Abstract
Sequencing of over 40 fungal and oomycete genomes has been completed. The next major challenge in modern fungal/oomycete biology is now to translate this plethora of genome sequence information into biological functions. Reverse genetics has emerged as a seminal tool for functional genomics investigations. Techniques utilized for reverse genetics like targeted gene disruption/replacement, gene silencing, insertional mutagenesis, and targeting induced local lesions in genomes will contribute greatly to the understanding of gene function of fungal and oomycete pathogens. This paper provides an overview on high-throughput reverse genetics approaches to decode fungal/oomycete genomes.
1. Introduction
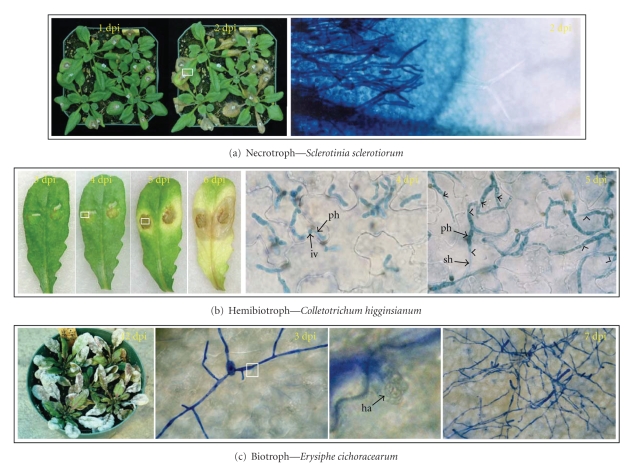
Fungal and oomycete phytopathogens are major constraints in global food production as they cause many of the world's most notorious plant diseases. These phytopathogens can be broadly divided into those that kill the host and feed on the cell contents (necrotrophs), those that require a living host to complete their life cycle (biotrophs), and those that act as both biotrophs and necrotrophs at different stages of infection (hemibiotrophs). Figure 1 illustrates three phytopathosystems (necrotrophic Arabidopsis-Sclerotinia sclerotiorum, biotrophic Arabidopsis-Erysiphe cichoracearum, and hemibiotrophic Arabidopsis-Colletotrichum higginsianum), which have been widely researched with the objective to understand molecular mechanisms underpinning pathogen development and pathogenesis.
Figure 1.
Arabidopsis—fungus interaction. (a) Necrotrophic interaction—Sclerotinia sclerotiorum infects Arabidopsis by killing host tissues in advance of hyphal colonization. Trypan blue stains necrotic dead tissues. (b) Hemibiotrophic interaction—Colletotrichum higginsianum initiates infection process by a biotrophic phase at 3–4 days post-inoculation (dpi), which is associated with infection vesicles (iv) and large primary hyphae (ph). Thin secondary hyphae (sh) appear in the necrotrophic phase at 5 dpi. (c) Biotrophic interaction—Erysiphe cichoracearum colonizes the plant surface by producing haustorium (ha) into epidermal cells for nutrient uptake and keeps the host cell in survival till the pathogen completes its life cycle by conidiation.
With the advent of high-throughput sequencing technologies, the number of fungal genomes sequenced is increasing rapidly. More than 40 fungal genomes have now been sequenced, of which 12 are plant pathogens: Botrytis cinerea (necrotroph, grey mould of grape, and other host species), Sclerotinia sclerotiorum (necrotroph, white mould of many host species), Fusarium graminearum (hemibiotroph, cereal head blight), Fusarium oxysporum (hemibiotroph, wilt diseases of many host species), Fusarium verticillioides (hemibiotroph, seed rot of corn), Magnaporthe oryzae (hemibiotroph, rice blast), Nectria haematococca (hemibiotroph, pea wilt), Mycosphaerella fijiensis (hemibiotroph, black leaf streak of banana), Mycosphaerella graminicola (hemibiotroph, leaf blotch of wheat), Puccinia graminis (biotroph, cereal rust), Stagonospora nodorum (necrotroph, wheat glume blotch), and Ustilago maydis (biotroph, corn smut). In addition, the genomes sequences of two oomycete pathogens have also been sequenced: Phytophthora ramorum (hemibiotroph, sudden oak death) and Phytophthora sojae (hemibiotroph, stem/root rot of soybean) [1].
In contrast to “structural genomics,” where the entire nucleotide sequence of an organism's genome is determined, “functional genomics” encompasses genome-wide experimental approaches to assess gene function by making use of the information and reagents provided by “structural genomics” [2]. With the exponential progress in structural genomics, the major undertaking in fungal/oomycete genomics is now to investigate the function of the large number of genes identified by in silico approaches. Reverse genetics, an approach to discover the function of genes, will contribute greatly in deciphering the molecular mechanisms underlying fungal/oomycete development (sporulation, spore germination, appressorium or infection structure formation, appressorium morphogenesis and penetration), fungal nutrition (uptake of nutrients, e.g., iron, phosphorous from the host milieu) and the interactions between fungi/oomycetes and their host plants (compatible or incompatible interactions). For the longest time, oomycetes were considered to belong to the fungi based on certain morphological similarities. Both fungi and oomycetes are characterized by filamentous vegetative growth, the production of mycelia, and formation of spores through asexual and sexual processes. Similarities also exist in infection structures and the mode of infection. However, cell walls of oomycetes primarily consist of cellulose rather than chitin, which is the main component of true fungal cell walls. Furthermore, hyphae of oomycetes are nonseptate and are diploid in their vegetative form, which distinguishes them from true fungi. Based on morphological differences and molecular evidence, it was found that oomycetes were related to heterokont algae and thus they were reclassified under the stramenopiles [3, 4]. Among the oomycetes, Phytophthora, Pythium, and Peronospora are well-known pathogens of high economic significance. These oomycete phytopathogens are equally destructive as fungal phytopathogens. Unless specified otherwise, fungal and oomycete phytopathogens will be referred to as phytopathogens hereafter in the paper.
Reverse genetics approaches have been extensively employed to unveil infection strategies of necrotrophic and hemibiotrophic phytopathogens at the molecular level. Reverse genetics cannot be applied to obligate biotrophs because suitable methods for the molecular analyses, such as in vitro cultivation, transformation, and gene disruption methods, are lacking. This has hindered the identification of corresponding genes in these species.
This paper provides an overview of reverse genetics approaches, such as targeted gene disruption/replacement (knock-out), gene silencing (knock-down), insertional mutagenesis, and targeting induced local lesions in genomes (TILLING), which can be applied to phytopathogens in order to determine gene function.
2. Reverse Genetics Approaches
2.1. Targeted Gene Disruption/Replacement (Knock-Out)
One of the most powerful approaches for dissecting gene function in phytopathogens is the study of the phenotypes of mutants in which a genomic locus has been altered by insertion (gene disruption) or replacement (gene replacement) with heterologous DNA [5–7]. Such reverse genetic approaches have become even more straightforward since increasing amounts of genomic sequence information have become available for a rising number of phytopathogen species.
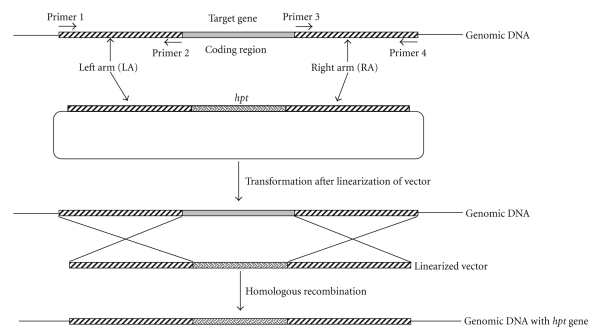
The targeted integration of DNA constructs by homologous recombination enables inactivation of genes by disruption, deletion, or replacement. Homologous recombination involves a reciprocal exchange of DNA sequences as found between two chromosomes that carry the same genetic loci. Homologous recombination between a target gene and the introduced DNA carrying its mutant allele results in targeted gene knock-out. This approach was first pioneered for the budding yeast Saccharomyces cerevisiae to decipher gene function [8]. Since then, it has been applied to several phytopathogens. Four types of gene knock-out approaches are used most commonly to elucidate the function of genes: simple disruption of the gene of interest by a resistance cassette; alteration of the expression of the candidate gene rather than its deletion; over-expression or miss-expression of a gene; gene replacement, which combines the second and third approaches. A targeted gene replacement strategy is shown in Figure 2.
Figure 2.
Schematic representation of a gene replacement strategy. The 5′- and 3′- flanking regions (left and right arms) of the target gene are PCR amplified from genomic DNA of wild-type strain and ligated into vector containing the hygromycin phosphotransferase (hpt) gene. This vector is then linearized using restriction enzyme and introduced into the protoplast of the wild-type strain through transformation. Homologous recombination replaces the gene of interest with hpt gene.
Kämper [9] developed a PCR-based technique to generate gene replacement mutants in Ustilago maydis. In this technique, the 5′ and 3′-regions of the target gene are first amplified by PCR, and then ligated directionally to a marker cassette via two distinct SfiI restriction sites, providing the flanking homologies needed for homologous recombination. The ligation product is then used as a template to amplify the replacement construct, which can be used directly for transformation of U. maydis. Brachmann et al. [10] described a versatile reverse genetics approach based on the technique developed by Kämper [9] to generate mutants of U. maydis. They constructed a comprehensive collection of insertion cassettes with the basic structure of an optional reporter gene (green fluorescent protein [gfp] gene), a resistance cassette (hpt gene), and an optional promoter cassette flanked by two SfiI recognition sites for PCR amplified gene fragment insertion. Using this approach, the authors generated two replacement mutants [10]. In both mutants, the endogenous promoter of the pheromone gene mfa1 drives expression of the gfp gene. Simultaneously, expression of the mfa1 open reading frame (ORF) is modulated either by the carbon source-regulated crg1 promoter or the nitrogen source-regulated nar1 promoter. The expression was induced by pheromone, and pheromone expression was only observed when the heterologous promoters were active. Cho et al. [11] developed a method for high-throughput targeted gene disruption for Alternaria brassicicola using linear minimal element (LME) constructs. Alternaria brassicicola causes black spot disease of cultivated Brassicaceae species and has been used frequently as a necrotrophic fungal pathogen for studies with Arabidopsis. To improve targeted gene disruption efficiency as well as to expedite gene disruption construct production, the authors used a short linear construct with minimal elements, an antibiotic resistance selectable marker gene, and a partial target gene. Using LME constructs, targeted gene knock-out efficiency was improved substantially compared with standard plasmid construct.
Targeted gene disruption has been applied to many phytopathogens, including M. oryzae [12–23], Colletotrichum gloeosporioides (causal agent of anthracnose disease) [24, 25], U. maydis [9, 10], A. brassicicola [11], Cochliobolus carbonum (northern corn leaf spot) [26], and F. graminearum [27]. Over the past decade M. oryzae (formerly Magnaporthe grisea) has emerged as a seminal model to elucidate mechanisms underlying fungal development, fungal-plant interactions, and pathogenicity and virulence. Using targeted gene disruption, many genes implicated in virulence and pathogenicity of the rice blast fungus have been characterized. Some of these pathogenicity genes are MPG1 (class I hydrophobin) [12], PMK1 (homologue of yeast Fus3/Kss1 MAP kinase) [13], MAC1 (adenylate cyclase) [14], Mps1 (homologue of yeast Slt7 MAP kinase kinase) [15], ABC1 (ATP-binding cassette 1) [16], GAS1 and GAS1 (homologues of gEgh16 of the powdery mildew fungus) [17], ACE1 (avirulence conferring enzyme 1) [18], CHM1 and MST20 (p21-activated kinases) [19], MMT1 (metallothionein 1) [20], ATG1 (serine and threonine kinase) [21], CUT2 (cutinase 2) [22], DSE1 (defense suppressor 1) [23], and COM1 (conidial morphology 1) [Dr. You-Liang Peng, The MOA Key Laboratory of Molecular Plant Pathology, China Agricultural University, Beijing, China (personal communication)].
Inserting a selectable marker gene like hpt into the gene of interest requires several restrictions, ligation, cloning and subcloning steps, and is time consuming. It makes this classical DNA recombinant technology inefficient for large-scale functional analyses of genes. To circumvent these limitations, Invitrogen has introduced the Gateway in vitro recombination cloning technology. Using this technology, high-throughput cloning of target genes for knock-out and knock-down (discussed in the next section) can now be achieved. The Gateway cloning system exploits the precise, site- specific recombination system utilized by bacteriophage lambda in order to shuttle DNA fragments between entry and destination vectors bearing compatible recombination sites while maintaining the ORF [28]. This technology allows researchers to construct multiple destination vectors for different purposes, such as expression in Escherichia coli or S. cerevisiae, from a single entry vector with inserted gene of interest [29]. Since its inception in functional genomics analyses, GATEWAY technology has been widely used to characterize many genes. Recently, Shafran et al. [28] developed two high throughput functional genomics tools for filamentous fungi based on the GATEWAY technology, namely, pTroya (Knock-down) and Gene Blast (Knock-out). The authors tested the utility of both tools on Colletotrichum gloeosporioides for loss of function analyses.
A major advantage of gene knock-out is its propensity to target a specific genetic region. However, the value of this conventional approach for generating knock-out strains is limited by its poor efficiency for homologous recombination (which varies considerably among phytopathogen species) due to nonhomologous (ectopic) integration of the transforming DNA, and the time required for construction of replacement vectors. Higher recombination efficiencies can be obtained by increasing the length of homologous DNA flanking the transformation marker, albeit this is a tedious process when standard molecular techniques are used for the construction of gene replacement cassettes. The majority of fungi consist of multicellular and/or multinuclear hyphae, and some of them have two or more genetically different nuclei in a common cytoplasm (heterokaryon). These characteristics of fungi make gene targeting complicated and inefficient.
2.2. RNA Interference (Knock-Down)
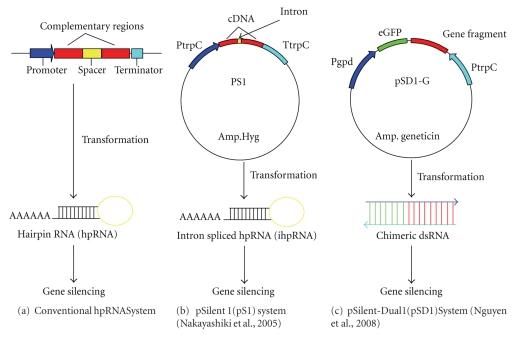
RNA interference-(RNAi-) based gene silencing (post-transcriptional gene silencing in plants and RNAi in animals) is an exciting strategy for reverse genetics [30]. RNAi is a technique in which double-stranded RNA (dsRNA) triggers the degradation of a homologous mRNA, thereby diminishing or abolishing gene expression. It was first discovered in the nematode Caenorhabditis elegans as a response to dsRNA, which resulted in sequence-specific gene silencing [31]. This technique is based on the generation of dsRNA molecules, which act as templates for Dicer ribonuclease III producing short interfering RNAs (siRNAs). These siRNAs are incorporated into the RNA-inducing silencing complex (RISC) and serve as sequence-specific guides that target corresponding mRNA molecules for destruction [32, 33]. RNA-mediated gene silencing methods that block the expression of genes at the post-transcriptional level have been identified in a few economically important phytopathogens like M. oryzae [34] and P. infestans [35–37]. The most common forms involve the introduction of antisense RNA, dsRNA, or sense transgenes (also called co-suppression in plants or quelling in fungi). RNAi is an important tool not only for elucidating the function of the many unknown genes but also for the identification of genes essential for phytopathogenic growth and pathogenesis [38]. The application of RNAi in phytopathogens is limited to a few species. Figure 3 illustrates three RNAi strategies (conventional hairpin RNAi, intron spliced hairpin RNAi, and chimeric double stranded RNA mediated silencing) that have been used in functional analyses of phytopathogens to date.
Figure 3.
RNAi strategies: (a) conventional hpRNAi, transcripts with complementary or near-complementary 20- to 50-base pair inverted repeats form double-stranded RNA (dsRNA) hairpins. These dsRNAs are processed into micro-RNAs that mediate mRNA degradation. (b) pSilent1 (heterogeneous nuclear RNA expressing vector system), it carries a hygromycin resistance cassette and a transcriptional unit for hairpin RNA expression with multiple cloning sites and a spacer of an intron sequence. (c) pSilent-Dual1 system (opposing dual promoter system), trpC and gpdA promoters were cloned in a convergent manner separated by multicloning site. For co-silencing, a 0.41 kb eGFP fragment was first inserted in pSD1, resulting in pSD1-G. Gene fragments of ~500 bp can be inserted into pSD1 vector, which will express corresponding chimeric double-stranded RNA, a template for homology-dependent degradation of the target mRNA.
Kadotani and colleagues [34] carried out a systematic analysis of RNA silencing in the blast fungus M. oryzae using expression of the enhanced green fluorescence protein (eGFP) gene as a reporter. They found that the accumulation of eGFP mRNA was drastically reduced in the silenced transformants. In addition, it was noticed that small interfering RNAs (siRNAs) were present only in the silenced transformants. These results indicated that RNA silencing operated in M. oryzae, which provided a new tool for genome-wide functional analysis of this fungus. Akihiro and colleagues [39] demonstrated the applicability of this targeted gene silencing as a useful reverse-genetics approach in Bipolaris oryzae, causal agent of brown leaf spot disease in rice. A polyketide synthase gene (PKS1) implicated in fungal melanin biosynthesis was targeted by gene silencing as a marker. The silencing vector encoding hairpin RNA (hpRNA) of the PKS1 fragment was constructed and introduced into the B. oryzae genomic DNA (Figure 3(a)). Silencing of the PKS1 gene resulted in reduction of PKS1 mRNA expression and albino phenotypes. More recently, Nguyen and colleagues [40] described a novel high-throughput approach for gene function analysis using RNAi, which provides an alternative to the gene knock-out by homologous recombination. The authors developed an RNA silencing vector, pSilent-Dual1 (pSD1) that carries two convergent dual promoters, the Aspergillus nidulans tryptophan promoter (PtrpC) and the A. nidulans glyceraldehyde-3-phosphate dehydrogenase promoter (Pgpd) (Figure 3(c)). Both promoters have been used to drive constitutive gene expression in a large number of filamentous fungi. A multicloning site (MCS) has been inserted between two promoters. The greatest merit of the pSD1 system over others, such as hpRNA or intron spliced hair-pin RNA (ihpRNA) (Figure 3(b)) silencing system is that it allows a single step cloning for generation of an RNAi construct. To facilitate efficient screening for silenced transformants, Nguyen and colleagues [40] incorporated the gfp gene into pSD1 system. It allows expression of a chimeric RNA and assessment of gene silencing efficiency by utilizing a recipient strain that produces GFP and therefore fluoresces green when using epifluorescence microscopy. A main bottleneck of this system is its lower silencing efficiency compared with hpRNA or ihpRNA-expressing RNA-silencing vectors. Formation of dsRNA in the pSD1 system requires physical annealing of two different RNA molecules in the target cells while that in the hpRNA systems is achieved by self-folding of inverted repeats within RNA molecule. The difference in dsRNA formation between the systems can be a major cause of the different silencing efficiencies. The authors generated a series of knock-down mutants of almost all known calcium related genes in the genome of M. oryzae and examined for phenotypical defects.
Gene knock-down requires relatively short stretches of sequence information. This is a major advantage for phytopathogens for which there is little sequence information available. As RNAi works at the mRNA level, its efficacy is not compromised by the presence of nontransformed nuclei or multicopy genes due to aneuploidy [41]. RNAi causes only a partial reduction in, but not a complete loss of, gene expression. Partial gene suppression is considered a main drawback of RNAi. However, it could be a merit where the effect of an essential gene on a phenotype is of interest. Gene knock-down offers a more convenient and effective tool, especially in combination with an inducible promoter that allows gene expression to be diminished at specific stages during development [42]. Another disadvantage of gene knock-down is that, as it requires only a short sequence, genes other than those targeted might be silenced. This causes unexpected changes in gene expression patterns (off-target effects). Testing for the possibility of off-target effects is simpler for phytopathogen species for which complete genome sequence data are available but remains elusive for those phytopathogens whose genomes have not been sequenced [41].
2.3. Insertional Mutagenesis
Insertional mutagenesis is a powerful tool to dissect the molecular mechanism of most genetically determined processes, including those in phytopathogens. Its main advantage is that it does not require a priori genome information. Therefore, it can be applied to those phytopathogens, whose genomes have not been sequenced yet. Classical genetic analysis approaches using mutagens such as chemicals or ultraviolet light have yielded a wealth of information on pathogen development and pathogenesis. These mutagens normally generate base pair deletions or substitutions, which can result in the loss or an alteration of gene function, and their relative lack of specificity allows saturation of a genome with mutations. Subsequent genetic analysis of mutant strains can, however, be time consuming because it usually encompasses isolation of the mutated gene by complementation using a genomic DNA library from the wild-type strain. This partly explains the increasing use of insertional mutagenesis by chromosomal integration of transforming DNA. The presence of a selectable marker in the transforming DNA can be used to establish linkage between the insertion and the observed phenotype, and to recover DNA representing the mutated allele for cloning and subsequent analyses [43].
Biocomputational analyses of sequenced genomes have extracted only a handful of genes. At this point, the challenge is to convert the available plethora of genomic sequences into meaningful biological information, which will require the large-scale construction of mutant libraries. Therefore, insertional mutagenesis techniques like Agrobacterium tumefaciens-mediated transformation (ATMT) and restriction enzyme mediated integration (REMI) have been developed.
Insertional mutagenesis by ATMT has been well established in the recent years. In the filamentous fungi, transformation with DNA that does not exhibit homology with the fungal genome results in heterologous integration of transforming DNA into the genome, which makes it possible to use the transforming DNA as an insertional mutagen to disrupt genes, and eventually assist in the study of plant disease [44]. Agrobacterium tumefaciens is a plant pathogenic bacterium capable of causing crown gall tumors on plants by transferring a part of its DNA (Transfer DNA; T-DNA), located on the tumor inducing plasmid, through a type IV secretion system to the hosts. Once inside the host, the T-DNA is targeted to the nucleus where it randomly integrates into the host genome. A full description of ATMT is beyond the scope of this paper, and for detailed description on ATMT, the reader is referred to Michielse et al. [45, 46]. ATMT has been widely exploited for transforming a number of phytopathogen species, such as Botrytis cinerea [47], Colletotrichum spp. (C. gloeosporiodes, C. lagenarium and C. trifolii) [48–50], Fusarium spp. (F. circinatum [51] and F. oxysporum [52]), Leptosphaeria spp. (L. maculans and L. biglobosa) [53], M. oryzae [52, 54, 55], Mycosphaerella graminicola [56], Venturia inaequalis [57], Pythium ultimum [58], and Phytophthora spp. (P. infestans and P. palmivora) [58]. Recently, Jeon and colleagues [55] carried out large-scale insertional mutagenesis of the M. oryzae strain KJ201 via ATMT to identify pathogenicity genes. They obtained 21,070 hygromycin-resistant mutants, which were tagged with T-DNA. Over 80% of the mutants were estimated to have a single copy of the T-DNA integrated into the genome. These mutants were then screened to detect disruption of seven phenotypic characters, such as fungal growth, pigmentation, conidiation, conidial morphology, conidial germination, appressorium formation, and pathogenicity. ATMT has been shown to have several advantages over conventional transformation methods like CaCl2/PEG-mediated transformation, lithium acetate-mediated transformation, particle bombardment, and electroporation. The principal advantage of ATMT over conventional transformation techniques is its versatility in choosing which starting material to transform [44]. Intact cells, such as conidia and mycelia, can be used as starting material, thereby eliminating the need to generate protoplasts. ATMT results in higher transformation efficiencies when compared with the above-mentioned transformation methods. T-DNA is an efficient substrate for homologous recombination, leading to relatively high gene knock-out frequencies. Another key advantage is that it generates a high percentage of transformants with a single copy insert of DNA, which facilitates the isolation of tagged genes [45]. These merits make ATMT a valuable tool to perform global or systematic mutational analyses in phytopathogens, either by targeted or insertional mutagenesis. ATMT is less suitable for generating strains for high protein production, due to largely single-copy T-DNA integration, whereas multiple gene copies are usually required for higher expression levels [59, 60].
The second insertional mutagenesis technique, REMI, can be used to generate random and targeted insertional mutations in phytopathogens. In REMI mutagenesis, linearized plasmid DNA is integrated into fungal protoplast in the presence of a restriction enzyme used to linearize the vector. The restriction enzyme targets the nucleus and induces double stranded breaks in the genome. As a result, plasmid integration takes place at the corresponding restriction sites in the genome, by recombining the ends of these breakages with the linearized plasmid [61]. The REMI technique was originally developed for S. cerevisiae [62]. Although REMI is an efficient tool for tagging and cloning pathogenicity genes from phytotopathogens, a substantial portion (20 to 100%) of generated mutants appears to be untagged by the transforming DNA. In spite of this, REMI has remained a powerful genetic tool for the past 15 years and has been used to mutagenize and tag genes in several phytopathogen species, including Cochliobolus heterostrophus [63], M. oryzae [64–66], U. maydis [67], Colletotrichum spp. (C. lindemuthianum and C. graminicola) [68], and Pyrenophora teres [69].
The biggest merit of this technique can be a significantly higher percentage of single-copy integration. However, REMI has some limitations. It requires fungal protoplast preparation, which is time consuming and laborious. Yield and viability of protoplasts are dependent on enzyme batches used to digest fungal cell walls and their ability to digest cell walls from different phytopathogens [70, 71]. REMI can also generate a significant number of different integration events, including single insertion with deletion of flanking restriction sites, nonhomologous integration in the absence of an appropriate restriction site, tandem insertion, and large genome deletions or inversions [72–77].
2.4. Tilling
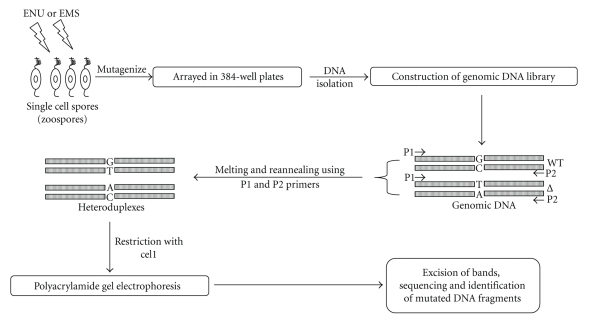
The genome sequence drafts of the five oomycetes Phytophthora sojae, P. ramorum, P. infestans, and P. capsici, and Hyaloperonospora parasitica have been completed [78]. The next major undertaking is now to transcribe this genome information into biological function. McCallum and colleagues [79] introduced a new reverse genetic strategy for plants known as Targeting Induced Local Lesions In Genomes (TILLING) that combines the efficiency of ethyl methanesulfonate-(EMS-) induced mutagenesis (chemical mutagenesis) with the ability of denaturing high-performance liquid chromatography (DHPLC) to detect base pair changes (G/C to A/T transition) by heteroduplex analysis. TILLING has emerged as an influential tool for functional genomics of phytopathogens. Lamour and colleagues [80] employed TILLING to isolate gene-specific mutants in Phytophthora spp. They constructed a library of 2400 ethylnitrosourea (ENU) mutants of P. sojae and screened for induced point mutations in the genes encoding a necrosis-inducing protein (PsojNIP) and a Phytophthora-specific phospholipase D (PsPXTMPLD). Homozygous mutants carrying a potentially deleterious missense mutation in PsojNIP and a premature stop codon in PsPXTM-PLD were identified. No phenotypical changes were observed in PsojNIP mutants; however PsPXTM-PLD mutants showed reduced mycelial growth. Figure 4 shows an illustrative TILLING strategy. Single cell uninucleated spores like zoospores of Phytophthora spp. are ideal for TILLING mutagenesis. These spores are mutated by ENU or EMS and then arrayed into 384-well plates. A genomic DNA library is constructed using DNA extracted from individual mutant colonies followed by PCR amplification using sequence specific forward (P1) and reverse (P2) primers. Purified PCR products are then heated and cooled down to form heteroduplexes between wild type (WT) and mutant (Δ) DNA strands. The heteroduplexes are restricted with the single strand specific endonuclease Cel1, which cuts 3′ ends of single base mismatches producing novel DNA fragments. These fragments are resolved on a gel by polyacrylamide gel electrophoresis (PAGE). Fragment bands are then excised from the gel, purified and sequenced to identify mutant colonies carrying an induced point mutation. Confirmed mutants are characterized to determine the impact of mutation on the phenotype.
Figure 4.
An illustrative TILLING strategy for phytopathogens. TILLING process encompasses the development and screening of a chemically mutagenized population from single cell uninucleated spores like zoospores to isolate individuals carrying induced mutations within the target gene.
The main disadvantage of insertional mutagenesis is a relatively low mutation rate, which makes it difficult to tag a specific gene [81]. Unlike insertional mutagenesis, chemical mutagenesis like EMS treatment brings about a high mutation frequency without apparent preferences for specific genomic regions. This method can also generate many alleles, which facilitates the recovery of null phenotypes [82]. Although the development of novel mutagenesis techniques may eventually make TILLING obsolete, at present, it remains the technique of choice for medium- to high-throughput reverse genetics analyses in many phytopathogens [83].
3. Concluding Remarks
With the recent expansion of phytopathogen genome sequence data banks, locus-to-phenotype or gene-to-phenotype reverse genetic tools, such as knock-out, RNAi, ATMT, REMI, and TILLING, have become increasingly attractive methods to elucidate the molecular basis of host-pathogen interactions (compatible or incompatible), phytopathogen development, and virulence and pathogenicity. These reverse genetics tools can efficiently decode genome information into biological information. In the post-genomic era, gene targeting (knock-out) by homologous recombination has become the most influential reverse genetics tool to identify gene function. However, knock-out remains elusive for those phytopathogens that show low homologous recombination. Furthermore, the multinucleate nature of filamentous phytopathogens represents another challenge for knock-out as well as insertional mutageneses, which rely on the isolation of homokaryotic transformants derived from a single transformation to study loss of function/null mutants. RNAi, which disrupts gene expression by targeting the mRNA rather than the gene, may offer a solution to both problems [41]. However, the application of RNAi in filamentous phytopathogens is still in the developmental stage and does not work efficiently in every phytopathogen. The next challenges in RNAi will be the assessment of the extent of off-target effects in phytopathogens and the development of an inducible RNAi system coupled with a strictly controlled promoter and a convenient inducer that are applicable to a wide range of filamentous phytopathogens [42]. Gene tagging using insertional mutagenesis tools provides high transformation frequency and random insertion as a single copy insert. In this regard, ATMT has a clear advantage over REMI as it shows relatively higher transformation frequency and does not require protoplast preparation. TILLING certainly adds to the arsenal of reverse genetics tools, but it may become obsolete once new mutagenesis techniques have been developed. All the reverse genetics tools described above have their own merits and demerits, and any one of them may be more effective for a particular phytopathogen while less suitable for others. Therefore, a careful planning is required to harness the advantage of reverse genetics tools prior to conduct functional analyses.
Acknowledgments
This work was supported by NSERC-CRD grants to Drs. S. Banniza and Y. Wei, and National Basic Research Program of China (973 Program) grant to Dr. Y. Peng. We extend our thanks to anonymous reviewers of this paper for many valuable comments.
References
- 1.Soanes DM, Richards TA, Talbot NJ. Insights from sequencing fungal and oomycete genomes: what can we learn about plant disease and the evolution of pathogenicity? The Plant Cell. 2007;19(11):3318–3326. doi: 10.1105/tpc.107.056663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hieter P, Boguski M. Functional genomics: it's all how you read it. Science. 1997;278(5338):601–602. doi: 10.1126/science.278.5338.601. [DOI] [PubMed] [Google Scholar]
- 3.Barr DJS. Evolution and kingdoms from the perspective of a mycologist. Mycologia. 1992;84:1–11. [Google Scholar]
- 4.Simpson AGB, Roger AJ. Eukaryotic evolution: getting to the root of the problem. Current Biology. 2002;12(20):R691–R693. doi: 10.1016/s0960-9822(02)01207-1. [DOI] [PubMed] [Google Scholar]
- 5.Timberlake WE, Marshall MA. Genetic engineering of filamentous fungi. Science. 1989;244:1313–1317. doi: 10.1126/science.2525275. [DOI] [PubMed] [Google Scholar]
- 6.Goffeau A. Yeast genes in search of functions. Nature. 1994;369:101–102. doi: 10.1038/369101a0. [DOI] [PubMed] [Google Scholar]
- 7.Wendland J. PCR-based methods facilitate targeted gene manipulations and cloning procedures. Current Genetics. 2003;44:115–123. doi: 10.1007/s00294-003-0436-x. [DOI] [PubMed] [Google Scholar]
- 8.Scherer S, Davis RW. Replacement of chromosome segments with altered DNA sequences constructed in vitro. Proceedings of the National Academy of Sciences of the United States of America. 1979;76(10):4951–4955. doi: 10.1073/pnas.76.10.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kämper J. A PCR-based system for highly efficient generation of gene replacement mutants in Ustilago maydis . Molecular Genetics and Genomics. 2004;271(1):103–110. doi: 10.1007/s00438-003-0962-8. [DOI] [PubMed] [Google Scholar]
- 10.Brachmann A, König J, Julius C, Feldbrügge M. A reverse genetic approach for generating gene replacement mutants in Ustilago maydis . Molecular Genetics and Genomics. 2004;272(2):216–226. doi: 10.1007/s00438-004-1047-z. [DOI] [PubMed] [Google Scholar]
- 11.Cho Y, Davis J, Kim KH, et al. A high throughput targeted gene disruption method for Alternaria brassicicola functional genomics using linear minimal element (LME) constructs. Molecular Plant Microbe Interaction. 2006;19(1):7–15. doi: 10.1094/MPMI-19-0007. [DOI] [PubMed] [Google Scholar]
- 12.Talbot NJ, Ebbole DJ, Hamer JE. Identification and characterization of MPG1, a gene involved in pathogenicity from the rice blast fungus Magnaporthe grisea . The Plant Cell. 1993;5(11):1575–1590. doi: 10.1105/tpc.5.11.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu JR, Hamer JE. MAP kinase and cAMP signaling regulate infection structure formation and pathogenic growth in the rice blast fungus Magnaporthe grisea . Genes & Development. 1996;10(21):2696–2706. doi: 10.1101/gad.10.21.2696. [DOI] [PubMed] [Google Scholar]
- 14.Choi W, Dean RA. The adenylate cyclase gene MAC1 of Magnaporthe grisea controls appressorium formation and other aspects of growth and development. The Plant Cell. 1997;9(11):1973–1983. doi: 10.1105/tpc.9.11.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu JR, Staiger CJ, Hamer JE. Inactivation of the mitogen-activated protein kinase Mps1 from the rice blast fungus prevents penetration of host ceils but allows activation of plant defence responses. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:12713–12718. doi: 10.1073/pnas.95.21.12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urban M, Bhargava T, Hamer JE. An ATP-driven efflux pump is a novel pathogenicity factor in rice blast disease. The EMBO Journal. 1999;18(3):512–521. doi: 10.1093/emboj/18.3.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xue C, Park G, Choi W, Zheng L, Dean RA, Xu J-R. Two novel fungal virulence genes specifically expressed in appressoria of the rice blast fungus. The Plant Cell. 2002;14(9):2107–2119. doi: 10.1105/tpc.003426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Böhnert HU, Fudal I, Dioh W, Tharreau D, Notteghem JL, Lebrun MH. A putative polyketide synthase/peptide synthetase from Magnaporthe grisea signals pathogen attack to resistant rice. The Plant Cell. 2004;16(9):2499–2513. doi: 10.1105/tpc.104.022715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Xue C, Bruno K, Nishimura M, Xu JR. Two PAK kinase genes, CHM1 and MST20, have distinct functions in Magnaporthe grisea . Molecular Plant-Microbe Interactions. 2004;17(5):547–556. doi: 10.1094/MPMI.2004.17.5.547. [DOI] [PubMed] [Google Scholar]
- 20.Tucker SL, Thornton CR, Tasker K, et al. A fungal metallothionein is required for pathogenicity of Magnaporthe grisea . The Plant Cell. 2004;16(6):1575–1588. doi: 10.1105/tpc.021279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X-H, Lu J-P, Zhang L, Dong B, Min H, Lin F-C. Involvement of a Magnaporthe grisea serine/threonine kinase gene, MgATG1, in Appressorium turgor and pathogenesis. Eukaryotic Cell. 2007;6(6):997–1005. doi: 10.1128/EC.00011-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skamnioti P, Gurr SJ. Magnaporthe grisea cutinase2 mediates appressorium differentiation and host penetration and is required for full virulence. The Plant Cell. 2007;19(8):2674–2689. doi: 10.1105/tpc.107.051219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chi M-H, Park S-Y, Kim S, Lee Y-H. A novel pathogenicity gene is required in the rice blast fungus to suppress the basal defenses of the host. PLoS Pathogens. 2009;5(4, article e1000401) doi: 10.1371/journal.ppat.1000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei Y-D, Shen W, Dauk M, Wang F, Selvaraj G, Zou J. Targeted gene disruption of glycerol-3-phosphate dehydrogenase in Colletotrichum gloeosporioides reveals evidence that glycerol is a significant transferred nutrient from host plant to fungal pathogen. The Journal of Biological Chemistry. 2004;279(1):429–435. doi: 10.1074/jbc.M308363200. [DOI] [PubMed] [Google Scholar]
- 25.Stephenson SA, Stephens CM, Maclean DJ, Manners JM. CgDN24: a gene involved in hyphal development in the fungal phytopathogen Colletotrichum gloeosporioides . Microbiological Research. 2005;160(4):389–397. doi: 10.1016/j.micres.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Görlach JM, Knaap EVD, Walton JD. Cloning and targeted disruption of MLG1, a gene encoding two of three extracellular mixed-linked glucanases of Cochliobolus carbonum . Applied and Environmental Microbiology. 1998;64(2):385–391. doi: 10.1128/aem.64.2.385-391.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenczmionka NJ, Schäfer W. The Gpmk1 MAP kinase of Fusarium graminearum regulates the induction of specific secreted enzymes. Current Genetics. 2005;47(1):29–36. doi: 10.1007/s00294-004-0547-z. [DOI] [PubMed] [Google Scholar]
- 28.Shafran H, Miyara I, Eshed R, Prusky D, Sherman A. Development of new tools for studying gene function in fungi based on the Gateway system. Fungal Genetics and Biology. 2008;45(8):1147–1154. doi: 10.1016/j.fgb.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 29.Abe A, Elegado EB, Sone T. Construction of pDESTR, a GATEWAY vector for gene disruption in filamentous fungi. Current Microbiology. 2006;52(3):210–215. doi: 10.1007/s00284-005-0238-0. [DOI] [PubMed] [Google Scholar]
- 30.Waterhouse PM, Graham MW, Wang M. Virus resistance and gene silencing in plants can be induced by simultaneous expression of sense and antisense RNA. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(23):13959–13964. doi: 10.1073/pnas.95.23.13959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans . Nature. 1998;391(6669):806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 32.Hammond SM, Boettcher S, Caudy AA, Kobayashi R, Hannon GJ. Argonaute2, a link between genetic and biochemical analyses of RNAi. Science. 2001;293(5532):1146–1150. doi: 10.1126/science.1064023. [DOI] [PubMed] [Google Scholar]
- 33.Hannon GJ. RNA interference. Nature. 2002;418:244–251. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- 34.Kadotani N, Nakayashiki H, Tosa Y, Mayama S. RNA silencing in the phytopathogenic fungus Magnaporthe oryzae . Molecular Plant-Microbe Interactions. 2003;16(9):769–776. doi: 10.1094/MPMI.2003.16.9.769. [DOI] [PubMed] [Google Scholar]
- 35.van West P, Kamoun S, van't Klooster JW, Govers F. Internuclear gene silencing in Phytophthora infestans . Molecular Cell. 1999;3(3):339–348. doi: 10.1016/s1097-2765(00)80461-x. [DOI] [PubMed] [Google Scholar]
- 36.Latijnhouwers M, Ligterink W, Vleeshouwers VGAA, West P, Govers F. A Galpha subunit controls zoospore motility and virulence in the potato late blight pathogen Phytophthora infestans . Molecular Microbiology. 2004;51:925–936. doi: 10.1046/j.1365-2958.2003.03893.x. [DOI] [PubMed] [Google Scholar]
- 37.Whisson SC, Avrova AO, West PV, Jones JT. A method for double-stranded RNA-mediated transient gene silencing in Phytophthora infestans . Molecular Plant Pathology. 2005;6(2):153–163. doi: 10.1111/j.1364-3703.2005.00272.x. [DOI] [PubMed] [Google Scholar]
- 38.De Backer MD, Raponi M, Arndt GM. RNA-mediated gene silencing in non-pathogenic and pathogenic fungi. Current Opinion in Microbiology. 2002;5(3):323–329. doi: 10.1016/s1369-5274(02)00319-3. [DOI] [PubMed] [Google Scholar]
- 39.Akihiro M, Makoto U, Sakae A, Junichi K. RNA-mediated gene silencing in the phytopathogenic fungus Bipolaris oryzae . FEMS Microbiology Letters. 2007;269(1):85–89. doi: 10.1111/j.1574-6968.2006.00606.x. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen QB, Kadotani N, Kasahara S, Tosa Y, Mayama S, Nakayashiki H. Systematic functional analysis of calcium-signalling proteins in the genome of the rice-blast fungus, Magnaporthe oryzae, using a high-throughput RNA-silencing system. Molecular Microbiology. 2008;68(6):1348–1365. doi: 10.1111/j.1365-2958.2008.06242.x. [DOI] [PubMed] [Google Scholar]
- 41.Weld RJ, Plummer KM, Carpenter MA, Ridgway HJ. Approaches to functional genomics in filamentous fungi. Cell Research. 2006;16(1):31–44. doi: 10.1038/sj.cr.7310006. [DOI] [PubMed] [Google Scholar]
- 42.Nakayashiki H, Nguyen QB. RNA interference: roles in fungal biology. Current Opinion in Microbiology. 2008;11(6):1–9. doi: 10.1016/j.mib.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Brown JS, Holden DW. Insertional mutagenesis of pathogenic fungi. Current Opinion in Microbiology. 1998;1(4):390–394. doi: 10.1016/s1369-5274(98)80054-4. [DOI] [PubMed] [Google Scholar]
- 44.Mullins ED, Chen X, Romaine P, Raina R, Geiser DM, Kang S. Agrobacterium-mediated transformation of Fusarium oxysporum: an efficient tool for insertional mutagenesis and gene transfer. Phytopathology. 2001;91(2):173–180. doi: 10.1094/PHYTO.2001.91.2.173. [DOI] [PubMed] [Google Scholar]
- 45.Michielse CB, Hooykaas PJJ, van den Hondel CA, Ram AFJ. Agrobacterium-mediated transformation as a tool for functional genomics in fungi. Current Genetics. 2005;48(1):1–17. doi: 10.1007/s00294-005-0578-0. [DOI] [PubMed] [Google Scholar]
- 46.Michielse CB, Hooykaas PJ, van den Hondel CA, Ram AF. Agrobacterium-mediated transformation of the filamentous fungus Aspergillus awamori . Nature Protocols. 2008;3(10):1671–1678. doi: 10.1038/nprot.2008.154. [DOI] [PubMed] [Google Scholar]
- 47.Rolland S, Jobic C, Fevre M, Bruel C. Agrobacterium-mediated transformation of Botrytis cinerea, simple purification of monokaryotic transformants and rapid conidia-based identification of the transfer-DNA host genomic DNA flanking sequences. Current Genetics. 2003;44(3):164–171. doi: 10.1007/s00294-003-0438-8. [DOI] [PubMed] [Google Scholar]
- 48.de Groot MJ, Bundock P, Hooykaas PJ, Beijersbergen AG. Agrobacterium-mediated transformation of filamentous fungi. Nature Biotechnology. 1998;16(9):839–842. doi: 10.1038/nbt0998-839. [DOI] [PubMed] [Google Scholar]
- 49.Tsuji G, Fujii S, Hirose N, Tsuge S, Shiraishi T, Kubo Y. Agrobacterium tumefaciens-mediated transformation for random insertional mutagenesis in Colletotrichum lagenarium . Journal of General Plant Pathology. 2003;69:230–239. [Google Scholar]
- 50.Takahara H, Tsuji G, Kubo Y, et al. Agrobacterium tumefaciens-mediated transformation as a tool for random mutagenesis in Colletotrichum trifolii . Journal of General Plant Pathology. 2004;70:93–96. [Google Scholar]
- 51.Covert SF, Kapoor P, Lee M-H, Briley A, Nairn CJ. Agrobacterium tumefaciens-mediated transformation of Fusarium circinatum . Mycological Research. 2001;105(3):259–264. [Google Scholar]
- 52.Khang C-H, Park S-Y, Lee Y-H, Kang S. A dual selection based, targeted disruption tool for Magnaporthe grisea and Fusarium oxysporum . Funagl Genetics and Biology. 2005;42(6):483–492. doi: 10.1016/j.fgb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 53.Eckerta M, Maguirea K, Urbana M, et al. Agrobacterium tumefaciens-mediated transformation of Leptosphaeria spp. and Oculimacula spp. with the reef coral gene DsRed and the jellyfish gene gfp. FEMS Microbiology Letters. 2008;112(3):407–413. doi: 10.1016/j.femsle.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 54.Rho HS, Kang S, Lee Y-H. Agrobacterium tumefaciens-mediated transformation of the plant pathogenic fungus, Magnaporthe grisea. Molecules and cells. 2001;12(3):407–411. [PubMed] [Google Scholar]
- 55.Jeon J, Park SY, Chi MH, et al. Genome-wide functional analysis of pathogenicity genes in the rice blast fungus. Nature Genetics. 2007;39(4):561–565. doi: 10.1038/ng2002. [DOI] [PubMed] [Google Scholar]
- 56.Zwiers LH, de Waard MA. Agrobacterium tumefaciens-mediated gene disruption in the phytopathogen Mycosphaerella graminicola . Current Genetics. 2001;39(5-6):388–393. doi: 10.1007/s002940100216. [DOI] [PubMed] [Google Scholar]
- 57.Fitzgerald AM, Mudge AM, Gleave AP, Plummer KM. Agrobacterium and PEG-mediated transformation of the phytopathogenic fungus Venturia inaequalis . Mycological Research. 2003;107:803–810. doi: 10.1017/s0953756203008086. [DOI] [PubMed] [Google Scholar]
- 58.Vijn I, Govers F. Agrobacterium tumefaciens-mediated transformation of the oomycete plant pathogen Phytophthora infestans . Molecular Plant Pathology. 2003;4(6):459–467. doi: 10.1046/j.1364-3703.2003.00191.x. [DOI] [PubMed] [Google Scholar]
- 59.Gouka RJ, Hooykaas PJJ, Bundock P, Musters W, Verrips CT, de Groot MJA. Transformation of Aspergillus awamori by Agrobacterium tumefaciens-mediated homologous recombination. Nature Biotechnology. 1999;17(6):598–601. doi: 10.1038/9915. [DOI] [PubMed] [Google Scholar]
- 60.Michielse CB, Ram AF, van den Hondel CA. The Aspergillus nidulans amdS gene as a marker for the identification of multicopy T-DNA integration events in Agrobacterium-mediated transformation of Aspergillus awamori . Current Genetics. 2004;45(6):399–403. doi: 10.1007/s00294-004-0500-1. [DOI] [PubMed] [Google Scholar]
- 61.Riggle PJ, Kumamoto CA. Genetic analysis in fungi using restriction-enzyme-mediated integration. Current Opinion in Microbiology. 1998;1(4):395–399. doi: 10.1016/s1369-5274(98)80055-6. [DOI] [PubMed] [Google Scholar]
- 62.Schiestl RH, Petes TD. Integration of DNA fragments by illegitimate recombination in Saccharomyces cerevisiae . Proceedings of the National Academy of Sciences of the United States of America. 1991;88(17):7585–7589. doi: 10.1073/pnas.88.17.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang G, Rose MS, Turgeon BG, Yoder OC. A polyketide synthase is required for fungal virulence and production of the polyketide T-toxin. Plant Physiology. 1996;8(11):2139–2150. doi: 10.1105/tpc.8.11.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shi Z, Christian D, Leung H. Enhanced transformation in Magnaporthe grisea by restriction enzyme mediated integration of plasmid DNA. Phytopathology. 1995;85(3):329–333. [Google Scholar]
- 65.Sweigard JA, Carroll AM, Farrall L, et al. Magnaporthe grisea pathogenicity genes obtained through insertional mutagenesis. Molecular Plant-Microbe Interaction. 1998;11:404–412. doi: 10.1094/MPMI.1998.11.5.404. [DOI] [PubMed] [Google Scholar]
- 66.Mitchell TK, Dean RA. The cAMP-dependent protein kinase catalytic subunit is required for appressorium formation and pathogenesis by the rice blast pathogen Magnaporthe grisea . The Plant Cell. 1995;7(11):1869–1878. doi: 10.1105/tpc.7.11.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bolker M, Böhnert HU, Braun KH, Gorl J, Kahmann R. Tagging pathogenicity genes in Ustilago maydis by restriction enzyme-mediated integration (REMI) Molecular and General Genetics. 1995;248(5):547–552. doi: 10.1007/BF02423450. [DOI] [PubMed] [Google Scholar]
- 68.Redman RS, Rodriguez RJ. Factors affecting the efficient transformation of Colletotrichum species. Experimental Mycology. 1994;18(3):230–246. [Google Scholar]
- 69.Maier FJ, Schafer W. Mutagenesis via insertional or restriction enzyme-mediated-integration (REMI) as a tool to tag pathogenicity related genes in plant pathogenic fungi. Biological Chemistry. 1999;380(7-8):855–864. doi: 10.1515/BC.1999.105. [DOI] [PubMed] [Google Scholar]
- 70.Te'o VS, Bergquist PL, Nevalainen KM. Biolistic transformation of Trichoderma reesei using the Bio-Rad seven barrels Hepta Adaptor system. Journal of Microbiological Methods. 2002;52(3):393–399. doi: 10.1016/s0167-7012(02)00126-4. [DOI] [PubMed] [Google Scholar]
- 71.Cvitanich C, Judelson HS. Stable transformation of the oomycete, Phytophthora infestans, using microprojectile bombardment. Current Genetics. 2003;42(4):228–235. doi: 10.1007/s00294-002-0354-3. [DOI] [PubMed] [Google Scholar]
- 72.Chung KR, Ehrenshaft M, Wetzel DK, Daub ME. Cercosporin-deficient mutants by plasmid tagging in the asexual fungus Cercospora nicotianae . Molecular Genetics and Genomics. 2003;270(2):103–113. doi: 10.1007/s00438-003-0902-7. [DOI] [PubMed] [Google Scholar]
- 73.Brown JS, Aufauvre-Brown A, Holden DW. Insertional mutagenesis of Aspergillus fumigatus . Molecular and General Genetics. 1998;259(3):327–335. doi: 10.1007/s004380050819. [DOI] [PubMed] [Google Scholar]
- 74.Thon MR, Nuckles EM, Vaillancourt LJ. Restriction enzyme mediated integration used to produce pathogenicity mutants of Colletotrichum graminicola . Molecular Plant-Microbe Interaction. 2000;13:1356–1365. doi: 10.1094/MPMI.2000.13.12.1356. [DOI] [PubMed] [Google Scholar]
- 75.Redman RS, Ranson JC, Rodriguez RJ. Conversion of the pathogenic fungus Colletotrichum magna to a nonpathogenic, endophytic mutualist by gene disruption. Molecular Plant-Microbe Interactions. 1999;12(11):969–975. [Google Scholar]
- 76.Rogers CW, Challen MP, Green JR, Whipps JM. Use of REMI and Agrobacterium-mediated transformation to identify pathogenicity mutants of the biocontrol fungus, Coniothyrium minitans . FEMS Microbiology Letters. 2004;241(2):207–214. doi: 10.1016/j.femsle.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 77.Balhadere PV, Foster AJ, Talbot NJ. Identification of pathogenicity mutants of the rice blast fungus Magnaporthe grisea by insertional mutagenesis. Molecular Plant-Microbe Interactions. 1999;12(2):129–142. [Google Scholar]
- 78.Tyler BM, Tripathy S, Zhang X, et al. Phytophthora genome sequences uncover evolutionary origins and mechanisms of pathogenesis. Science. 2006;313:1261–1266. doi: 10.1126/science.1128796. [DOI] [PubMed] [Google Scholar]
- 79.McCallum CM, Comai L, Greene EA, Henikoff S. Targeting induced local lesions IN genomes (TILLING) for plant functional genomics. Plant Physiology. 2000;123(2):439–442. doi: 10.1104/pp.123.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lamour KH, Finley L, Hurtado-Gonzales O, Gobena D, Tierney M, Meijer HJG. Targeted gene mutation in Phytophthora spp. Molecular Plant-Microbe Interactions. 2006;19(12):1359–1367. doi: 10.1094/MPMI-19-1359. [DOI] [PubMed] [Google Scholar]
- 81.Lindsay DS, Lenz SD, Cole RA, Dubey JP, Blagburn BL. Mouse model for central nervous system Neospora caninum infections. Journal of Parasitology. 1995;81(2):313–315. [PubMed] [Google Scholar]
- 82.Meinke DW. Molecular genetics of plant embryogenesis. Annual Review of Plant Physiology and Plant Molecular Biology. 1995;46:369–394. [Google Scholar]
- 83.Gilchrist EJ, Haughn GW. TILLING without a plough: a new method with applications for reverse genetics. Current Opinion in Plant Biology. 2005;8(2):211–215. doi: 10.1016/j.pbi.2005.01.004. [DOI] [PubMed] [Google Scholar]