 [DOI] [PubMed] [Google Scholar]
- Richards JD, Cubeddu L, Roberts J, Liu H, White MF (2008) The archaeal XPB protein is a ssDNA-dependent ATPase with a novel partner. J Mol Biol 376: 634–644 [DOI] [PubMed] [Google Scholar]
- Riedl T, Hanaoka F, Egly JM (2003) The comings and goings of nucleotide excision repair factors on damaged DNA. EMBO J 22: 5293–5303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy R, Schaeffer L, Humbert S, Vermeulen W, Weeda G, Egly JM (1994) The DNA-dependent ATPase activity associated with the class II basic transcription factor BTF2/TFIIH. J Biol Chem 269: 9826–9832 [PubMed] [Google Scholar]
- Sancar A (1996) DNA excision repair. Annu Rev Biochem 65: 43–81 [DOI] [PubMed] [Google Scholar]
- Schaeffer L, Moncollin V, Roy R, Staub A, Mezzina M, Sarasin A, Weeda G, Hoeijmakers JHJ, Egly JM (1994) The ERCC2/DNA repair protein is associated with the class II BTF2/TFIIH transcription factor. EMBO J 13: 2388–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer L, Roy R, Humbert S, Moncollin V, Vermeulen W, Hoeijmakers JH, Chambon P, Egly JM (1993) DNA repair helicase: a component of BTF2 (TFIIH) basic transcription factor. Science 260: 58–63 [DOI] [PubMed] [Google Scholar]
- Sijbers AM, de Laat WL, Ariza RR, Biggerstaff M, Wei YF, Moggs JG, Carter KC, Shell BK, Evans E, de Jong MC, Rademakers S, de Rooij J, Jaspers NG, Hoeijmakers JH, Wood RD (1996) Xeroderma pigmentosum group F caused by a defect in a structure-specific DNA repair endonuclease. Cell 86: 811–822 [DOI] [PubMed] [Google Scholar]
- Sugasawa K, Ng J, Masutani C, Iwai S, van der Spek P, Eker A, Hanaoka F, Bootsma D, Hoeijmakers J (1998) Xeroderma pigmentosum group C protein complex is the initiator of global genome nucleotide excision repair. Mol Cell 2: 223–232 [DOI] [PubMed] [Google Scholar]
- Sung P, Higgins D, Prakash L, Prakash S (1988) Mutation of lysine-48 to arginine in the yeast RAD3 protein abolishes its ATPase and DNA helicase activities but not the ability to bind ATP. EMBO J 7: 3263–3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapias A, Auriol J, Forget D, Enzlin J, Scharer O, Coin F, Coulombe B, Egly J (2004) Ordered conformational changes in damaged DNA induced by nucleotide excision repair factors. J Biol Chem 279: 19074–19083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirode F, Busso D, Coin F, Egly JM (1999) Reconstitution of the transcription factor TFIIH: assignment of functions for the three enzymatic subunits, XPB, XPD, and cdk7. Mol Cell 3: 87–95 [DOI] [PubMed] [Google Scholar]
- Tuteja N, Tuteja R (2004) Unraveling DNA helicases. Motif, structure, mechanism and function. Eur J Biochem 271: 1849–1863 [DOI] [PubMed] [Google Scholar]
- Volker M, Mone MJ, Karmakar P, van Hoffen A, Schul W, Vermeulen W, Hoeijmakers JH, van Driel R, van Zeeland AA, Mullenders LH (2001) Sequential assembly of the nucleotide excision repair factors in vivo. Mol Cell 8: 213–224 [DOI] [PubMed] [Google Scholar]
- von Hippel P (2004) Helicases become mechanistically simpler and functionally more complex. Nat Struct Mol Biol 11: 494–496 [DOI] [PubMed] [Google Scholar]
- Winkler GS, Araujo SJ, Fiedler U, Vermeulen W, Coin F, Egly JM, Hoeijmakers JH, Wood RD, Timmers HT, Weeda G (2000) TFIIH with inactive XPD helicase functions in transcription initiation but is defective in DNA repair. J Biol Chem 275: 4258–4266 [DOI] [PubMed] [Google Scholar]
- Wolski SC, Kuper J, Hanzelmann P, Truglio JJ, Croteau DL, Van Houten B, Kisker C (2008) Crystal structure of the FeS cluster-containing nucleotide excision repair helicase XPD. PLoS Biol 6: e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi M, Masutani C, Maekawa T, Sugasawa K, Ohkuma Y, Hanaoka F (2000) The xeroderma pigmentosum group C protein complex XPC-HR23B plays an important role in the recruitment of transcription factor IIH to damaged DNA. J Biol Chem 275: 9870–9875 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1S
Supplementary data 1
Review Process File