Abstract
AIM: To investigate the anti-oxidant and anti-neutrophil recruitment effects of rectal d-alpha (d-α) tocopherol administration on mild and moderately active ulcerative colitis (UC).
METHODS: Fifteen patients with mild and moderately active ulcerative colitis were enrolled in an open-label study of d-α tocopherol enema (8000 U/d) for 12 wk. All patients were receiving concomitant therapy with 5-aminosalicylic acid derivatives (5-ASA) and/or immunomodulator medications. Endoscopic evaluation was performed at baseline and after 4th and 12th weeks. Disease activity was measured with the Mayo disease activity index (DAI) and remission was defined as DAI of ≤ 2 with no blood in stool. Clinical response was defined as a DAI reduction of ≥ 2.
RESULTS: At the end of 12th week, the average DAI score significantly decreased compared to the beginning of the study (2.3 ± 0.37 vs 8 ± 0.48, P < 0.0001). One patient was withdrawn after 3 wk for being unavailable to follow-up. On the 4th week of therapy, 12 patients showed clinical response, 3 of whom (21.4%) achieving remission. After 12 wk, all 14 patients responded clinically to the therapy and remission was induced in 9 of them (64%). No patient reported adverse events or was hospitalized due to worsened disease activity.
CONCLUSION: This preliminary report suggests that rectal d-α tocopherol may represent a novel therapy for mild and moderately active UC. The observed results might be due to the anti-inflammatory and anti-oxidative properties of vitamin E.
Keywords: Vitamin E, Ulcerative colitis, Inflammatory bowel disease, Enema, Activity index
INTRODUCTION
Inflammatory bowel disease (IBD) comprises a group of chronic, lifelong, immuno-inflammatory disorders, characterized by flare-ups due to increased inflammatory activity of the intestinal mucosa, interspersed with asymptomatic periods of remission[1]. Although the etiology of IBD remains unknown, it is believed that the generation of an exaggerated intestinal immune response to otherwise innocuous stimuli plays a key role in the pathophysiology of this intestinal disorder[2]. IBD is mainly characterized by upregulation of synthesis and release of different proinflammatory mediators, including reactive oxygen and nitrogen metabolites, eicosanoids, platelet-activating factor and cytokines. All of these mediators actively contribute to the pathogenic cascade that initiates and perpetuates the inflammatory response of the gut. Thus, the treatment strategy for IBD focuses on eliminating these causal inflammatory triggers and mediators.
Unfortunately no disease-specific treatment for IBD has yet emerged, and the best strategy to effectively downregulate the exacerbated immune response is likely to interfere with multiple stages of the inflammatory cascade[3]. In fact, the drugs currently used for the management of human IBD, i.e. 5-aminosalicylic acid derivatives, immunosuppressives and systemic or local glucocorticoids, exert their beneficial effects through a combination of different mechanisms[4]. On the other hand, even the most effective drugs used in inflammatory bowel disease are only successful in about two-thirds of patients[5,6], while these drugs are not devoid of potentially serious side effects that limit their use in a further substantial proportion of patients[7,8].
It is now well established that vitamin E is a major lipophilic antioxidant in cellular membranes with excellent antioxidant activities[9,10] which protects membrane lipids from peroxidation[11,12] by scavenging not only chain carrying peroxyl radicals but also singlet oxygen and superoxide anion radicals[13]. This is especially interesting in the case of ulcerative colitis (UC), considering the pivotal role of oxygen free radicals in the genesis of mucosal damage. Additionally, the production of reactive oxygen species increases by the colonic mucosa in patients with UC[14-16]; and inhibition of lipid peroxidation or scavenging of oxygen free radicals produces valuable preventive and therapeutic strategies for IBD[12].
Thus, given the recent evidence suggesting anti-inflammatory properties for vitamin E[17,18], d-alpha (d-α) tocopherol, as the dominant vitamin E isomer in plasma with the highest biopotency, may be expected to reduce the development of tissue injury in IBD. In support of this hypothesis, we report the preliminary results of an ongoing open-label case series study on clinical and endoscopic changes of disease severity in patients with active UC who received daily rectal doses of d-α tocopherol for at least 12 wk.
MATERIALS AND METHODS
Inclusion and exclusion criteria
We recruited 15 volunteer UC patients (5 males, 10 females) between February 2006 and February 2008, seen in our university-based gastroenterology practice. The study protocol was submitted to the university ethics committee and written informed consent was obtained from all participating patients. All enrolled patients had active disease, limited to sigmoid at the beginning of the study. In order to minimize observation errors, all diagnoses were made on the basis of a combination of clinical, endoscopic and histological criteria by a single faculty member gastroenterologist. Only patients with mild and moderately active UC despite a minimum of 4 wk of therapy with at least 3 g/d of an oral 5-ASA compound were eligible for inclusion to whom we permitted concomitant therapy with azathioprine or 6-mercaptopurine as long as the patient had been receiving the medication for a minimum of 24 wk and was on a stable dose for a minimum of 12 wk before enrolment. No patient received concomitant therapy with corticosteroids or rectally administered therapies of any kind. Use of anti-diarrheal medications was not permitted and no patient had taken additional non-steroidal anti-inflammatory drugs (NSAIDs) and/or antibiotics during the 3 mo preceding their enrolment.
Patients were excluded if they had evidence of infectious colitis, history of an active malignancy, previous surgical procedure except for cholecystectomy or appendicitis, and contraindication to flexible sigmoidoscopy or biopsy. Further exclusion criteria included present or recent pregnancy, and concomitant serious illness such as history of diabetes mellitus, hypertension, severe liver disease and cardiac failure.
Study design
Disease activity was assessed using Mayo Disease Activity Index (DAI)[19]. This index is calculated by summing the scores of four factors, each of which are graded on a scale from 0 to 3. The four features of disease activity are stool frequency, bleeding, physician’s assessment of disease activity, and mucosal appearance. The maximum potential score is 12 points, with mild and moderate disease activity defined as a score below 10. Only patients with mild or moderate active UC based on DAI score were selected.
A primary clinical and colonoscopic evaluation was performed in all cases. Patients were then assessed clinically every 2 wk, and the following endoscopic evaluations were performed after 4th and 12th weeks. Therefore, it was not possible to calculate a DAI score at each time point. However, we computed a modified DAI (mDAI) score that includes all components of the full DAI score except the endoscopic appearance of the bowel. The maximum potential score in the mDAI is 9.
Meticulous laboratory evaluations were conducted for all participants at the time of recruitment, including determination of the hematocrit value, white blood cell and platelet count, liver function tests, ERS, stool exam and culture, and serum level of vitamin E. Serum samples were stored at -80°C until analysis. Vitamin E was measured, after extraction with methanol, by HPLC, with UV detection at 294 nm. Methanol, deionized water and butanol (90:4:6) were used as mobile phase and the column was Eurospher 100 C8 (4.6 mm × 25 cm). Re-evaluation of these tests was done each month until completion of study[20].
All patients were trained to rectally apply the sufficient amount of liquid vitamin E (0912, NOW Foods, IL, USA) equivalent to 8000 IU (15 mL) using specific instrument for enema (Enema irrigator disposable, Model: D-201, Taiwan Snatch Co., Taiwan; Figure 1) every night at home and were advised to lie for at least 15 min in left supine position after administration. During the first 12 wk all patients were given diary sheets containing multiple choice questions on stool characteristics including the number of defecation per day, stool consistency and type of blood excretion at each defecation episode. Patients were asked to complete each sheet on its day and bring them back at next visit (every 2 wk). After the first 12 wk we stopped obtaining diary sheets from patients and continued the follow-up by monthly visits only and endoscopic studies every 3 mo. This second phase of study is still in progress.
Figure 1.
Specific instrument for enema (Enema irrigator disposable). Fifteen mL of the liquid vitamin E was rectally administered in our patients using this disposable enema irrigator.
Definition of outcomes
Patients with a final DAI score of ≤ 2 points were considered to have achieved remission. A clinical response was defined as a reduction in the DAI of ≥ 2 points. Clinical relapse was defined as the occurrence or worsening of symptoms, accompanied by an increase in the DAI score to 4, necessitating a change in therapy (addition of rectal therapies such corticosteroids or 5-ASA, surgery, etc). Refractory patients were those who had no significant improvement from their baseline, despite 12 wk of drug application.
Statistical analysis
Descriptive data are reported as percentages and medians and ranges. For statistical analysis, the 1-way analysis of variance (ANOVA) followed by Tukey post hoc was used. When appropriate, The Student t distribution was employed to compare 2 groups. Average DAI and mDAI scores at different time points were compared using the Mann-Whitney U test. All analyses used two sided tests of statistical significance with a significance level of 0.05.
RESULTS
Fifteen patients (5 males) were enrolled in the study and one patient left the study due to permanent change of his city of residence. Therefore the analysis was performed on 14 patients. The age of patients was 33 ± 9.6 years (mean ± SE) ranging from 21 to 55 years old, and the duration of UC was 4.7 ± 1.6 years. At enrolment, all patients had active disease with mild and moderate disease activity restricted to sigmoid (30 cm maximum involvement). All patients were receiving treatment at the time of entry into the study, consisting of sulphasalazine in 4 cases and mesalazine in the others. Two patients were also receiving azathioprine. No patient was on corticosteroids, antimicrobials or NSAIDS. At enrollment, the median Mayo DAI score was 8 (range, 4 to 10). Only 3 patients had mild disease activity, while the other 11 patients had moderate UC. On the 12th week, 9 patients had disease of mild severity and 5 had moderately active disease (Figure 2).
Figure 2.
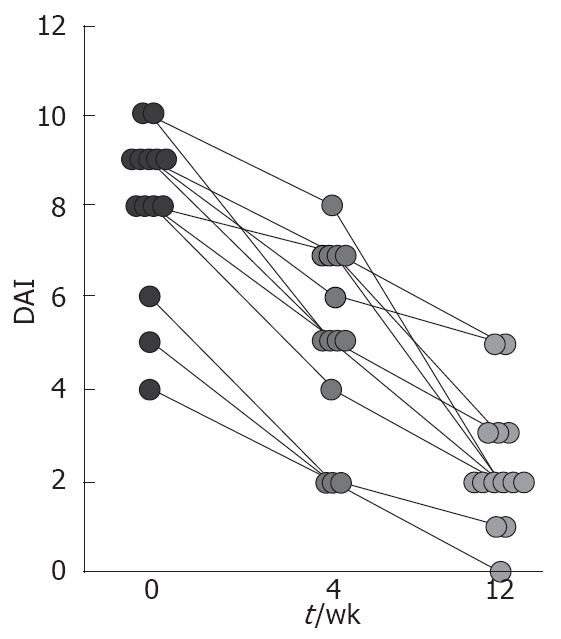
Individual changes in disease activity from week 0 to 12, assessed by the Mayo Disease Activity Index, in subjects who received d-α Tocopherol enema. One patient who was withdrawn early from the study is not included. There was a significant decrease in the mean DAI score for patients after 4th and 12th weeks of therapy (P < 0.0001).
As summarized in Figure 2, clinical response was observed in 12 patients on the 4th week while clinical remission was induced only in 3 patients. After 12 wk, all patients responded clinically to therapy, with 9 of them going to clinical remission. In a secondary analysis, we examined the change in the DAI and mDAI scores over time (Figures 2 and 3). The mean DAI score before and on the 4th and 12th week of therapy were 8 ± 0.48, 5.1 ± 0.54 and 2.3 ± 0.37, respectively. Mann-Whitney U test revealed significant decrease in the mean DAI scores on 4th and 12th week of therapy comparing to the baseline (P = 0.01 and P < 0.0001, respectively) as shown in Figure 2. The average mDAI score started to decrease significantly on second week (Figure 3). As shown in Figure 4, mDAI score was significantly lower than at baseline for patients by week 4 and remained significantly lower for the remainder of the study (P < 0.0001 at weeks 4, 6, 8, 10 and 12, respectively). During the course of study there was no case of worsening disease activity or report of serious adverse event.
Figure 3.
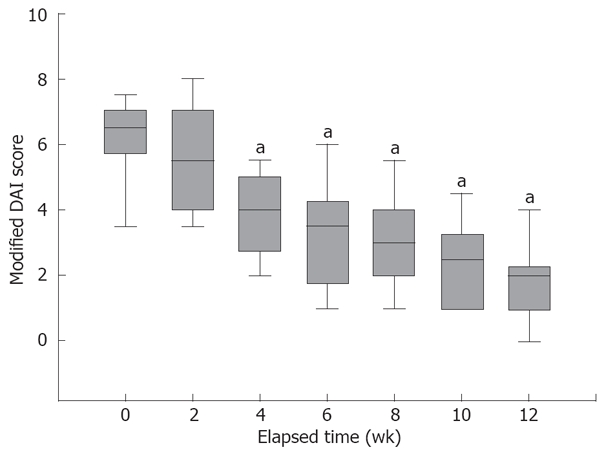
Change in the modified DAI. A modified DAI score, including all components of the complete DAI other than the endoscopic appearance is calculated at each observation point. By week 4 the mean modified DAI score significantly decreased compared to baseline (week 0) scores. aP < 0.0001 vs baseline.
Figure 4.
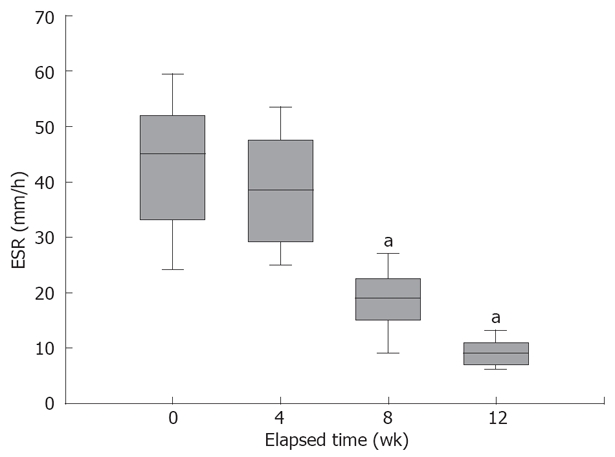
Individual changes in ESR from week 0 to 12, in our patients. There was a significant decrease in the mean ESR on the 8th and the 12th week of therapy compared to the beginning of the study. aP < 0.0001 vs baseline (0 wk).
At the end of 12 wk, 12 patients elected to continue to receive d-α tocopherol, while 2 patients left the survey due to personal reasons despite achieving a desirable response and satisfaction with therapy. All patients are assessed monthly for symptoms of disease activity and undergo endoscopic studies every 3 mo. The overall follow-up of patients in our study is approaching its mean of 8 mo by the time of composing this article. Interestingly, there have been no recurrences in patients who remained on therapy, while 2 flare-ups occurred in patients who left the study, after 4 wk and 7 wk.
As shown in Figure 4 the mean value of baseline ESR was 42.26 ± 11.7 while it was 9.33 mm/h on 12th week, showing a significant decrease (P < 0.0001). Eleven patients had moderate anemia at baseline (defined as haematocrit < 35 in men and < 40 in women) for which supplemental iron and folic acids were prescribed. At the end of the 12 wk, a low haematocrit was detected in only 3 patients, and it increased from 35.3 ± 6 to 43.9 ± 6 after 12 wk. The serum level of α-tocopherol did not change significantly after 12 wk compared to that of baseline (7.2 ± 4.2 and 6.7 ± 1.1, respectively), and all patients had serum levels less than or within the normal range (5-12 μg/dL).
DISCUSSION
This case series provides evidence suggesting that local administration of d-α tocopherol might exert protective effects against UC in patients with mild and moderately active disease. In our study, rectal administration of d-α tocopherol significantly reduced the clinical disease activity indices and eliminated further need to corticosteroid therapy in otherwise non-responsive UC patients.
Recent studies demonstrated that plasma levels of α-tocopherol decrease after infection, trauma, burns, and inflammatory reactions, indicating that this antioxidant is exhausted during acute tissue injury[21,22]. Similar concept exists for inflammatory bowel disease, indicating that anti-oxidants are intimately involved in the process of active IBD, for example ingested high doses of vitamin E as an anti-oxidant along with other anti-oxidants reduce the extent of tissue damage in IBD, and tissue antioxidant levels are shown to be decreased in UC patients[23-25]. Systemic α-tocopherol in conjunction with selenium is also shown to reduce the severity of IBD in chemically induced colitis in rats[26]. Therefore, it is tempting to hypothesize that increasing the exposure of affected intestinal mucosa to this anti-oxidant might strengthen the defense capacity of the affected gut.
Vitamin E, particularly α-tocopherol, exerts a number of non-anti-oxidant functions, some associated with inhibition of protein kinase C (PKC) such as inhibition of platelet aggregation, and others independent of PKC such as the expression of intercellular adhesion molecule-1 (ICAM-1, CD54), integrins and CD36[27,28]. α-tocopherol also inhibits respiratory burst in human macrophages via a mechanism involving PKC inhibition, followed by attenuation of p47 (phox) phosphorylation and membrane translocation[29].
Increased NF-κB activity is found in inflamed intestinal mucosa; and factors implicated in IBD, such as TNF-α, LPS, and IL-1, are potent activators of NF-κB[30]. On the other hand, reactive oxygen species, the hypothetical mediators of IBD flare-ups, have been implicated in the stimulation of the signal transduction pathway involving NF-κB. Many current therapies for IBD act at least in part through the inhibition of NF-κB or through inhibition of signals that activate NF-κB. Interestingly, α-tocopherol is proved to inhibit NF-κB activation in rat Kupffer cells and a human monocytic cell line[31,32].
One of the proinflammatory genes regulated by NF-κB is ICAM-1 (cell surface glycoprotein, playing a critical role in mediating leukocyte-endothelial and a marker of active inflammation). Gulubova et al[33] demonstrated a marked upregulation of endothelial E-selectin, ICAM-1, and VCAM-1 expression in the inflamed colonic mucosa and submucosa in active UC. Infiltration of neutrophils into colonic mucosa is a central event in the acute phase of UC for which cell adhesion molecules are necessary[34]. Accordingly, by blocking the activity of NF-κB, which leads to decreased production of proinflammatory cytokines, anti-inflammatory properties of α-tocopherol might be explained. Therefore, α-tocopherol in high local doses might also affect the binding affinity between the neutrophil and the endothelial cell by decreasing the expression of adhesion molecules on the endothelial cells.
Systemic markers of inflammation, such as ESR, CRP, platelet count and white blood cell count are commonly used in clinical practice, but correlation to ongoing intestinal inflammation is poor[35]. Therefore, we monitored the consecutive changes in ESR (Figure 4) and complete blood count (CBC) of our patients. These results show a significant conversion of ESR and quantitative increase in haematocrit.
To the best of our knowledge, this is the first data on remission inducing properties of vitamin E. This study also confirms the feasibility and acceptability of rectal administration of vitamin E in patients with IBD. Except for one patient who left the study due to difficulty for follow-up, all patients completed the three month course of study required for primary assessments. Furthermore, 12 patients are still taking medication and participate in the regular evaluations. By the time of composing this article, the average course of treatment is approaching 8 mo. Disease activity was rated as inactive (clinical remission) in 64%. Comparing these results with the beginning of study demonstrates a significant reduction in disease severity of UC patients (P < 0.0001) starting after the 4th week of therapy. As shown in Figure 3, symptoms started to improve after 3 wk. This result is comparable with conventional therapies for IBD such as mesalazine or sulfasalazine. Overall satisfaction of patients despite the uncomfortable method of nightly rectal administration of d-α tocopherol reflects that the desirable outcomes outweigh its difficulty in application.
Elevated and toxic levels of plasma vitamin E were a concern in our study, since there was no previous study measuring the systemic absorption of vitamin E when applied intrarectally. Thus, plasma levels of vitamin E were measured to eliminate concerns about vitamin E overdosage and results revealed that the mean vitamin E level was not significantly different from that of before the study. This shows that despite the high doses of rectal vitamin E administered every night, little is absorbed and no concern remains about the overdosage of vitamin E in patients.
In the setting of a case series study we only recruited UC patients with strictly defined criteria. Our hypothesis was the increased local effects of vitamin E in UC, in which the pathology is mainly restricted to mucosal layer. In addition to the substantial physical and financial burden of IBD on patients, it is often difficult to continue treatment due to decreased therapeutic effects or adverse reactions over time[7,8]. In this regard, use of vitamin E may expand the choices available for treatment of IBD, simplifying prescription or therapeutic technique.
The nature of our study casts limitations to implicate definite clinical results from this report, since case series cannot measure and do not eliminate the placebo effect. However, the best expected placebo effect is reported to be 16%-52% in rectal therapies of IBD which is still far lower than our results[36]. Best UC therapies only have 60% to 80% success rates, while we gained 100% clinical response with over 60% remission induction in our patients on the 12th week. This is especially important considering that these results are achieved without prescribing any synthetic agent, and we eliminated the need to corticosteroids in patients otherwise resistance to therapy.
Taken together, case series like this study are best used as a source of hypotheses for investigation by stronger study designs. Thus, future researchs should aim at testing the efficacy of natural vitamin E enema in a well controlled study to measure its exact effect on reducing risk of flare-ups with the minimum confounding factors. This evidence suggests that vitamin E reduced the development of colon inflammation. The observed effect seems to be due to antioxidative and anti-inflammatory effects of vitamin E which is potently taking effect by local administration. Based on our preliminary results vitamin E might show considerable promise as a new therapeutic modality for IBD.
COMMENTS
Background
The exact etiology of inflammatory bowel disease (IBD) is not yet understood, however it is believed that the generation of an exaggerated intestinal immune response to otherwise innocuous stimuli plays a key role in the pathophysiology of this intestinal disorder. IBD is mainly characterized by upregulation of synthesis and release of different pro-inflammatory mediators all of which actively contribute to the pathogenic cascade that initiates and perpetuates the inflammatory response of the gut. Thus, the current treatment strategy for IBD focuses on eliminating these causal inflammatory triggers and mediators.
Research frontiers
Vitamin E is a major lipophilic antioxidant in cellular membranes with excellent antioxidant activities which protects membrane lipids from peroxidation by scavenging not only chain carrying peroxyl radicals but also singlet oxygen and superoxide anion radicals. This is especially interesting in case of ulcerative colitis (UC), considering the pivotal role of oxygen free radicals in the genesis of mucosal damage. Given the recent evidence suggesting anti-inflammatory properties for Vitamin E, d-alpha (d-α) tocopherol, as the dominant vitamin E isomer in plasma with the highest biopotency, may be expected to reduce the development of tissue injury in UC.
Innovations and breakthroughs
This case series provides evidence for the first time that local administration of d-α tocopherol might exert protective effects against UC. The authors have carefully followed the serial alterations of patients’ disease activity index, along with few other markers of disease severity and have shown that rectal administration of d-α tocopherol significantly reduces the clinical disease activity indices which eliminated further need to corticosteroid therapy in otherwise non-responsive UC patients with mild and moderately active disease. The observed effect seems to be due to antioxidant and anti-inflammatory effects of vitamin E which is potently taking effect by local administration.
Applications
The results of this interesting study suggests that natural antioxidants like d-α tocopherol might show considerable promise as new therapeutic modalities for IBD, with apparently lower side effects and complications compared to the current therapies.
Peer review
This is the first study to address the immunoregulatory effects of d-α tocopherol in a dominant Th1 response disease. The outstanding results demonstrate that natural isomer of vitamin E, reduces the extent of macroscopic mucosal damage and clinical severity of related syndromes in UC patients, when applied intra-rectally for as short as 4 wk.
Acknowledgments
The personnel of Pars Hospital, Tehran, are thanked for the collaboration. Authors thank Dr. Taha Gholipour (Tehran University of Medical Sciences) for kindly accepting to review the manuscript. This work was supported by a research grant provided by the Tehran University of Medical Sciences.
Footnotes
Supported by Research grant provided by the Tehran University of Medical Sciences
Peer reviewer: Peter R Gibson, Professor, Department of Medicine, Monash University, Box Hill Hospital, Box Hill 3128, Australia
S- Editor Zhong XY L- Editor Negro F E- Editor Lin YP
References
- 1.Keefer L, Keshavarzian A. Feasibility and acceptability of gut-directed hypnosis on inflammatory bowel disease: a brief communication. Int J Clin Exp Hypn. 2007;55:457–466. doi: 10.1080/00207140701506565. [DOI] [PubMed] [Google Scholar]
- 2.Camuesco D, Galvez J, Nieto A, Comalada M, Rodriguez-Cabezas ME, Concha A, Xaus J, Zarzuelo A. Dietary olive oil supplemented with fish oil, rich in EPA and DHA (n-3) polyunsaturated fatty acids, attenuates colonic inflammation in rats with DSS-induced colitis. J Nutr. 2005;135:687–694. doi: 10.1093/jn/135.4.687. [DOI] [PubMed] [Google Scholar]
- 3.Kho YH, Pool MO, Jansman FG, Harting JW. Pharmacotherapeutic options in inflammatory bowel disease: an update. Pharm World Sci. 2001;23:17–21. doi: 10.1023/a:1011268302386. [DOI] [PubMed] [Google Scholar]
- 4.Belluzzi A. Polyunsaturated fatty acids (n-3 PUFAs) and inflammatory bowel disease (IBD): pathogenesis and treatment. Eur Rev Med Pharmacol Sci. 2004;8:225–229. [PubMed] [Google Scholar]
- 5.Kamm MA. Review article: maintenance of remission in ulcerative colitis. Aliment Pharmacol Ther. 2002;16 Suppl 4:21–24. doi: 10.1046/j.1365-2036.16.s4.4.x. [DOI] [PubMed] [Google Scholar]
- 6.Xu CT, Meng SY, Pan BR. Drug therapy for ulcerative colitis. World J Gastroenterol. 2004;10:2311–2317. doi: 10.3748/wjg.v10.i16.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stein RB, Hanauer SB. Comparative tolerability of treatments for inflammatory bowel disease. Drug Saf. 2000;23:429–448. doi: 10.2165/00002018-200023050-00006. [DOI] [PubMed] [Google Scholar]
- 8.Nakajima H, Munakata A, Yoshida Y. Adverse effects of sulfasalazine and treatment of ulcerative colitis with mesalazine. J Gastroenterol. 1995;30 Suppl 8:115–117. [PubMed] [Google Scholar]
- 9.Burton GW, Traber MG. Vitamin E: antioxidant activity, biokinetics, and bioavailability. Annu Rev Nutr. 1990;10:357–382. doi: 10.1146/annurev.nu.10.070190.002041. [DOI] [PubMed] [Google Scholar]
- 10.Hajiani M, Golestani A, Shariftabrizi A, Rastegar R, Payabvash S, Salmasi AH, Dehpour AR, Pasalar P. Dose-dependent modulation of systemic lipid peroxidation and activity of anti-oxidant enzymes by vitamin E in the rat. Redox Rep. 2008;13:60–66. doi: 10.1179/135100008X259114. [DOI] [PubMed] [Google Scholar]
- 11.Burton GW, Joyce A, Ingold KU. First proof that vitamin E is major lipid-soluble, chain-breaking antioxidant in human blood plasma. Lancet. 1982;2:327. doi: 10.1016/s0140-6736(82)90293-8. [DOI] [PubMed] [Google Scholar]
- 12.Isozaki Y, Yoshida N, Kuroda M, Takagi T, Handa O, Kokura S, Ichikawa H, Naito Y, Okanoue T, Yoshikawa T. Effect of a novel water-soluble vitamin E derivative as a cure for TNBS-induced colitis in rats. Int J Mol Med. 2006;17:497–502. [PubMed] [Google Scholar]
- 13.Fukuzawa K, Gebicki JM. Oxidation of alpha-tocopherol in micelles and liposomes by the hydroxyl, perhydroxyl, and superoxide free radicals. Arch Biochem Biophys. 1983;226:242–251. doi: 10.1016/0003-9861(83)90290-4. [DOI] [PubMed] [Google Scholar]
- 14.Lih-Brody L, Powell SR, Collier KP, Reddy GM, Cerchia R, Kahn E, Weissman GS, Katz S, Floyd RA, McKinley MJ, et al. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig Dis Sci. 1996;41:2078–2086. doi: 10.1007/BF02093613. [DOI] [PubMed] [Google Scholar]
- 15.McKenzie SJ, Baker MS, Buffinton GD, Doe WF. Evidence of oxidant-induced injury to epithelial cells during inflammatory bowel disease. J Clin Invest. 1996;98:136–141. doi: 10.1172/JCI118757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamada T, Grisham MB. Role of neutrophil-derived oxidants in the pathogenesis of intestinal inflammation. Klin Wochenschr. 1991;69:988–994. doi: 10.1007/BF01645144. [DOI] [PubMed] [Google Scholar]
- 17.Fox ES, Brower JS, Bellezzo JM, Leingang KA. N-acetylcysteine and alpha-tocopherol reverse the inflammatory response in activated rat Kupffer cells. J Immunol. 1997;158:5418–5423. [PubMed] [Google Scholar]
- 18.Yoshida N, Yoshikawa T, Manabe H, Terasawa Y, Kondo M, Noguchi N, Niki E. Vitamin E protects against polymorphonuclear leukocyte-dependent adhesion to endothelial cells. J Leukoc Biol. 1999;65:757–763. doi: 10.1002/jlb.65.6.757. [DOI] [PubMed] [Google Scholar]
- 19.Naber AH, de Jong DJ. Assessment of disease activity in inflammatory bowel disease; relevance for clinical trials. Neth J Med. 2003;61:105–110. [PubMed] [Google Scholar]
- 20.Combs GF Jr. The Vitamins: Fundamental Aspects in Nutrition and Health. Second ed. San Diego: Academic Press; 1998. p. 618. [Google Scholar]
- 21.Suntres ZE, Shek PN. Treatment of LPS-induced tissue injury: role of liposomal antioxidants. Shock. 1996;6 Suppl 1:S57–S64. [PubMed] [Google Scholar]
- 22.Demling R, LaLonde C, Ikegami K, Picard L, Nayak U. Alpha-tocopherol attenuates lung edema and lipid peroxidation caused by acute zymosan-induced peritonitis. Surgery. 1995;117:226–231. doi: 10.1016/s0039-6060(05)80090-x. [DOI] [PubMed] [Google Scholar]
- 23.D'Odorico A, Bortolan S, Cardin R, D'Inca' R, Martines D, Ferronato A, Sturniolo GC. Reduced plasma antioxidant concentrations and increased oxidative DNA damage in inflammatory bowel disease. Scand J Gastroenterol. 2001;36:1289–1294. doi: 10.1080/003655201317097146. [DOI] [PubMed] [Google Scholar]
- 24.Koch TR, Yuan LX, Stryker SJ, Ratliff P, Telford GL, Opara EC. Total antioxidant capacity of colon in patients with chronic ulcerative colitis. Dig Dis Sci. 2000;45:1814–1819. doi: 10.1023/a:1005517824877. [DOI] [PubMed] [Google Scholar]
- 25.Buffinton GD, Doe WF. Depleted mucosal antioxidant defences in inflammatory bowel disease. Free Radic Biol Med. 1995;19:911–918. doi: 10.1016/0891-5849(95)94362-h. [DOI] [PubMed] [Google Scholar]
- 26.Ademoglu E, Erbil Y, Tam B, Barbaros U, Ilhan E, Olgac V, Mutlu-Turkoglu U. Do vitamin E and selenium have beneficial effects on trinitrobenzenesulfonic acid-induced experimental colitis. Dig Dis Sci. 2004;49:102–108. doi: 10.1023/b:ddas.0000011610.47179.0b. [DOI] [PubMed] [Google Scholar]
- 27.Azzi A, Ricciarelli R, Zingg JM. Non-antioxidant molecular functions of alpha-tocopherol (vitamin E) FEBS Lett. 2002;519:8–10. doi: 10.1016/s0014-5793(02)02706-0. [DOI] [PubMed] [Google Scholar]
- 28.Breyer I, Azzi A. Differential inhibition by alpha- and beta-tocopherol of human erythroleukemia cell adhesion: role of integrins. Free Radic Biol Med. 2001;30:1381–1389. doi: 10.1016/s0891-5849(01)00541-x. [DOI] [PubMed] [Google Scholar]
- 29.Cachia O, Benna JE, Pedruzzi E, Descomps B, Gougerot-Pocidalo MA, Leger CL. alpha-tocopherol inhibits the respiratory burst in human monocytes. Attenuation of p47(phox) membrane translocation and phosphorylation. J Biol Chem. 1998;273:32801–32805. doi: 10.1074/jbc.273.49.32801. [DOI] [PubMed] [Google Scholar]
- 30.Ekstrand-Hammarstrom B, Osterlund C, Lilliehook B, Bucht A. Vitamin E down-modulates mitogen-activated protein kinases, nuclear factor-kappaB and inflammatory responses in lung epithelial cells. Clin Exp Immunol. 2007;147:359–369. doi: 10.1111/j.1365-2249.2006.03285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blackwell TS, Blackwell TR, Holden EP, Christman BW, Christman JW. In vivo antioxidant treatment suppresses nuclear factor-kappa B activation and neutrophilic lung inflammation. J Immunol. 1996;157:1630–1637. [PubMed] [Google Scholar]
- 32.Naraghi M, Deroee AF, Ebrahimkhani M, Kiani S, Dehpour A. Nitric oxide: a new concept in chronic sinusitis pathogenesis. Am J Otolaryngol. 2007;28:334–337. doi: 10.1016/j.amjoto.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 33.Gulubova MV, Manolova IM, Vlaykova TI, Prodanova M, Jovchev JP. Adhesion molecules in chronic ulcerative colitis. Int J Colorectal Dis. 2007;22:581–589. doi: 10.1007/s00384-006-0236-0. [DOI] [PubMed] [Google Scholar]
- 34.Strober W, Kelsall B, Fuss I, Marth T, Ludviksson B, Ehrhardt R, Neurath M. Reciprocal IFN-gamma and TGF-beta responses regulate the occurrence of mucosal inflammation. Immunol Today. 1997;18:61–64. doi: 10.1016/s0167-5699(97)01000-1. [DOI] [PubMed] [Google Scholar]
- 35.Cellier C, Sahmoud T, Froguel E, Adenis A, Belaiche J, Bretagne JF, Florent C, Bouvry M, Mary JY, Modigliani R. Correlations between clinical activity, endoscopic severity, and biological parameters in colonic or ileocolonic Crohn's disease. A prospective multicentre study of 121 cases. The Groupe d'Etudes Therapeutiques des Affections Inflammatoires Digestives. Gut. 1994;35:231–235. doi: 10.1136/gut.35.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyers S, Janowitz HD. The "natural history" of ulcerative colitis: an analysis of the placebo response. J Clin Gastroenterol. 1989;11:33–37. doi: 10.1097/00004836-198902000-00008. [DOI] [PubMed] [Google Scholar]


