Abstract
Replicative polymerases are tethered to DNA by sliding clamps for processive DNA synthesis. Despite attachment to a sliding clamp, the polymerase on the lagging strand must cycle on and off DNA for each Okazaki fragment. In the ‘collision release' model, the lagging strand polymerase collides with the 5′ terminus of an earlier completed fragment, which triggers it to release from DNA and from the clamp. This report examines the mechanism of collision release by the Escherichia coli Pol III polymerase. We find that collision with a 5′ terminus does not trigger polymerase release. Instead, the loss of ssDNA on filling in a fragment triggers polymerase to release from the clamp and DNA. Two ssDNA-binding elements are involved, the τ subunit of the clamp loader complex and an OB domain within the DNA polymerase itself. The τ subunit acts as a switch to enhance polymerase binding at a primed site but not at a nick. The OB domain acts as a sensor that regulates the affinity of Pol III to the clamp in the presence of ssDNA.
Keywords: lagging strand, Okazaki fragment, replication fork, replisome, sliding clamp
Introduction
Chromosomal replicases are distinguished from repair DNA polymerases by their multi-subunit composition, rapid synthetic rate and high processivity (Benkovic et al, 2001; McHenry, 2003; O'Donnell, 2006). High processivity derives from a ring-shaped sliding clamp that is assembled onto DNA by a clamp loader machine. A DNA polymerase held to DNA by a mobile sliding clamp is nicely suited to highly processive synthesis on the leading strand of the replication fork but stands in contrast to the actions required on the lagging strand, which is synthesized as a series of Okazaki fragments in the direction opposite fork progression. Therefore, the lagging strand polymerase must rapidly dissociate after extension of each Okazaki fragment and recycle to a new upstream RNA primer to start the next fragment (Kornberg and Baker, 1992).
Biochemical studies in diverse systems show that highly processive replicases rapidly recycle from one Okazaki fragment to the next, including Escherichia coli Pol III, eukaryotic Pol δ and bacteriophages T4 and T7 (O'Donnell, 1987; Hacker and Alberts, 1994; Stukenberg et al, 1994; Lee et al, 2006; Yang et al, 2006; Langston and O'Donnell, 2008). However, the mechanism that triggers the replicase to dissociate on reaching the end of an Okazaki fragment is poorly understood.
In this report, we examine the detailed mechanism of collision release in the E. coli system. The E. coli replicase, DNA polymerase III holoenzyme (Pol III HE), consists of two molecules of the heterotrimeric Pol III core (α, DNA polymerase; ɛ, 3′–5′ exonuclease; and θ) that attach to the two τ subunits within the clamp loader (γ1τ2δ1δ′1χ1 ψ1) to form Pol III*; association of Pol III* with the β clamp forms the HE (McHenry, 2003; Johnson and O'Donnell, 2005). Studies in the E. coli system show that Pol III HE undergoes two types of recycling. In one polymerase-recycling process, Pol III HE remains tightly bound to DNA by the β clamp during processive synthesis, but Pol III* quickly releases from β on completing a DNA fragment, thereby releasing Pol III* to recycle to a new primed site (O'Donnell, 1987; Stukenberg et al, 1994). This recycling process is referred to as ‘collision release' as Pol III HE collides with the 5′ terminus of a downstream Okazaki fragment. Once released, Pol III* rapidly reassociates with a new β clamp that has been assembled onto an RNA primer synthesized by primase.
The second mechanism of polymerase recycling involves the release of Pol III* from β prematurely, before the Okazaki fragment is complete (Li and Marians, 2000; McInerney and O'Donnell, 2004). This mechanism is also observed in the T4 and T7 systems, in which it appears to be signalled by priming or clamp assembly on new RNA primers and is therefore referred to as ‘premature release' or ‘signalling release' (Li and Marians, 2000; Lee et al, 2006; Yang et al, 2006; Hamdan et al, 2009). The existence of two polymerase-recycling mechanisms on the lagging strand may reflect the importance of keeping lagging strand synthesis coupled with leading strand synthesis during chromosome duplication.
Mechanistic studies of collision release in the E. coli system have shown that the τ subunit helps disengage Pol III from DNA and β on completing replication to a nick (Leu et al, 2003; Lopez de Saro et al, 2003). Although the exact mechanism by which τ functions is not known, it is suggested that τ may be located near the active site in the Pol III α subunit in which it can recognize DNA structural changes on completing an Okazaki fragment (Leu et al, 2003). In fact, the τ subunit binds the C-terminal region of α (Kim and McHenry, 1996), and structural studies show that this region is close to the polymerase active site (Bailey et al, 2006; Wing et al, 2008). The C-terminal region of E. coli Pol III α subunit contains two β-binding sites; one at the C-terminus and another 240 residues internal to the C-terminus (Lopez de Saro et al, 2003; Dohrmann and McHenry, 2005). The internal site is essential for processive function with β and is nicely positioned to bind the clamp in the α structure (Bailey et al, 2006; Wing et al, 2008). The extreme C-terminal β-binding site in α helps it to function with β and is required for function with τ (Dohrmann and McHenry, 2005; Lamers et al, 2006).
This study focuses on the mechanism of collision release in the E. coli system. We first examine whether Pol III* recognizes the 5′ terminus to trigger collision release. The results show that 5′ terminal recognition is not involved in collision release. Hence, we shifted our focus to the role of ssDNA as a possible trigger for the release process, as ssDNA is lost on complete conversion to dsDNA. There exist two ssDNA-binding elements within Pol III*. One is an OB domain within α that binds template ssDNA and is positioned near the active site (Bailey et al, 2006; Wing et al, 2008). It is suggested that on completing an Okazaki fragment, the OB domain triggers a conformational change in Pol III that disconnects it from β (Wing et al, 2008). The other ssDNA-binding element in Pol III* is the τ subunit. Our earlier studies on τ show that it binds to ssDNA and has a function in collision release (Leu et al, 2003).
Collision release requires Pol III to disconnect from two substrates, DNA and the β clamp. This study shows that the OB domain and τ subunit have distinct functions in collision release. The OB domain is a sensor that modulates the affinity of Pol III to the β clamp in response to ssDNA. Furthermore, we show that the τ subunit is a DNA switch that strengthens Pol III* binding to primed DNA, but not completed DNA (i.e. dsDNA or DNA with a nick). Thus, on completing an Okazaki fragment, both the τ switch and OB sensor are activated, and this relaxes the grip of Pol III* to DNA (τ subunit) and to the β clamp (OB domain), resulting in the release of Pol III* from β and DNA.
Results
Pol III HE does not recognize the 5′ terminus as a signal to disengage from β
To address whether Pol III HE recognizes a 5′ terminal duplex as a signal to release from the β clamp and DNA, we blocked the 5′ terminus using a site-specific DNA-binding protein, Epstein–Barr virus origin binding protein 1 (EBNA1), which fully occludes its recognition sequence (Bochkarev et al, 1996). We placed the EBNA1 site at the extreme 5′ terminus of a DNA primer annealed to a 7.2-kb primed circular M13mp18 ssDNA (illustrated in Figure 1A). Pol III HE extends the primer full circle, resulting in a nick on reaching the 5′ terminus of the same primer. If Pol III HE must recognize the 5′ terminus to release from β, EBNA1 should prevent the recognition process and stop Pol III* from dissociating from β and DNA.
Figure 1.
Pol III HE does not recognize a 5′ terminus for collision release. (A) Pol III* and β were assembled onto a 7.2-kb primed M13mp18 ssDNA in the presence (or absence) of EBNA1, which binds to the extreme 5′ nucleotides of the 46-mer primed site. In a separate reaction, β is assembled onto a five-fold excess of a challenge primed M13Gori ssDNA (8.6 kb). Reactions were mixed, replication was initiated and timed aliquots were analysed in a native agarose gel. In control reactions, lanes 1–4, β was not assembled onto acceptor M13Gori DNA. Lanes 5–8 are reactions in the absence of EBNA1, and lanes 9–12 contained EBNA1. The 7.2- and 8.6-kb products were quantified using a phosphorimager and are shown in the plot as the ratio of replicated 8.6 kb acceptor RFII DNA over replicated 7.2 kb RFII donor DNA versus time. (B) The 5′ terminus of the primer is modified with either biotin (plus or minus streptavidin), or psoralen (see scheme). Reactions were performed and the 7.2- and 8.6-kb RFII products were quantified as described in (A).
To monitor Pol III* release from β and DNA on completing replication, we performed a challenge assay using circular primed DNAs of different sizes. The 7.2-kb primed circular donor ssDNA contains an EBNA1 site. The challenge DNA is an 8.6-kb primed M13Gori ssDNA circle (O'Donnell, 1987; Stukenberg et al, 1994; Turner and O'Donnell, 1995). Pol III HE is first assembled onto the donor DNA in the presence or absence of EBNA1 along with only two dNTPs to prevent chain elongation. In a separate reaction, the β clamp is assembled onto a five-fold excess of 8.6 kb primed challenge DNA using the γ-complex clamp loader. The two reactions are then mixed and replication is initiated by adding the remaining dNTPs and α32P-dTTP.
Pol III HE is processive and rapidly converts the 7.2-kb DNA into a circular duplex (i.e. RFII). If Pol III HE must recognize the 5′ terminus for collision release, then EBNA1 should prevent this recognition and Pol III* will not release and transfer to the 8.6-kb challenge DNA. On the other hand, if Pol III HE does not need to recognize the 5′ terminus it should still undergo collision release and cycle to the challenge DNA to produce an 8.6-kb RFII product. A control reaction confirms that the challenge DNA is only replicated when it is pre-loaded with a β clamp (Figure 1A, lanes 1–4).
In the absence of EBNA1, Pol III* dissociates on completing the 7.2-kb DNA and readily cycles to the 8.6-kb challenge DNA (Figure 1A, lanes 5–8). In the presence of EBNA1, the rate of 8.6 kb RFII product formation is the same as in the absence of EBNA1 (Figure 1A, lanes 9–12 and quantitation to the right), indicating that occluding the 5′ terminus does not prevent the release of Pol III*. To exclude the possibility that Pol III HE binds the 5′ terminus by displacing EBNA1, we used 32P-labelled EBNA1 and analysed whether Pol III HE displaces it on replicating the circular DNA (Supplementary Figure S1). The results show that Pol III HE does not displace 32P-EBNA1 from DNA on completing replication, confirming that Pol III HE does not have access to the 5′ terminus in the challenge experiments.
It is possible that Pol III* dissociates from β through a conformational change that occurs as a consequence of bumping into a protein block, rather than by triggering the collision release mechanism. Two lines of evidence indicate that a block to forward progression does not destabilize Pol III HE. First, Pol III* remains stably attached to β on DNA after encountering a template lesion, indicating that a sudden block to forward motion does not induce the polymerase to dissociate (McInerney and O'Donnell, 2007). Second, when an E. coli Pol III HE collides with an in-line RNA polymerase, the replisome remains bound to DNA, whereas it displaces the RNA polymerase and takes over the mRNA primer to continue DNA synthesis (Pomerantz and O'Donnell, 2008). This result indicates that encounter of a protein block per se does not induce the polymerase to release from DNA. Nevertheless, we cannot rigorously exclude that a conformational change occurs when the polymerase hits the EBNA1 block, inducing polymerase dissociation.
To further support that 5′ end recognition is not required for collision release, we repeated the cycling experiments but used an oligonucleotide that was biotinylated at the 5′ terminus in the presence or absence of streptavidin (see scheme in Figure 1B). The result shows that after Pol III HE collides with the streptavidin bound to the 5′ terminus, it cycles to the challenge 8.6 kb DNA at the same rate as when it collides with an unmodified primer (Figure 1B). We also used a primer containing a bulky 5′ psoralen moiety and again observe transfer of polymerase to the challenge DNA (Figure 1B). These results support the conclusion that collision release by Pol III HE does not require recognition of a 5′ terminus.
Pol III* can dissociate from the 3′ terminus before it dissociates from the β clamp
If recognition of the 5′ terminus does not trigger collision release, it is possible that Pol III* detaches from the 3′ terminus before it dissociates from the β clamp. If so, Pol III*-β may remain together and slide along duplex DNA, as suggested by an earlier study (O'Donnell and Kornberg, 1985). To test whether Pol III* dissociates from β and DNA on colliding with a 5′ terminus, we observed its behaviour on encountering a short duplex in its path, during primer extension of a 5.4-kb φX174 ssDNA (see scheme in Figure 2A). We developed a DNA trap to capture any Pol III* that dissociates, thereby preventing it from reassociating with β on the downstream primer #2. The trap consists of 11-fold excess 7.2 kb M13mp18 ssDNA primed with a 3′ dideoxyoligonucleotide onto which β was assembled using the γ-complex clamp loader. The 3′ dideoxy terminus prevents extension and subsequent polymerase release. We used Pol III* containing an exonuclease-deficient ɛ subunit to prevent removal of the 3′ dideoxynucleotide. As a test of the trap, Pol III* and β were added to a mixture of the singly primed φX174 ssDNA and the excess trap M13mp18 ssDNA. The results (Figure 2A) show that the trap substrate is highly effective at sequestering free Pol III* from solution.
Figure 2.
Pol III HE releases DNA before it releases from β. (A) The reaction scheme to test effectiveness of a trap for free Pol III* is illustrated at the top. The trap is a 7.2-kb circular 3′dideoxy-terminated primed ssDNA, preloaded with a β clamp. Analysis of products in a native agarose gel at different amounts of added trap is shown to the left and quantification of DNA synthesis is shown in the plot to the right. (B) The experiment to test whether Pol III* stays bound to β after colliding with the downstream primer #2 is illustrated at the top. Pol III HE was assembled onto primer #1, and then the trap DNA (containing a preloaded clamp) was added along with primer #2 (as indicated). Replication was initiated and quenched at the indicated times followed by analysis in an alkaline agarose gel.
If Pol III* releases from β on colliding with the 5′ terminus of primer #2, the polymerase will be sequestered by the trap, leaving only the 2.3-kb product. In contrast, if Pol III* remains bound to β on colliding with the 5′ terminus, the polymerase may slide over the duplex and extend primer #2 to form the 3.4-kb product. The experiment to examine whether Pol III* dissociates from β immediately on encountering a downstream primer is shown in Figure 2B. First, sub-stoichiometric Pol III HE was assembled onto the 5.4-kb φX174 ssDNA primed only with primer #1 (in the presence of two dNTPs to prevent extension). Then a second oligonucleotide (primer #2) is rapidly annealed to the φX174 ssDNA downstream of primer #1. Short oligonucleotides are known to anneal rapidly (<1 min) to SSB-coated ssDNA (O'Donnell and Kornberg, 1985). When dNTPs are added, Pol III HE will extend primer #1 until it collides with the 5′ terminus of downstream primer #2. If Pol III* releases from β and DNA, it will be trapped by β on the challenge DNA and only the 2-kb extension product of primer #1 will be observed. On the other hand, if Pol III* releases from DNA but stays attached to β, it will slide with β over primer #2, reattach to DNA and extend primer #2 to form the 3.4-kb segment.
The results of the experiment show that in the presence of primer #2, a 2-kb product appears, rapidly followed by a 3.4-kb product (Figure 2B). This result indicates that on colliding with primer #2, Pol III* can stay attached to β, release from the 3′ terminus of the 2-kb product, and slide over primer #2, whereas staying bound to β. Quantitation of the replication products, shown below the gel, indicates that 35% of the time Pol III* remains bound to β and diffuses over primer #2. The presence of a small amount of full-length 5.4 kb product in reactions containing primer #2 is a background due to a small proportion of DNA templates that primer #2 does not hybridize to during the short 1-min annealing time. A control experiment without the trap shows about 75% extension of primer #2 after 60 s (Supplementary Figure S2). Hence, a significant amount of Pol III* remains bound to β, whereas it slides over primer #2. It is unclear whether the portion of Pol III* that dissociates from β does so before, or during β diffusion over the downstream primer. In summary, the results indicate that Pol III* does not necessarily release precisely at the site of collision with a 5′ terminus and supports the conclusion that the 5′ terminus is not the primary signal for collision release.
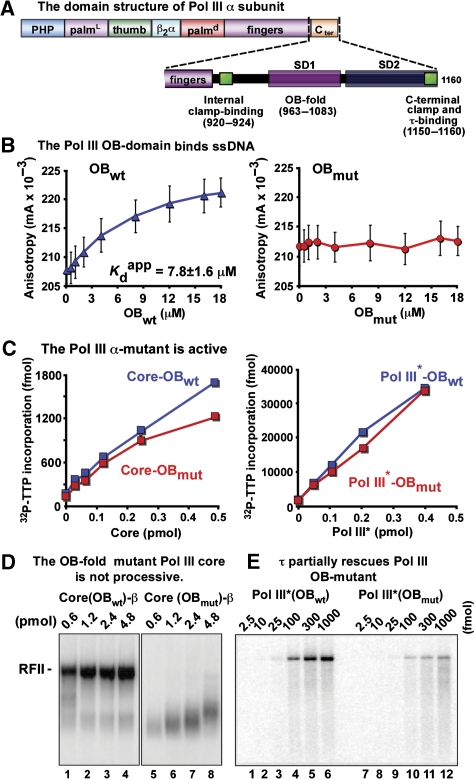
The OB domain of Pol III α subunit is needed for processive function with the β clamp
The experiments so far indicate that another trigger besides 5′ duplex recognition underlies collision release of Pol III* from β and DNA. On completing an Okazaki fragment, the ssDNA template strand is completely converted into duplex DNA, so the loss of contacts between the polymerase and ssDNA could bring about collision release. An invariant property of C-family DNA polymerases is the presence of an OB domain (Figure 3A). The crystal structure of Pol III α subunit reveals that the OB domain lies near the active site in which it can interact with template ssDNA just ahead of the polymerase (Bailey et al, 2006; Wing et al, 2008). This is also observed in the structure of Pol C from a gram-positive bacterium (Evans et al, 2008). Recent biochemical data show that the OB domain of E. coli α subunit binds ssDNA, but its function during DNA replication is not understood (McCauley et al, 2008). On the basis of the structure of Thermus aquaticus α subunit, it has been hypothesized that the OB domain in α may act as a sensor for the presence of the ssDNA template and that the loss of the OB–ssDNA interaction may trigger release of Pol III from β when synthesis of a fragment is completed (Bailey et al, 2006).
Figure 3.
The OB-fold within Pol III α subunit binds ssDNA and is needed for processive synthesis. (A) Scheme of the domain structure of Pol III α subunit. The expanded C-terminal region shows the OB domain and sequences that bind β and τ. (B) Fluorescence anisotropy DNA-binding assay. The isolated wt OB domain (left), or the mutant OB domain (right), is titrated into a reaction containing 5′-fluorescent labelled DNA. (C) Analysis of wt (squares) and OB-mutant (circles) Pol III core (left plot) and Pol III* (right plot) in β-independent assays using gapped DNA. (D) Native agarose gel product analysis of β-dependent replication assays using primed M13mp18 ssDNA and either wt Pol III core (lanes 1–4) or OB-mutant Pol III core (lanes 5–8). (E) As in (D) except using wt Pol III* (lanes 1–6) or OB-mutant Pol III* (lanes 7–12).
To examine the role of the OB domain in Pol III HE function, we expressed and purified isolated wt and mutant OB domains (residues 962–1083) of Pol III α. The mutant form contained substitutions of three amino acids that are proposed to participate in ssDNA binding (R1004S, K1009S and R1010S) (Lamers et al, 2006). To determine whether these substitutions inactivate ssDNA binding, we used a DNA oligonucleotide labelled at the 5′ terminus with a fluorophore and measured the anisotropy change with increasing amounts of Pol III α OB domain. The results show that the wt OB domain binds to the ssDNA oligonucleotide with an apparent Kd value of 7.8±1.6 μM (Figure 3B, left panel), whereas ssDNA binding by the mutant OB domain was undetectable (Figure 3B, right panel). We also determined that neither the wt nor the mutant OB domain interacts with duplex DNA (Supplementary Figure S3).
Next, we mutated the same OB-domain residues in the full-length α subunit and used it to reconstitute OB-mutant Pol III core and OB-mutant Pol III*. The reconstituted mutant complexes are nearly as active as their wt counterparts on activated calf thymus DNA. These substrates do not require processive activity for nucleotide incorporation, allowing to monitor catalytic activity independent of the β clamp (Figure 3C). This result indicates that ssDNA binding by the OB domain is not essential for DNA synthesis. On its own, the isolated OB-mutant α subunit is about half as active as wt α (Supplementary Figure S4), indicating that the additional subunits in Pol III core and Pol III* may stabilize the active site architecture or compensate for the reduced affinity of the OB-mutant Pol III α subunit to the DNA substrate.
To examine the effect of α subunit OB-domain mutations on processive function with β, we assembled Pol III core-β or OB-mutant Pol III core-β on primed 7.2 kb M13mp18 ssDNA with only two dNTPs (the γ complex was used to assemble β onto DNA), then replication was initiated on adding the remaining dNTPs along with α32P-dTTP. Wild-type Pol III core-β rapidly converts the 7.2-kb substrate to a RFII circular duplex (Figure 3D, lanes 1–4). In contrast, the OB-mutant Pol III core-β yields only short products, indicating that the OB-mutant Pol III core is not processive with β (lanes 5–8, quantitation is in Supplementary Figure 5A). Hence, ssDNA binding by the OB domain of α is required for processive function of Pol III core with β.
In summary, these results show that the OB domain of Pol III α binds ssDNA (Figure 3B) and that ssDNA binding is not required for intrinsic DNA polymerase activity (Figure 3C) but is required for processive function with the β clamp (Figure 3D). We show later in this report that ssDNA binding by the OB domain is required for optimal binding of α to β specifically at a primer/terminus, and that this accounts for the deficiency of the OB-mutant Pol III in function with β.
The τ subunit partially rescues the OB-domain mutant
The defect in processivity of the OB-mutant Pol III core may be explained by an altered affinity of Pol III core for DNA or the β clamp (or both). The τ subunit of the clamp loader is known to increase the binding of Pol III core to the β clamp on DNA (Stukenberg and O'Donnell, 1995), and therefore one may predict that the τ subunit will partially compensate for ssDNA-binding mutations in the OB domain of Pol III core. To examine this possibility, we tested the α OB mutant reconstituted into Pol III* (which contains τ) in β-dependent assays using primed M13mp18 ssDNA. The results, in Figure 3E lanes 7–12, show that the OB-mutant Pol III*-β produces full-length RFII duplex products, although it is less efficient and generates many short products compared with wt Pol III*-β (Figure 3E, lanes 1–6; see Supplementary Figure 5B for quantitation). Hence, the τ subunit partially rescues the defective processivity of the OB-mutant Pol III core in β-dependent synthesis.
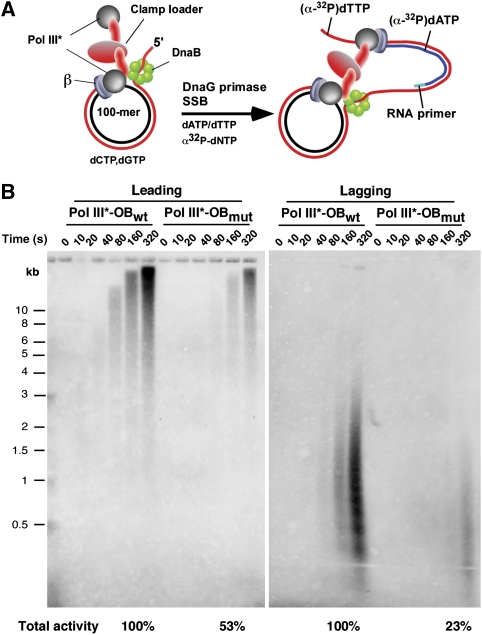
OB-mutant Pol III* is more deficient on the lagging strand than the leading strand
The results obtained thus far indicate that the OB–ssDNA interaction is needed for processive synthesis of Pol III core with β, and thus one may expect that the OB mutant will not function properly on either the leading or the lagging strand. However, there may be no ssDNA on the leading strand, as leading strand synthesis does not require SSB (Mok and Marians, 1987). In this case, leading strand synthesis should not be hindered by OB mutations that eliminate ssDNA binding. Furthermore, the leading strand polymerase connects to DnaB helicase through the τ subunit (Dallmann et al, 2000), and this polymerase-helicase connection may confer processivity to the leading strand polymerase as shown earlier in the T4 and T7 systems (Xi et al, 2005; Johnson et al, 2007).
To explore this issue, replisomes were reconstituted with DnaB and either the wt or OB-mutant Pol III HE on a synthetic 100-mer rolling circle replication fork substrate. Each strand of the synthetic minicircle contains only three nucleotides so that leading and lagging strands can be specifically labelled in separate reactions using either [α32P] dTTP or [α32P] dATP, respectively. The replisome is assembled by first loading DnaB on the 5′ dT40 tail of the minicircle, and then Pol III HE is assembled onto the leading strand in the presence of dCTP and dGTP to prevent fork movement (see scheme in Figure 4A). After 4 min, replication is initiated on adding dATP, dTTP, SSB, DnaG primase, the four rNTPs and the appropriate radiolabelled dNTP. Timed aliquots are analysed in alkaline agarose gels (Figure 4B).
Figure 4.
The OB-mutant Pol III HE is deficient in lagging strand synthesis. (A) Scheme of replisome assembly on a minicircle replication fork substrate using DnaB helicase and wt or OB-mutant Pol III HE (B) Leading (left panel) and lagging (right panel) strands can be selectively labelled depending on whether α-32P dATP or α-32P dTTP is present during synthesis. Aliquots were removed at the indicated times and analysed in a 1.2% alkaline agarose gel followed by autoradiography. The relative level of DNA synthesis at 320 s is shown at the bottom of the gel.
The results show that replisomes containing the OB-mutant Pol III HE give lower synthesis on both leading and lagging strands compared with wt Pol III HE (Figure 4B), yet the lagging strand is more affected by the OB mutation than the leading strand. The fact that leading strand synthesis is impaired by the OB mutation suggests that ssDNA may be present on the leading strand ahead of the polymerase. Alternatively, the OB mutations may impair assembly of Pol III HE into the replisome or alter a property of Pol III HE function in the context of the replisome that is not divulged in assays using primed M13mp18 ssDNA.
Earlier studies have shown that Okazaki fragment size is determined by fork speed and primase concentration (Wu et al, 1992). The gel analysis in Figure 4B shows that the speed of the leading strand OB-mutant replisome is the same as the wt replisome. Therefore, as primase concentration is held constant in these experiments, the lagging strand should be primed at the same frequency and should produce similar sized Okazaki fragments. However, the results clearly show a decrease in Okazaki fragment size from 1 to 0.5 kb for the OB-mutant replisome (Figure 4B, right). Furthermore, quantitation of the products shows a much greater reduction of lagging strand synthesis by the OB-mutant replisome than of leading strand synthesis (23% of wt versus 53% of wt, respectively, Figure 4B, bottom). Therefore, the lagging strand OB-mutant Pol III probably releases from most Okazaki fragments well before they are complete.
Binding of the OB domain to ssDNA regulates the affinity of Pol III for β
To understand why the OB-mutant Pol III core is less processive with β, we developed fluorescence-based assays to measure the affinity of wt and OB-mutant Pol III for DNA and the β clamp. To measure the affinity of Pol III core for the β clamp, Pol III core was reconstituted using a θ subunit labelled with AlexaFlor594 (FRET donor) and the β clamp was labelled using AlexaFlor647 (FRET acceptor). FRET assays were performed by titrating β into a constant amount of Pol III core, and reactions were excited at 594 nm. Representative fluorescence emission spectra during a titration of β into wt Pol III core are shown in the left panel of Figure 5A. Binding of β to Pol III core gives a FRET signal, manifested by a decrease in fluorescence intensity at 612 nm and a corresponding fluorescence intensity increase at 680 nm (due to emission from the acceptor that is excited by resonance energy transfer from the donor). Figure 5A (right panel) shows the fluorescence intensity change of wt or OB-mutant Pol III core at increasing β concentration. Quantitation of the results shows that wt and OB-mutant Pol III core bind to β with similar affinity. Hence, the OB mutations do not affect binding of Pol III core to the β clamp in the absence of DNA.
Figure 5.
The OB domain regulates the affinity of Pol III for β in response to binding DNA. (A) FRET assay to measure the affinity of wt or OB-mutant Pol III core for β. Overlay of corrected emission spectra are shown to the left for wt Pol III core binding to β (excitation 594 nm; emission: 600–720 nm). Plots for binding of wt and OB-mutant Pol III core to β are shown to the right. (B) Fluorescent DNA-binding assay for wt and OB-mutant Pol III core for primed DNA in the absence (left) or presence (right) of β. (C) Results of fluorescent DNA-binding assays for wt and OB-mutant Pol III core for primed and nicked DNA in the presence of β.
To measure the affinity of wt and OB-mutant Pol III core for primer/template DNA, we used a 34/62-mer primed site containing a fluorescent moiety on the template strand, 21 residues from the 5′ terminus. This substrate registers a change in the fluorescence intensity on binding Pol III core. Titrations of wt and OB-mutant Pol III core into reactions containing the primed template are shown in the left panel of Figure 5B. Although the OB domain interacts with ssDNA (Figure 3B), we observed no significant difference in affinity between wt and OB-mutant Pol III core for the primed site, suggesting that ssDNA interaction by the OB domain does not directly stabilize Pol III on DNA in the absence of β.
As the OB-mutant Pol III core is clearly defective in function with β (Figure 3D) yet binds to β in the absence of DNA with similar affinity to wt Pol III core (Figure 5A), it seemed possible that the OB mutant may be defective in binding to β when the clamp is on DNA. To test this we measured the affinity of wt and OB-mutant Pol III core for a primed site in the presence of β (Figure 5B, right panel). As β can slide off the short linear DNA, the effect of β will be underestimated in this assay. Nonetheless, we observed a five-fold enhancement in affinity of wt Pol III core for the β–DNA complex relative to DNA alone, compared with only two-fold enhancement for OB-mutant Pol III core. These results indicate that when the OB domain binds template ssDNA, it increases the affinity of Pol III core for β. To confirm that this effect is specific for a primer/template, we measured the affinity of wt and OB-mutant Pol III core for a fluorescently labelled nicked substrate in the presence of the β clamp; as expected there was no significant difference between wt and OB-mutant Pol III binding to nicked DNA (Figure 5C). The measured Kd values of wt and mutant OB Pol III core binding to a nick, are comparable to the Kd value of OB-mutant Pol III core binding to a primed substrate (Figure 5B, left panel).
To determine whether Pol III begins to lose its affinity for β and the DNA template before reaching the terminal nucleotide, we measured the affinity of wt Pol III core to a synthetic 62-mer DNA substrate designed to contain ssDNA gaps of defined length (Supplementary Figure S6). We observe that the affinity of Pol III core for β and DNA decreases gradually as the ssDNA gap is brought close to a nick. This result is consistent with the position of the OB domain in the Taq α-subunit structure, which indicates that the OB domain interacts with ssDNA several residues ahead of the polymerase active site (Wing et al, 2008) We presume that the koff is slow relative to the rate of synthesis, accounting for the observed synthesis of Pol III* HE to the last nucleotide of a primed M13mp18 circular substrate (Stukenberg et al, 1994).
Given the importance of ssDNA binding to the OB domain for Pol III-β stability, it is conceivable that a 5′ ssDNA tail in trans could interact with the OB domain to stabilize Pol III* at a nick. This has been examined earlier using Pol III* with β on a circular dsDNA template containing a 40 nucleotide 5′ ssDNA flap (Yuzhakov et al, 1996). The 5′ flap did not stabilize the attachment of Pol III* to β on the DNA, indicating that the architecture of the OB domain does not accommodate ssDNA in trans.
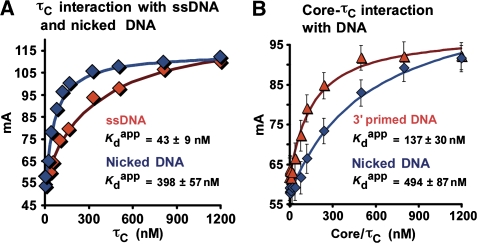
The τ subunit enhances the affinity of Pol III core for a primed site over a nicked site
Our earlier studies have shown that τ increases the affinity of Pol III core for β on primed DNA (Leu et al, 2003). In the experiments of Figure 6A, we examined the DNA-binding properties of the isolated τ subunit using fluorescence anisotropy to determine the affinity of τ for ssDNA and for nicked DNA. For these experiments, we used the C-terminal region of τ subunit (τC), which contains the DNA-binding site (Leu et al, 2003), and retains high affinity to α (Gao and McHenry, 2001). The result shows that τC binds ssDNA about nine-fold tighter than nicked DNA.
Figure 6.
τ increases the affinity of Pol III for primed DNA more than nicked DNA. Fluorescent DNA-binding assay for τC and Pol III core–τC complex. (A) τC binding to ssDNA (blue diamonds) and nicked DNA (red diamonds). (B) Pol III core–τC complex binding to primed DNA (triangles) and a nicked DNA (diamonds).
Next, we used 5′-fluorescent labelled DNA templates to measure the effect of τC on Pol III core binding to a primed site and a nicked site (in the absence of β). The results show that τC enhances the affinity of Pol III core for primed DNA about four-fold (compare Figures 5B and 6B). The affinity of Pol III core for nicked DNA is essentially the same in the absence and presence of τC (compare Figures 5C and 6B). These results may explain how τ can enhance the grip of Pol III to primed DNA, yet enable Pol III to release from DNA on completing replication. Specifically, τ increases the affinity of Pol III core to a primed site through binding ssDNA, but τ does not bind strongly to a nick, and therefore this binding enhancement is lost when ssDNA is converted to dsDNA.
Discussion
E. coli Pol III HE derives its high processivity from the β clamp, which encircles duplex DNA and tethers the polymerase to the primed template during replication (Kong et al, 1992). However, during lagging strand synthesis, Pol III HE must rapidly come on and off DNA during each cycle of Okazaki fragment synthesis. The scarcity of Pol III HE in the cell (10–20 molecules/cell) makes this balancing act all the more important. The speed of the replication fork (∼650 ntd/s) (Breier et al, 2005), coupled with the 1–2 kb length of each Okazaki fragment, indicates that Okazaki fragments are synthesized about once every 2–4 s. How does a processive replicase that is tightly bound to DNA by a sliding clamp rapidly come on and off DNA?
5′ end recognition is not required for collision release from β
Pol III HE displays diametrically opposed properties of tight binding to DNA for processivity, but rapid dissociation on completing an Okazaki fragment to a nick (Stukenberg et al, 1994). This process has been termed ‘collision release' because the replicase collides with a 5′ duplex on filling an ssDNA gap (i.e. completing an Okazaki fragment). One model to explain collision release is that Pol III HE recognizes the 5′ terminal duplex that it collides with, thereby signalling a conformational change that severs the connection of Pol III* to the β clamp. Indeed, the crystal structure of the Pol III α subunit reveals regions of positive charge near the DNA polymerase active site that could conceivably be used to recognize a 5′ duplex (Lamers et al, 2006).
This study provides three different experimental results that argue against 5′ end recognition to trigger collision release by Pol III*. First, Pol III* still releases from β on finishing replication when the 5′ duplex is occluded by EBNA1; second, Pol III* still releases from β when it collides with a 5′ biotin moiety, even in the presence of streptavidin. And third, when Pol III* collides with a 5′ terminus it can remain attached to β and diffuse on dsDNA away from the 5′ terminus before dissociating, and therefore release does not need to occur at the collision site. These lines of evidence indicate that Pol III HE does not recognize the 5′ terminus to trigger collision release from β and DNA.
Collision release is signalled by loss of ssDNA
Bacterial replicases belong to the C-family of DNA polymerases, including the gram-negative Pol III α subunit and gram-positive Pol C. An invariant property of C-family polymerases is the presence of an OB domain (Zhao et al, 2006). Crystal structures of T. aquaticus Pol III α subunit and Streptomyces griseus Pol C bound to a primed site shows that the OB domain binds the ssDNA template just ahead of the polymerase active site (Evans et al, 2008; Wing et al, 2008).
In E. coli and T. aquaticus α, the OB domain is located in the C-terminal region, which also contains the β clamp binding sites. It has been suggested that binding of ssDNA to the OB domain leads to a conformational change in α and alters its affinity for the clamp (Bailey et al, 2006). Consistent with this hypothesis, we find here that mutations that disrupt ssDNA binding to the OB domain lack high processivity with β (Figure 2). We also show that the OB-mutant Pol III is defective in binding to β on DNA (Figure 5). Hence, one may infer that on completing an Okazaki fragment to a nick, the OB domain no longer binds ssDNA, and the unfilled OB domain acts as a sensor to lower the affinity of Pol III to β. We also show that the τ subunit binds ssDNA and increases the affinity of Pol III for a primed site, similarly as β does (Figure 5B); but τ does not bind a nick or increase the affinity of Pol III for a nick (Figure 6). Thus, on filling an ssDNA gap to a nick, τ will no longer hold Pol III to DNA.
A simple model that combines the observations of this report with the crystal structure of the α subunit is illustrated in Figure 7. Panel A shows the protein components of the replication fork, highlighting the lagging strand polymerase τ–β interactions. When Pol III core is bound to the primed site, the OB domain binds template ssDNA and this results in a conformation that presents the internal β-binding site in α to bind to β (‘On-DNA' conformation, Figure 7A). On completing a segment of DNA, the ssDNA is converted to duplex DNA and the interactions of the OB domain and τ to ssDNA are disrupted (Figure 7B). Loss of ssDNA binding by the OB domain and τ results in Pol III dissociation from the 3′ terminus (‘Off-DNA' conformation, Figure 7C). The OB sensor, without bound ssDNA, triggers a conformation change that lowers the affinity of Pol III to β. The interaction of β to Pol III is not released immediately at the nick, which allows Pol III-β to slide along the duplex DNA (Figure 7C) before Pol III dissociation from β (Figure 7D).
Figure 7.
Model of polymerase collision release from DNA during lagging strand replication. (A) Diagram of the E. coli replisome at a replication fork. Pol III HE contains two Pol III cores, one for each strand, and each are bound to a β clamp. The clamp loader contains several subunits, including two τ subunits that bind Pol III cores and DnaB helicase, a hexamer that encircles the lagging strand. Collision release occurs repeatedly on the lagging strand (highlighted). Pol III α subunit connects to β through a C-terminal arm to which the OB domain is attached. The τ subunit also binds the C-terminal region of α. Binding of the OB domain and τ to ssDNA ahead of the active site leads to tight interaction with DNA and β (‘On-DNA' conformation). (B) On finishing an Okazaki fragment, the OB domain and τ no longer bind ssDNA as the ssDNA is converted to dsDNA. Pol III α releases from DNA when τ has no ssDNA to bind. When the OB sensor has no ssDNA, it promotes a conformation change that decreases the affinity of α for β. (C) The α–β complex diffuses on dsDNA (‘Off-DNA' conformation) (D) Pol III departs from β.
Earlier studies indicate that τ helps Pol III to recycle by facilitating the separation of Pol III from the β subunit (Leu et al, 2003). The τ subunit is known to bind the extreme C-terminus of Pol III α subunit (Kim and McHenry, 1996), and therefore is quite close to the OB domain. Perhaps this proximity of τ to the OB domain helps it to support the function of the OB domain in polymerase release from β. The C-terminus of Pol III can also bind β, and facilitates its function with the clamp (Lopez de Saro et al, 2003; Dohrmann and McHenry, 2005).
Implications for polymerase recycling on the lagging strand in eukaryotes
Several features of the E. coli replicase generalize to eukaryotes. The eukaryotic replication machinery uses the PCNA clamp for processivity during synthesis (Stillman, 2008). The PCNA clamp is structurally related to the E. coli β clamp and is loaded onto DNA by a clamp loader (replication factor C) similar in structure and function to the E. coli γ-complex clamp loader (Bowman et al, 2004). In contrast to bacteria, eukaryotes use two different replicases for the leading and lagging strands, Pol ɛ and Pol δ, respectively, both of which function with PCNA (Stillman, 2008).
The lagging strand is synthesized in discontinuous manner in all cells, implying a need for rapid recycling of the chromosomal replicase during lagging strand replication. Studies of Pol δ, the eukaryotic lagging strand enzyme, show that its processivity is enhanced by the PCNA clamp (Chilkova et al, 2007), although human Pol δ appears less processive than yeast Pol δ (Podust et al, 1995; Maga and Hubscher, 2003; Langston and O'Donnell, 2008). One may presume that Pol δ (especially the highly processive yeast Pol δ) has the capability of undergoing collision release like E. coli Pol III HE. Indeed, we have recently shown that Saccharomyces cerevisiae Pol δ disengages from PCNA specifically on completing replication of a ssDNA gap (Langston and O'Donnell, 2008).
The presence of OB domains in regulatory subunits of DNA polymerases has been observed in at least two other systems. The recent crystal structure of a co-complex of the two small non-catalytic subunits of yeast Pol δ reveals an OB domain in the Pol 31 subunit (Baranovskiy et al, 2008). Pol 31 tightly interacts with the catalytic subunit of Pol δ and its presence is essential for cell survival (Sugimoto et al, 1995). In addition, two components of the telomere-binding complex in human cells, Pot1 and TPP1, contain one or more OB domains and these proteins have been shown to greatly increase the processivity of telomerase (Lei et al, 2003; Wang et al, 2007). Whether polymerase processivity and collision release in these systems is sensed and regulated through OB domains, as shown here for the E. coli replicase, remains an interesting question for future studies to address.
Materials and methods
Reagents and proteins
Proteins were purified as described: α, ɛ, γ, τ, δ, δ′, χ, ψ, θ, β (Onrust et al, 1995), and SSB (Yao et al, 2000). Pol III* ((αɛθ)2τ2γδδ′χψ), Pol III core (αɛθ) and γ complex (γ3δδ'χψ) were reconstituted as described (Onrust et al, 1995). When indicated, Pol III* was assembled using an ɛ mutant (D12A and E14A), which eliminates the 3′–5′ exonuclease activity. α OB domain (α residues 962–1083), OB-mutant (R1004S/K1009S/R1010S) domain, and α containing the same OB mutations along with a short N-terminal hexa-histidine tag were purified as described (Lamers et al, 2006). Replication buffer is 20 mM Tris–HC1 (pH 7.5), 4% glycerol, 0.1 mM EDTA, 40 μg/ml BSA, 5 mM DTT, 8 mM MgCl2, 0.5 mM ATP, 60 μM dGTP and 60 μM dCTP.
DNA-replication assays
Reactions contained 72 ng primed M13mp18 ssDNA (30 fmol), 60 μM dCTP and dGTP, 0.5 mM ATP, 32 μg SSB (2 nmol), 11 ng (135 fmol) β2 and 22 ng (31 fmol) Pol III* or Pol III core (wt or OB mutant) at the indicated concentrations, in 25 μl replication buffer. Reactions were incubated at 37°C (Pol III*) or 30°C (Pol III core) for 5 min, and then 60 μM dATP, 20 μM dTTP and 1 μCi α32P-dTTP were added to initiate replication. DNA synthesis was quenched after 20 s (Pol III*) or 1 min (Pol III core) by adding 25 μl of 40 mM EDTA and 1% SDS. Synthesis was quantified as described (Rowen and Kornberg, 1978). Quenched reactions were analysed in a 1% TBE-agarose gel, and visualized using a PhosphorImager (Molecular Dynamics). Reactions using gapped DNA were performed similarly except 2.2 μg activated calf thymus DNA (Sigma) replaced the M13mp18 ssDNA, no SSB or β was added, and reactions were initiated on adding wt or OB-mutant Pol III core (0–500 fmol) followed by incubation at 37°C for 5 min before quenching and quantifying total DNA synthesis. Rolling circle replication reactions (final volume 25 μl) contained 100 fmol 100-mer minicircle DNA (McInerney and O'Donnell, 2007), 0.5 mM ATP and 4 pmol DnaB in replication buffer for 30 s at 37°C before addition of 60 μM dCTP, dGTP, 370 fmol β2 and 100 fmol of Pol III*. Reactions were incubated at 37°C for 4 min before addition of 13 pmol SSB, 50 μM each of CTP, GTP, UTP, 60 μM dATP, 10 μM dTTP, 1 μCi α32P-dTTP and 150 pmol DnaG to initiate replication. Reactions were processed as described above.
Polymerase-cycling assays
Initial (donor) primed 7.2 kb M13mp18 ssDNA (containing an EBNA1 site) was prepared by annealing a DNA 30-mer (unmodified or 5′ modification with biotin or psoralen), or a 46-mer containing the 18-bp EBNA1 site at the extreme 5′ terminus. The challenge 8.6 kb M13Gori ssDNA was primed with a DNA 30-mer. Donor reactions were assembled in 47 μl of replication buffer containing 144 ng (60 fmol) primed 7.2 kb M13mp18 ssDNA, 1.6 μg (84 pmol) SSB, 22 ng (270 fmol) β2, and 44 ng (62 fmol) Pol III*, followed by a 3-min incubation at 37°C. When present, EBNA1 (7.5 pmol) was added to primed M13mp ssDNA and incubated 1 min before adding other proteions. Challenge DNA reactions were assembled in 47 μl of replication buffer containing 720 ng 8.6 kb challenge primed M13Gori ssDNA, 4 μg (53 pmol) SSB, 66 ng (815 fmol) β, and 10 ng (52 fmol) γ complex followed by 3 min at 37°C. Replication was initiated by mixing the donor with challenge reactions and adding 6 μl of 1.5 mM dATP, 0.5 mM [α-32P]dTTP. Aliquots were removed and processed as described above.
Fluorescent OB domain DNA-binding assays
Increasing amounts of wt or mutant OB domains were titrated into reactions containing 1 μM ssDNA (Cy5-28-mer) or dsDNA (Cy5-28/28-mer) in 20 mM Tris–HCl pH 7.5, 10% glycerol, 75 mM NaCl, 0.5 mM EDTA, 20 mM arginine, 30 mM glutamate, at 23°C. Cy5-28-mer was (5′-Cy5-CCCATCGTATACGAAGGGAGTCGACTGG-3′). Cy5-dsDNA was prepared by annealing the above oligonucleotide with its complementary 28-mer. Anisotropy was measured in a PTI spectrofluorimeter configured in the T-format and using the time-trace function to generate 35 data points, which were averaged. Anisotropy is defined by anisotropy=(IVV−IVH)/(IVV+2IVH), where IVV and IVH are the intensities of the vertical and horizontal components of the emitted light using vertical polarized excitation. Differences in the response of the detector to vertical and horizontal polarized light were corrected (G factor) automatically by the spectrofluorimeter.
Fluorescent Pol III core DNA-binding assays
Analysis of Pol III core plus and minus τ were as follows. Increasing amounts of Pol III core or Pol III core–τC complex (0–1.5 μM final concentration) were titrated into reactions containing 10 nM of either fluorescent primed template [34/62(5′F)], or singly nicked dsDNA [34/28/62(5′F)] in 20 mM Tris–HCl pH 7.5, 10% glycerol, 25 mM NaCl, 0.5 mM EDTA, 1 mM DTT. After 30 min at 23°C, anisotropy measurements were performed as described above. Control titrations were performed using only τC. Pol III core-DNA-β binding reactions used an internal fluorescein label on the 62-mer [62(iF): 5′-CCCATCGTATACGAAGGGAGTCGACTGAGCTAGCTAGC/iF-T/CTATAGCTAGCTAGCTAGCT] annealed to a complementary 34-mer to form a 28-mer 5′overhang. Pol III core/τC-DNA-binding reactions used the same 62-mer sequence, but 5′-end labelled with fluorescein, annealed either to the 34-mer, to form a 28-mer 5′overhang, or with the 34-mer and a complementary 28-mer, to form a nicked duplex [34/28/62(5′F)].
Analysis of Pol III core binding to β was as follows. Increasing amounts of wt or OB-mutant Pol III core (0–1.5 μM final concentration) were titrated into reactions containing 10 nM fluorescent primed template [34/62(iF)] in 20 mM Tris–HCl pH 7.5, 10% glycerol, 75 mM NaCl, 1 mM DTT, 0.5 mM EDTA (performed in the absence or presence of 50 nM β). After 20 min at 23°C, samples were excited at 490 nm and emission (500–600 nm) was recorded using a PTI spectrofluorometer. For data analysis, binding isotherms were constructed under the simplifying assumption that the DNA concentration is adequately below the Kd such that total added protein is equivalent to free protein. The equilibrium titration data were fit to a single-site binding model.
FRET Pol III core β-binding assay
Pol III core was reconstituted using a θ subunit labelled with AlexaFlor594 (FRET donor) and the β clamp was labelled using AlexaFlor647 (FRET acceptor) using maleimide chemistry (Invitrogen, Molecular Probes). Reaction contained 100 nM wt or OB-mutant Pol III core and variable amounts of β2 (0–1800 nM) in 60 μl of 20 mM Tris–HCl (pH 7.5), 1 mM DTT, 0.5 mM EDTA. Reactions were incubated for 15 min at 22°C and then analysed in a PTI spectrofluorimeter. Fluorescence resulting from direct (i.e. non-sensitized) β2-AlexaFlor647 excitation was eliminated by subtracting the spectra of β2-AlexaFlor647 in the absence of Pol III core.
Analysis of Pol III HE sliding over an intramolecular duplex
Reactions contained 141 fmol of φX174 ssDNA pre-annealed with primer #1, 75 fmol Pol III*, 6.2 pmol β2, 132 pmol SSB and were pre-incubated at 30°C for 2 min in replication buffer containing 60 μM dCTP and dGTP. The trap was prepared by loading 32 pmol β clamp onto 840 fmol M13mp18: Pr3BddC using 2.5 pmol γ complex in replication buffer containing 60 μM dATP, 20 μM dTTP and 1 μCi [α-32P]dTTP. Primer #2 was annealed for 1 min as described (O'Donnell and Kornberg, 1985) before initiating replication on adding the M13mp18 trap (final volume 25 μl). Reactions were incubated at 30°C for the indicated times and DNA products were analysed in a 1.2% alkaline agarose gel. Effectiveness of the trap was assessed by mixing reactions before initiating synthesis on adding Pol III*. Primers for φX174 ssDNA were primer #1 (30-mer) that anneals to map position 2794–2823, and primer #2 (15-mer) that anneals to map position 791–805. The trap used a 3′dd 30-mer annealed to M13mp18 ssDNA: (5′-GTTAAAGGCCGCTTTTGCGGGATCGTCACddC-3′).
Supplementary Material
Supplementary Figures S1–S6
Review Process File
Acknowledgments
We are grateful to Lance Langston for helpful suggestions and critical reading of the manuscript. We also thank Maija Skangalis and other members of the laboratory for help in purifying some of the proteins used in this study. This work was supported by a grant from the NIH (GM38839).
Footnotes
The authors declare that they have no conflict of interest.
References
- Bailey S, Wing RA, Steitz TA (2006) The structure of T. aquaticus DNA polymerase III is distinct from eukaryotic replicative DNA polymerases. Cell 126: 893–904 [DOI] [PubMed] [Google Scholar]
- Baranovskiy AG, Babayeva ND, Liston VG, Rogozin IB, Koonin EV, Pavlov YI, Vassylyev DG, Tahirov TH (2008) X-ray structure of the complex of regulatory subunits of human DNA polymerase delta. Cell Cycle (Georgetown, TX) 7: 3026–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkovic SJ, Valentine AM, Salinas F (2001) Replisome-mediated DNA replication. Ann Rev Biochem 70: 181–208 [DOI] [PubMed] [Google Scholar]
- Bochkarev A, Barwell JA, Pfuetzner RA, Bochkareva E, Frappier L, Edwards AM (1996) Crystal structure of the DNA-binding domain of the Epstein-Barr virus origin-binding protein, EBNA1, bound to DNA. Cell 84: 791–800 [DOI] [PubMed] [Google Scholar]
- Bowman GD, O'Donnell M, Kuriyan J (2004) Structural analysis of a eukaryotic sliding DNA clamp-clamp loader complex. Nature 429: 724–730 [DOI] [PubMed] [Google Scholar]
- Breier AM, Weier HU, Cozzarelli NR (2005) Independence of replisomes in Escherichia coli chromosomal replication. Proc Natl Acad Sci USA 102: 3942–3947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilkova O, Stenlund P, Isoz I, Stith CM, Grabowski P, Lundstrom EB, Burgers PM, Johansson E (2007) The eukaryotic leading and lagging strand DNA polymerases are loaded onto primer-ends via separate mechanisms but have comparable processivity in the presence of PCNA. Nucleic Acids Res 35: 6588–6597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallmann HG, Kim S, Pritchard AE, Marians KJ, McHenry CS (2000) Characterization of the unique C terminus of the Escherichia coli tau DnaX protein. Monomeric C-tau binds alpha AND DnaB and can partially replace tau in reconstituted replication forks. J Biol Chem 275: 15512–15519 [DOI] [PubMed] [Google Scholar]
- Dohrmann PR, McHenry CS (2005) A bipartite polymerase-processivity factor interaction: only the internal beta binding site of the alpha subunit is required for processive replication by the DNA polymerase III holoenzyme. J Mol Biol 350: 228–239 [DOI] [PubMed] [Google Scholar]
- Evans RJ, Davies DR, Bullard JM, Christensen J, Green LS, Guiles JW, Pata JD, Ribble WK, Janjic N, Jarvis TC (2008) Structure of PolC reveals unique DNA binding and fidelity determinants. Proc Natl Acad Sci USA 105: 20695–20700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D, McHenry CS (2001) tau binds and organizes Escherichia coli replication through distinct domains. Partial proteolysis of terminally tagged tau to determine candidate domains and to assign domain V as the alpha binding domain. J Biol Chem 276: 4433–4440 [DOI] [PubMed] [Google Scholar]
- Hacker KJ, Alberts BM (1994) The rapid dissociation of the T4 DNA polymerase holoenzyme when stopped by a DNA hairpin helix. A model for polymerase release following the termination of each Okazaki fragment. J Biol Chem 269: 24221–24228 [PubMed] [Google Scholar]
- Hamdan SM, Loparo JJ, Takahashi M, Richardson CC, van Oijen AM (2009) Dynamics of DNA replication loops reveal temporal control of lagging-strand synthesis. Nature 457: 336–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, O'Donnell M (2005) Cellular DNA replicases: components and dynamics at the replication fork. Ann Rev Biochem 74: 283–315 [DOI] [PubMed] [Google Scholar]
- Johnson DE, Takahashi M, Hamdan SM, Lee SJ, Richardson CC (2007) Exchange of DNA polymerases at the replication fork of bacteriophage T7. Proc Natl Acad Sci USA 104: 5312–5317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DR, McHenry CS (1996) Biotin tagging deletion analysis of domain limits involved in protein-macromolecular interactions. Mapping the tau binding domain of the DNA polymerase III alpha subunit. J Biol Chem 271: 20690–20698 [DOI] [PubMed] [Google Scholar]
- Kong XP, Onrust R, O'Donnell M, Kuriyan J (1992) Three-dimensional structure of the beta subunit of E. coli DNA polymerase III holoenzyme: a sliding DNA clamp. Cell 69: 425–437 [DOI] [PubMed] [Google Scholar]
- Kornberg A, Baker TA (1992) DNA Replication, 2nd edn., New York: W.H. Freeman [Google Scholar]
- Lamers MH, Georgescu RE, Lee SG, O'Donnell M, Kuriyan J (2006) Crystal structure of the catalytic alpha subunit of E. coli replicative DNA polymerase III. Cell 126: 881–892 [DOI] [PubMed] [Google Scholar]
- Langston L, O'Donnell M (2008) DNA Polymerase delta is highly processive with PCNA and undergoes collision release upon completing DNA. J Biol Chem 283: 29522–29531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JB, Hite RK, Hamdan SM, Xie XS, Richardson CC, van Oijen AM (2006) DNA primase acts as a molecular brake in DNA replication. Nature 439: 621–624 [DOI] [PubMed] [Google Scholar]
- Lei M, Podell ER, Baumann P, Cech TR (2003) DNA self-recognition in the structure of Pot1 bound to telomeric single-stranded DNA. Nature 426: 198–203 [DOI] [PubMed] [Google Scholar]
- Leu FP, Georgescu R, O'Donnell M (2003) Mechanism of the E. coli tau processivity switch during lagging-strand synthesis. Mol Cell 11: 315–327 [DOI] [PubMed] [Google Scholar]
- Li X, Marians KJ (2000) Two distinct triggers for cycling of the lagging strand polymerase at the replication fork. J Biol Chem 275: 34757–34765 [DOI] [PubMed] [Google Scholar]
- Lopez de Saro FJ, Georgescu RE, O'Donnell M (2003) A peptide switch regulates DNA polymerase processivity. Proc Natl Acad Sci USA 100: 14689–14694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maga G, Hubscher U (2003) Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J Cell Sci 116 (Pt 15): 3051–3060 [DOI] [PubMed] [Google Scholar]
- McCauley MJ, Shokri L, Sefcikova J, Venclovas C, Beuning PJ, Williams MC (2008) Distinct double- and single-stranded DNA binding of E. coli replicative DNA polymerase III alpha subunit. ACS Chem Biol 3: 577–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry CS (2003) Chromosomal replicases as asymmetric dimers: studies of subunit arrangement and functional consequences. Mol Microbiol 49: 1157–1165 [DOI] [PubMed] [Google Scholar]
- McInerney P, O'Donnell M (2004) Functional uncoupling of twin polymerases: mechanism of polymerase dissociation from a lagging-strand block. J Biol Chem 279: 21543–21551 [DOI] [PubMed] [Google Scholar]
- McInerney P, O'Donnell M (2007) Replisome fate upon encountering a leading strand block and clearance from DNA by recombination proteins. J Biol Chem 282: 25903–25916 [DOI] [PubMed] [Google Scholar]
- Mok M, Marians KJ (1987) The Escherichia coli preprimosome and DNA B helicase can form replication forks that move at the same rate. J Biol Chem 262: 16644–16654 [PubMed] [Google Scholar]
- O'Donnell M (2006) Replisome architecture and dynamics in Escherichia coli. J Biol Chem 281: 10653–10656 [DOI] [PubMed] [Google Scholar]
- O'Donnell ME (1987) Accessory proteins bind a primed template and mediate rapid cycling of DNA polymerase III holoenzyme from Escherichia coli. J Biol Chem 262: 16558–16565 [PubMed] [Google Scholar]
- O'Donnell ME, Kornberg A (1985) Dynamics of DNA polymerase III holoenzyme of Escherichia coli in replication of a multiprimed template. J Biol Chem 260: 12875–12883 [PubMed] [Google Scholar]
- Onrust R, Finkelstein J, Turner J, Naktinis V, O'Donnell M (1995) Assembly of a chromosomal replication machine: two DNA polymerases, a clamp loader, and sliding clamps in one holoenzyme particle. III. Interface between two polymerases and the clamp loader. J Biol Chem 270: 13366–13377 [DOI] [PubMed] [Google Scholar]
- Podust VN, Podust LM, Muller F, Hubscher U (1995) DNA polymerase delta holoenzyme: action on single-stranded DNA and on double-stranded DNA in the presence of replicative DNA helicases. Biochemistry 34: 5003–5010 [DOI] [PubMed] [Google Scholar]
- Pomerantz RT, O'Donnell M (2008) The replisome uses mRNA as a primer after colliding with RNA polymerase. Nature 456: 762–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowen L, Kornberg A (1978) Primase, the dnaG protein of Escherichia coli. An enzyme which starts DNA chains. J Biol Chem 253: 758–764 [PubMed] [Google Scholar]
- Stillman B (2008) DNA polymerases at the replication fork in eukaryotes. Mol Cell 30: 259–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stukenberg PT, O'Donnell M (1995) Assembly of a chromosomal replication machine: two DNA polymerases, a clamp loader, and sliding clamps in one holoenzyme particle. V. Four different polymerase-clamp complexes on DNA. J Biol Chem 270: 13384–13391 [DOI] [PubMed] [Google Scholar]
- Stukenberg PT, Turner J, O'Donnell M (1994) An explanation for lagging strand replication: polymerase hopping among DNA sliding clamps. Cell 78: 877–887 [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Sakamoto Y, Takahashi O, Matsumoto K (1995) HYS2, an essential gene required for DNA replication in Saccharomyces cerevisiae. Nucleic Acids Res 23: 3493–3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J, O'Donnell M (1995) Cycling of Escherichia coli DNA polymerase III from one sliding clamp to another: model for lagging strand. Methods Enzymol 262: 442–449 [DOI] [PubMed] [Google Scholar]
- Wang F, Podell ER, Zaug AJ, Yang Y, Baciu P, Cech TR, Lei M (2007) The POT1-TPP1 telomere complex is a telomerase processivity factor. Nature 445: 506–510 [DOI] [PubMed] [Google Scholar]
- Wing RA, Bailey S, Steitz TA (2008) Insights into the replisome from the structure of a ternary complex of the DNA polymerase III alpha-subunit. J Mol Biol 382: 859–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CA, Zechner EL, Reems JA, McHenry CS, Marians KJ (1992) Coordinated leading- and lagging-strand synthesis at the Escherichia coli DNA replication fork. V. Primase action regulates the cycle of Okazaki fragment synthesis. J Biol Chem 267: 4074–4083 [PubMed] [Google Scholar]
- Xi J, Zhang Z, Zhuang Z, Yang J, Spiering MM, Hammes GG, Benkovic SJ (2005) Interaction between the T4 helicase loading protein (gp59) and the DNA polymerase (gp43): unlocking of the gp59-gp43-DNA complex to initiate assembly of a fully functional replisome. Biochemistry 44: 7747–7756 [DOI] [PubMed] [Google Scholar]
- Yang J, Nelson SW, Benkovic SJ (2006) The control mechanism for lagging strand polymerase recycling during bacteriophage T4 DNA replication. Mol Cell 21: 153–164 [DOI] [PubMed] [Google Scholar]
- Yao N, Hurwitz J, O'Donnell M (2000) Dynamics of beta and proliferating cell nuclear antigen sliding clamps in traversing DNA secondary structure. J Biol Chem 275: 1421–1432 [DOI] [PubMed] [Google Scholar]
- Yuzhakov A, Turner J, O'Donnell M (1996) Replisome assembly reveals the basis for asymmetric function in leading and lagging strand replication. Cell 86: 877–886 [DOI] [PubMed] [Google Scholar]
- Zhao XQ, Hu JF, Yu J (2006) Comparative analysis of eubacterial DNA polymerase III alpha subunits. Genomics Proteomics Bioinformatics 4: 203–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures S1–S6
Review Process File