Abstract
Endoplasmic reticulum-associated degradation (ERAD) is a cellular pathway for the disposal of misfolded secretory proteins. This process comprises recognition of the misfolded proteins followed by their retro-translocation across the ER membrane into the cytosol in which polyubiquitination and proteasomal degradation occur. A variety of data imply that the protein import channel Sec61p has a function in the ERAD process. Until now, no physical interactions between Sec61p and other essential components of the ERAD pathway could be found. Here, we establish this link by showing that Hrd3p, which is part of the Hrd-Der ubiquitin ligase complex, and other core components of the ERAD machinery physically interact with Sec61p. In addition, we study binding of misfolded CPY* proteins to Sec61p during the process of degradation. We show that interaction with Sec61p is maintained until the misfolded proteins are ubiquitinated on the cytosolic side of the ER. Our observations suggest that Sec61p contacts an ERAD ligase complex for further elimination of ER lumenal misfolded proteins.
Keywords: CPY*, ERAD, retro-translocation, Sec61p, ubiquitin ligase
Introduction
The occurrence of mistakes is inherent in biological systems. Misfolding of proteins is frequent and has deleterious effects in cells (Aridor and Hannan, 2000; Dobson, 2003). Therefore, protein quality control and elimination pathways have evolved. One such elimination pathway is connected with the endoplasmic reticulum (ER), the organelle responsible for import, folding and distribution of secretory proteins to their site of action. This pathway, termed as ER-associated degradation (ERAD), comprises the retro-translocation of misfolded proteins across the ER membrane into the cytosol, their polyubiquitination and proteasomal degradation (Plemper and Wolf, 1999; Kostova and Wolf, 2003; Meusser et al, 2005; Römisch, 2005; Nakatsukasa and Brodsky, 2008; Schäfer et al, 2008; Vembar and Brodsky, 2008). Depending on the localization of the misfolded domain, proteins are degraded through different ERAD pathways (Vashist and Ng, 2004; Carvalho et al, 2006; Nakatsukasa and Brodsky, 2008). All these pathways have in common that they require the ubiquitin conjugating enzyme Ubc7p and a complex consisting of the AAA–ATPase Cdc48p, Ufd1p and Npl4p as well as the proteasome for elimination of misfolded proteins. Proteins carrying cytosolic misfolded domains or degradation signals are degraded by the ERAD-C (cytosolic) pathway, which depends in addition on the ER membrane-located ubiquitin ligase Doa10p (Huyer et al, 2004; Vashist and Ng, 2004). The central component of the ERAD-L (lumen) and ERAD-M (membrane) pathway is the Hrd-Der ubiquitin ligase complex of the ER membrane. This complex is necessary for the recognition and ubiquitination process of proteins carrying misfolded domains in the lumen or in the membrane of the ER (Vashist and Ng, 2004; Carvalho et al, 2006). It consists of the ubiquitin ligase Der3p (also known as Hrd1p), which exposes a RING finger domain to the cytoplasm (Bays et al, 2001; Deak and Wolf, 2001) and of Hrd3p carrying a long N-terminal domain in the ER lumen (Hampton et al, 1996; Plemper et al, 1999a). Recognition of misfolded ERAD-L glycoproteins includes binding to Hrd3p assisted by the chaperone Kar2p and the lectin Yos9p in the ER lumen (Kim et al, 2005; Denic et al, 2006; Gauss et al, 2006a). Thereafter, the misfolded proteins are transported into the cytosol, which requires transfer across the ER membrane and their subsequent polyubiquitination by the Der3p ubiquitin ligase (Hiller et al, 1996; Bordallo et al, 1998). In the cytosol, the AAA–ATPase Cdc48p and its substrate recruiting factors, Ufd1p and Npl4p, provide the driving force for final extraction of polyubiquitinated misfolded proteins from the ER membrane (Bays et al, 2001; Ye et al, 2001; Braun et al, 2002; Jarosch et al, 2002; Rabinovich et al, 2002). Thus, the ligase Doa10p and the Hrd-Der ligase complex in conjunction with the Cdc48 complex are central parts of the ER retro-translocation machineries.
The discussion of the retro-translocation channel
The nature of the retro-translocation channel is still under discussion. Several data point at the ER membrane proteins Derlin-1 and Sec61p as possible candidates for the channel-forming protein for retro-translocation. The data concerning Derlin-1 as a candidate were mostly obtained from studies in mammalian cells. In cytomegalovirus-infected mammalian cells, newly synthesized, ER membrane-localized MHC class I molecules are re-directed into the cytosol and thereby interact with p97 and Derlin-1 (Lilley and Ploegh, 2004; Ye et al, 2004), the mammalian homologues of yeast Cdc48 and Der1p, respectively (Knop et al, 1996). This finding led to the proposal that Derlin-1 is a central component of the retro-translocation channel. Furthermore, data obtained by an in vitro fluorescence approach have also shown an involvement of Derlin-1 in retro-translocation without the participation of mammalian Sec61α (Wahlman et al, 2007). These observations are in contrast to an earlier study that favoured Sec61p as the retro-translocon (Wiertz et al, 1996). Indeed, due to its function as the import channel into the ER, Sec61p was early shortlisted as a possible retro-translocation channel for misfolded proteins. In yeast, indications for a role of Sec61p in ERAD are based on a variety of genetic data. Conditional, temperature-sensitive sec61 mutant strains showed a disturbed degradation of the misfolded ER-model substrate CPY*, derivatives of CPY* and of mutated pro-α-factor (Pilon et al, 1997, 1998; Plemper et al, 1997; Willer et al, 2008). Genetic interaction studies also point to an involvement of Sec61p in the ERAD process (Plemper et al, 1999a). An additional connection with ER-associated proteolysis is indicated by the fact that Sec61p interacts with the 19S sub-complex of the proteasome (Ng et al, 2007). All these data reflect a participation of Sec61p in retro-translocation of misfolded proteins in yeast, but so far, no interaction of Sec61p with known core components of the ERAD machinery could be detected.
In this work, we investigated the physical interactions of Sec61p with substrates and components of the ERAD-L pathway. We show the interaction of Sec61p with Hrd3p and other ERAD core components. We show that fully glycosylated and, therefore, completely imported soluble ERAD substrates bind to Sec61p. Interestingly, deletions of Hrd3p or Der3p lead to an enhanced binding of the ERAD substrate CPY* to Sec61p. This indicates that the association of CPY* to Sec61p is retained until ubiquitination of the misfolded proteins on the cytosolic side of the ER takes place. Our data provide strong evidence that Sec61p is a physical part of the ERAD machinery for further transport and subsequent degradation of misfolded ER lumenal proteins.
Results
Sec61p is associated with core components of the ERAD-L pathway
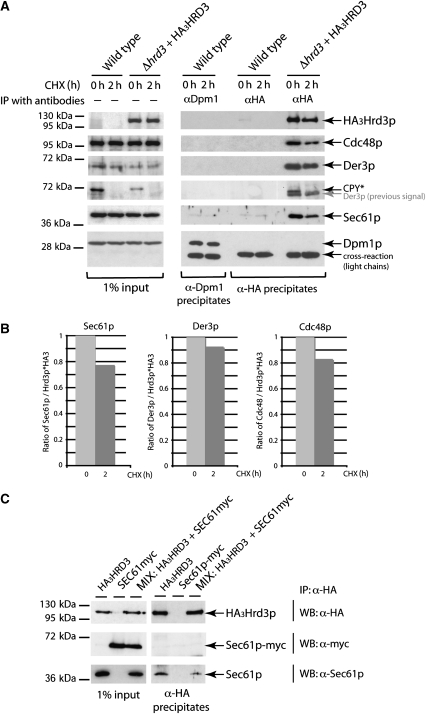
Any participation of Sec61p in the degradation of misfolded proteins should require an association with the ERAD machinery. Therefore, we wanted to know whether any physical contact between components and substrates of the ERAD pathway and Sec61p exits. To address this question, we isolated the ERAD component Hrd3p, which is part of the Hrd–ubiquitin ligase complex, and analysed it for the presence of Sec61p. We precipitated functional aminoterminally HA-tagged Hrd3p (HA3Hrd3p) from digitonin-solubilized microsomal extracts using specific α-HA antibodies. As Hrd3p is an ER resident membrane protein, one may assume that it is inserted into the membrane through the Sec61p translocon during import. To exclude that any detectable interaction between Sec61p and ER membrane proteins occurs during the import process, we blocked new protein synthesis for 2 h with cycloheximide (CHX) before immunoprecipitation. This treatment allows complete transfer of already existing polypeptides into the ER. In immunoprecipitates of HA3Hrd3p without addition of CHX to cells (time zero), we observed an association of Sec61p with HA3Hrd3p (Figure 1A). The association also exists after 2 h CHX treatment (Figure 1A), even though the amount of Sec61p is somewhat reduced after 2 h (Figure 1B). The continued presence of the Sec61p–Hrd3p interaction even after 2 h of halt of protein synthesis points to a functional association of both proteins independent from Hrd3p import. Besides Sec61p, we detected already known interaction partners of Hrd3p such as the ubiquitin ligase Der3p as well as Cdc48p (Figure 1A). The amount of Der3p interacting with Hrd3p after 2 h of CHX addition is only marginally reduced, indicating a rather stable interaction over time (Figure 1B). Interestingly, association of Cdc48p with the Hrd complex is decreased after 2 h CHX treatment of the cells. This observation can be explained by the fact that Cdc48p is in a dynamic relation to the Hrd complex and seems to be removed from the ER membrane for delivery of ubiquitinated proteins to the proteasome (Alberts et al, 2009). We observed a similar decrease in the Sec61p–Hrd3p contact (Figure 1A and B), indicating that this interaction might also be a dynamic process. As expected, we also found the misfolded ERAD-L substrate CPY* interacting with HA3Hrd3p in cells without CHX treatment. However, after 2 h of CHX addition to cells, CPY* is completely degraded, whereas Sec61p is still associated with HA3Hrd3p (Figure 1A). The association might be mediated by further interacting proteins or by endogenous misfolded proteins still present in the ER.
Figure 1.
Sec61p is associated with components of the ERAD pathway. (A) Immunoprecipitation experiment using a plasmid encoded, N-terminally HA3-tagged version of Hrd3p. Digitonin-solubilized microsomal proteins were prepared from wild type and Δhrd3+HA3Hrd3p strains (input). Half of the cells were pre-treated with CHX for 2 h, whereas the other half was left untreated. HA3Hrd3p and its interaction partners were isolated by immunoprecipitation with α-HA-antibodies and ProteinA-Sepharose (α-HA precipitates). The precipitates were analysed by immunoblotting with the indicated antibodies. Dpm1p serves as a negative control as it does not interact with Sec61p and was precipitated using specific α-Dpm1p antibodies. When detecting the CPY* signal, the earlier signal of Der3p could still be seen in α-HA precipitates (indicated in grey letters). (B) Ratios of Der3p, Cdc48 and Sec61p to HA3Hrd3p obtained by quantification of the corresponding immunoblot signals of the precipitates shown in (A) using the program ImageJ 1.41o. (C) ‘MIX' experiment to exclude formation of artificial Sec61p containing protein complexes. Cells from strains expressing either Sec61p-myc and endogenous Hrd3p or untagged Sec61p and HA3Hrd3p were subjected to immunoprecipitation as described above. In the MIX, sample cells of these two strains were mixed before immunoprecipitation.
To ensure that the observed interaction of HA3Hrd3p with Sec61p has not arisen from an unspecific binding of Sec61p to ER membrane proteins, we immunoprecipitated dolichol phosphate mannose synthase (Dpm1p) and analysed for Sec61p interactions. Dpm1p is a stable ER membrane protein with one transmembrane domain similar to Hrd3p. Only insignificant Sec61p background signals were immunoprecipitated with α-Dpm1p (Figure 1A). Similarly, insignificant Sec61p signals were found in α-HA precipitates of wild-type cells confirming the specificity of the applied immunoprecipitation approach. To exclude the possibility of artificial interactions of Sec61p to other proteins during membrane solubilization and precipitation, we performed the following experiment. Cells of two different strains expressing either Sec61p–myc and endogenous Hrd3p or untagged Sec61p and HA3Hrd3p were mixed. Thereafter, membrane proteins were solubilized and subjected to immunoprecipitation as described before followed by immunoblot analysis. Although endogenous Sec61p was bound to HA3Hrd3p, no Sec61p–myc was present in the precipitates (Figure 1C). This excludes that the applied experimental procedure results in isolation of Sec61p interactions due to formation of an artefact.
Sec61p loses contact to misfolded proteins during the degradation process
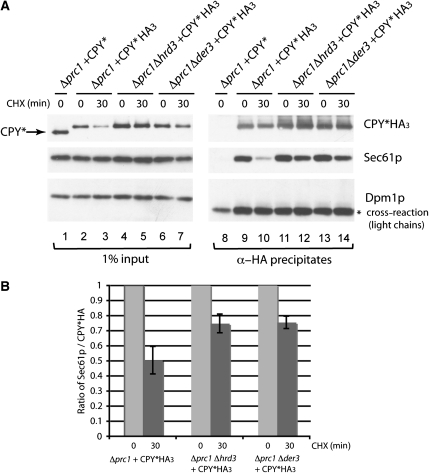
To characterize the contact between Sec61p and a misfolded protein during its degradation at the ER membrane, the ERAD-L substrate CPY*HA3 was used as a bait. In yeast cells, several pools of CPY* proteins exist, localized at different positions at the ER membrane. CPY* contacts Sec61p during the import step, is quickly recognized as misfolded and thereafter bound to ERAD components on the ER lumenal side. An additional pool of CPY* could reside in the retro-translocation channel and in a partly ubiquitinated form on the cytosolic side, but still in contact with the ER membrane. To enrich the endogenous amount of CPY* in the stage of misfolding and retro-translocation, cells were treated for 30 min with CHX. This treatment still allows import of CPY* into the ER, which is completed within 5 min, whereas protein degradation by the ERAD pathway continues (Taxis et al, 2002). As the half-life of misfolded CPY* is about 20 min (Hiller et al, 1996), all CPY* molecules present after 30 min of CHX treatment should be on their way to degradation. For analysis, cells deleted for the coding sequence of CPY (Δprc1) and complemented with a plasmid encoding CPY*HA3 were treated with and without CHX. Microsomal CPY*HA3 extracts were subjected to immunoprecipitation followed by immunoblot analysis. We found an interaction of CPY*HA3 with Sec61p even after 30 min of CHX treatment of cells (Figure 2A, lanes 9 and 10). During degradation of CPY*HA3 over a time period of 30 min, the association of Sec61p with CPY*HA3 decreased, but still exists. This association points to an import independent contact between Sec61p and CPY*HA3.
Figure 2.
Sec61p loses contact to misfolded proteins during the degradation process. (A) Immunoprecipitation experiment using CPY*HA3 as a bait. Digitonin-solubilized membrane proteins of isolated microsomes were prepared from Δprc1, Δprc1Δhrd3 and Δprc1Δder3 strains expressing either CPY* or CPY*HA3 (input). The cells were pre-treated for 0 and 30 min with CHX. After precipitation using specific α-HA antibodies, the samples (α-HA precipitates) were analysed by immunoblotting with the indicated antibodies. The stars indicate unspecific cross-reactions. (B) Ratio of Sec61p to CPY*HA3 obtained by quantification of the corresponding immunoblot signals of the immunopreciptates shown in (A) using the program ImageJ 1.41o. (A). The error bars represent the s.e.m. of three independent experiments.
The contact between Sec61p and CPY* is enhanced in ERAD mutant strains
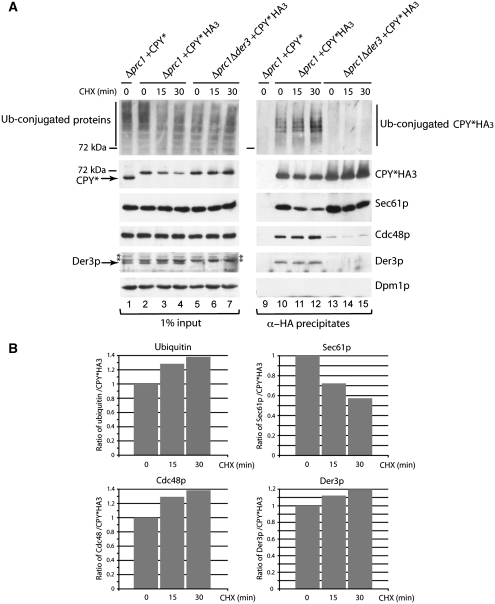
It is known that a deletion of the ubiquitin ligase Der3p leads to a complete block of CPY* degradation. In a Δder3 strain, the misfolded protein is not retrograde transported into the cytosol and accumulates in the ER (Bordallo et al, 1998). Indeed, we observed an accumulation of CPY*HA3 in the microsomal membrane extracts of Δprc1Δder3 and Δprc1Δhrd3 strains after 30 min of CHX addition (Figure 2A, lanes 4–7). Interestingly, after this 30 min treatment, deletions of Der3p and of its complex partner Hrd3p lead to enhanced interactions of Sec61p with CPY*HA3 at the ER membrane (Figure 2A, lanes 11–14). The ratio of quantified Sec61p and CPY*HA3 signals revealed an increase of this contact of about 25% in Δprc1Δhrd3 and Δprc1Δder3 strains after 30 min of CHX addition in comparison to the wild-type situation in a Δprc1 strain (Figure 2B). These data suggest that the contact of Sec61p to misfolded CPY*HA3 persits until ubiquitination of CPY*HA3 by the Der3p ligase has occurred, implying an association of CPY*HA3 with Sec61p during transport out of the ER. In agreement with this, we observed increased relative amounts of polyubiquitinated CPY*HA3 in the immunoprecipitates of wild-type cells over a time period of 0, 15 and 30 min after CHX addition, whereas the interaction of CPY*HA3 with Sec61p again decreased with time (Figure 3A, lanes 10–12, and 3B). We also found the known ERAD components Cdc48p and Der3p in the immunoprecipitates (Figure 3A, lanes 10–12), which also show an increased contact with CPY*HA3 after CHX treatment (Figure 3A and B). Deletions of DER3 disturb the contact between ER lumenal misfolded proteins and the Cdc48 complex in the cytosol (Gauss et al, 2006b), resulting in the observed weak Cdc48 signal in CPY*HA3 immunoprecipitates (Figure 3A, lanes 13–15). The enhanced signals of ubiquitin, Cdc48p and Der3p together with CPY*HA3 confirm that we isolated in this experiment a pool of CPY*, which is on its way out of the ER after 15 and 30 min of CHX addition to cells.
Figure 3.
The pool of ubiquitinated CPY* at the ER membrane increases with time, whereas Sec61p loses contact to CPY*. (A) The immunoprecipitation experiment was carried out as described in Figure 2A using Δprc1 and Δprc1Δder3 strains expressing either CPY* or CPY*HA3 (input). The cells were pre-treated for 0, 15 and 30 min with CHX. The immunoblot analysis included the detection of ubiquitinated proteins. The stars indicate unspecific cross-reactions. (B) Ratio of the ubiquitin, Sec61p, Cdc48 and Der3p signals to the CPY*HA3 signals in immunoprecipitates of Δprc1+CPY*HA3 shown in (A) using the program ImageJ 1.41o.
Sec61p is necessary for degradation of the CPY* moiety of the membrane protein CTL*myc
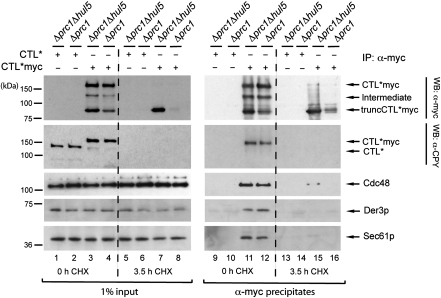
To analyse an interaction between Sec61p and a misfolded membrane protein, we performed additional immunoprecipitation experiments and isolated CTL*myc. CTL*myc consists of an ER lumenal CPY* moiety, the last transmembrane segment of Pdr5p and a cytosolically located, myc-tagged 3-isopropylmalate dehydrogenase (Leu2p). Recently, we have shown that CTL*myc is degraded by the ERAD system in a step-wise manner (Figure 4) (Kohlmann et al, 2008). Besides the ubiquitin ligase Der3p and the AAA–ATPase Cdc48p, membrane extraction and complete degradation of CTL*myc requires the E4 ligase Hul5p. The absence of Hul5p leads to the appearance of a stable fragment of CTL*myc, truncCTL*myc (Figure 4). TruncCTL*myc is still membrane embedded and contains the myc-tagged Leu2 protein domain localized in the cytosol, but has lost most of its CPY* moiety (Kohlmann et al, 2008). Immunoprecipitation experiments showed that full-length CTL*myc interacts with the ERAD components Der3p and Cdc48p during degradation, whereas the membrane-embedded fragment truncCTL*myc did not exhibit any interaction with the ERAD machinery (Kohlmann et al, 2008). Therefore, we tested whether CTL*myc and truncCTL*myc interact with Sec61p. We isolated CTL*myc with α-myc antibodies and observed, besides the interaction with Der3p and Cdc48, also an association of CTL*myc with Sec61p (Figure 4, lanes 11 and 12). Interestingly, the membrane-embedded truncCTL*myc, which has lost the contact to Der3p and Cdc48, also does not associate with Sec61p (Figure 4, compare lanes 11 and 12 with lanes 15 and 16). Thus, we conclude that Sec61p is necessary for degradation of the misfolded ER lumenal CPY* moiety of CTL*myc. The remaining membrane-embedded part of the protein may traverse a different route.
Figure 4.
CTL*myc interacts with Sec61p until the CPY* moiety of CTL*myc is degraded. Cells of the yeast strains Δprc1 and Δprc1Δhul5 expressing either plasmid encoded CTL* or CTL*myc were treated with CHX for 0 and 3.5 h. This enables the accumulation of the fragment truncCTL*myc in a Δprc1Δhul5 strain (lane 7). Solubilized microsomal proteins were subjected to immunoprecipitation using α-myc antibodies (α-myc precipitates) and were analysed by immunoblot analysis with the indicated antibodies. Except for the interaction studies of Sec61p, this figure was earlier published in Kohlmann et al (2008). CTL*myc interacts with Sec61p during its degradation (lanes 11 and 12) as well as the known components of the ERAD machinery, Der3p and Cdc48 (Kohlmann et al, 2008).
Fully imported and glycosylated proteins are associated with Sec61p
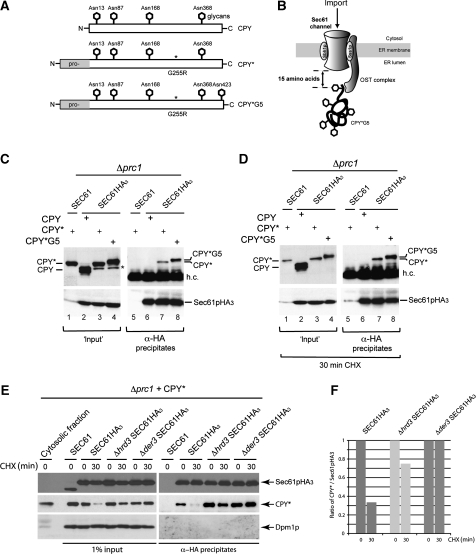
By isolating CPY*HA3, we were able to detect an interaction of CPY* with Sec61p, Cdc48 and the ERAD-L ubiquitin ligase Der3p (Figure 3A, lanes 10–12). To address these interactions from the Sec61p side, we performed immunoprecipitation experiments using carboxyterminally HA-tagged Sec61p (Sec61pHA3) expressed from genomically integrated SEC61-HA3. In addition, we expressed either the plasmid encoded vacuolar carboxypeptidase Y (CPY) or mutated versions in a strain deleted for the coding sequence of endogenous CPY (Δprc1). The CPY species are all post-translationally imported as pre-pro-forms in an unfolded state into the ER lumen through the Sec61p channel. There, the pre-(signal) sequences are cleaved off and the proteins are glycosylated and folded. Properly folded wild-type CPY is further processed in the ER and the Golgi apparatus and finally delivered to the vacuole in which the enzyme is matured by cleavage of its pro-sequence (Müller and Müller, 1981; Stevens et al, 1982) In contrast to wild-type CPY, the mutated versions CPY* and CPY*G5 are unable to fold properly. These proteins are recognized as misfolded, retained in the ER and degraded by the ERAD pathway (Hiller et al, 1996; Plemper et al, 1999b). Although CPY* is glycosylated at four different sites, the CPY* derivative CPY*G5 contains an additional glycosylation site positioned at three amino acids from the C-terminus (Figure 5A) (Plemper et al, 1999b). During import through the Sec61p channel, the proteins are glycosylated by the oligosaccharyl-transferase (OST) complex. The spatial gap between the active site of the OST complex on imported polypeptides and the Sec61p import channel was identified to be 15 amino-acid residues (Figure 5B) (Whitley et al, 1996). As CPY*G5 is glycosylated at all five sites, one can conclude that its import into the ER has been fully completed and contact with Sec61p during import has been lost.
Figure 5.
Fully glycosylated proteins are associated with Sec61p. (A) Schematic representation of carboxypeptidase Y (CPY), point mutated CPY G255R (CPY*) and CPY* including an additional glycan at the amino-acid position 423 (CPY*G5). (B) Import model of CPY*G5. The active site of the OST complex is located in a distance of ∼15 residues from the end of the hydrophobic transmembrane segments of Sec61p. (C) Immunoprecipitation experiment using a C-terminally HA3-tagged version of Sec61p. Digitonin-solubilized microsomal proteins (input) were prepared from strains deleted for the coding sequence of CPY (Δprc1) and expressing either plasmid encoded CPY, CPY* or CPY*G5. Sec61pHA3 was precipitated with α-HA antibodies and ProteinA–Sepharose (α-HA precipitates) and analysed by immunoblotting using specific α-CPY and α-Sec61p antibodies. The star indicates pre-pro-forms of CPY* and CPY*G5; h.c. indicates a cross-reaction with the heavy chains of the antibodies used for precipitation (D) Immunoprecipitation experiment carried out as described above. The cells were pre-treated with CHX for 30 min at 30°C. (E) Immunoprecipitation experiment using the strains SEC61HA3, Δhrd3 SEC61HA3 and Δder3 SEC61HA3. All strains carry in addition a Δprc1 deletion and plasmid encoded CPY*. The deletion of the core components of the Hrd–ubiquitin ligase complex, Der3p and Hrd3p, leads to an enhanced interaction of CPY* with Sec61p. (F) Ratio of immunoblot signals of CPY* to Sec61p signals of immunoprecipitates shown in (E).
For immunoprecipitation experiments, functional microsomal Sec61pHA3 was isolated from a Δprc1Sec61pHA3 strain expressing either CPY, CPY* or CPY*G5 (Figure 5C, lanes 2–4). CPY* and CPY*G5 can be clearly immunoprecipitated with Sec61pHA3 (Figure 5C, lanes 7 and 8). In contrast, no interaction was observed with wild-type CPY or with its pro-forms (Figure 5C, lane 6), indicating a normal import of CPY in a strain carrying the HA-tagged version of Sec61p. In the microsomal extracts (input), we detected an additional CPY* and CPY*G5 signal in the Δprc1Sec61pHA3 strain (Figure 5C, lanes 3 and 4, indicated by a star) compared with the CPY* signal in Δprc1 (Figure 5C, lane 1). Most likely, these additional signals represent unglycosylated pre-pro-forms of CPY* and CPY*G5. Such forms can sometimes be detected and disappear when import is completed, as it is for instance after the addition of CHX to the cells for 30 min (Figure 5D, lanes 3 and 4). Interestingly, neither the pre-pro-forms of mutated CPY*, CPY*G5 nor of wild-type CPY were found in the Sec61pHA3 immunoprecipitates (Figure 5C, lanes 6–8). Obviously, these forms are rapidly imported and have, therefore, only a short and non-detectable contact with Sec61pHA3. The observation that only fully glycosylated, misfolded CPY* species are bound to Sec61pHA3 indicates that this binding occurs after their complete import and glycosylation in the ER.
The OST complex interacts directly with the protein Sss1p, which is part of the trimeric Sec61p complex (Scheper et al, 2003). One might argue that the observed association of mutated CPY* and CPY*G5 with Sec61pHA3 may be the result of an indirect interaction through the OST complex. To exclude this possibility, cells were treated with CHX for 30 min before immunoprecipitation as described before, allowing complete import of soluble CPY* and CPY*G5 into the ER lumen. Under these conditions, we still observe an association of misfolded CPY* and CPY*G5 with Sec61pHA3 (Figure 5D, lanes 7 and 8). For quantitative analysis of this association, we analysed the samples side by side on one SDS gel (Figure 5E) and calculated the ratio between the Sec61p and CPY* immunoblot signals (Figure 5F). We observed a decrease of this binding, which we also found earlier when isolating Sec61p through CPY*HA3 (Figure 2A). Deletion of the ubiquitin ligase Der3p and its complex partner Hrd3p leads to enhanced interaction between Sec61pHA3 and CPY* at the ER membrane (Figure 5E), which we also observed in the earlier analysis of CPY*HA3 immunoprecipitates (Figure 2A). This association is not mediated through the pre-sequence of CPY*, which may have contact with Sec61p until cleavage by the signal peptidase occurs (Johnson et al, 1987; Ng et al, 1996). The molecular mass of soluble, pre-sequence cleaved CPY* (Figure 5E, cytosolic fraction) is identical with the molecular mass of microsomal, ER membrane-associated CPY* found in the input fractions and in the immunoprecipitates (Figure 5E). Thus, Sec61pHA3 must be considered to be associated with pre-sequence cleaved CPY*. We were unable to detect Cdc48 or Der3p in the Sec61pHA3 immunoprecipitates (data not shown). Interestingly, this import independent Sec61p–CPY* contact at the ER membrane seems to exist even without the presence of Hrd3p (Figures 2A and 5E), which was proposed to be necessary for the recruitment of misfolded proteins in the ERAD process (Denic et al, 2006; Gauss et al, 2006b). The absence of the Hrd-Der ligase complex as well as Cdc48 in Sec61pHA3 immunoprecipitates may point to the existence of a protein complex composed of Sec61p and misfolded proteins without known ERAD components. This complex seems to exist in addition to the described Sec61p containing Hrd complex (Figure 1A).
Discussion
Sec61p is part of the ERAD machinery
Several genetic studies implicated a function of Sec61p in the ERAD process for elimination of misfolded proteins (Pilon et al, 1997; Plemper et al, 1997, 1999a). Recent data using a novel sec61-3 mutant strain and co-translationally imported CPY* derivatives corroborated that a functional Sec61p is essential for the elimination process of ERAD-L substrates (Willer et al, 2008). Up to now, no interaction between Sec61p and known ERAD components could be determined on a biochemical basis. Therefore, we investigated the physical contact between components and substrates of the ERAD pathway with Sec61p. The essential role of Sec61p in the import process of proteins into the ER complicates the analysis of an additional function of Sec61p in the ERAD process. As most of the ERAD components have to be imported into the ER, detection of an interaction between Sec61p and ERAD substrates or ERAD components such as Hrd3p might represent only contact during the import step. To analyse completely imported proteins, we pre-treated the cells with CHX before immunoprecipitation experiments. This treatment prevents new protein synthesis, whereas the import of already existing polypeptides and the ERAD process continues. Even after the addition of CHX, we observed an association of Sec61p with the Hrd-Der ligase complex and Cdc48p using HA3Hrd3p as a bait (Figure 1A). Furthermore, we confirmed this interaction by isolating the misfolded ERAD-L substrate CPY*HA3 together with Sec61p, Cdc48 and the ubiquitin ligase Der3p (Figure 3A). As Der3p is always connected to its complex partner Hrd3p (Plemper et al, 1999a; Gardner et al, 2000; Denic et al, 2006), Hrd3p can also be assumed to be present in the immunoprecipitates. Thus, we propose that Sec61p is a dynamic part of the Hrd-Der ligase complex. In an additional experiment using Sec61pHA3 as a bait, the interaction between Sec61p and misfolded CPY* and CPY*G5 was proven (Figure 5C and D). However, in the same experiment, we did not observe any Cdc48 or Der3p signals (data not shown). One explanation for this observation might reside in the existence of a protein complex, including Sec61p and misfolded proteins, but excluding the Hrd-Der ligase complex. Interestingly, the association between CPY* and Sec61p is not disrupted in a Δhrd3 strain (Figures 2A and 5E). This observation is somewhat surprising because Hrd3p is suggested to be the central binding partner for misfolded proteins in the ER lumen, acting before the retro-translocation event (Kim et al, 2005; Gauss et al, 2006a). Therefore, we expected disruption of the Sec61p–CPY* interaction in a Δhrd3 strain. Instead, the amount of Sec61p associated with CPY* is increased in Δhrd3 and Δder3 strains (Figures 2A, 3A and 5E). Obviously, binding of misfolded CPY* to Sec61p can be independent of the presence of the Hrd-Der ligase complex. This observation may underline the possibility of the existence of a second Sec61p–CPY*-containing complex. One may assume that binding between Sec61p and CPY* continues until ubiquitination by the Der3p ligase takes place. Indeed, in the presence of Der3p, Sec61p loses contact with CPY* during the degradation process when it is ubiquitinated and retro-translocated to the cytosolic side of the ER (Figure 3A). Thus, one function of Sec61p in the ERAD-L pathway might reside in a process upstream of the function of the Hrd-Der ligase complex. We observed that solely the ER lumenal CPY* moiety of the misfolded membrane protein CTL*myc contacts Sec61p during degradation, whereas the part of CTL*myc containing the transmembrane region fused with a cytosolic Leu2p-myc loses contact to Sec61p (Figure 4). Due to this finding, one might conclude that the functional capacity of Sec61p might be restricted to misfolded proteins of the ER lumen, whereas the handling of certain membrane spanning protein stretches for degradation might be executed by other devices.
Different Sec61p functions in translocation
Any translocation mode of the protein-conducting channel Sec61p is defined through its interaction with additional proteins. Main partners for co-translational import of soluble and membrane proteins are ribosomes. Binding of ribosomes to Sec61p enables the transfer of nascent polypeptide chains into the ER lumen as well as the lateral insertion of proteins into the ER membrane during their synthesis (Osborne et al, 2005). Post-translational import of already synthesized polypeptides requires the assembly of a hetero-heptameric Sec61p complex in the ER membrane and the assistance of the chaperone Kar2p in the ER lumen (Deshaies et al, 1991; Panzner et al, 1995; Matlack et al, 1999; Rapoport, 2008). Interestingly, the mode of action can be altered under ER stress. Through the association of cytosolic chaperones, the translocation channel is reprogrammed from import to co-translational proteasomal degradation of apolipoprotein B (Pariyarath et al, 2001; Fisher and Ginsberg, 2002; Oyadomari et al, 2006). This implies that Sec61p can change its transport directionality. Such a switch in the directionality of Sec61p was also suggested for the retro-translocation step of misfolded ERAD substrates (Plemper et al, 1997, 1998, 1999a; Pilon et al, 1998; Römisch, 1999; Meusser et al, 2005). One may expect that docking of ERAD core components to Sec61p leads to reprogramming of the channel and to transport of misfolded proteins to the cytosol. Indeed, we could show that Sec61p is associated with Hrd3p, Der3p and Cdc48p, which are all components required for the degradation of misfolded lumenal ER proteins similar to CPY*. Thus, our results support the idea that the mode of Sec61p can be switched into one or the other direction by the assistance of additional recruited partners. Nevertheless, our data also allow different interpretations about the nature of the retro-translocation channel. When Der3p is missing, ubiquitination and retro-translocation of misfolded proteins is disturbed, whereas their interaction with Sec61p still persists (Figures 2A, 3A and 5E). Therefore, one might speculate that the Hrd-Der ligase complex forms a channel whereby the function of Sec61p resides in the delivery of misfolded proteins to the Hrd complex. This delivery function could also include the formation of a hybrid channel composed of Sec61p and the Hrd-Der ligase complex. The retro-translocation process might also require the assistance of additional ER membrane spanning proteins. Sec61p seems to be highly dynamic having a multitude of options to deal with the needs of the cell to guarantee a functional ER. From our biochemical and the existing genetic data, we conclude that one of such options resides in its participation in the ERAD-L process for elimination of misfolded proteins.
Materials and methods
Yeast and plasmids
Media preparation, genetic and molecular biology techniques were carried out using standard methods (Guthrie and Fink, 1991; Ausubel et al, 1992). The Saccharomyces cerevisiae strains W303Δprc1 and W303Δprc1Δhul5 were described before (Plemper et al, 1998, 1999b; Kohlmann et al, 2008). The double deletion strains W303Δprc1Δhrd3 and W303Δprc1Δder3 were generated by tetrade analysis after crossing W303Δprc1∷LEU2 with W303prc1-1Δder3∷HIS3Δhrd3∷HIS3 (Bordallo et al, 1998). Epitope tagging of Sec61pHA3 and Sec61pmyc were achieved by homologous recombination as described in Knop et al (1999) generating the strains W303Δprc1∷LEU2, SEC61-HA3-HIS3MX and W303prc1-1, SEC61-MYC9-TRPMX. W303Δprc1∷LEU2Δder3∷HIS3,SEC61-HA3-HIS3MX and W303Δprc1∷LEU2Δhrd3∷HIS3,SEC61-HA3-HIS3MX were generated by crossing W303Δprc1∷LEU2,SEC61-HA3-HIS3MX with W303Δprc1∷LEU2Δder3∷HIS3 and W303Δprc1∷LEU2Δhrd3∷HIS3, respectively, and subsequent tetrade dissection. Plasmids encoding for CPY*G5, CPY*HA3, CTL* and CTL*myc were described earlier (Plemper et al, 1999b; Taxis et al, 2003; Medicherla et al, 2004; Kohlmann et al, 2008). HA3Hrd3p was subcloned as a 3.3-kb KpnI/SacI fragment from pYS14 (Saito et al, 1999) into pRS314 (Sikorski and Hieter, 1989). For generation of pRS316-CPY, a 2.6-kb PCR fragment was generated using genomic W303-DNA as template and the oligonucleotides fwdCPY (5′AACTGCAGGAATTCTACGGTATGTGTGGCGGTTA3′) and revCPY (5′ACGCTCG AGGTCGACCGTGCCCAAAGCTTGATTAC3′). This fragment was EcoRI/SalI cloned into pRS316.
Antibodies
Monoclonal antibodies specific for detection of Dpm1p (Molecular Probes through Invitrogen), c-myc (clone 9E10, Sigma-Aldrich) and HA epitope (clone 16B12, Covance, CA) were used in immunoprecipitation experiments and for immunodetection. For the detection of CPY (Rockland, PA) and Der3p, specific polyclonal antibodies were used (Bordallo et al, 1998). Monoclonal ubiquitin antibodies (P4G7) were purchased from Covance (CA). αCdc48 and αSec61p antibodies were provided by T Sommer.
Immunoprecipitation and immunoblotting
Immunoprecipitation experiments were performed as described in Kohlmann et al (2008). The cells were pre-treated with CHX (150 μg/1 OD600 of cells) at 30°C for 15, 30 min, 2 or 3.5 h, as indicated in the results and figures. Washed microsomal membranes of 150–250 OD600 of cells were used for the solubilization step with 1% digitonin. Standard methods were used for SDS–PAGE and immunoblot analysis (Ausubel et al, 1992).
Quantification of immunoblot signals
The corresponding signals of the immunoprecipitates were quantified using the program ImageJ 1.41o. The immunoblot signals were normalized to the signals of proteins used as baits similar to HA3Hrd3p (Figure 1A), CPY*HA3 (Figures 2A and 3A) or Sec61pHA3 (Figure 5E) respectively, resulting in the presented ratios in Figures 1B, 2B, 3B and 5F.
Acknowledgments
We thank Thomas Sommer for the generous gift of antibodies, Li Xiao for cloning of Hrd3p and strains. We appreciate the valuable comments by Elena Martinez-Benitez, Ruth Menssen-Franz, Alexandra Stolz and the Wolf group members on this paper. The work was supported by the Deutsche Forschungsgemeinschaft (Bonn, Germany) and the EU Network of Excellence, RUBICON.
References
- Alberts SM, Sonntag C, Schäfer A, Wolf DH (2009) Ubx4 modulates Cdc48 activity and influences degradation of misfolded proteins of the endoplasmic reticulum. J Biol Chem 284: 16082–16089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aridor M, Hannan LA (2000) Traffic jam: a compendium of human diseases that affect intracellular transport processes. Traffic 1: 836–851 [DOI] [PubMed] [Google Scholar]
- Ausubel FM, Kingston RE, Seidman FG, Struhl K, Moore DD, Brent R, Smith FA (eds) (1992) Current Protocols in Molecular Biology. New York: Greene, Publishing & Wiley Intersciences [Google Scholar]
- Bays NW, Gardner RG, Seelig LP, Joazeiro CA, Hampton RY (2001) Hrd1p/Der3p is a membrane-anchored ubiquitin ligase required for ER-associated degradation. Nat Cell Biol 3: 24–29 [DOI] [PubMed] [Google Scholar]
- Bordallo J, Plemper RK, Finger A, Wolf DH (1998) Der3p/Hrd1p is required for endoplasmic reticulum-associated degradation of misfolded lumenal and integral membrane proteins. Mol Biol Cell 9: 209–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun S, Matuschewski K, Rape M, Thoms S, Jentsch S (2002) Role of the ubiquitin-selective CDC48(UFD1/NPL4) chaperone (segregase) in ERAD of OLE1 and other substrates. EMBO J 21: 615–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho P, Goder V, Rapoport TA (2006) Distinct ubiquitin-ligase complexes define convergent pathways for the degradation of ER proteins. Cell 126: 361–373 [DOI] [PubMed] [Google Scholar]
- Deak PM, Wolf DH (2001) Membrane topology and function of Der3/Hrd1p as a ubiquitin-protein ligase (E3) involved in endoplasmic reticulum degradation. J Biol Chem 276: 10663–10669 [DOI] [PubMed] [Google Scholar]
- Denic V, Quan EM, Weissman JS (2006) A luminal surveillance complex that selects misfolded glycoproteins for ER-associated degradation. Cell 126: 349–359 [DOI] [PubMed] [Google Scholar]
- Deshaies RJ, Sanders SL, Feldheim DA, Schekman R (1991) Assembly of yeast Sec proteins involved in translocation into the endoplasmic reticulum into a membrane-bound multisubunit complex. Nature 349: 806–808 [DOI] [PubMed] [Google Scholar]
- Dobson CM (2003) Protein folding and misfolding. Nature 426: 884–890 [DOI] [PubMed] [Google Scholar]
- Fisher EA, Ginsberg HN (2002) Complexity in the secretory pathway: the assembly and secretion of apolipoprotein B-containing lipoproteins. J Biol Chem 277: 17377–17380 [DOI] [PubMed] [Google Scholar]
- Gardner RG, Swarbrick GM, Bays NW, Cronin SR, Wilhovsky S, Seelig L, Kim C, Hampton RY (2000) Endoplasmic reticulum degradation requires lumen to cytosol signaling. Transmembrane control of Hrd1p by Hrd3p. J Cell Biol 151: 69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauss R, Jarosch E, Sommer T, Hirsch C (2006a) A complex of Yos9p and the HRD ligase integrates endoplasmic reticulum quality control into the degradation machinery. Nat Cell Biol 8: 849–854 [DOI] [PubMed] [Google Scholar]
- Gauss R, Sommer T, Jarosch E (2006b) The Hrd1p ligase complex forms a linchpin between ER-lumenal substrate selection and Cdc48p recruitment. EMBO J 25: 1827–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie C, Fink GR (eds) (1991) In Methods in Enzymology, Guide to Yeast Genetics and Molecular Biology, Vols 350 and 351. San Diego: Academic Press [PubMed] [Google Scholar]
- Hampton RY, Gardner RG, Rine J (1996) Role of 26S proteasome and HRD genes in the degradation of 3-hydroxy-3-methylglutaryl-CoA reductase, an integral endoplasmic reticulum membrane protein. Mol Biol Cell 7: 2029–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller MM, Finger A, Schweiger M, Wolf DH (1996) ER degradation of a misfolded luminal protein by the cytosolic ubiquitin-proteasome pathway. Science 273: 1725–1728 [DOI] [PubMed] [Google Scholar]
- Huyer G, Piluek WF, Fansler Z, Kreft SG, Hochstrasser M, Brodsky JL, Michaelis S (2004) Distinct machinery is required in Saccharomyces cerevisiae for the endoplasmic reticulum-associated degradation of a multispanning membrane protein and a soluble luminal protein. J Biol Chem 279: 38369–38378 [DOI] [PubMed] [Google Scholar]
- Jarosch E, Taxis C, Volkwein C, Bordallo J, Finley D, Wolf DH, Sommer T (2002) Protein dislocation from the ER requires polyubiquitination and the AAA-ATPase Cdc48. Nat Cell Biol 4: 134–139 [DOI] [PubMed] [Google Scholar]
- Johnson LM, Bankaitis VA, Emr SD (1987) Distinct sequence determinants direct intracellular sorting and modification of a yeast vacuolar protease. Cell 48: 875–885 [DOI] [PubMed] [Google Scholar]
- Kim W, Spear ED, Ng DT (2005) Yos9p detects and targets misfolded glycoproteins for ER-associated degradation. Mol Cell 19: 753–764 [DOI] [PubMed] [Google Scholar]
- Knop M, Finger A, Braun T, Hellmuth K, Wolf DH (1996) Der1, a novel protein specifically required for endoplasmic reticulum degradation in yeast. EMBO J 15: 753–763 [PMC free article] [PubMed] [Google Scholar]
- Knop M, Siegers K, Pereira G, Zachariae W, Winsor B, Nasmyth K, Schiebel E (1999) Epitope tagging of yeast genes using a PCR-based strategy: more tags and improved practical routines. Yeast 15: 963–972 [DOI] [PubMed] [Google Scholar]
- Kohlmann S, Schäfer A, Wolf DH (2008) Ubiquitin ligase Hul5 is required for fragment-specific substrate degradation in endoplasmic reticulum-associated degradation. J Biol Chem 283: 16374–16383 [DOI] [PubMed] [Google Scholar]
- Kostova Z, Wolf DH (2003) For whom the bell tolls: protein quality control of the endoplasmic reticulum and the ubiquitin-proteasome connection. EMBO J 22: 2309–2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley BN, Ploegh HL (2004) A membrane protein required for dislocation of misfolded proteins from the ER. Nature 429: 834–840 [DOI] [PubMed] [Google Scholar]
- Matlack KE, Misselwitz B, Plath K, Rapoport TA (1999) BiP acts as a molecular ratchet during posttranslational transport of prepro-alpha factor across the ER membrane. Cell 97: 553–564 [DOI] [PubMed] [Google Scholar]
- Medicherla B, Kostova Z, Schaefer A, Wolf DH (2004) A genomic screen identifies Dsk2p and Rad23p as essential components of ER-associated degradation. EMBO Rep 5: 692–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meusser B, Hirsch C, Jarosch E, Sommer T (2005) ERAD: the long road to destruction. Nat Cell Biol 7: 766–772 [DOI] [PubMed] [Google Scholar]
- Müller M, Müller H (1981) Synthesis and processing of in vitro and in vivo precursors of the vacuolar yeast enzyme carboxypeptidase Y. J Biol Chem 256: 11962–11965 [PubMed] [Google Scholar]
- Nakatsukasa K, Brodsky JL (2008) The recognition and retrotranslocation of misfolded proteins from the endoplasmic reticulum. Traffic 9: 861–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng DT, Brown JD, Walter P (1996) Signal sequences specify the targeting route to the endoplasmic reticulum membrane. J Cell Biol 134: 269–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng W, Sergeyenko T, Zeng N, Brown JD, Romisch K (2007) Characterization of the proteasome interaction with the Sec61 channel in the endoplasmic reticulum. J Cell Sci 120: 682–691 [DOI] [PubMed] [Google Scholar]
- Osborne AR, Rapoport TA, van den Berg B (2005) Protein translocation by the Sec61/SecY channel. Annu Rev Cell Dev Biol 21: 529–550 [DOI] [PubMed] [Google Scholar]
- Oyadomari S, Yun C, Fisher EA, Kreglinger N, Kreibich G, Oyadomari M, Harding HP, Goodman AG, Harant H, Garrison JL, Taunton J, Katze MG, Ron D (2006) Cotranslocational degradation protects the stressed endoplasmic reticulum from protein overload. Cell 126: 727–739 [DOI] [PubMed] [Google Scholar]
- Panzner S, Dreier L, Hartmann E, Kostka S, Rapoport TA (1995) Posttranslational protein transport in yeast reconstituted with a purified complex of Sec proteins and Kar2p. Cell 81: 561–570 [DOI] [PubMed] [Google Scholar]
- Pariyarath R, Wang H, Aitchison JD, Ginsberg HN, Welch WJ, Johnson AE, Fisher EA (2001) Co-translational interactions of apoprotein B with the ribosome and translocon during lipoprotein assembly or targeting to the proteasome. J Biol Chem 276: 541–550 [DOI] [PubMed] [Google Scholar]
- Pilon M, Romisch K, Quach D, Schekman R (1998) Sec61p serves multiple roles in secretory precursor binding and translocation into the endoplasmic reticulum membrane. Mol Biol Cell 9: 3455–3473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon M, Schekman R, Romisch K (1997) Sec61p mediates export of a misfolded secretory protein from the endoplasmic reticulum to the cytosol for degradation. EMBO J 16: 4540–4548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plemper RK, Böhmler S, Bordallo J, Sommer T, Wolf DH (1997) Mutant analysis links the translocon and BiP to retrograde protein transport for ER degradation. Nature 388: 891–895 [DOI] [PubMed] [Google Scholar]
- Plemper RK, Bordallo J, Deak PM, Taxis C, Hitt R, Wolf DH (1999a) Genetic interactions of Hrd3p and Der3p/Hrd1p with Sec61p suggest a retro-translocation complex mediating protein transport for ER degradation. J Cell Sci 112(Pt 22): 4123–4134 [DOI] [PubMed] [Google Scholar]
- Plemper RK, Deak PM, Otto RT, Wolf DH (1999b) Re-entering the translocon from the lumenal side of the endoplasmic reticulum. Studies on mutated carboxypeptidase yscY species. FEBS Lett 443: 241–245 [DOI] [PubMed] [Google Scholar]
- Plemper RK, Egner R, Kuchler K, Wolf DH (1998) Endoplasmic reticulum degradation of a mutated ATP-binding cassette transporter Pdr5 proceeds in a concerted action of Sec61 and the proteasome. J Biol Chem 273: 32848–32856 [DOI] [PubMed] [Google Scholar]
- Plemper RK, Wolf DH (1999) Retrograde protein translocation: ERADication of secretory proteins in health and disease. Trends Biochem Sci 24: 266–270 [DOI] [PubMed] [Google Scholar]
- Rabinovich E, Kerem A, Frohlich KU, Diamant N, Bar-Nun S (2002) AAA-ATPase p97/Cdc48p, a cytosolic chaperone required for endoplasmic reticulum-associated protein degradation. Mol Cell Biol 22: 626–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport TA (2008) Protein transport across the endoplasmic reticulum membrane. FEBS J 275: 4471–4478 [DOI] [PubMed] [Google Scholar]
- Römisch K (1999) Surfing the Sec61 channel: bidirectional protein translocation across the ER membrane. J Cell Sci 112(Pt 23): 4185–4191 [DOI] [PubMed] [Google Scholar]
- Römisch K (2005) Endoplasmic reticulum-associated degradation. Annu Rev Cell Dev Biol 21: 435–456 [DOI] [PubMed] [Google Scholar]
- Saito Y, Yamanushi T, Oka T, Nakano A (1999) Identification of SEC12, SED4, truncated SEC16, and EKS1/HRD3 as multicopy suppressors of ts mutants of Sar1 GTPase. J Biochem 125: 130–137 [DOI] [PubMed] [Google Scholar]
- Schäfer A, Kostova Z, Wolf DH (2008) Endoplasmic reticulum protein quality control and degradation. In The Ubiquitin-Proteasome System and Disease, Mayer RJ, Ciechanover A, Rechsteiner M (eds), Vol. 4 123–133. Weinheim, Germany: Wiley-VHC [Google Scholar]
- Scheper W, Thaminy S, Kais S, Stagljar I, Romisch K (2003) Coordination of N-glycosylation and protein translocation across the endoplasmic reticulum membrane by Sss1 protein. J Biol Chem 278: 37998–38003 [DOI] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P (1989) A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122: 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens T, Esmon B, Schekman R (1982) Early stages in the yeast secretory pathway are required for transport of carboxypeptidase Y to the vacuole. Cell 30: 439–448 [DOI] [PubMed] [Google Scholar]
- Taxis C, Hitt R, Park SH, Deak PM, Kostova Z, Wolf DH (2003) Use of modular substrates demonstrates mechanistic diversity and reveals differences in chaperone requirement of ERAD. J Biol Chem 278: 35903–35913 [DOI] [PubMed] [Google Scholar]
- Taxis C, Vogel F, Wolf DH (2002) ER-golgi traffic is a prerequisite for efficient ER degradation. Mol Biol Cell 13: 1806–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashist S, Ng DT (2004) Misfolded proteins are sorted by a sequential checkpoint mechanism of ER quality control. J Cell Biol 165: 41–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vembar SS, Brodsky JL (2008) One step at a time: endoplasmic reticulum-associated degradation. Nat Rev Mol Cell Biol 9: 944–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlman J, DeMartino GN, Skach WR, Bulleid NJ, Brodsky JL, Johnson AE (2007) Real-time fluorescence detection of ERAD substrate retrotranslocation in a mammalian in vitro system. Cell 129: 943–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley P, Nilsson IM, von Heijne G (1996) A nascent secretory protein may traverse the ribosome/endoplasmic reticulum translocase complex as an extended chain. J Biol Chem 271: 6241–6244 [DOI] [PubMed] [Google Scholar]
- Wiertz EJ, Tortorella D, Bogyo M, Yu J, Mothes W, Jones TR, Rapoport TA, Ploegh HL (1996) Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature 384: 432–438 [DOI] [PubMed] [Google Scholar]
- Willer M, Forte GM, Stirling CJ (2008) Sec61p is required for ERAD-L: genetic dissection of the translocation and ERAD-L functions of Sec61p using novel derivatives of CPY. J Biol Chem 283: 33883–33888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Meyer HH, Rapoport TA (2001) The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature 414: 652–656 [DOI] [PubMed] [Google Scholar]
- Ye Y, Shibata Y, Yun C, Ron D, Rapoport TA (2004) A membrane protein complex mediates retro-translocation from the ER lumen into the cytosol. Nature 429: 841–847 [DOI] [PubMed] [Google Scholar]