Abstract
Carbonic anhydrase IX (CAIX) is frequently expressed in human tumors and serves as a marker for hypoxia. Further, CAIX expression is considered a predictor of poor survival in many, but not all, cancer types. Herein, we compare the specificity of two CAIX antibodies: the M75, monoclonal antibody which recognizes an epitope in the N-terminus and a commercially available polyclonal antibody generated against a C-terminal peptide (NB100-417). Western blot analysis of multiple breast cell lines revealed that the polyclonal antibody detected both membrane-bound and soluble proteins. The M75 antibody recognized only the membrane-bound species, which is presumed to be CAIX. These data were confirmed in an aggressive prostate cell line. We further compared these antibodies in prostate tumors by immunohistochemistry. Staining with NB100 was comparable to that of the M75 antibody, but only at high dilution. Otherwise, cytoplasmic staining was also noted. Two-dimensional gel electrophoresis followed by mass spectrometric analysis revealed that the cytoplasmic protein detected by NB100 is β-tubulin. This cross-reactivity could lead to false positives for CAIX expression in samples where cytosolic proteins are present.
Keywords: CAIX, β-tubulin, antibody-specificity, breast cancer cells, prostate cancer cells, hypoxia
Introduction
Carbonic anhydrase IX (CAIX) is a membrane-bound form of the carbonic anhydrase (CA) family of zinc metalloenzymes that catalyzes the reversible conversion between carbon dioxide and bicarbonate. The CA family members participates in the regulation of pH, CO2 and HCO3 transport, and water and electrolyte balance [1].
Expression of CAIX is associated with tumor cell hypoxia in a variety of human tumors [2], including breast [3; 4] and urologic cancers [5-7]. CAIX is a membrane glycoprotein in which the catalytic domain, along with a unique N-terminal, proteoglycan domain, faces the extracellular milieu [8]. CAIX is upregulated by hypoxia [9] and its gene is a target of hypoxia-inducible factor-1 (HIF1α) [10]. One of the striking features of cancer cells is their ability to acidify their environment and the orientation of CAIX suggests that it may serve as one of the mechanisms by which cancer cells regulate extracellular pH and induce cytoplasmic alkalinization [11]. Multiple studies have shown that the expression of CAIX in breast tumors, as well as other solid tumors, is associated with poor prognosis [3; 4; 12; 13] Thus, CAIX is being used clinically as a diagnostic tool which has implications for therapy and patient outcome. This demands the most careful analysis of CAIX expression as it may directly impact patient care.
In the 1980’s, Oosterwijk et al. generated a monoclonal antibody (G 250) against a cell surface protein expressed by renal carcinoma cells [6]. Using molecular cloning, this antibody was shown to bind to CAIX [7]. Later, Pastorekova et al. developed a monoclonal antibody against a 54/58 kDa protein called MN expressed endogenously in a human mammary tumor cell line [14]. This antibody was also shown to target CAIX [15]. The specific epitope for the G250 antibody is unknown, but it has excellent specificity for CAIX in immunohistochemical analysis. The M75 (considered the gold standard for CAIX identification) recognizes the extracellular proteoglycan-like domain and is useful for western blotting, immunoprecipitation, and immunohistochemistry. CAIX antibodies are now available commercially. One of the first companies to offer this product was Novus Biologicals (Littleton, CO). Their polyclonal antibody was generated against a peptide in the C-terminus, a domain which faces the cytoplasmic compartment. R&D Systems (Minneapolis, MN) also has a number of monoclonal and polyclonal antibodies available. In this paper, we compare the specificity of the polyclonal antibody from Novus Biologicals (NB100-417) with that of the monoclonal antibody, M75. In three different breast cell lines and a prostate cell line, our data show that NB100 recognizes a protein(s) not detected by M75. We identified the major “non-specific” protein as the cytoskeletal protein, β-tubulin, which is not sensitive to hypoxia. We also analyzed prostate xenograft tumor tissue by immunohistochemical analysis and found both membrane and cytoplasmic staining with NB100, although at high dilution it has specificity similar to the M75 antibody. Together, these data suggest that identification of CAIX using NB100 could lead to false-positives in research samples but more importantly in human tissue. This argues for caution with diagnostic samples.
2. Material and Methods
2.1. Cell lines and cell culture
The MDA-MB-231 cell line (Kevin Brown, University of Florida) was plated at a density of 1,000 cells/cm2 DMEM (Gibco, 12100-061) containing 10% FBS (Valley Biomedical, #BS3033). The T47D line (Keith Robertson, University of Florida) was plated at a density of 2,000 cells/cm2 McCoy’s medium (Gibco, #16600) containing 10% FBS and 0.2 units/mL bovine insulin (Elanco, #4020). The MCF10A line was purchased from ATCC and plated at a density of 2,000 cells/cm2 in Mammary Epithelial Basal medium (Cambrex Bioscience, #CC3151 ) supplemented with 0.1 ug/mL cholera toxin (Calbiochem, #227035). PC-3 human prostate cancer cells were obtained from ATCC. The cells were maintained in Nutrient Mixture F-12 Ham (Sigma-Aldrich, St. Louis MO) supplemented with 2 mM L-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, and 10% fetal bovine serum (Hyclone, Logan UT). Normoxic and desferoxamine (DFO)- treated cells were incubated in a humidified atmosphere at 37°C in 5% CO2. Hypoxic conditions (1% O2, 5% CO2, and balance N2) were maintained in humidified Modulator Incubator Chambers (MIC-101, Billups-Rothenberg, Inc).
2.2 Mouse xenograft tumors
Male athymic nu/nu mice (6-8 wks old) (Harlan, Indianapolis, IN) were maintained under specific pathogen-free conditions in facilities approved by the AAALAC and in accordance with current standards of the U.S. Department of Agriculture, U.S. Department of Health and Human Services, and the National Institutes of Health. Mouse experiments were performed with approval from the Institutional Animal Care and Use Committee of the University of Florida. For implantation of PC-3 cancer cells, 3×106 cells suspended in 0.1 ml buffered Matrigel (1mg/ml) were injected subcutaneously in the right lower flank area. Tumor length (L) and width (W) were recorded twice weekly and tumor volumes calculated according to the formula L × (W)2 × 0.523. At 7 weeks, the tumors were resected and fixed in 10% buffered formalin for 24 hours.
2.3 Immunohistochemistry
Immunohistochemical staining on paraffin-embedded samples was performed using the mouse monoclonal M-75 antibody (Siemens Medical Diagnostic) against CAIX or a rabbit polyclonal against CAIX (Novus Biologicals, NB100-417). For both antibodies, tissue sections were deparaffinized with xylene and graded ethanol, then endogenous peroxidase was blocked using 3% hydrogen peroxide in methanol for 30 min. Antigen retrieval was performed by incubating slides in 10 mM citric acid buffer pH 6.0 for 15 min at 95°C.
For NB100, sections were blocked for 5 hours with blocking buffer (PBS with 1% BSA, 1% goat serum and 1.5% horse serum). Sections were incubated overnight at room temperature with primary antibody (1:1000, 1:3000 or 1:5000 in blocking buffer + 0.02% Tween 20). The next day, sections were incubated in biotinylated anti-rabbit IgG (Vector Laboratories, Burlingame CA, 1:400) for 3 hours at room temperature, followed by avidin-biotin complex (Vectastain Elite ABC Kit; Vector Labs) and DAB substrate (Vector Labs).
For the M75 mouse monoclonal antibody, the M.O.M.™ Kit (Vector Labs) was used to reduce background staining related to interactions between the mouse monoclonal and mouse cells that might infiltrate the tumor. Briefly, sections were blocked for 1 hour with M.O.M. blocking reagent, washed with PBS and incubated 5 minutes in M.O.M. Protein Concentrate. Sections were then incubated for 30 minutes in primary antibody (1:5000 in Protein Concentrate), washed with PBS, and incubated for 10 minutes in M.O.M. Biotinylated Anti-Mouse IgG Reagent, followed by avidin-biotin complex (Vectastain Elite ABC Kit; Vector Labs) and DAB substrate (Vector Labs).
All slides were counterstained with Gill’s hematoxylin, dehydrated and coverslips mounted with Permount. Stained slides were photographed using a Zeiss Axioplan 2 imaging microscope and Openlab 5.0.3 Beta Improvision® software.
2.4. Cell Lysate Preparation
Lysates were prepared after DFO or hypoxia treatment by cooling cells on ice, washing with ice-cold PBS (10 mM phosphate salts, 120mM NaCl, pH 7.4), and extracting in lysis buffer [1% Triton X-100, 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 0.5 mM sodium orthovanadate, 25 mM NaF, and protease inhibitor (Roche Diagnostics)]. Cell extracts were exposed to centrifugation at 16,300 × g for 15 minutes at 4°C. The cleared supernatants were designated as cell lysates. Protein concentration was determined using a modification of the Lowry assay [16].
2.5. Cytoplasmic and Total Membrane Isolation
Subconfluent, MDA-MB-231 cells were washed 3 times with 5 mL Krebs Ringer Phosphate (KRP) buffer (pH 7.4) at 37°C and incubated for 10 min. Cells were then homogenized in a Tris-based buffer (TESp) containing 20 mM Tris-HCL (pH7.4), 255 mM sucrose, 1 mM EDTA and protease inhibitor (Roche Diagnostics). The cytoplasmic and total membrane fractions were separated by centrifugation at 212,000 × g for 1 hour. The pellet was washed twice and recollected by centrifugation. The final pellet was resuspended in a small volume of TESp.
2.6. Western Blot Analysis
One hundred μg of cell lysates, total membrane, or cytoplasmic fractions were loaded onto 10% polyacrylamide SDS-gels under reducing conditions. Proteins were separated by electrophoresis and transferred to nitrocellulose. After blocking with 5% nonfat milk in TBS, the membranes were probed with a rabbit polyclonal anti CAIX (Novus Biologicals, NB100-417) or the mouse monoclonal antibody, M75 (E. Oosterwijk), followed by horseradish peroxidase-linked secondary antibodies (Sigma) and detection by enhanced chemiluminescence (Amersham).
2.7. Two-dimensional gel electrophoresis
Two-dimensional gel electrophoresis was performed as previously described by Semple-Rowland et al. [17] Briefly, a concentrated cytoplasmic protein fraction in TES, was diluted with an equal volume of isoelectric focusing (IEF) sample solution (6.4% NP-40, 6.5 mM DTT). Proteins (up to 400 μg in 200 μl) were mixed with a 4% acrylamide solution containing 9M urea, 2% NP-40, and 2% carrier ampholytes (pH 3-10, Sigma). The gels were cast in glass tubes (13×3mm). The anodic and cathodic buffers were 10 mM phosphoric acid and 20 mM sodium hydroxide, respectively. Electrophoresis was carried out at 350 V for 18 hours and 800 V for 2 hours. The gels were removed from the tubes and either embedded in agarose on the top of an SDS-PAGE gel for the second dimension. After the second electrophoretic step, the slab gel were either stained with Coomassi blue (20% ethanol, 10% glacial acid, 0.2 % Coomassi blue R-250) or processed for western blotting. The pH gradient from the first dimension was determined by running a tube gel with 200 μl TES:IEF sample solution in place of the protein sample. The gel was cut into 0.5 cm pieces, soaked in 1 mL dH2O for at least 2 hours, and analyzed for pH.
2.8. Mass spectrometric protein identification
The “spots” on the Coomassi-stained gel were aligned with western blots on which proteins were identified by reaction with the NB100 antibody. These spots were excised and treated with trypsin. The peptide fragments were identifed by LC-MS/MS on a hybrid quadrupole-TOF mass spectrometer (QSTAR, Applied Biosystems) at the Interdisciplinary Center for Biotechnology Research at University of Florida. Mass spectra were extracted by ABI Analyst version 1.1. Samples were analyzed by Mascot (Matrix Science, London; version 2.0.01).
3. Results and Discussion
3.1. CAIX expression in breast cancer cell lines in response to hypoxia
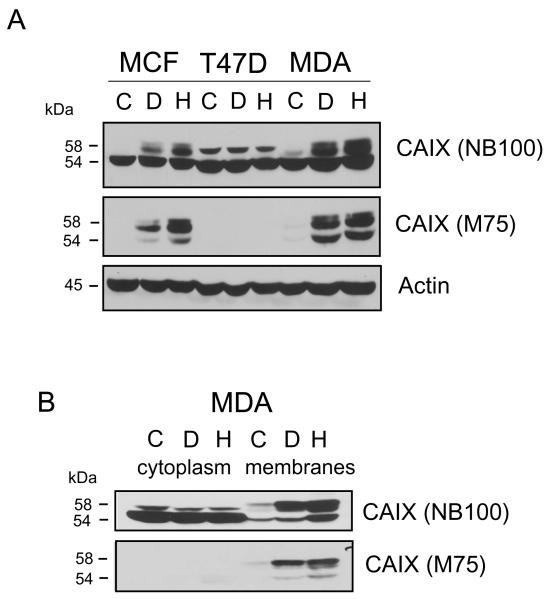
Cells were grown to about 75% subconfluence. Cells were then treated with DFO (to mimic hypoxia) or exposed to 1% oxygen for 16 hours. Cell lysates were analyzed by immunoblotting using two different CAIX antibodies: anti-CAIX antibody from Novus (NB100) or the mouse monoclonal, M75 antibody (a gift from E. Oosterwijk). In western blots using NB100, CAIX was apparently detected in all three breast cell lines (Figure 1A). In the MCF10A non-transformed epithelial breast line, we observed three protein bands. The upper band migrated as a doublet at about 58kDa. The bottom band appeared to be a single protein, migrating at about 54kDa. This appeared to be consistent with the description of CAIX migration from previously published data [15]. However, only the upper doublet appeared to be responsive to DFO and hypoxia. In the T47D cells, only two bands were observed, neither of which showed response to DFO or hypoxia. In the MDA-MB-231 cells, three bands were once again detected. Again, only the upper doublet appeared to show a response to DFO and hypoxia. After stripping, the membrane was reprobed with M75. The difference in the results was striking. In MCF10A cells, no protein was observed in controls, but three bands were detected in response to DFO and hypoxia. No protein was recognized by M75 in T47D cells, under any condition. In MDA-MB-231 cells, there was little M75-reactive protein in controls, but expression was significantly enhanced by DFO treatment or hypoxia. Because of the responsiveness to hypoxia and the high specificity of M75 for CAIX, our data suggest that the NB100 antibody might be interacting with a non-specific protein which overlaps with the migration of CAIX.
Figure 1. Detection of CAIX in breast cell lines using M75 and NB100 antibodies.
Panel A. Subconfluent cells were lysed after exposure to DFO or hypoxia, as described in the methods. Equal protein (100μg) was analyzed by western blot analysis using CAIX antibodies: the M75 monoclonal antibody and NB100, the polyclonal antibody from Novus Biologicals. Actin was used as a loading control. MCF= MCF10A cells, T47D cells, MDA=MDA-MB-231, C=control; D=DFO, H=hypoxia. These data are representative of at least three independent experiments. Panel B. Subconfluent MDA-MB-231 cells were collected and separated into a total membrane and cytoplasmic fraction after exposure or not to DFO and hypoxia. Equal protein (100μg) was analyzed by western blot analysis for CAIX using M75 or NB100. C=control; D=DFO, H=hypoxia. These data are representative of at least three independent experiments.
3.2. Sub-cellular localization of the non-specific protein(s)
CAIX is a transmembrane protein and well recognized as a hypoxia-inducible protein. We have shown previously that MDA-MB-231 cells do not express CAIX in the subconfluent state [18] We felt that the lack of CAIX expression would be an advantage in identifying the non-specific protein(s). So we used subconfluent MDA-MB-231 cells to determine sub-cellular localization of the apparent non-specific protein(s) identified by NB100. Figure 1B shows a western blot of cytoplasmic and membrane proteins identified by NB100 and M75. NB100 recognized a 54 kDa cytoplasmic protein that did not respond to DFO or hypoxia. However, both the 58 kDa doublet, and a 54 kDa protein could be detected in the membrane fraction. All were induced by DFO or hypoxia. On re-probing, the M75 antibody did not recognize any cytoplasmic protein. Proteins identified in the membrane fraction by M75 were clearly similar to those identified by NB100. Taken together, these data suggested that the non-specific protein(s) are localized to the cytoplasmic fraction and migrate at the same molecular weight as does the 54kDa form of membrane-bound CAIX.
3.3 Identification of the non-specific protein(s)
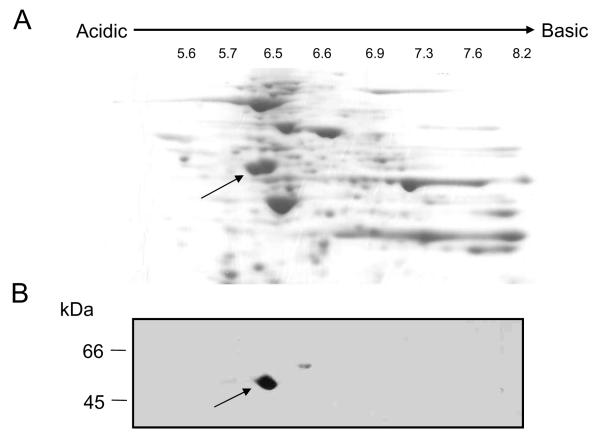
To provide better resolution of the non-specific protein(s), we isolated cytoplasmic proteins from subconfluent MDA-MB-231 cells and separated them using two-dimensional gel electrophoresis (Figure 2A). Immunoblotting using NB100 identified an acidic protein with a pI of about 6.0 (Figure 2B). The corresponding protein in the Coomassie-stained gel was excised, trypsin-treated, and applied to a mass spectrometer to identify the peptide fragments. Twentyseven unique tryptic digest fragments matched the sequence of tubulin and predominantly β—tubulin (Table 1).
Figure 2. Separation of cytoplasmic proteins by two-dimensional electrophoresis.
Panel A: A cytoplasmic fraction from subconfluent MDA cells was separated by two-dimensional electrophoresis. The gel was stained with Coomassi blue. The arrow points to the protein spot recognized by NB100. Panel B: Proteins were transferred to nitrocellulose membranes and immunoblotted with NB100.
Table 1.
Identification of tubulin by mass spectrometry.
| Protein name | Molecular Weight kDa |
Numbers of unique peptides |
|---|---|---|
| TUBB Tubulin beta chain | 50 | 27 |
| TUBA1C Tubulin alpha-1C chain | 50 | 15 |
| TUBB2C Tubulin beta-2C chain | 50 | 5 |
| KRT10 Keratin, type I cytoskeletal | 60 | 2 |
| TUBB3 Tubulin beta-3 chain1 | 50 | 8 |
| TUBB6 46 kDa protein | 46 | 6 |
| EEF1A1 Elongation factor 1-alpha | 50 | 4 |
| KRT1 Keratin, type II cytoskeletal 1 | 66 | 1 |
| HSP90AA1 heat shock protein 90kDa alpha (cytosolic), | 98 | 7 |
| TUBA4A Tubulin alpha-4A chain2 | 50 | 3 |
| RBBP7 Histone-binding protein RBBP7 | 48 | 4 |
| TUBB2A Tubulin beta-2A chain | 50 | 2 |
| TFG Protein TFG | 43 | 3 |
| ATP5B ATP synthase subunit beta, mitochondrial precursor | 57 | 2 |
| HSP90AB1 85 kDa protein | 85 | 2 |
| Putative uncharacterized protein (Fragment) | 17 | 2 |
| PPM1F Protein phosphatase 1F | 50 | 2 |
3.4. Confirmation of identity of non-specific protein by western blotting
To confirm the identity of β-tubulin, the nitrocellulose membrane of the 2D-PAGE gel was stripped and re-probed for β-tubulin expression using an anti-β-tubulin antibody (Sigma). The same spot detected by NB100 was recognized by the β-tubulin antibody (data not shown). The cytoplasmic protein detected by NB100 (Figure 1B), also tested positive when probed with the β-tubulin antibody (data not shown).
Identification of CAIX in PC-3 prostate cancer cells
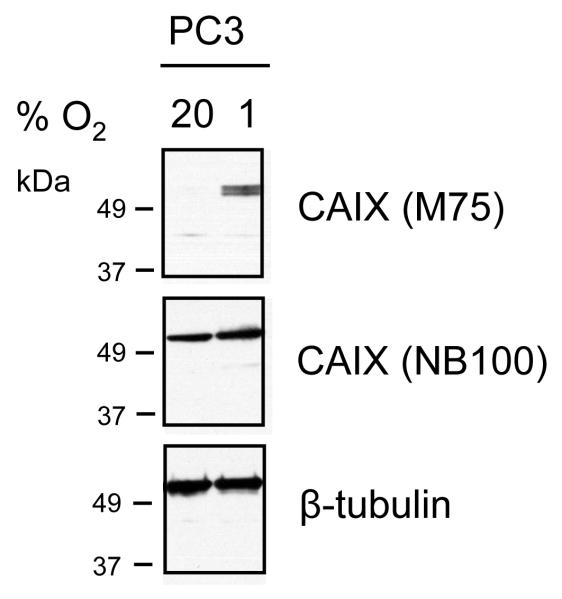
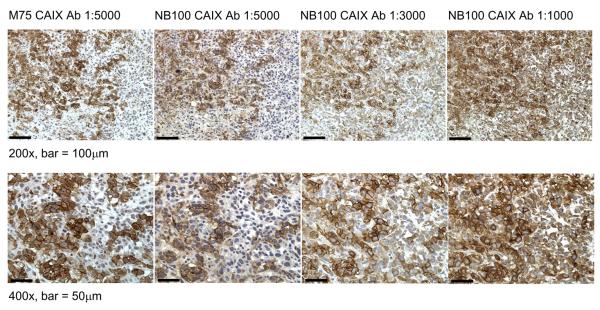
To demonstrate that the results described above may cross cell types, we examined the expression of CAIX in the aggressive prostate cancer cell line, PC-3 (Figure 3). Cells were exposed to normoxic or hypoxic conditions and then lysed for western blot analysis. Detection with M75 showed strong hypoxic induction. Identification with NB100 showed bands of similar intensity in cells from both normoxic and hypoxic conditions. Tubulin migrated similarly to this protein. We also examined the expression of CAIX in xenograft tumors grown from PC-3 cells in mice (Figure 4). Strong membrane staining was observed with the M75 antibody at 1:5000 dilution. NB100 showed both membrane and cytoplasmic staining with NB100 at 1:1000 dilution, the dilution recommended by the manufacturer. At the higher dilution of 1:5000, specificity was similar to that of the M75 antibody. This suggests that background staining with the NB-100 antibody at lower dilutions could be interpreted as a false-positive in human tissue because of non-specific interactions.
Figure 3. Expression of CAIX and tubulin in PC-3 prostate cancer cells.
PC-3 cells were cultured under normoxic or hypoxic conditions (1% O2 for 48 hours). Cell lysates (15ug) were separated on 10% SDS-PAGE gels and transferred to nitrocellulose membranes. Blots were probed with antibodies to tubulin (1:1000) and CAIX (NB100 and M75, both at 1:5000 dilution).
Figure 4. Detection of CAIX in xenograft tumors grown from PC-3 cells in mice.
Mice were injected subcutaneously in the flank with 3×106 PC-3 prostate cancer cells and tumors were resected on Day 39. Immunohistochemical staining of Formalin-fixed, paraffin-embedded sections was performed using antibodies to CAIX (NB100 at 1:1000, 1:3000 and 1:5000 dilutions; M75 at 1:5000 dilution). Antigen retrieval was performed by incubating slides in 10 mM citric acid buffer pH 6.0, as described in Methods. Stained sections were photographed at using a Zeiss Axioplan 2 imaging microscope and Openlab 5.0.3 Beta Improvision® software.
CAIX is normally expressed in a limited number of normal tissues, primarily the epithelial cells of the stomach and intestines [19]. However, CAIX is strongly expressed in numerous tumors, predominantly in carcinomas that are mostly derived from tissues that do not normally express CAIX. Recently, it has been shown that CAIX expression is associated with poor prognosis. Thus, it is crucial in making a diagnostic prediction that an extremely specific anti-CAIX antibody is used in immunohistochemical analysis of human tissue. A recent study has suggested that the NB100 antibody can be used in immunohistochemical analysis in clear cell renal cell carcinoma as a diagnostic and potentially a prognostic marker [20]. However, in our study, we provide evidence that the NB100 anti-CAIX antibody recognizes at least one protein in addition to CAIX. The peptide that was used to make the polyclonal antibody derives from sequence in the C-terminus of CAIX (RGTKGGVSYR). Alignment of this sequence against that of full length human β-tubulin selects a possible match: RGLKMAVTFI. However, it seems unlikely that sufficient identity exists to allow cross-reactivity between the CAIX-specific antibody and β-tubulin. In conclusion, this antibody is useful for membrane preparations and perhaps immunohistochemistry at high dilution, but caution should still be exercised with clinical samples.
Acknowledgments
This study is supported by individual grants from the Thomas H. Maren Foundation [SCF and KTS] and NIH (KTS; CA102386). The authors thank Xiaowei Gu for her excellent work in cell culture.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- [1].Parkkila S. An Overview of the Distribution and Function of Carbonic Anhydrases in Mammals. In: Chegwidden WR, Carter N, Edwards Y, editors. The Carbonic Anhydrases: New Horizons. Birkhauser Verlag; Basel, Switzerland: 2000. pp. 76–93. [Google Scholar]
- [2].Potter C, Harris AL. Hypoxia Inducible Carbonic Anhydrase IX, Marker of Tumor Hypoxia. Survival Pathway and Therapy Target Cell Cycle. 2004;3:164–167. [PubMed] [Google Scholar]
- [3].Chia SK, Wykoff CC, Watson PH, Han C, Leek RD, Pastorek J, Gatter KC, Ratcliffe P, Harris AL. Prognostic Significance of a Novel Hypoxia-Regulated Marker, Carbonic Anhydrase IX, in Invasive Breast Cancer. J.Clin.Oncol. 2005;19:3660–3668. doi: 10.1200/JCO.2001.19.16.3660. [DOI] [PubMed] [Google Scholar]
- [4].Hussain SA, Ganesan R, Reynolds G, Gross L, Stevens A, Pastorek J, Murray PG, Perunovic B, Anwar MS, Billingham L, James ND, Spooner D, Poole CJ, Rea DW, Palmer DH. Hypoxia-regulated Carbonic Anhydrase IX Expression Is Associated with Poor Survival in Patients with Invasive Breast Cancer. Br.J.Cancer. 2007;96:104–109. doi: 10.1038/sj.bjc.6603530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ord JJ, Agrawal S, Thamboo TP, Roberts I, Campo L, Turley H, Han C, Fawcett DW, Kulkarni LP, Cranston D, Harris AL. An Investigation into the Prognostic Significance of Necrosis and Hypoxia in High Grade and Invasive Bladder Cancer. J.Urology. 2007;178:677–682. doi: 10.1016/j.juro.2007.03.112. [DOI] [PubMed] [Google Scholar]
- [6].Oosterwijk E, Ruiter DJ, Hoedemaeker Ph.J., Pauwels EKJ, Jonas U, Zwartendijk I, Warnaar SO. Monclonal Antibody G 250 Recognizes a Determinant Present in Renal-Cell Carcinoma and Absent from Normal Kidney. Int.J.Cancer. 1986;38:489–494. doi: 10.1002/ijc.2910380406. [DOI] [PubMed] [Google Scholar]
- [7].Grabmaier K, Vissers JLM, de Weijert MCA, Oosterwijk-Wakka JC, Bokhoven AV, Brakenhoff RH, Noessner E, Mulders PA, Merkx G, Figdor CG, Adema GJ, Oosterwijk E. Molecular Cloning and Immunogenicity of Renal Cell Carcinoma-Associated Antigen G250. Int.J.Cancer. 2000;85:865–870. doi: 10.1002/(sici)1097-0215(20000315)85:6<865::aid-ijc21>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- [8].Wykoff CC, Beasley NJP, Watson PH, Turner KJ, Pastorek J, Sibtain A, Wilson GD, Turley H, Talks KL, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL. Hypoxia-inducible Expression of Tumor-associated Carbonic Anhydrase. Cancer Res. 2000;60:7075–7083. [PubMed] [Google Scholar]
- [9].Wykoff CC, Beasley N, Watson PH, Campo L, Chia SK, English R, Pastorek J, Sly WS, Ratcliffe P, Harris AL. Expression of Hypoxia-Inducible and Tumor-Associated Carbonic Anhydrases in Ductal Carcinoma in Situ of the Breast. Am.J.Pathol. 2001;158:1011–1019. doi: 10.1016/S0002-9440(10)64048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sowter HM, Raval R, Moore J, Ratcliffe PJ, Harris AL. Predominant Role of Hypoxia-Inducible Transcription Factor (Hif)-1α versus Hif-2α in Regulation of the Transcriptional Response to Hypoxia. Cancer Res. 2003;63:6130–6134. [PubMed] [Google Scholar]
- [11].Chiche J, Ilc K, Laferriere J, Trottier E, Dayan F, Mazure NM, Brahimi-Horn MC, Pouyssegur J. Hypoxia-Inducible Carbonic Anhydrase IX and XII Promote Tumor Cell Growth by Counteracting Acidosis through the Regulation of the Intracellular pH. Cancer Res. 2009;69:358–368. doi: 10.1158/0008-5472.CAN-08-2470. [DOI] [PubMed] [Google Scholar]
- [12].Swinson DEB, Jones JL, Richardson D, Wykoff C, Turley H, Pastorek J, Taub N, Harris AL, O’Bryne KJ. Carbonic Anhydrase IX Expression, a Novel Surrogate Marker of Tumor Hypoxia, Is Associated with a Poor Prognosis in Non-Small-Cell Lung Cancer. J.Clin.Oncol. 2003;21:473–482. doi: 10.1200/JCO.2003.11.132. [DOI] [PubMed] [Google Scholar]
- [13].Trastour C, Benizri E, Ettore F, Ramaioli A, Chamorey E, Pouyssegur J, Berra E. HIF-1α and CA IX Staining in Invasive Breast Carcinomas: Prognosis and Treatment Outcome. Int.J.Cancer. 2007;120:1451–1458. doi: 10.1002/ijc.22436. [DOI] [PubMed] [Google Scholar]
- [14].Pastorekova S, Zavadova Z, Kostal M, Babusikova O, Zavada J. A Novel Quasi-viral Agent, MaTu, is a Two-Component System. 1992. pp. 620–626. Anonymous. [DOI] [PubMed]
- [15].Pastorekova S, Parkkila S, Parkkila A, Opavsky R, Zelnik V, Saarnio J, Pastorek J. Carbonic Anhydrase IX, MN/CAIX: Analysis of Stomach Complementary DNA Sequence and Expression in Human and Rat Alimentary Tracts Gastroenterology. 1997;112:398–408. doi: 10.1053/gast.1997.v112.pm9024293. [DOI] [PubMed] [Google Scholar]
- [16].Markwell MAK, Haas SM, Lieber LL, Tolbert NE. A Modification of the Lowry Procedure to Simplify Protein Determination in Membrane and Lipoprotein Samples. Anal.Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- [17].Semple-Rowland SL, Adamus G, Cohen RJ, Ulshafer RJ. A Reliable Two-dimensional Gel Electrophoresis Procedure for Separating Neural Proteins Electrophoresis. 1991;12:307–312. doi: 10.1002/elps.1150120414. [DOI] [PubMed] [Google Scholar]
- [18].Li Y, Wang H, Oosterwijk E, Tu C, Shiverick KT, Silverman DN, Frost SC. Expression and Activity of Carbonic Anhydrase IX Is Associated with Metabolic Dysfunction in MDA-MB-231. Breast Cancer Cells Cancer Investigation. 2009 doi: 10.1080/07357900802653464. 10.1080/06357900802653464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Saarnio J, Parkkila S, Parkkila A-K, Waheed A, Casey MC, Zhou XY, Pastoreková S, Pastorek J, Karttunen T, Haukipuro K, Kairaluoma MI, Sly WS. Immunohistochemistry of Carbonic Anhydrase Isozyme IX (MN/CA IX) in Human Gut Reveals Polarized Expression in Epithelial Cells with the Highest Proliferative Capacity. J.Histochem.Cytochem. 1998;46:497–504. doi: 10.1177/002215549804600409. [DOI] [PubMed] [Google Scholar]
- [20].Al-Ahmadie HA, Alden D, Qin L, Olgac S, Fine SW, Gopalan A, Russo P, Motzer RJ, Reuter VE, Tickoo SK. Carbonic Anhydrase IX Expression in clear Cell Renal Cell Carcinoma: An Immunohistochemical Study Comparing 2 Antibodies. Am.J.Surg.Pathol. 2008;32:377–382. doi: 10.1097/PAS.0b013e3181570343. [DOI] [PubMed] [Google Scholar]