Abstract
The recent discovery of CD4+ T cells characterized by secretion of interleukin (IL)-17 (TH17 cells) and the naturally occurring regulatory FOXP3+ CD4 T cell (nTreg) has had a major impact on our understanding of immune processes not readily explained by the TH1/TH2 paradigm. TH17 and nTreg cells have been implicated in the pathogenesis of human autoimmune diseases, including multiple sclerosis, rheumatoid arthritis, inflammatory bowel disease and psoriasis1,2. Our recent data and the work of others demonstrated that transforming growth factor-β (TGF-β) and IL-6 are responsible for the differentiation of naive mouse T cells into TH17 cells, and it has been proposed that IL-23 may have a critical role in stabilization of the TH17 phenotype3-5. A second pathway has been discovered in which a combination of TGF-β and IL-21 is capable of inducing differentiation of mouse TH17 cells in the absence of IL-6 (refs 6-8). However, TGF-β and IL-6 are not capable of differentiating human TH17 cells2,9 and it has been suggested that TGF-β may in fact suppress the generation of human TH17 cells10. Instead, it has been recently shown that the cytokines IL-1β, IL-6 and IL-23 are capable of driving IL-17 secretion in short-term CD4+ T cell lines isolated from human peripheral blood11, although the factors required for differentiation of naive human CD4 to TH17 cells are still unknown. Here we confirm that whereas IL-1β and IL-6 induce IL-17A secretion from human central memory CD4+ T cells, TGF-β and IL-21 uniquely promote the differentiation of human naive CD4+ T cells into TH17 cells accompanied by expression of the transcription factor RORC2. These data will allow the investigation of this new population of TH17 cells in human inflammatory disease.
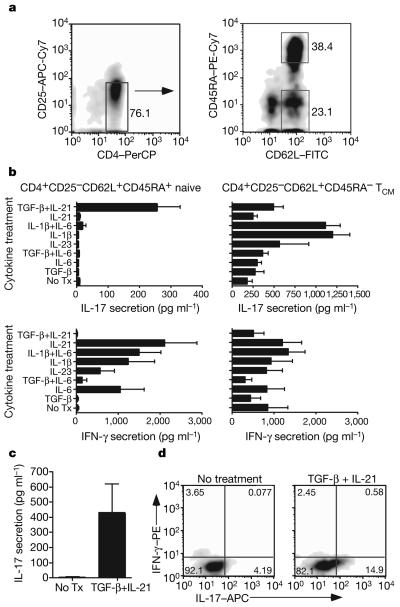
To better understand regulation of IL-17A secretion from human CD4+ T cells, we used a strategy that would allow us to evaluate the effects of various combinations of cytokine on expansion of TH17 cells from memory T cells versus differentiation of naive CD4+ lymphocytes into TH17 cells. Specifically, we used high-speed flow cytometry for sorting these two distinct populations of CD4+ T cells from the peripheral blood of healthy subjects: CD4+CD25− CD62L+CD45RAhi cells highly enriched for naive T cells and CD4+CD25−CD62L+CD45RA− cells enriched for central memory T cells (TCM; Fig. 1a). All cells enriched for a naive or a central memory phenotype expressed the chemokine receptor CCR7 (data not shown). These two T cell populations were then stimulated with plate-bound anti-CD3 and soluble anti-CD28 monoclonal antibodies for 7 days in serum-free medium containing different combinations of cytokines implicated in CD4+ TH17 cell differentiation.
Figure 1. TGF-β and IL-21 promote TH17 differentiation from naive CD4+ T cells.
a, CD4+ T cells were sorted into populations enriched for naive or central memory T helper cells. b, IFN-γ and IL-17A secretion is shown from T cells stimulated for 7 days in the presence of the indicated cytokines. Standard deviation using T cells from three unrelated subjects is represented. TGF-β- and IL-21-induced IL-17 secretion is highly significant (P < 0.01). Tx, treatment. c, IL-17 secretion from naive T cells from six different donors is represented (mean±s.e.m.). d, Intracellular expression of IL-17 and IFN-γ from one of five experiments is shown.
As reported previously, the cytokine IL-1β induced the greatest amount of IL-17A secretion from TCM (Fig. 1b). The addition of IL-6 alone had little effect on induction of IL-17A, and when added with IL-1β had no additive or synergistic effect on IL-17A production. Addition of IL-23 was also able to modestly enhance IL-17A secretion from TCM. However, IL-1β alone or together with IL-6 failed to induce IL-17A secretion from naive CD4+ T cells. In marked contrast, a combination of TGF-β and IL-21 was uniquely able to induce TH17 differentiation. Whereas IL-21, IL-1β or IL-6 induced significant amounts of interferon-γ (IFN-γ) secretion from naive T cells, the addition of TGF-β with IL-21 suppressed IFN-γ secretion and induced differentiation of TH17 cells. Whereas we did observe variability in the extent of TH17 differentiation among unrelated healthy donors (Fig. 1c), we always observed induction of IL-17A after differentiation in the presence of TGF-β with IL-21. Intracytoplasmic staining demonstrated, in agreement with enzyme-linked immunosorbent assay (ELISA) results, that the combination of TGF-β and IL-21 differentiated CD4+ T cells that secreted only IL-17A and no IFN-γ (Fig. 1d). A recent study12 has demonstrated that the pathogenicity of mouse IL-17-secreting T cells is influenced by whether they also secrete IL-10. Using intracytoplasmic staining, we failed to observe any IL-10 when naive CD4+ T cells were differentiated in the presence of TGF-β and IL-21 (data not shown).
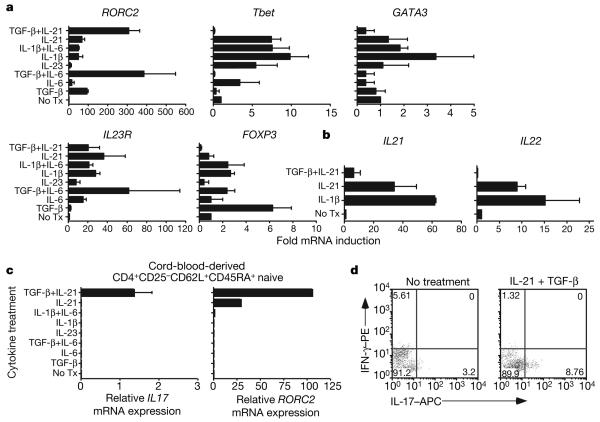
RORC2 is the human homologue of mouse RORγt—a transcription factor critical for the differentiation of mouse IL-17-secreting T cells. Thus, to understand the molecular mechanisms involved in differentiation of human TH17 cells, we used quantitative PCR with reverse transcription (RT–PCR) to evaluate messenger RNA levels of RORC2 and other molecules implicated in TH17 differentiation. The combination of TGF-β and IL-21 induced high levels of RORC2 (Fig. 2a), consistent with their ability to induce IL-17A secretion from naive human CD4+ T cells. It was of particular interest that the combination of TGF-β and IL-6 that induces TH17 differentiation in mouse T cells also induced expression of RORC2 in naive human CD4 cells. Because this combination of cytokines did not, however, induce IL-17A secretion, these data indicate that expression of RORC2 in humans is not in itself sufficient to induce IL-17 production, and that another as yet unidentified transcription factor in combination with RORC2, perhaps the human homologue of mouse RORα (ref. 13), may be required to induce IL-17A-secreting TH17 cells.
Figure 2. TGF-β and IL-21 induce RORC2 in naive CD4+ T cells.
a, mRNA expression levels (fold-induction relative to T cells without exogenous cytokines) of RORC2, Tbet, GATA3, IL23R and FOXP3 are shown after naive T cells were differentiated as indicated. b, Fold-induction±s.e.m. (n = 3) of IL21 and IL22 are represented. c, Mean expression±s.e.m. of IL17A and RORC2 are shown (n = 3) for naive T cells obtained from cord blood. d, Shown is intracytoplasmic staining of IL-17 and IFN-γ from cord blood naive T cells after 7 days of differentiation. Similar results were seen in another independent assay.
T-bet (also known as TBX21) is the master regulator for TH1 cells secreting IFN-γ, whereas GATA3 induces TH2, IL-4 secreting CD4 cells. Messenger RNA expression levels of Tbet were highly concordant with amounts of IFN-γ secretion and were consistent with our findings that whereas TGF-β and IL-21 induce TH17 cell differentiation with RORC2 expression, TGF-β suppresses the induction of T-bet by IL-21. Similarly, there was no induction of GATA3 with TGF-β and IL-21. The cytokines IL-6, IL-21 and IL-1β but not TGF-β induced IL-23 receptor upregulation in stimulated naive CD4+ T cells. We also examined the expression of the Treg transcription factor FOXP3. As has been previously reported in both mouse and human systems, FOXP3 was induced by TGF-β1. Induction of FOXP3 was inhibited by IL-6 and to a greater extent by IL-21—transcription factors that induce RORC2. Thus, although the induction of RORC2 and FOXP3 transcription factors was highly similar between mouse and human naive CD4 cells, the induction of IL-17A by IL-6 in combination with TGF-β is discordant between the species.
We and others have shown previously that IL-21 secreted by mouse CD4+ T cells can induce the secretion of IL-21 in an autocrine amplification loop6-8,14. Thus, we examined whether human IL-21 similarly induced IL-21 secretion from naive CD4+ T cells; we also evaluated the effects of a combination of TGF-β with IL-21 and IL-1β, given the ability of these cytokines to induce IL-17 from naive and central memory CD4+ T cells. Consistent with results in mice, IL-21 upregulated IL-21, although IL-1β induced even greater amounts of IL21 mRNA (Fig. 2b). In contrast to what has been observed in mice, IL-21 also increased IL22 mRNA levels in naive CD4+ T cells in the absence of any exogenous IL-23. However, TGF-β suppressed the expression of IL21 and IL22 mRNA induced by IL-21 (Fig. 2b). These data further highlight similar yet subtle differences between human and mouse CD4+ T cells, because whereas IL-21 induces IL21 and IL22, differentiation to TH17 cells with TGF-β inhibits the expression of these cytokines.
To confirm the unique function of TGF-β and IL-21 in the differentiation of TH17 cells from naive human CD4+ T cells, we sorted CD4+CD25−CD62L+CD45RAhi cells from human cord blood. As expected, a higher proportion of CD4 cells in the cord blood exhibited this naive phenotype relative to peripheral blood obtained from healthy adult subjects (data not shown). TGF-β and IL-21 induced the upregulation of IL17A and RORC2 mRNA (Fig. 2c). Although IL-21 alone modestly induced RORC2, only TGF-β and IL-21 were able to induce IL17A mRNA. When given a very strong in vitro stimulus, naive CD4+ T cells sorted from cord blood secreted IL-17A protein (Fig. 2d). These data further indicate that TGF-β and IL-21 are critical in the differentiation of both human and mouse TH17 cells.
Collectively, our data refine and extend our understanding of the regulation of IL-17A secretion from human CD4+ T cells and define the conditions required for human TH17 cell differentiation. We confirm recent reports that IL-1β together with IL-6 (ref. 9) or IL-23 (ref. 2) can induce IL-17A secretion, which is most apparent in the human memory CD4+ T cell subset. A combination of TGF-β plus IL-21 is required for the differentiation of TH17 cells from naive T cells. It is important to note that to observe TH17 differentiation from naive human CD4+ T cells, appropriate amounts of both IL-21 and TGF-β are needed: addition of IL-21 alone must be sufficient to upregulate IFN-γ secretion, and the amount of TGF-β added must inhibit IL-21-induced IFN-γ secretion (see Methods for details). TH17 cells differentiated under these conditions are also notable for secretion of IL-17A in the absence of IFN-γ. In summary, our data together with previous reports in humans suggest that IL-1β and IL-6 induced during the early stages of an inflammatory response may act on memory T cells to promote IL-17 and IL-21 secretion, with induced IL-21 able to synergize with TGF-β to promote differentiation of TH17 cells from naive CD4+ T cells. IL-23 may serve to expand or stabilize the phenotype of previously differentiated TH17 cells. These experiments allow for the characterization of human inflammatory TH17 responses associated with infection and autoimmune diseases.
METHODS SUMMARY
Cell sorting
PBMCs were obtained from the peripheral blood of healthy subjects or from cord blood (AllCells) in compliance with institutional review board (IRB) protocols. CD4+ T cells were subsequently isolated by negative selection using magnetic beads (Miltenyi Biotech). Naive (CD25−CD62L+CD45RAhi) and central memory (CD25−CD62L+CD45RA−) CD4+ T cells were obtained by staining with the following antibodies: CD62L–FITC, CD4–PerCP, CD45RA–PE–Cy7, CD25–APC–Cy7 (BD Pharmingen) and were sorted on a FACS Aria (BD Biosciences).
Differentiation assays
Naive or central memory CD4+ T cells were stimulated with plate-bound anti-CD3 and soluble CD28 monoclonal antibodies in serum-free X-VIVO15 medium (Biowhittaker) and cytokines (IL-6, 25 ng ml−1; TGF-β1, 5 ng ml−1; IL-1β, 12.5 ng ml−1; IL-21, 25 ng ml−1; IL-23, 25 ng ml−1) for a period of 7 days, at which point supernatants were collected and tested by ELISA for IFN-γ (BD Biosciences) or IL-17A (eBioscience) using paired antibodies. We have observed that concentrations of TGF-β ranging from 0.1 ng ml−1 to 10 ng ml−1 in the presence of IL-21 promote TH17 differentiation, whereas 50 ng ml−1 TGF-β suppresses differentiation. Intracytoplasmic staining was performed using standard methodologies and anti-IL-17–APC (R&D Systems) and anti-IFN-γ-PE or anti-IL-10–PE (BD Biosciences) antibodies.
Real-time PCR
All primers and probes were obtained from Applied Biosystems and used according to standard methodologies.
METHODS
Differentiation assays
Plates (96-well U-bottom) were coated with 1.5 μg ml−1 anti-CD3 monoclonal antibody (eBioscience, clone OKT3) in a volume of 50 ml of PBS and were incubated overnight (16 h) at 4 °C. For T cells isolated from cord blood, a 96-well plate was pre-coated overnight at 4 °C with 3 μg ml−1 anti-CD3 monoclonal antibody (UCHT1, BD Biosciences). Antibody solution was then removed, plates were rinsed once with serum-free X-VIVO 15 medium (Biowhittaker), and naive or central memory CD4+ T cells (5 × 104 per well) were stimulated in serum-free X-VIVO15 medium with soluble CD28 (BD Biosciences, clone 28.2) monoclonal antibody (1.0 μg ml−1) and cytokines (IL-6, 25 ng ml−1; TGF-β1, 5 ng ml−1; IL-1β, 12.5 ng ml−1; IL-21, 25 ng ml−1; IL-23, 25 ng ml−1) for a period of 7 days in the absence of IL-2. The cytokines IL-6, IL-1β, IL-23 and TGFβ1 (catalogue number 240-B-002) were all obtained from R&D systems. The TGFβ1 was not acid-treated before addition. IL-21 was purchased from Cell Sciences (catalogue number CRI 172A). Supernatants were collected and tested by ELISA for IFN-γ (Endogen) or IL-17A (human IL-17A ELISA kit from eBioscience or human IL-17 duoset from R&D systems) using paired antibodies. We have observed that concentrations of TGF-β1 ranging from 0.1 ng ml−1 to 10 ng ml−1 in the presence of IL-21 promote TH17 differentiation, with lower doses of TGFβ1 associated with induction of a higher proportion of IL-17-producing cells than higher doses; use of 50 ng ml−1 TGF-β1 suppresses differentiation and IL-17 secretion. Intracytoplasmic staining was performed using standard methodologies and anti-IL-17-APC (R&D Systems) and anti-IFN-γ-PE or anti-IL-10-PE (BD Biosciences) antibodies. Cells were stimulated with phorbol 12-myristate 13-acetate (PMA; 10 ng ml−1), iono-mycin (0.5 μg ml−1) and Golgistop for 5 h at 37 °C before intracellular staining.
Real-time PCR
After removing supernatants from wells in differentiation assays, 250 ml per well of lysis buffer was added, at which point RNA was isolated using a RNeasy mini kit (Qiagen). Total RNA was converted to complementary DNA using Taqman Reverse transcription reagents (Applied Biosystems). Quantitative PCR was performed using a 7500 Fast Real-time PCR system (Applied Biosystems). All primers and probes were obtained from Applied Biosystems and used according to standard methodologies.
Acknowledgements
We thank D. Kozoriz for assistance with flow cytometric cell sorting. Supported by grants from the NIH (D.A.H., V.K.K.), the National Multiple Sclerosis Society (D.A.H., V.K.K.), the Juvenile Diubetes Research Foundation (D.A.H., V.K.K.), and the American Cancer Society (D.E.A.). D.A.H. and V.K.K. are recipients of a Javit2 Investigator award from the NIH.
Footnotes
Reprints and permissions information is available at www.nature.com/reprints.
Full Methods and any associated references are available in the online version of the paper at www.nature.com/nature.
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
References
- 1.Kikly K, Liu L, Na S, Sedgwick JD. The IL-23/Th(17) axis: therapeutic targets for autoimmune inflammation. Curr. Opin. Immunol. 2006;18:670–675. doi: 10.1016/j.coi.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Wilson NJ, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nature Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 3.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 4.Mangan PR, et al. Transforming growth factor-β induces development of the TH17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 5.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nurieva R, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 8.Zhou L, et al. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nature Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 9.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17-producing human T helper cells. Nature Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 10.Evans HG, Suddason T, Jackson I, Taams LS, Lord GM. Optimal induction of T helper 17 cells in humans requires T cell receptor ligation in the context of Toll-like receptor-activated monocytes. Proc. Natl Acad. Sci. USA. 2007;104:17034–17039. doi: 10.1073/pnas.0708426104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laurence A, O'Shea JJ. TH-17 differentiation: of mice and men. Nature Immunol. 2007;8:903–905. doi: 10.1038/ni0907-903. [DOI] [PubMed] [Google Scholar]
- 12.McGeachy MJ, et al. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell-mediated pathology. Nature Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 13.Yang XO, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors RORα and RORγ. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei L, Laurence A, Elias KM, O'Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J. Biol. Chem. 2007;282:34605–34610. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]