Abstract
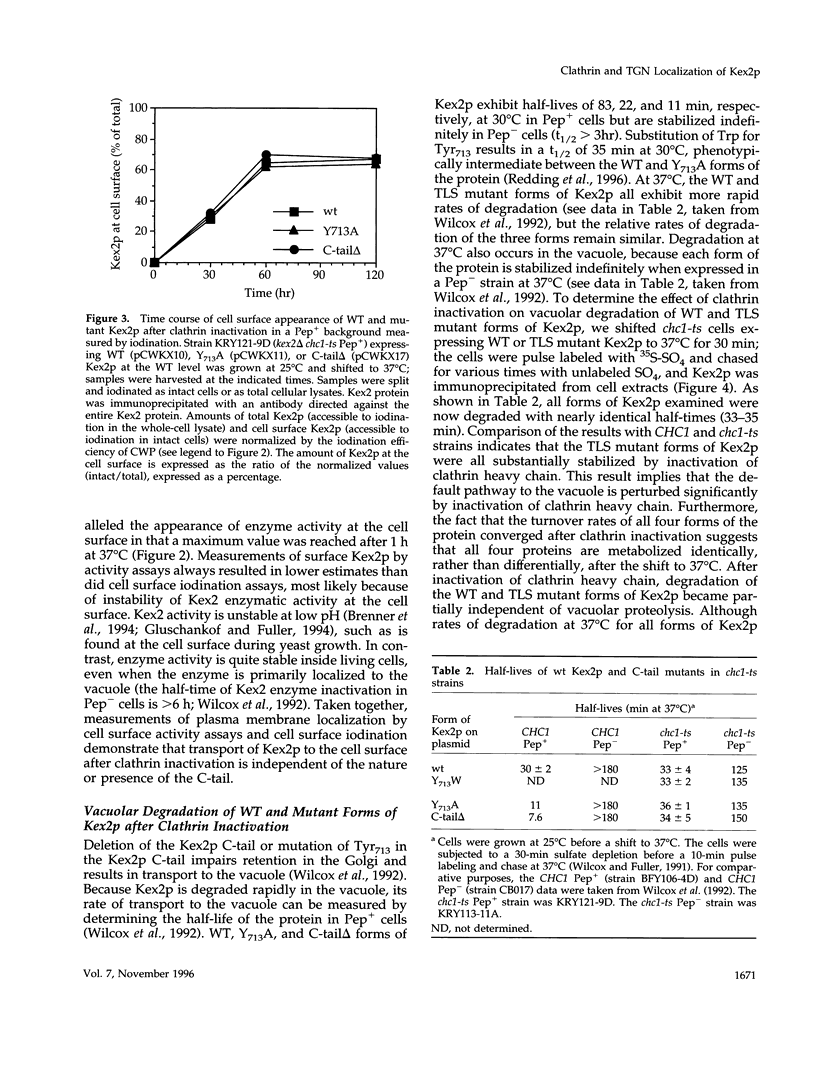
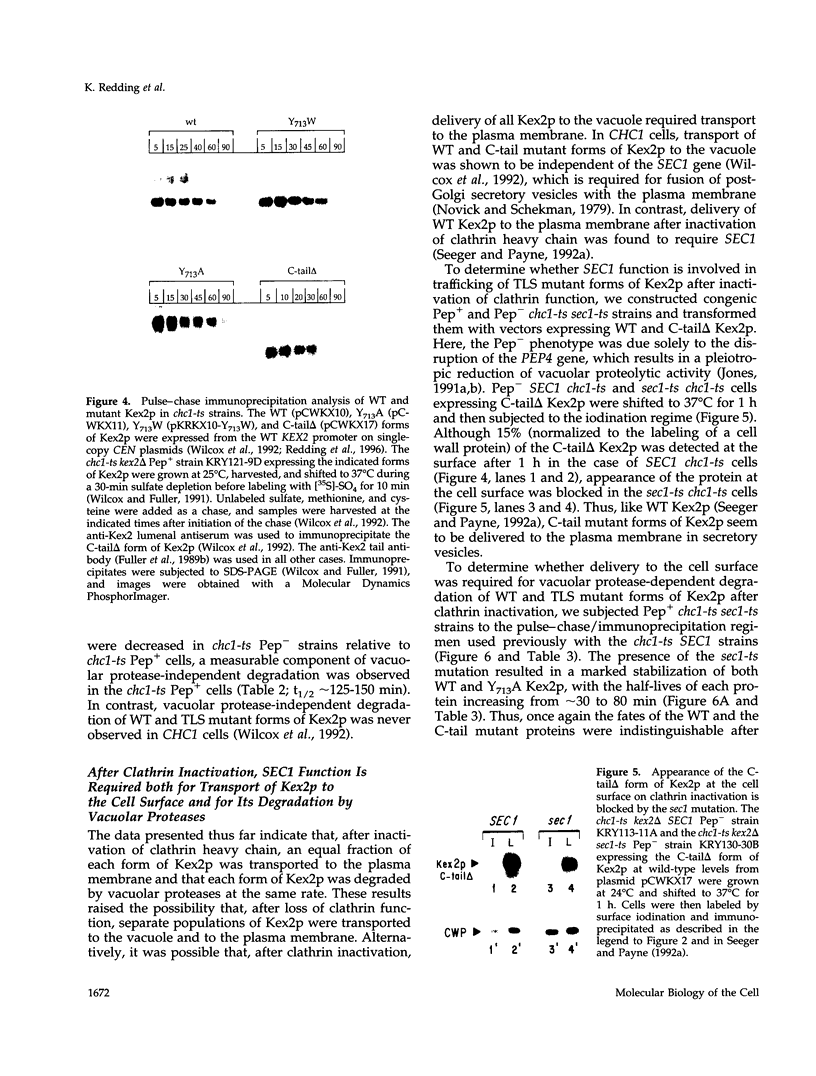
Localization of Kex2 protease (Kex2p) to the yeast trans-Golgi network (TGN) requires a TGN localization signal (TLS) in the Kex2p C-terminal cytosolic tail. Mutation of the TLS accelerates transport of Kex2p to the vacuole by an intracellular (SEC1-independent) pathway. In contrast, inactivation of the clathrin heavy-chain gene CHC1 results in transport of Kex2p and other Golgi membrane proteins to the cell surface. Here, the relationship of the two localization defects was assessed by examining the effects of a temperature-sensitive CHC1 allele on trafficking of wild-type (WT) and TLS mutant forms of Kex2p. Inactivation of clathrin by shifting chc1-ts cells to 37 degrees C caused WT and TLS mutant forms of Kex2p to behave identically. All forms of Kex2p appeared at the plasma membrane within 30-60 min of the temperature shift. TLS mutant forms of Kex2p were stabilized, their half-lives increasing to that of wild-type Kex2p. After inactivation of clathrin heavy chain, vacuolar protease-dependent degradation of all forms of Kex2p was blocked by a sec1 mutation, which is required for secretory vesicle fusion to the plasma membrane, indicating that transport to the cell surface was required for degradation by vacuolar proteolysis. Finally, after clathrin inactivation, all forms of Kex2p were degraded in part by a vacuolar protease-independent pathway. After inactivation of both chc1-ts and sec1-ts, Kex2 was degraded exclusively by this pathway. We conclude that the effects of clathrin inactivation on Kex2p localization are independent of the Kex2p C-terminal cytosolic tail. Although these results neither prove nor rule out a direct interaction between the Kex2 TLS and a clathrin-dependent structure, they do imply that clathrin is required for the intracellular transport of Kex2p TLS mutants to the vacuole.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beltzer J. P., Spiess M. In vitro binding of the asialoglycoprotein receptor to the beta adaptin of plasma membrane coated vesicles. EMBO J. 1991 Dec;10(12):3735–3742. doi: 10.1002/j.1460-2075.1991.tb04942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos K., Wraight C., Stanley K. K. TGN38 is maintained in the trans-Golgi network by a tyrosine-containing motif in the cytoplasmic domain. EMBO J. 1993 May;12(5):2219–2228. doi: 10.1002/j.1460-2075.1993.tb05870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner C., Bevan A., Fuller R. S. Biochemical and genetic methods for analyzing specificity and activity of a precursor-processing enzyme: yeast Kex2 protease, kexin. Methods Enzymol. 1994;244:152–167. doi: 10.1016/0076-6879(94)44013-1. [DOI] [PubMed] [Google Scholar]
- Brewer C. B., Roth M. G. A single amino acid change in the cytoplasmic domain alters the polarized delivery of influenza virus hemagglutinin. J Cell Biol. 1991 Aug;114(3):413–421. doi: 10.1083/jcb.114.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant N. J., Boyd A. Immunoisolation of Kex2p-containing organelles from yeast demonstrates colocalisation of three processing proteinases to a single Golgi compartment. J Cell Sci. 1993 Nov;106(Pt 3):815–822. doi: 10.1242/jcs.106.3.815. [DOI] [PubMed] [Google Scholar]
- Burgers P. M., Percival K. J. Transformation of yeast spheroplasts without cell fusion. Anal Biochem. 1987 Jun;163(2):391–397. doi: 10.1016/0003-2697(87)90240-5. [DOI] [PubMed] [Google Scholar]
- Casanova J. E., Apodaca G., Mostov K. E. An autonomous signal for basolateral sorting in the cytoplasmic domain of the polymeric immunoglobulin receptor. Cell. 1991 Jul 12;66(1):65–75. doi: 10.1016/0092-8674(91)90139-p. [DOI] [PubMed] [Google Scholar]
- Cereghino J. L., Marcusson E. G., Emr S. D. The cytoplasmic tail domain of the vacuolar protein sorting receptor Vps10p and a subset of VPS gene products regulate receptor stability, function, and localization. Mol Biol Cell. 1995 Sep;6(9):1089–1102. doi: 10.1091/mbc.6.9.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A., Bussey H. Yeast Kex1p is a Golgi-associated membrane protein: deletions in a cytoplasmic targeting domain result in mislocalization to the vacuolar membrane. J Cell Biol. 1992 Dec;119(6):1459–1468. doi: 10.1083/jcb.119.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupers P., Veithen A., Kiss A., Baudhuin P., Courtoy P. J. Clathrin polymerization is not required for bulk-phase endocytosis in rat fetal fibroblasts. J Cell Biol. 1994 Nov;127(3):725–735. doi: 10.1083/jcb.127.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damke H., Baba T., Warnock D. E., Schmid S. L. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994 Nov;127(4):915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damke H., Baba T., van der Bliek A. M., Schmid S. L. Clathrin-independent pinocytosis is induced in cells overexpressing a temperature-sensitive mutant of dynamin. J Cell Biol. 1995 Oct;131(1):69–80. doi: 10.1083/jcb.131.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. S., Brake A. J., Thorner J. Intracellular targeting and structural conservation of a prohormone-processing endoprotease. Science. 1989 Oct 27;246(4929):482–486. doi: 10.1126/science.2683070. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Brake A., Thorner J. Yeast prohormone processing enzyme (KEX2 gene product) is a Ca2+-dependent serine protease. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1434–1438. doi: 10.1073/pnas.86.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. S., Sterne R. E., Thorner J. Enzymes required for yeast prohormone processing. Annu Rev Physiol. 1988;50:345–362. doi: 10.1146/annurev.ph.50.030188.002021. [DOI] [PubMed] [Google Scholar]
- Gaynor E. C., te Heesen S., Graham T. R., Aebi M., Emr S. D. Signal-mediated retrieval of a membrane protein from the Golgi to the ER in yeast. J Cell Biol. 1994 Nov;127(3):653–665. doi: 10.1083/jcb.127.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman J. N., Conibear E., Pearse B. M. Specificity of binding of clathrin adaptors to signals on the mannose-6-phosphate/insulin-like growth factor II receptor. EMBO J. 1989 Apr;8(4):1041–1047. doi: 10.1002/j.1460-2075.1989.tb03471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluschankof P., Fuller R. S. A C-terminal domain conserved in precursor processing proteases is required for intramolecular N-terminal maturation of pro-Kex2 protease. EMBO J. 1994 May 15;13(10):2280–2288. doi: 10.1002/j.1460-2075.1994.tb06510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham T. R., Seeger M., Payne G. S., MacKay V. L., Emr S. D. Clathrin-dependent localization of alpha 1,3 mannosyltransferase to the Golgi complex of Saccharomyces cerevisiae. J Cell Biol. 1994 Nov;127(3):667–678. doi: 10.1083/jcb.127.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskovits J. S., Burgess C. C., Obar R. A., Vallee R. B. Effects of mutant rat dynamin on endocytosis. J Cell Biol. 1993 Aug;122(3):565–578. doi: 10.1083/jcb.122.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey J. S., Peters P. J., Yuan L. C., Bonifacino J. S. Localization of TGN38 to the trans-Golgi network: involvement of a cytoplasmic tyrosine-containing sequence. J Cell Biol. 1993 Mar;120(5):1123–1135. doi: 10.1083/jcb.120.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. F., Chan W., Kornfeld S. Cation-dependent mannose 6-phosphate receptor contains two internalization signals in its cytoplasmic domain. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10010–10014. doi: 10.1073/pnas.87.24.10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. W. Tackling the protease problem in Saccharomyces cerevisiae. Methods Enzymol. 1991;194:428–453. doi: 10.1016/0076-6879(91)94034-a. [DOI] [PubMed] [Google Scholar]
- Jones E. W. Three proteolytic systems in the yeast saccharomyces cerevisiae. J Biol Chem. 1991 May 5;266(13):7963–7966. [PubMed] [Google Scholar]
- Julius D., Brake A., Blair L., Kunisawa R., Thorner J. Isolation of the putative structural gene for the lysine-arginine-cleaving endopeptidase required for processing of yeast prepro-alpha-factor. Cell. 1984 Jul;37(3):1075–1089. doi: 10.1016/0092-8674(84)90442-2. [DOI] [PubMed] [Google Scholar]
- Kean L. S., Fuller R. S., Nichols J. W. Retrograde lipid traffic in yeast: identification of two distinct pathways for internalization of fluorescent-labeled phosphatidylcholine from the plasma membrane. J Cell Biol. 1993 Dec;123(6 Pt 1):1403–1419. doi: 10.1083/jcb.123.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ktistakis N. T., Thomas D., Roth M. G. Characteristics of the tyrosine recognition signal for internalization of transmembrane surface glycoproteins. J Cell Biol. 1990 Oct;111(4):1393–1407. doi: 10.1083/jcb.111.4.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladinsky M. S., Kremer J. R., Furcinitti P. S., McIntosh J. R., Howell K. E. HVEM tomography of the trans-Golgi network: structural insights and identification of a lace-like vesicle coat. J Cell Biol. 1994 Oct;127(1):29–38. doi: 10.1083/jcb.127.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon S. K., Jones E. W. Clathrin requirement for normal growth of yeast. Science. 1987 Oct 23;238(4826):504–509. doi: 10.1126/science.3116672. [DOI] [PubMed] [Google Scholar]
- Marcusson E. G., Horazdovsky B. F., Cereghino J. L., Gharakhanian E., Emr S. D. The sorting receptor for yeast vacuolar carboxypeptidase Y is encoded by the VPS10 gene. Cell. 1994 May 20;77(4):579–586. doi: 10.1016/0092-8674(94)90219-4. [DOI] [PubMed] [Google Scholar]
- Munn A. L., Silveira L., Elgort M., Payne G. S. Viability of clathrin heavy-chain-deficient Saccharomyces cerevisiae is compromised by mutations at numerous loci: implications for the suppression hypothesis. Mol Cell Biol. 1991 Aug;11(8):3868–3878. doi: 10.1128/mcb.11.8.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson K. K., Lemmon S. K. Suppressors of clathrin deficiency: overexpression of ubiquitin rescues lethal strains of clathrin-deficient Saccharomyces cerevisiae. Mol Cell Biol. 1993 Jan;13(1):521–532. doi: 10.1128/mcb.13.1.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr S. F., Conibear E., Stevens T. H. Golgi and vacuolar membrane proteins reach the vacuole in vps1 mutant yeast cells via the plasma membrane. J Cell Biol. 1995 Apr;129(1):35–46. doi: 10.1083/jcb.129.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr S. F., Roberts C. J., Stevens T. H. Membrane protein retention in the yeast Golgi apparatus: dipeptidyl aminopeptidase A is retained by a cytoplasmic signal containing aromatic residues. J Cell Biol. 1993 Jun;121(6):1197–1209. doi: 10.1083/jcb.121.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Schekman R. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1858–1862. doi: 10.1073/pnas.76.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H., Stewart J., Fournier M. C., Bosshart H., Rhee I., Miyatake S., Saito T., Gallusser A., Kirchhausen T., Bonifacino J. S. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995 Sep 29;269(5232):1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Hasson T. B., Hasson M. S., Schekman R. Genetic and biochemical characterization of clathrin-deficient Saccharomyces cerevisiae. Mol Cell Biol. 1987 Nov;7(11):3888–3898. doi: 10.1128/mcb.7.11.3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne G. S., Schekman R. A test of clathrin function in protein secretion and cell growth. Science. 1985 Nov 29;230(4729):1009–1014. doi: 10.1126/science.2865811. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Schekman R. Clathrin: a role in the intracellular retention of a Golgi membrane protein. Science. 1989 Sep 22;245(4924):1358–1365. doi: 10.1126/science.2675311. [DOI] [PubMed] [Google Scholar]
- Phan H. L., Finlay J. A., Chu D. S., Tan P. K., Kirchhausen T., Payne G. S. The Saccharomyces cerevisiae APS1 gene encodes a homolog of the small subunit of the mammalian clathrin AP-1 complex: evidence for functional interaction with clathrin at the Golgi complex. EMBO J. 1994 Apr 1;13(7):1706–1717. doi: 10.1002/j.1460-2075.1994.tb06435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper R. C., Cooper A. A., Yang H., Stevens T. H. VPS27 controls vacuolar and endocytic traffic through a prevacuolar compartment in Saccharomyces cerevisiae. J Cell Biol. 1995 Nov;131(3):603–617. doi: 10.1083/jcb.131.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prill V., Lehmann L., von Figura K., Peters C. The cytoplasmic tail of lysosomal acid phosphatase contains overlapping but distinct signals for basolateral sorting and rapid internalization in polarized MDCK cells. EMBO J. 1993 May;12(5):2181–2193. doi: 10.1002/j.1460-2075.1993.tb05866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rad M. R., Phan H. L., Kirchrath L., Tan P. K., Kirchhausen T., Hollenberg C. P., Payne G. S. Saccharomyces cerevisiae Apl2p, a homologue of the mammalian clathrin AP beta subunit, plays a role in clathrin-dependent Golgi functions. J Cell Sci. 1995 Apr;108(Pt 4):1605–1615. doi: 10.1242/jcs.108.4.1605. [DOI] [PubMed] [Google Scholar]
- Raymond C. K., Howald-Stevenson I., Vater C. A., Stevens T. H. Morphological classification of the yeast vacuolar protein sorting mutants: evidence for a prevacuolar compartment in class E vps mutants. Mol Biol Cell. 1992 Dec;3(12):1389–1402. doi: 10.1091/mbc.3.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redding K., Holcomb C., Fuller R. S. Immunolocalization of Kex2 protease identifies a putative late Golgi compartment in the yeast Saccharomyces cerevisiae. J Cell Biol. 1991 May;113(3):527–538. doi: 10.1083/jcb.113.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C. J., Nothwehr S. F., Stevens T. H. Membrane protein sorting in the yeast secretory pathway: evidence that the vacuole may be the default compartment. J Cell Biol. 1992 Oct;119(1):69–83. doi: 10.1083/jcb.119.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti T., Cardelli J. A., Niswonger M. L., O'Halloran T. J. Clathrin heavy chain functions in sorting and secretion of lysosomal enzymes in Dictyostelium discoideum. J Cell Biol. 1994 Jul;126(2):343–352. doi: 10.1083/jcb.126.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Garg C., Böker C., Nadimpalli S. K., von Figura K., Hille-Rehfeld A. Tail-specific antibodies that block return of 46,000 M(r) mannose 6-phosphate receptor to the trans-Golgi network. J Cell Biol. 1993 Aug;122(3):541–551. doi: 10.1083/jcb.122.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger M., Payne G. S. A role for clathrin in the sorting of vacuolar proteins in the Golgi complex of yeast. EMBO J. 1992 Aug;11(8):2811–2818. doi: 10.1002/j.1460-2075.1992.tb05348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger M., Payne G. S. Selective and immediate effects of clathrin heavy chain mutations on Golgi membrane protein retention in Saccharomyces cerevisiae. J Cell Biol. 1992 Aug;118(3):531–540. doi: 10.1083/jcb.118.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A., Carpenter G. Interaction of activated EGF receptors with coated pit adaptins. Science. 1993 Jul 30;261(5121):612–615. doi: 10.1126/science.8342026. [DOI] [PubMed] [Google Scholar]
- Sosa M. A., Schmidt B., von Figura K., Hille-Rehfeld A. In vitro binding of plasma membrane-coated vesicle adaptors to the cytoplasmic domain of lysosomal acid phosphatase. J Biol Chem. 1993 Jun 15;268(17):12537–12543. [PubMed] [Google Scholar]
- Stepp J. D., Pellicena-Palle A., Hamilton S., Kirchhausen T., Lemmon S. K. A late Golgi sorting function for Saccharomyces cerevisiae Apm1p, but not for Apm2p, a second yeast clathrin AP medium chain-related protein. Mol Biol Cell. 1995 Jan;6(1):41–58. doi: 10.1091/mbc.6.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens T. H., Rothman J. H., Payne G. S., Schekman R. Gene dosage-dependent secretion of yeast vacuolar carboxypeptidase Y. J Cell Biol. 1986 May;102(5):1551–1557. doi: 10.1083/jcb.102.5.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoorvogel W., Oorschot V., Geuze H. J. A novel class of clathrin-coated vesicles budding from endosomes. J Cell Biol. 1996 Jan;132(1-2):21–33. doi: 10.1083/jcb.132.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan P. K., Davis N. G., Sprague G. F., Payne G. S. Clathrin facilitates the internalization of seven transmembrane segment receptors for mating pheromones in yeast. J Cell Biol. 1993 Dec;123(6 Pt 2):1707–1716. doi: 10.1083/jcb.123.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. C., Brewer C. B., Roth M. G. Vesicular stomatitis virus glycoprotein contains a dominant cytoplasmic basolateral sorting signal critically dependent upon a tyrosine. J Biol Chem. 1993 Feb 15;268(5):3313–3320. [PubMed] [Google Scholar]
- Thomas D. C., Roth M. G. The basolateral targeting signal in the cytoplasmic domain of glycoprotein G from vesicular stomatitis virus resembles a variety of intracellular targeting motifs related by primary sequence but having diverse targeting activities. J Biol Chem. 1994 Jun 3;269(22):15732–15739. [PubMed] [Google Scholar]
- Vater C. A., Raymond C. K., Ekena K., Howald-Stevenson I., Stevens T. H. The VPS1 protein, a homolog of dynamin required for vacuolar protein sorting in Saccharomyces cerevisiae, is a GTPase with two functionally separable domains. J Cell Biol. 1992 Nov;119(4):773–786. doi: 10.1083/jcb.119.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida T. A., Graham T. R., Emr S. D. In vitro reconstitution of intercompartmental protein transport to the yeast vacuole. J Cell Biol. 1990 Dec;111(6 Pt 2):2871–2884. doi: 10.1083/jcb.111.6.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida T. A., Huyer G., Emr S. D. Yeast vacuolar proenzymes are sorted in the late Golgi complex and transported to the vacuole via a prevacuolar endosome-like compartment. J Cell Biol. 1993 Jun;121(6):1245–1256. doi: 10.1083/jcb.121.6.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox C. A., Fuller R. S. Posttranslational processing of the prohormone-cleaving Kex2 protease in the Saccharomyces cerevisiae secretory pathway. J Cell Biol. 1991 Oct;115(2):297–307. doi: 10.1083/jcb.115.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
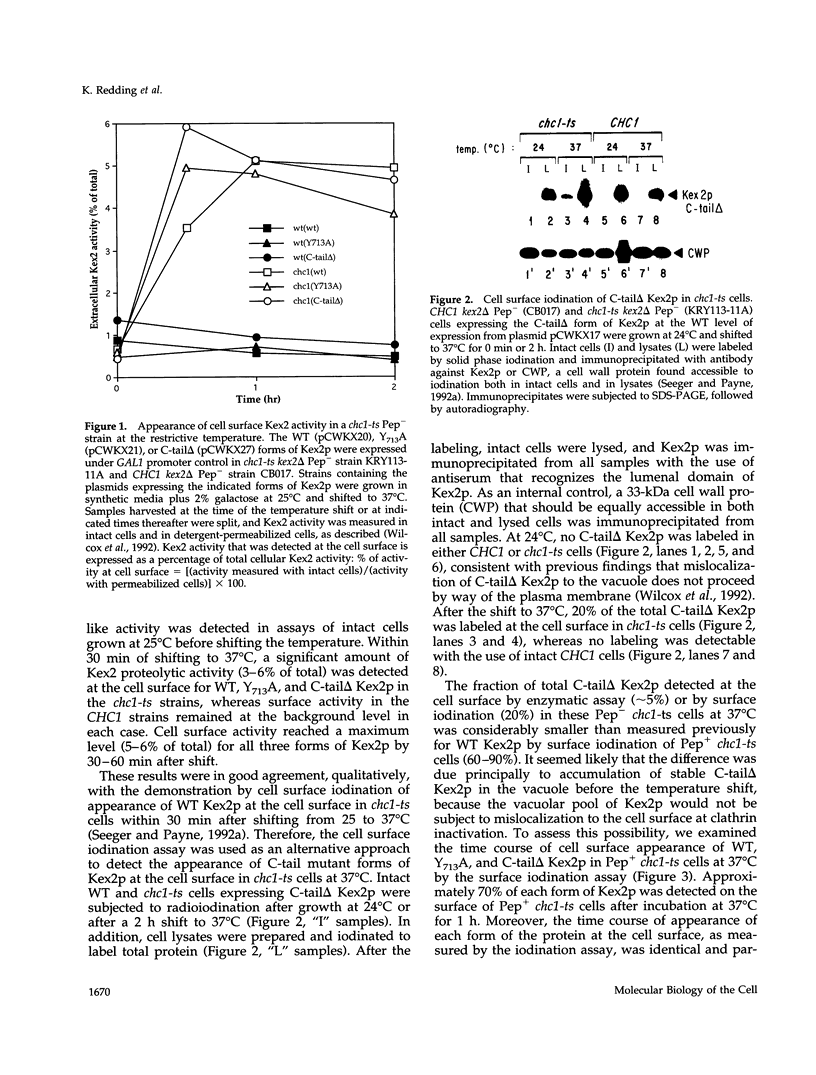
- Wilcox C. A., Redding K., Wright R., Fuller R. S. Mutation of a tyrosine localization signal in the cytosolic tail of yeast Kex2 protease disrupts Golgi retention and results in default transport to the vacuole. Mol Biol Cell. 1992 Dec;3(12):1353–1371. doi: 10.1091/mbc.3.12.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsbach K., Payne G. S. Vps1p, a member of the dynamin GTPase family, is necessary for Golgi membrane protein retention in Saccharomyces cerevisiae. EMBO J. 1993 Aug;12(8):3049–3059. doi: 10.1002/j.1460-2075.1993.tb05974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bliek A. M., Redelmeier T. E., Damke H., Tisdale E. J., Meyerowitz E. M., Schmid S. L. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J Cell Biol. 1993 Aug;122(3):553–563. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]