Abstract
The epithelial cells of multicellular organisms form highly organized tissues specialized for the tasks of protection, secretion and absorption, all of which require tight regulation of the core processes of cell polarity and tissue architecture. Disruption of these core processes is a critical feature of epithelial tumors. Cell polarity and tissue architecture are intimately linked, as proteins controlling cell shape are also responsible for proper localization and assembly of cell-cell junctions and three-dimensional tissue organization. The extracellular matrix underlying epithelial tissues supports tissue architecture and suppresses malignant growth through regulation of cell adhesion and activation of protective signaling cascades. Emerging evidence is uncovering the mechanisms by which polarity pathways alter the way epithelial cells organize and interact with the tissue microenvironment to promote aberrant growth and invasion during tumorigenesis. We discuss how cell polarity pathways regulate cell-cell junctions and highlight the new insights gained by investigating the role played by polarity pathways during transformation of epithelial cells.
Introduction
Normal epithelial cell structure and organization is lost early during tumorigenesis. We are far from developing an understanding of the molecular mechanisms by which cell and tissue structure is lost during carcinogenesis, however, we are beginning to understand how epithelial cells establish structure and undergo morphogenesis during development. Cell-cell adhesions play critical roles during establishment and maintenance of cell structure and tissue organization and hence understanding how they are regulated is likely to provide novel insights into the mechanisms by which cell and tissue structure is lost in carcinoma.
Epithelia in glandular structures contain an apical membrane that faces the lumen and a basolateral surface that interacts with the neighboring cells and the basement membrane. This asymmetric organization is referred to as apical-basal cell polarity and is a characteristic trait of epithelial cells. Cell-cell adhesions are mediated by different types of junctional complexes, including tight junctions (TJ), adherens junctions (AJ), gap junctions and desmosomes. These junctions are comprised of transmembrane proteins with extracellular domains that mediate interactions between neighboring cells and intracellular surfaces that facilitate interaction with signaling molecules and cytoskeletal proteins. In polarized epithelial cells, the junctional complexes are asymmetrically localized. For example, TJ are located at the apical-basal border and act to separate the apical and basolateral membrane domains, hold adjacent cells together and create an impermeant fluid barrier between cells [1]. Adherens junctions are located basal to the tight junctions and are considered as primary determinants of cell-cell adhesion [2]. The mechanisms by which cells develop cell-cell junctions and localize proteins to create the intracellular asymmetry are an active area of investigation. Most of our understanding of the molecular mechanisms by which cell polarity is established and maintained stems from genetic studies in model organisms and biochemical studies in cultured epithelial cells. This review will focus on how cell polarity pathways regulate the establishment and maintenance of cell-cell junctions and highlight the new insights gained on initiation and progression of carcinoma by investigating the role played by polarity pathways during transformation of epithelial cells.
Cell junctions and polarity pathways
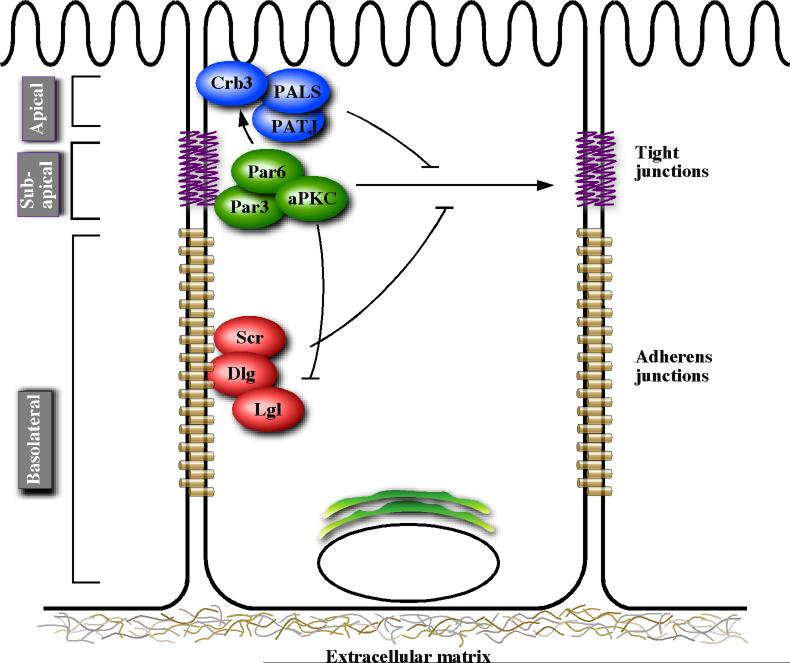
The spatially asymmetric localization of these junctional complexes are mediated by an evolutionarily conserved class of proteins that are herein referred to as polarity proteins [3]. Functional analysis of the polarity determinants in a broad array of model organisms has resulted in their placement into three functional groups: the Crumbs complex, the Scribble complex and the Par complex. The apical domain is specified by the Crumbs complex, which is made up of the transmembrane protein Crumbs (Crb) and intracellular signaling adaptors PALS1 (Protein associated with lin-7) and PATJ (PALS1-associated tight junction protein) [4]. The basolateral domain is thought to be specified by the Scribble complex, consisting of signaling adaptors Scribble (Scr), Discs large (Dlg) and Lethal giant larvae (Lgl) [5]. The sub-apical domain that defines the apical-basal border is specified by the Par (Partitioning defective) complex, which consists of Par3, Par6, atypical protein kinase C (aPKC) and Cdc42 [6]. These protein complexes cross regulate each other during epithelial polarization. For example, in addition to its role in regulating tight junctions formation, Par6 interacts with the Crumbs complex [7] and aPKC negatively regulates Lgl [8-10] (Figure 1).
Figure 1.
A simplified view of polarity protein complexes. The figure depicts subcellular localization of polarity proteins along the apical-basal axis of polarized epithelia and positive (arrows) and negative (blunt head) interactions between the polarity complexes.
Cell polarity proteins regulate tight junctions
Tight junctions are composed of transmembrane proteins such as occludins and claudins and intracellular proteins such as Zonula occludens 1 (ZO-1) that coalesce apical to adherens junctions and seal the spaces between neighboring epithelial cells, separate apical and basal membrane domains, and interact with the cytoskeletal network [1]. The Par3/Par6/aPKC complex localizes to mammalian TJs and is required for TJ assembly and maintenance [11,12]. Overexpression of Par6 in Madin-Darby canine kidney (MDCK) epithelial cells disrupts the localization of Par3 at cell-cell adhesion sites and alters TJ structure as measured by mislocalization of the TJ marker ZO-1 (zona occludens-1) [12]. Par3 mediates it's effects on TJ formation through a direct interaction with junctional adhesion molecule (JAM), a TJ component that associates with ZO-1 [13]. Par3 localizes to the apical region of TJs and overexpression of Par3 in MDCK cells leads to rapid onset of transepithelial electrical resistance (TER), a hallmark of TJ function [14]. In addition, loss of Par3 disrupts TJ formation, and rescue experiments provided evidence that Par3 coordinates TJ assembly through its C-terminus, independently of interaction with Par6, JAM and aPKC [15]. However, inhibition of aPKC activity, through the expression of a dominant negative aPKC mutant, leads to aberrant redistribution of Par3 and ZO-1 during TJ assembly [16], suggesting that aPKC is required for TJ formation.
The nature and identity of targets of the Par complex that are required for TJ assembly are only beginning to be understood. The C-terminal region of Par3 binds directly to the Rac guanine nucleotide exchange factor (GEF), Tiam1. Loss of Tiam1 alone disrupts TJ formation in epidermal keratinocytes [17]. While Tiam1 is not required for development of primordial junction complexes, it is required for maturation of tight junctions in a Rac1 activation dependent manner [17]. It is likely that Par3 localizes Tiam1 to sites of developing cell-cell adhesions and activates Rac to promote TJ maturation [15]. Par3 also regulates actin dynamics at the TJ through inhibition of LIM kinase 2 (LIMK2) activity, which leads to activation of cofilin and promotion of TJ formation [18].
Phosphorylation of Par complex components plays critical roles in the regulation of TJ maintenance. Activation of epidermal growth factor receptor (EGFR) signaling leads to phosphorylation of Par3 by c-Src and c-Yes and subsequent dissociation of the Par3/LIMK2 interaction [19]. The protein phosphatase PP2A accumulates at TJs, binds to aPKC and inhibits aPKC activity, leading to impaired TJ assembly [20]. Similarly, the protein phosphatase PP1 associates with Par3 and regulates the Par3/aPKC interaction [21]. These results underscore the possibility that signaling pathways that alter TJ dynamics interact with and post transcriptionally modify the Par polarity proteins.
Par complex also interacts with other polarity complexes. Expression of Crb3 in a human mammary epithelial cell line, MCF-10A, which lacks TJs, induces de novo TJ formation, highlighting the importance of this complex in TJ assembly [22]. Crb3 can either interact directly with Par6 or indirectly through PALS1 [7,23]. Expression of a dominant negative PATJ in MDCK cells led to redistribution of PALS1 and aPKC away from TJs [7]. Par complex proteins also interact with the basolateral polarity proteins. For example, aPKC phosphorylates and inactivates Lgl [8-10] and Lgl is required for the disassembly of Par3-Par6-aPKC complex in remodeling epithelia [24]. Thus, polarity proteins form an intricate signaling system within epithelial cells to assemble and maintain TJ integrity.
Cell polarity proteins regulate adherens junctions
The role polarity proteins play during AJ biogenesis and function is only begining to be understood. Several polarity proteins have been implicated in AJ formation and maintenance through regulation of E-cadherin. The basolateral polarity protein Scr is recruited to AJs in an E-cadherin-mediated manner, and loss of E-cadherin in human colorectal adenocarcinoma Caco-2 cells results in Scr mislocalization [25]. Loss of Scr decreased cell adhesion in a cell aggregation assay, although no changes were observed in levels of cellular E-cadherin. However, cells lacking Scr were deficient in binding to tissue culture plates coated with the extracellular domain of E-cadherin, suggesting a role for Scr in E-cadherin-mediated cell-cell adhesion. There has been no evidence of a direct interaction between Scribble and the E-cadherin complex, so it is likely that Scribble regulates E-cadherin in an indirect manner. Similarly, Dlg co-localizes with E-cadherin and knockdown of Dlg in Caco-2 cells inhibited the localization of E-cadherin and F-actin at cell junctions [26]. At the AJ, Dlg binds directly to PI3K (phosphatidylinositol 3-kinase) and is required for E-cadherin-mediated signaling. Finally, loss of PALS1 in MDCK cells disrupts AJ formation and E-cadherin localization [27]. In PALS1-deficient cells E-cadherin is retained in intracellular puncta suggesting a role for PALS1 in E-cadherin exocytosis to the plasma membrane. Thus, analysis of cell polarity proteins are providing novel insights into the mechanisms by which AJ are formed and maintained.
Cell polarity, epithelial morphogenesis and cancer initiation
In addition to regulating cell junctions, cell polarity pathways also play important roles during morphogenesis of epithelial cells. Disruption of polarity, by overexpression or loss of polarity proteins, induces defective morphogenesis. When plated on a bed of extracellular matrix, the MDCK and MCF-10A cells undergo a programmed morphogenetic process that results in formation of three dimensionally organized glandular structures composed of polarized epithelial cells surrounding a central lumen. These model systems have been used extensively to study the role played by polarity proteins during three-dimensional morphogenesis. Overexpression of Crumbs or downregulation of Junctional adhesion molecule-A (JAM-A) in MDCK cells significantly delays establishment of apical polarity in monolayer cultures and blocks lumen formation in 3D cysts [28,29]. Par6 and aPKC are required for lumen formation in MDCK cysts, regulating both polarization and cell death through a pathway involving glycogen synthase kinase 3β [30]. We have shown that deregulation of Scribble in MCF-10A cells, while only producing moderate effects on establishment of apical-basal polarity, significantly blocks morphogenesis by inhibiting cell death during lumen formation in 3D structures [31]. Loss of Lgl re-orients the apical membrane to the basal surface and blocks lumen formation in MDCK cells [24] demonstrating that disruption of polarity proteins can significantly affect morphogenesis. Furthermore, components of the polarity complex such as Rac1 and Cdc42 are critical regulators of MDCK and Caco2 3D morphogenesis where they play critical roles during polarization of MDCK cells and establishment of normal lumen in 3D cysts [32-34].
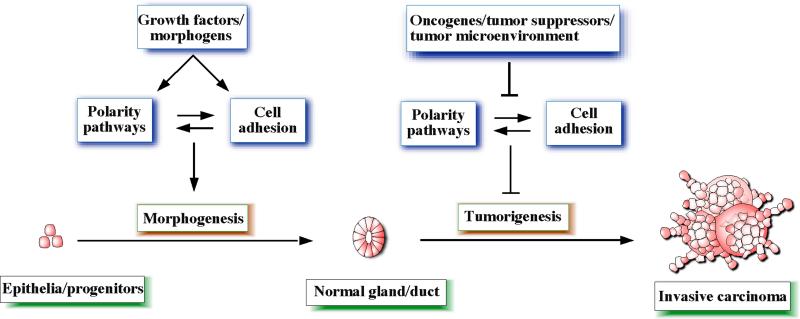
Consistent with the role for polarity proteins in tissue morphogenesis, loss of polarity proteins can initiate tumorigenesis in animal models of cancer. For example, downregulation of Scribble is sufficient to induce initiation of mammary tumors in an immortalized pluripotent mouse mammary epithelial cell line that harbors a mutant allele of the tumor suppressor gene p53 [31]. Loss of Crb3 is required for loss of contact inhibition of mouse kidney epithelial cells [28,29] and loss of Lgl1 results in severe dysplasia in the mouse brain [35]. Loss of the polarity proteins can directly deregulate cell adhesion processes, which in turn will disrupt morphogenesis and promote tumorigenesis. Consistent with this notion, loss of E-cadherin cooperates with p53 to induce invasive mouse mammary tumors [36]. Thus, cell polarity pathways are likely to constitute a new class of tumor suppressors, disruption of which can initiate tumorigenesis (Figure 2).
Figure 2.
A model that attempts to summarize the relationships between cell polarity, cell adhesion, morphogenesis and tumorigenesis pathways.
Cell polarity pathways function downstream of oncogenes
Oncogenes transform cells by deregulating multiple processes including cell proliferation and apoptosis proliferative pathways and disrupting of apoptosis. In addition, oncogenes have been known to disrupt cell polarity and architecture of epithelial cells, although the mechanisms by which this process is accomplished are just beginning to be uncovered. While high levels of expression of v-Src are sufficient to transform MDCK cells, low levels of v-Src in MDCK cells are unable to induce anchorage independent growth. However, cells expressing low levels of v-Src have defective cell-cell junctions and are unable to undergo normal 3D morphogenesis, suggesting that the ability of epithelial cells to form proper cell-cell junctions and undergo normal morphogenesis is exquisitely sensitive to the presence of aberrant oncogenic signals [37]. In addition, aberrant expression of genes associated with cell transformation such as v-K-ras, RhoA, Rac1, Raf-1 and β-catenin affect cell polarity and morphogenesis [38-41]. Several other oncogenes and soluble factors have been shown to disrupt cell polarity, including hepatocyte growth factor (HGF) [42] and the insulin-like growth factor receptor (IGFR) [43]. Interestingly, not all oncogenes have the ability to disrupt polarity. For example, activation of c-Myc or expression of Cyclin D1 does not induce disruption of polarity in mammary epithelial cells [44-46].
The precise mechanisms by which oncogenes disrupt epithelial polarity are only beginning to be understood. We demonstrated that the oncogene ErbB2 disrupts apical polarity of epithelial cells and this property of ErbB2 requires an interaction with the Par6 polarity protein [47]. Activation of ErbB2 causes mislocalization of ZO-1 and Par6 from the apical-lateral border, increases TJ permeability, and dissociates Par3 from the Par6/aPKC complex. ErbB2 associates with Par6 and this interaction is required for ErbB2 to disrupt polarity, 3D morphogenesis and inhibition of apoptosis. Interestingly, the ErbB2-Par6 pathway is not required for ErbB2 to induce cell proliferation, demonstrating that the ability of ErbB2 to disrupt polarity can be uncoupled from its ability to induce cell proliferation.
ErbB2 also cooperates with the β4 integrin to disrupt tight junction organization through activation of signal transducer and activator of transcription 3 (STAT3) [48]. Expression of a dominant negative β4 integrin blocked the ability of ErbB2 to disrupt TJ in a STAT3-dependent manner. However, inhibition of the ErbB2-β4 pathways had no effect on the ability of ErbB2 to activate Erk and also to induce cell proliferation, further demonstrating that ErbB2 uses separate mechanisms to induce cell proliferation and disrupt cell-cell adhesions. Together these observations demonstrate that disruption of cell-cell junctions and induction of uncontrolled proliferation are regulated by separate pathways during oncogene-induced transformation of mammalian epithelial cells.
Cell polarity pathways and tumor progression
Defects in cell and tissue polarity are recognized hallmarks of advanced epithelial tumors. The mechanisms by which oncogenes regulate polarity proteins during cancer progression are now beginning to emerge [49]. The first indication that loss of cell polarity genes cooperate with oncogene activation to induce tumor progression was demonstrated in Drosophila, where flies expressing an activated form of Ras were screened for secondary mutations that would lead to metastatic growth [50]. This screen identified several polarity proteins, including Scr, Dlg and Lgl, whose loss induced noninvasive Ras tumors to spread. Subsequently, cooperation between loss of Scr and Ras or Raf has been observed to cause invasive growth in mammalian cells in a MAPK-dependent manner [51].
Transforming growth factor β (TGFβ) cooperates with oncogenes to induce epithelial to mesenchymal transition (EMT) [52]. Loss of polarity and disruption cell-cell adhesion is associated with cells undergoing EMT and is thought to be a critical step during metastatic tumor progression. TGFβ-induced disruption of TJ and EMT in a mouse mammary epithelial cell line, NMuMG, requires an interaction between TGFβ receptor I and Par6 [53]. Upon TGFβ stimulation, TGFβRII is recruited to this complex where it phosphorylates Par6 at Ser345. This phosphorylation is required for the ability of TGFβ to disrupt TJs. In this context, Par6 functions as a scaffolding protein to facilitate an interaction between Smurf1, an E3 ubiquitin ligase, and RhoA to promote localized degradation of RhoA, a required step for TJ disruption [53]. In rat proximal epithelial cells, TGFβ disrupts polarity through downregulation of Par3 and mislocalization of the Par6/aPKC complex [54], the precise mechanism for this process is unknown. Interestingly, Snail, a transcriptional repressor that induces EMT, can target polarity proteins upon TGFβ stimulation [55]. Overexpression of Snail in MDCK cells leads to dissociation of both the Par and Crumbs complexes from TJs. In addition, Snail binds to the promoter region of Crb3 and directly represses Crb3 promoter activation in response to TGFβ [55]. The transcriptional repressor ZEB1, another inducer of EMT, inhibits transcription of several polarity proteins including Crb3, PATJ and Lgl2 [56,57]. Thus, multiple regulators of EMT require an interaction with polarity proteins demonstrating a role for polarity proteins during tumor progression.
Summary
Polarity pathways regulate important functions during formation and maintenance of cell-cell junctions and during morphogenesis. In addition, cell polarity pathways are emerging as critical regulators of initiation and progression of carcinoma by functioning as tumor suppressors, downstream of oncogenes, or promoters of the metastatic process (Figure 2). It is highly likely that further analysis of cell polarity proteins and the pathways they control will identify novel biomarkers and potential drug targets for managing and treating patients with carcinoma.
Acknowledgements
We would like to thank Jim Duffy for the artwork. SKM was supported by CA098830 and CA105388 grants from NCI; BC075024 and Era of Hope Scholar award from DOD Breast Cancer Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
* of special interest
** of outstanding interest
- 1.Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- 2.Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199–3214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- 3.Nelson WJ. Adaptation of core mechanisms to generate cell polarity. Nature. 2003;422:766–774. doi: 10.1038/nature01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bazellieres E, Assemat E, Arsanto JP, Le Bivic A, Massey-Harroche D. Crumbs proteins in epithelial morphogenesis. Front Biosci. 2009;14:2149–2169. doi: 10.2741/3368. [DOI] [PubMed] [Google Scholar]
- 5.Yamanaka T, Ohno S. Role of Lgl/Dlg/Scribble in the regulation of epithelial junction, polarity and growth. Front Biosci. 2008;13:6693–6707. doi: 10.2741/3182. [DOI] [PubMed] [Google Scholar]
- 6.Macara IG. Parsing the polarity code. Nat Rev Mol Cell Biol. 2004;5:220–231. doi: 10.1038/nrm1332. [DOI] [PubMed] [Google Scholar]
- 7*.Hurd TW, Gao L, Roh MH, Macara IG, Margolis B. Direct interaction of two polarity complexes implicated in epithelial tight junction assembly. Nat Cell Biol. 2003;5:137–142. doi: 10.1038/ncb923. [* of special interestThis paper uncovered interactions between distinct polarity complexes in the assembly and maintenance of tight junctions, underscoring the interconnectedness of cell polarity pathways in the determination of cell shape] [DOI] [PubMed] [Google Scholar]
- 8*.Betschinger J, Mechtler K, Knoblich JA. The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature. 2003;422:326–330. doi: 10.1038/nature01486. [* of special interestThis study demonstrates an interaction between the apical polarity Par complex and the lateral polarity protein Lgl] [DOI] [PubMed] [Google Scholar]
- 9*.Yamanaka T, Horikoshi Y, Sugiyama Y, Ishiyama C, Suzuki A, Hirose T, Iwamatsu A, Shinohara A, Ohno S. Mammalian Lgl forms a protein complex with PAR-6 and aPKC independently of PAR-3 to regulate epithelial cell polarity. Curr Biol. 2003;13:734–743. doi: 10.1016/s0960-9822(03)00244-6. [* of special interestThis study demonstrates an interaction between the apical polarity Par complex and the lateral polarity protein Lgl] [DOI] [PubMed] [Google Scholar]
- 10*.Plant PJ, Fawcett JP, Lin DC, Holdorf AD, Binns K, Kulkarni S, Pawson T. A polarity complex of mPar-6 and atypical PKC binds, phosphorylates and regulates mammalian Lgl. Nat Cell Biol. 2003;5:301–308. doi: 10.1038/ncb948. [* of special interestThis study demonstrates an interaction between the apical polarity Par complex and the lateral polarity protein Lgl] [DOI] [PubMed] [Google Scholar]
- 11.Izumi Y, Hirose T, Tamai Y, Hirai S, Nagashima Y, Fujimoto T, Tabuse Y, Kemphues KJ, Ohno S. An atypical PKC directly associates and colocalizes at the epithelial tight junction with ASIP, a mammalian homologue of Caenorhabditis elegans polarity protein PAR-3. J Cell Biol. 1998;143:95–106. doi: 10.1083/jcb.143.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joberty G, Petersen C, Gao L, Macara IG. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol. 2000;2:531–539. doi: 10.1038/35019573. [DOI] [PubMed] [Google Scholar]
- 13.Ebnet K, Suzuki A, Horikoshi Y, Hirose T, Meyer Zu Brickwedde MK, Ohno S, Vestweber D. The cell polarity protein ASIP/PAR-3 directly associates with junctional adhesion molecule (JAM). Embo J. 2001;20:3738–3748. doi: 10.1093/emboj/20.14.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirose T, Izumi Y, Nagashima Y, Tamai-Nagai Y, Kurihara H, Sakai T, Suzuki Y, Yamanaka T, Suzuki A, Mizuno K, et al. Involvement of ASIP/PAR-3 in the promotion of epithelial tight junction formation. J Cell Sci. 2002;115:2485–2495. doi: 10.1242/jcs.115.12.2485. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Macara IG. Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat Cell Biol. 2005;7:262–269. doi: 10.1038/ncb1226. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki A, Yamanaka T, Hirose T, Manabe N, Mizuno K, Shimizu M, Akimoto K, Izumi Y, Ohnishi T, Ohno S. Atypical protein kinase C is involved in the evolutionarily conserved par protein complex and plays a critical role in establishing epithelia-specific junctional structures. J Cell Biol. 2001;152:1183–1196. doi: 10.1083/jcb.152.6.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mertens AE, Rygiel TP, Olivo C, van der Kammen R, Collard JG. The Rac activator Tiam1 controls tight junction biogenesis in keratinocytes through binding to and activation of the Par polarity complex. J Cell Biol. 2005;170:1029–1037. doi: 10.1083/jcb.200502129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen X, Macara IG. Par-3 mediates the inhibition of LIM kinase 2 to regulate cofilin phosphorylation and tight junction assembly. J Cell Biol. 2006;172:671–678. doi: 10.1083/jcb.200510061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Du D, Fang L, Yang G, Zhang C, Zeng R, Ullrich A, Lottspeich F, Chen Z. Tyrosine phosphorylated Par3 regulates epithelial tight junction assembly promoted by EGFR signaling. Embo J. 2006;25:5058–5070. doi: 10.1038/sj.emboj.7601384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nunbhakdi-Craig V, Machleidt T, Ogris E, Bellotto D, White CL, 3rd, Sontag E. Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J Cell Biol. 2002;158:967–978. doi: 10.1083/jcb.200206114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Traweger A, Wiggin G, Taylor L, Tate SA, Metalnikov P, Pawson T. Protein phosphatase 1 regulates the phosphorylation state of the polarity scaffold Par-3. Proc Natl Acad Sci U S A. 2008;105:10402–10407. doi: 10.1073/pnas.0804102105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fogg VC, Liu CJ, Margolis B. Multiple regions of Crumbs3 are required for tight junction formation in MCF10A cells. J Cell Sci. 2005;118:2859–2869. doi: 10.1242/jcs.02412. [DOI] [PubMed] [Google Scholar]
- 23.Lemmers C, Michel D, Lane-Guermonprez L, Delgrossi MH, Medina E, Arsanto JP, Le Bivic A. CRB3 binds directly to Par6 and regulates the morphogenesis of the tight junctions in mammalian epithelial cells. Mol Biol Cell. 2004;15:1324–1333. doi: 10.1091/mbc.E03-04-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamanaka T, Horikoshi Y, Izumi N, Suzuki A, Mizuno K, Ohno S. Lgl mediates apical domain disassembly by suppressing the PAR-3-aPKC-PAR-6 complex to orient apical membrane polarity. J Cell Sci. 2006;119:2107–2118. doi: 10.1242/jcs.02938. [DOI] [PubMed] [Google Scholar]
- 25.Navarro C, Nola S, Audebert S, Santoni MJ, Arsanto JP, Ginestier C, Marchetto S, Jacquemier J, Isnardon D, Le Bivic A, et al. Junctional recruitment of mammalian Scribble relies on E-cadherin engagement. Oncogene. 2005;24:4330–4339. doi: 10.1038/sj.onc.1208632. [DOI] [PubMed] [Google Scholar]
- 26.Laprise P, Viel A, Rivard N. Human homolog of disc-large is required for adherens junction assembly and differentiation of human intestinal epithelial cells. J Biol Chem. 2004;279:10157–10166. doi: 10.1074/jbc.M309843200. [DOI] [PubMed] [Google Scholar]
- 27.Wang Q, Chen XW, Margolis B. PALS1 regulates E-cadherin trafficking in mammalian epithelial cells. Mol Biol Cell. 2007;18:874–885. doi: 10.1091/mbc.E06-07-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karp CM, Tan TT, Mathew R, Nelson D, Mukherjee C, Degenhardt K, Karantza-Wadsworth V, White E. Role of the polarity determinant crumbs in suppressing mammalian epithelial tumor progression. Cancer Res. 2008;68:4105–4115. doi: 10.1158/0008-5472.CAN-07-6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rehder D, Iden S, Nasdala I, Wegener J, Brickwedde MK, Vestweber D, Ebnet K. Junctional adhesion molecule-a participates in the formation of apico-basal polarity through different domains. Exp Cell Res. 2006;312:3389–3403. doi: 10.1016/j.yexcr.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Kim M, Datta A, Brakeman P, Yu W, Mostov KE. Polarity proteins PAR6 and aPKC regulate cell death through GSK-3beta in 3D epithelial morphogenesis. J Cell Sci. 2007;120:2309–2317. doi: 10.1242/jcs.007443. [DOI] [PubMed] [Google Scholar]
- 31*.Zhan L, Rosenberg A, Bergami KC, Yu M, Xuan Z, Jaffe AB, Allred C, Muthuswamy SK. Deregulation of scribble promotes mammary tumorigenesis and reveals a role for cell polarity in carcinoma. Cell. 2008;135:865–878. doi: 10.1016/j.cell.2008.09.045. [* of special interestThis paper uncovered interactions between distinct polarity complexes in the assembly and maintenance of tight junctions, underscoring the interconnectedness of cell polarity pathways in the determination of cell shape] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Brien LE, Jou TS, Pollack AL, Zhang Q, Hansen SH, Yurchenco P, Mostov KE. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell Biol. 2001;3:831–838. doi: 10.1038/ncb0901-831. [DOI] [PubMed] [Google Scholar]
- 33.Martin-Belmonte F, Yu W, Rodriguez-Fraticelli AE, Ewald AJ, Werb Z, Alonso MA, Mostov K. Cell-polarity dynamics controls the mechanism of lumen formation in epithelial morphogenesis. Curr Biol. 2008;18:507–513. doi: 10.1016/j.cub.2008.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaffe AB, Kaji N, Durgan J, Hall A. Cdc42 controls spindle orientation to position the apical surface during epithelial morphogenesis. J Cell Biol. 2008;183:625–633. doi: 10.1083/jcb.200807121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35*.Klezovitch O, Fernandez TE, Tapscott SJ, Vasioukhin V. Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Dev. 2004;18:559–571. doi: 10.1101/gad.1178004. [* of special interestThis study describes that loss of polarity can initiate dysplastic growth in brain] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Derksen PW, Liu X, Saridin F, van der Gulden H, Zevenhoven J, Evers B, van Beijnum JR, Griffioen AW, Vink J, Krimpenfort P, et al. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell. 2006;10:437–449. doi: 10.1016/j.ccr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 37*.Warren SL, Nelson WJ. Nonmitogenic morphoregulatory action of pp60v-src on multicellular epithelial structures. Mol Cell Biol. 1987;7:1326–1337. doi: 10.1128/mcb.7.4.1326. [* of special interestThis report was among the first to demonstrate that oncogenic activation could disrupt cell polarity in epithelial cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schoenenberger CA, Zuk A, Kendall D, Matlin KS. Multilayering and loss of apical polarity in MDCK cells transformed with viral K-ras. J Cell Biol. 1991;112:873–889. doi: 10.1083/jcb.112.5.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jou TS, Schneeberger EE, Nelson WJ. Structural and functional regulation of tight junctions by RhoA and Rac1 small GTPases. J Cell Biol. 1998;142:101–115. doi: 10.1083/jcb.142.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li D, Mrsny RJ. Oncogenic Raf-1 disrupts epithelial tight junctions via downregulation of occludin. J Cell Biol. 2000;148:791–800. doi: 10.1083/jcb.148.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naishiro Y, Yamada T, Takaoka AS, Hayashi R, Hasegawa F, Imai K, Hirohashi S. Restoration of epithelial cell polarity in a colorectal cancer cell line by suppression of beta-catenin/T-cell factor 4-mediated gene transactivation. Cancer Res. 2001;61:2751–2758. [PubMed] [Google Scholar]
- 42.Balkovetz DF, Pollack AL, Mostov KE. Hepatocyte growth factor alters the polarity of Madin-Darby canine kidney cell monolayers. J Biol Chem. 1997;272:3471–3477. doi: 10.1074/jbc.272.6.3471. [DOI] [PubMed] [Google Scholar]
- 43.Yanochko GM, Eckhart W. Type I insulin-like growth factor receptor over-expression induces proliferation and anti-apoptotic signaling in a three-dimensional culture model of breast epithelial cells. Breast Cancer Res. 2006;8:R18. doi: 10.1186/bcr1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reichmann E, Schwarz H, Deiner EM, Leitner I, Eilers M, Berger J, Busslinger M, Beug H. Activation of an inducible c-FosER fusion protein causes loss of epithelial polarity and triggers epithelial-fibroblastoid cell conversion. Cell. 1992;71:1103–1116. doi: 10.1016/s0092-8674(05)80060-1. [DOI] [PubMed] [Google Scholar]
- 45.Fialka I, Schwarz H, Reichmann E, Oft M, Busslinger M, Beug H. The estrogen-dependent c-JunER protein causes a reversible loss of mammary epithelial cell polarity involving a destabilization of adherens junctions. J Cell Biol. 1996;132:1115–1132. doi: 10.1083/jcb.132.6.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswamy SK, Brugge JS. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell. 2002;111:29–40. doi: 10.1016/s0092-8674(02)01001-2. [DOI] [PubMed] [Google Scholar]
- 47**.Aranda V, Haire T, Nolan ME, Calarco JP, Rosenberg AZ, Fawcett JP, Pawson T, Muthuswamy SK. Par6-aPKC uncouples ErbB2 induced disruption of polarized epithelial organization from proliferation control. Nat Cell Biol. 2006;8:1235–1245. doi: 10.1038/ncb1485. [** of outstanding interestThis publication describes a mechanism by which an oncogene disrupts epithelial polarity, and provided evidence that oncogene induced changes in cell proliferation and changes in cell polarity can be uncoupled.] [DOI] [PubMed] [Google Scholar]
- 48.Guo W, Pylayeva Y, Pepe A, Yoshioka T, Muller WJ, Inghirami G, Giancotti FG. Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell. 2006;126:489–502. doi: 10.1016/j.cell.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 49.Tanos B, Rodriguez-Boulan E. The epithelial polarity program: machineries involved and their hijacking by cancer. Oncogene. 2008;27:6939–6957. doi: 10.1038/onc.2008.345. [DOI] [PubMed] [Google Scholar]
- 50**.Pagliarini RA, Xu T. A genetic screen in Drosophila for metastatic behavior. Science. 2003;302:1227–1231. doi: 10.1126/science.1088474. [** of outstanding interestThis study demosntrates that polarity proteins cooperate with oncogenes to induce tumorigenesis and malignant progression in Drosophila.] [DOI] [PubMed] [Google Scholar]
- 51.Dow LE, Elsum IA, King CL, Kinross KM, Richardson HE, Humbert PO. Loss of human Scribble cooperates with H-Ras to promote cell invasion through deregulation of MAPK signalling. Oncogene. 2008 doi: 10.1038/onc.2008.219. [DOI] [PubMed] [Google Scholar]
- 52.Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 53**.Ozdamar B, Bose R, Barrios-Rodiles M, Wang HR, Zhang Y, Wrana JL. Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science. 2005;307:1603–1609. doi: 10.1126/science.1105718. [** of outstanding interestThis paper identifies a role Par6 polarity protein in TGFβ-induced epithelial to mesenchymal transition.] [DOI] [PubMed] [Google Scholar]
- 54.Wang X, Nie J, Zhou Q, Liu W, Zhu F, Chen W, Mao H, Luo N, Dong X, Yu X. Downregulation of Par-3 expression and disruption of Par complex integrity by TGF-beta during the process of epithelial to mesenchymal transition in rat proximal epithelial cells. Biochim Biophys Acta. 2008;1782:51–59. doi: 10.1016/j.bbadis.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 55.Whiteman EL, Liu CJ, Fearon ER, Margolis B. The transcription factor snail represses Crumbs3 expression and disrupts apico-basal polarity complexes. Oncogene. 2008;27:3875–3879. doi: 10.1038/onc.2008.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aigner K, Dampier B, Descovich L, Mikula M, Sultan A, Schreiber M, Mikulits W, Brabletz T, Strand D, Obrist P, et al. The transcription factor ZEB1 (deltaEF1) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene. 2007;26:6979–6988. doi: 10.1038/sj.onc.1210508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spaderna S, Schmalhofer O, Wahlbuhl M, Dimmler A, Bauer K, Sultan A, Hlubek F, Jung A, Strand D, Eger A, et al. The transcriptional repressor ZEB1 promotes metastasis and loss of cell polarity in cancer. Cancer Res. 2008;68:537–544. doi: 10.1158/0008-5472.CAN-07-5682. [DOI] [PubMed] [Google Scholar]