Abstract
Endovascular treatment of intracranial dural arteriovenous fistulas can be very challenging. Conventional endovascular treatment approaches include transarterial or transvenous access from the common femoral artery or vein, respectively. A novel approach is described for endovascular treatment of an aggressive type 4 dural arteriovenous fistula. This patient, who had recently suffered an associated intracranial hemorrhage, harbored a dural arteriovenous fistula involving his transverse dural venous sinus that did not allow for conventional transarterial or transvenous embolization routes. The clinical history and technical details are provided.
Endovascular treatment options for intracranial dural arteriovenous fistulas (dAVFs) include transarterial and transvenous routes using coils, particulates, or liquid embolic agents (1–6). Generally, endovascular treatment is reserved for those dAVFs that exhibit aggressive features such as increased intracranial pressure, intracranial hemorrhage, or cortical venous drainage. When anatomically feasible, retrograde transvenous coil embolization of dAVFs is highly effective, assuming the proximal venous pouch can be occluded. In cases when standard retrograde transvenous access is not possible or when the arterial anatomy allows for close microcatheter approximation to the site of fistulous connection, coils or liquid embolic agents can be placed transarterially for ablation of the fistula (1–4).
A case is presented in which standard transvenous and transarterial access routes for embolization were not available. A technique for ultrasound-guided puncture of an arterialized extracranial venous pouch with transosseous microcatheter navigation through a mastoid emissary vein (MEV) is described. This is offered as a useful alternative to other endovascular approaches for treating dAVFs.
CASE STUDY
A 60-year-old man presented with a 4-week history of headaches and difficulty reading. He had no significant past medical history and was not taking any medications for a chronic medical condition. His physical examination demonstrated normal strength and sensation in all four extremities. His vision was intact to confrontation, but he did have decreased visual acuity. A pulsatile bruit was audible with auscultation over the left retroauricular region, and his left occipital artery was asymmetrically enlarged and hyperdynamic compared with the contralateral side.
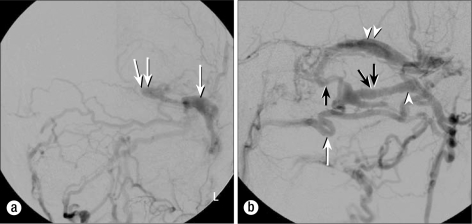
Initial magnetic resonance imaging of the head demonstrated a parenchymal hematoma in the left occipital lobe with multiple prominent flow voids at the base of the skull, suggesting a possible vascular malformation as the cause for his hemorrhage. A cerebral angiogram in anteroposterior (Figure 1a) and lateral views (Figure 1b) revealed an aggressive type 4 dAVF involving the left transverse dural venous sinus (TS) with multiple sites of arterial communication. The TS was isolated and drained into multiple intracranial superficial leptomeningeal and deep parenchymal veins. Additionally, the arterialized TS drained through an enlarged transosseous venous channel in the left temporal bone into an elongated venous pouch along the left occipital pericranium. The transosseous venous channel was most likely an enlarged MEV originating near the junction of the transverse and sigmoid sinuses. The extracranial venous pouch subsequently drained into numerous smaller veins of the suboccipital venous plexus. The TS had no connection with the torcular, and the sigmoid sinus/jugular bulb was occluded. There was extensive fistulization along the TS from multiple branches of the occipital, ascending pharyngeal, and middle meningeal arteries.
Figure 1.
Digital subtraction angiogram of the left external carotid artery before the procedure. (a) The anteroposterior projection demonstrates the dural fistula with early opacification of the left transverse sinus (arrow). Note that the transverse sinus does not communicate with the torcular and ends blindly (double arrow). (b) The lateral projection demonstrates the occipital artery (single white arrow), mastoid emissary vein (single black arrow), extracalvarial venous pouch (white arrowhead), and transverse sinus (double white arrowhead). The eventual puncture site for access to the venous pouch is indicated by the double black arrows.
Technique
The patient was placed under general anesthesia. Using standard technique, a 4F sheath was placed in the left common femoral artery. A 4F diagnostic catheter was advanced into the left external carotid artery for angiographic control runs. This catheter was connected to a continuous flush. The patient's head was then positioned facing the right in order to allow access to the left retromastoid region. This area was prepared in standard sterile fashion.
Biplane working projection left external carotid artery angiography was performed. Using native fluoroscopic roadmap guidance and sterile ultrasound, the arterialized venous pouch lying along the left retromastoid calvarium was punctured with a 21-gauge micropuncture needle. Using color-flow Doppler, the enlarged occipital artery was clearly differentiated from the adjacent arterialized pouch, allowing for precise puncture of the venous pouch. An 0.018″ stainless steel mandril wire was advanced through the needle and coiled in the anterior aspect of the extracalvarial venous pouch. Over this mandril wire, a 10-cm-long 4F micropuncture coaxial sheath was advanced into the pouch. Through the micropuncture sheath, an 0.018″ glidewire was then advanced through the enlarged MEV and into the left TS. The micropuncture coaxial sheath was then advanced through the transosseous MEV and into the anterior aspect of the left TS. The inner catheter of the micropuncture sheath set was removed, and the outer catheter was attached to a Tuohy-Borst valve and connected to a continuous flush. It was secured to the patient's neck with multiple clear adhesive strips to assure stable positioning.
Over an 0.014″ microwire, a microcatheter was then navigated to the most posterior aspect of the arterialized left TS. The TS was then embolized with multiple spherical 0.015″, 0.012″, and 0.010″ platinum coils as well as multiple helical 0.012″ platinum coils. The microcatheter was systematically pulled back as the TS was densely packed from back to front with coils. Although initially considered, liquid embolics were not utilized because of the relatively tenuous microcatheter access. The use of detachable coils ensured precise packing of the entire segment. This was accomplished without leaving an isolated patent segment that could not be reached for additional embolization despite continued dangerous intracranial venous drainage. After packing the TS anteriorly to the level of the MEV, the microcatheter was removed.
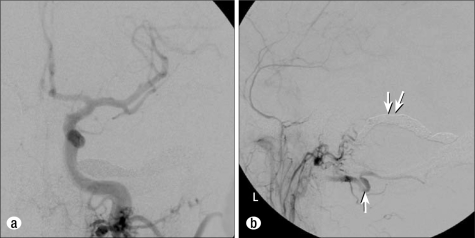
A final left common carotid artery angiogram was performed in anteroposterior (Figure 2a) and lateral views (Figure 2b). This demonstrated complete obliteration of the dAVF. No persistent arteriovenous shunting was present. The micropuncture sheath was removed, and light manual pressure was applied to the retromastoid puncture site. The patient awakened from anesthesia in stable condition with no headache or new neurological deficit. He was observed overnight in the hospital and discharged home the next day in stable condition. Follow-up angiography 9 months after embolization revealed persistent closure of the fistula, and the patient had no new symptoms.
Figure 2.
Postembolization digital subtraction angiogram of the left common carotid artery. (a) The anteroposterior projection demonstrates obliteration of the fistula. (b) The lateral projection demonstrates no evidence of residual dAVF. The left occipital artery is much slower (white arrow) because it no longer feeds the dAVF. Dense coil packing of the transverse sinus extending to the level of the mastoid emissary vein is noted (double white arrow).
DISCUSSION
The most common location for dAVFs is within the dura associated with the transverse and sigmoid dural venous sinuses. In those dAVF cases with aggressive features, open surgery, radiosurgery, endovascular embolization, or a combined multimodality treatment approach is warranted (7, 8). Historically, endovascular treatment of these fistulas was preferably performed from a retrograde venous approach with guide catheter positioning in the internal jugular vein or one of its tributaries. More recently, transarterial embolization with coils and liquid embolic agents has become a popular endovascular treatment option when anatomically feasible.
Chronic occlusion of the ipsilateral internal jugular vein precluded traditional retrograde transvenous embolization in this case. Similarly, because the posterior aspect of the TS did not communicate with the torcular, access from the contralateral jugular vein was not possible. Given the multiple sites of arterial input to the fistula covering a long segment of the TS, transarterial cure with liquid embolic agents was considered very unlikely. This case demonstrates the feasibility of coil embolization of dAVFs from a transosseous emissary vein approach using ultrasound guidance. In certain cases, this novel approach may provide the only endovascular access or offer significant advantages over other endovascular approaches for the treatment of dAVFs.
References
- 1.Layton KF, Nelson MD, Kallmes DF. Transarterial coil embolization of the venous component of aggressive type 4 dural arteriovenous fistulas. AJNR Am J Neuroradiol. 2006;27(4):750–752. [PMC free article] [PubMed] [Google Scholar]
- 2.van Rooij WJ, Sluzewski M, Beute GN. Tentorial artery embolization in tentorial dural arteriovenous fistulas. Neuroradiology. 2006;48(10):737–743. doi: 10.1007/s00234-006-0118-8. [DOI] [PubMed] [Google Scholar]
- 3.Cognard C, Januel AC, Silva NA, Jr, Tall P. Endovascular treatment of intracranial dural arteriovenous fistulas with cortical venous drainage: new management using Onyx. AJNR Am J Neuroradiol. 2008;29(2):235–241. doi: 10.3174/ajnr.A0817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nogueira RG, Dabus G, Rabinov JD, Eskey CJ, Ogilvy CS, Hirsch JA, Pryor JC. Preliminary experience with Onyx embolization for the treatment of intracranial dural arteriovenous fistulas. AJNR Am J Neuroradiol. 2008;29(1):91–97. doi: 10.3174/ajnr.A0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim DJ, Kim DI, Suh SH, Kim J, Lee SK, Kim EY, Chung TS. Results of transvenous embolization of cavernous dural arteriovenous fistula: a single-center experience with emphasis on complications and management. AJNR Am J Neuroradiol. 2006;27(10):2078–2082. [PMC free article] [PubMed] [Google Scholar]
- 6.van Rooij WJ, Sluzewski M, Beute GN. Dural arteriovenous fistulas with cortical venous drainage: incidence, clinical presentation, and treatment. AJNR Am J Neuroradiol. 2007;28(4):651–655. [PMC free article] [PubMed] [Google Scholar]
- 7.Giller CA, Barnett DW, Thacker IC, Hise JH, Berger BD. Multidisciplinary treatment of a large cerebral dural arteriovenous fistula using embolization, surgery, and radiosurgery. Proc (Bayl Univ Med Cent) 2008;21(3):255–257. doi: 10.1080/08998280.2008.11928405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White JB, Layton KF, Evans AJ, Tong FC, Jensen ME, Kallmes DF, Dion JE, Cloft HJ. Transorbital puncture for the treatment of cavernous sinus dural arteriovenous fistulas. AJNR Am J Neuroradiol. 2007;28(7):1415–1417. doi: 10.3174/ajnr.A0663. [DOI] [PMC free article] [PubMed] [Google Scholar]