Abstract
A 69-year-old man was diagnosed as having myasthenia gravis (MG) in September 2004, and treated with thymectomy and prednisolone. He was then diagnosed as having steroid-induced diabetes mellitus, and received sulfonylurea (SU) therapy in May 2005. An alpha- glucosidase inhibitor (αGI) was added in March 2006, resulting in good glycemic control. He experienced symptoms of abdominal distention, increased flatus, and constipation in October 2007, and was admitted into our hospital in late November with hematochezia. Plain abdominal radiography revealed small linear radiolucent clusters in the wall of the colon. Computed tomography (CT) showed intramural air in the sigmoid colon. Colonoscopy revealed multiple smooth surfaced hemispherical protrusions in the sigmoid colon. The diagnosis of pneumatosis cystoides intestinalis (PCI) was made on the basis of these findings. As the αGI voglibose was suspected as the cause of this patient’s PCI, treatment was conservative, ceasing voglibose, with fasting and fluid supplementation. The patient progressed well, and was discharged 2 wk later. Recently, several reports of PCI associated with αGI therapy have been published, predominantly in Japan where αGIs are commonly used. If the use of αGIs becomes more widespread, we can expect more reports of this condition on a global scale. The possibility of PCI should be considered in diabetic patients complaining of gastrointestinal symptoms, and the gastrointestinal tract should be thoroughly investigated in these patients.
Keywords: Alpha-glucosidase inhibitor, Colonoscopy, Diabetes mellitus, Pneumatosis cystoides intestinalis, Voglibose
INTRODUCTION
Pneumatosis cystoides intestinalis (PCI) is a rare condition in which multiple submucosal or subserosal pneumocystis develop in the submucosa or in subserosa of the colon[1,2]. The etiological mechanisms are unclear, although PCI has been reported to develop in association with raised intra-abdominal pressure due to ileus surgery[3-5], colonoscopy[6], pulmonary diseases such as chronic bronchitis and emphysema[7], trichloroethylene exposure[8], connective tissue disorders[9,10], the use of immunosuppressants[11], and ingestion of carbohydrates such as lactulose[12] and sorbitol[13]. Recently, the development of PCI during treatment with alpha-glucosidase inhibitors (αGIs), a new class of anti-diabetic agents, has been reported[14,15]. Our literature search yielded only 13 cases of PCI associated with αGI therapy[14-26]. Herein, we present a case depicting αGI as the probable cause of PCI, along with a review of the literature.
CASE REPORT
A 69-year-old man was diagnosed as having severe myasthenia gravis (MG) in September 2004, and treated with prednisolone (5 mg/d) from October of that year. He underwent thymectomy in March 2005. Hyperglycemia was detected in May 2005, leading to the diagnosis of steroid-induced diabetes mellitus, and sulfonylurea (SU) therapy was commenced immediately. As his blood sugar could not be controlled, αGI was prescribed in March 2006, resulting in good glycemic control. He claimed to have experienced abdominal distension, increased flatus and constipation, and noticed small amounts of bright rectal bleeding as early as mid-October 2007, but did nothing about it. The amount of rectal bleeding increased in late November that year, and he was referred to our hospital for investigation and treatment.
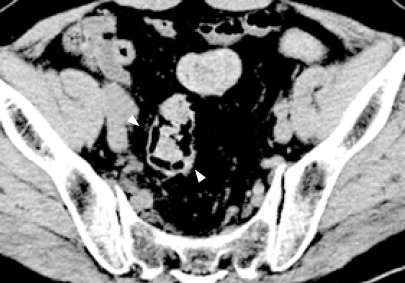
Laboratory investigations revealed no abnormalities in white blood cell (WBC) count, hemoglobin (Hb), or C-reactive protein, and HbA1c was slightly elevated to 6.0%. Plain abdominal radiography revealed small linear radiolucent gas collections along the wall of the colon (Figure 1). Unenhanced computed tomography (CT) of the abdomen showed intramural air in the sigmoid colon, and free gas in the peritoneal cavity around the sigmoid colon (Figure 2). Colonoscopy revealed multiple smooth surfaced small hemispherical protrusions in the sigmoid colon, and endoscopic ultrasonography (EUS) demonstrated highly echogenic submucosal lesions with acoustic shadows (Figure 3). The diagnosis of PCI was made on the basis of these findings.
Figure 1.
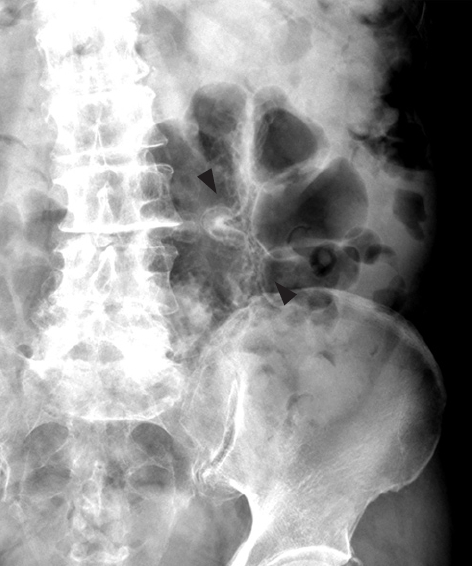
Plain radiography of the abdomen on admission revealing small linear and round radiolucent clusters in the wall of the colon (black arrows).
Figure 2.
Computed tomography (CT) scanning of the abdomen on admission revealing intramural gas in the sigmoid colon (white arrows).
Figure 3.
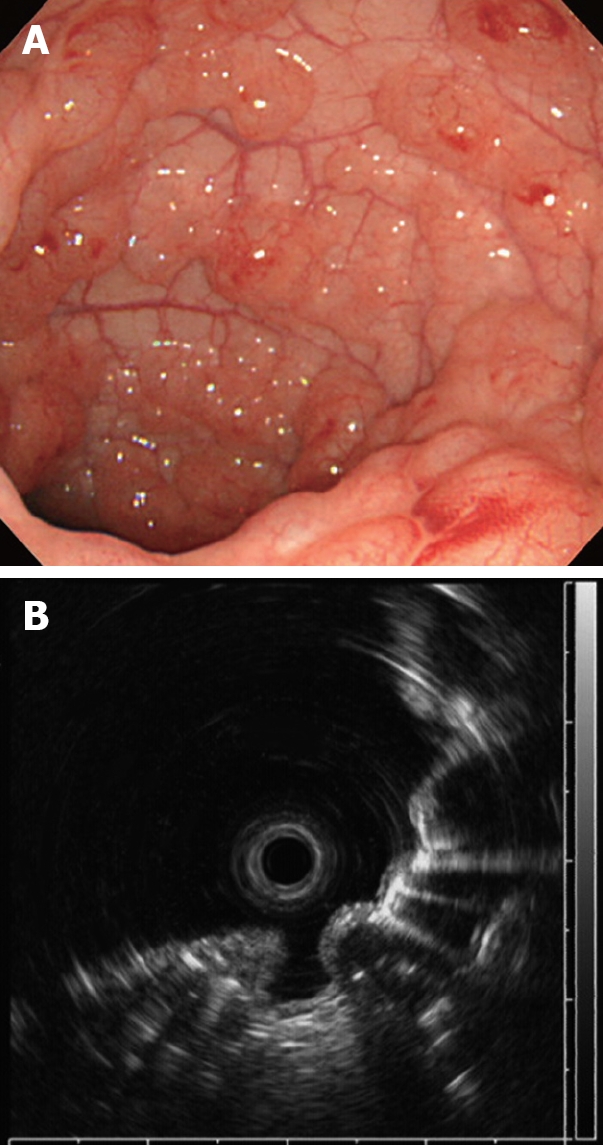
Colonoscopy on admission showing multiple round and smooth-surfaced elevated lesions like submucosal tumors in the sigmoid colon (A) and endoscopic ultrasonography (EUS) revealing hyperechoic lesions and acoustic shadows in the submucosal layer (B).
As voglibose was suspected to be the cause of this patient’s PCI, conservative treatment was administered, including ceasing the voglibose, along with fasting and fluid supplementation. The patient progressed well and plain abdominal radiography 2 wk later showed that the linear collections of gas along the wall of the colon were disappeared, and consequently the patient was discharged. Colonoscopy 3 mo later showed complete resorption of the pneumocystis throughout the sigmoid colon, leaving white scars. EUS confirmed disappearance of the submucosal acoustic shadows, indicating the resolution of PCI.
DISCUSSION
PCI, first reported by Du Vernoi[27] in 1730, is a rare condition in which multiple submucosal or subserosal pneumocystis develop in the submucosa or subserosa of the colon. It was previously thought to occur most frequently in the ileum, but with the recent increase in the number of barium enemas and colonoscopies performed, PCI now reportedly affects the colon more commonly.
There are some recent reports on PCI associated with αGI therapy[14-26]. The mechanism is thought to involve intestinal gas production through fermentation- by the intestinal flora-of carbohydrates whose absorption is inhibited by αGI. Along with peristaltic hypofunction associated with diabetes mellitus, this leads to raised intraluminal pressure, allowing the gas-producing bacteria to invade the colonic mucosa through breaks in the mucosal integrity, forming pneumocystis[14,15].
Our review of the medical literature between 1983 and 2008 yielded 7 cases of PCI associated with αGI therapy in PubMed [English language; 14, 15, 18, 21, 22, 25, 26], and 6 in Japana Centra Revuo Medicina [Japanese language; 16, 17, 19, 20, 23, 24]. The details of these cases, totaling 14 with the addition of our present case, are shown in Tables 1 and 2. All but 1 of the 14 cases was reported in Japan. About 30% of the Japanese diabetics are prescribed αGIs, which are rarely administered in Western countries where fats account for a larger proportion of the caloric intake than carbohydrates[28,29]. The preponderance of Japanese reports on αGI-associated PCI is not surprising as the Japanese market accounts for 98% of the total sales of voglibose, and 34% of those for acarbose.
Table 1.
A summary of previously reported cases of pneumatosis cystoides intestinalis (PCI) after an alpha-glucosidase inhibitor (αGI) treatment
| Case No. | Author | Age | Chief complaint | The αGI agent | Disease other than diabetes mellitus | Concomitant drug | Prescription of αGI after PCI onset | Treatment |
| Reference number | Sex | Quantity of αGI | Outcome | |||||
| Yr | Dosage period of αGIprior to PCI onset | Duration to the outcome | ||||||
| 1 | Hayakawa et al | 64 | Abdominal distention | Voglibose | Unknown | Insulin | Discontinuation | Conservative treatment |
| 14 | F | 0.6 mg/day | Healing | |||||
| 1999 | 1 mo | 4 d | ||||||
| 2 | Azami | 87 | Abdominal distention | Acarbose | Hypothyroidism | SU | Discontinuation | Conservative treatment |
| 15 | F | Appetite loss | 150 mg/day | Healing | ||||
| 2000 | 1 yr | 5 d | ||||||
| 3 | Maeda et al | 55 | Abdominal distention | Acarbose | Pemphigus vulgaris | Insuline steroid Immunosupressant | Continuation | Conservative treatment |
| 16 | F | 300 mg/day | Healing | |||||
| 2002 | 42 d | 141 d | ||||||
| 4 | Tachibana et al | 73 | Abdominal distention | Acarbose | Henoch-Schonlein | SU Steroid | Discontinuation | Conservative treatment |
| 17 | F | 150 mg/day | purpura nephritis | Healing | ||||
| 2002 | 8 yr | 28 d | ||||||
| 5 | Yanaru et al | 61 | Abdominal distention | Voglibose | Unknown | SU | Discontinuation | Conservative treatment |
| 18 | M | Constipation | 0.6 mg/day | Healing | ||||
| 2002 | Hematochezia | 5 yr | 28 d | |||||
| 6 | Matsuda et al | 62 | Abdominal pain | Voglibose | Lung cancer | Morphine sulfate | Unknown | Operation |
| 19 | M | Unknown | Remission | |||||
| 2004 | Unknown | 16 d | ||||||
| 7 | Nagahara et al | 66 | Left abdominal pain | Acarbose | Unknown | Unknown | Discontinuation | Conservative treatment |
| 20 | M | Unknown | Healing | |||||
| 2006 | 11 yr | 21 d | ||||||
| 8 | Hisamoto et al | 56 | No abdominal symptoms | Voglibose | Interstitial pneumonia | Steroid | Discontinuation | Conservative treatment |
| 21 | M | 0.6 mg/day | Healing | |||||
| 2006 | 7 d | 7 d | ||||||
| 9 | Furio et al | 64 | Abdominal pain | Acarbose | Unknown | Insulin | Discontinuation | Conservative |
| 22 | F | Diarrhea | Unknown | treatment | ||||
| 2006 | Tenesmus | 3 yr | Healing | |||||
| Weight loss | 15 d | |||||||
| 10 | Miyagawa et al | 65 | Abdominal pain | Voglibose | Gastric cancer | SU | Continuation→Discontinuation | Conservative treatment |
| 23 | M | Diarrhea | 0.6 mg/day | Healing | ||||
| 2006 | 6 years | 120 d | ||||||
| 11 | Yasuoka et al | 75 | Abdominal distention | Voglibose | Lung cancer | SU | Discontinuation | Conservative treatment |
| 24 | M | 0.6 mg/day | Rectal carcinoid | Healing | ||||
| 2007 | 10 yr | 20 d | ||||||
| 12 | Maeda et al | 72 | Rt lower abdominal pain | Voglibose | Minimal change disease | Insulin | Discontinuation | Conservative treatment |
| 25 | F | 0.9 mg/day | Steroid | Healing | ||||
| 2007 | 3 yr | Immunosupressant | 7 d | |||||
| 13 | Saito et al | 53 | Abdominal distention | Voglibose | Dermatomyositis | Steroid | Discontinuation | Conservative treatment |
| 26 | F | Nausea | 0.6 mg/day | Immunosupressant | Healing | |||
| 2007 | 1 mo | 21 d | ||||||
| 14 | Our case | 69 | Abdominal distention | Voglibose | Myasthenia gravis | SU Steroid | Discontinuation | Conservative treatment |
| M | Hematochezia | 0.6 mg/day | Healing | |||||
| 2008 | 1 yr 8 mo | 14 d |
Table 2.
Imaging findings in previously reported cases of PCI after an αGI treatment
| Case | Author | Plain radiography of the abdomen | Computed tomography of the abdomen | Barium enema | Colonoscopy |
| No. | Reference number | ||||
| Year | |||||
| 1 | Hayakawa et al | Distention of the ascending and proximal transverse colon with cystic radiolucencies, indicating intramural gas | Subserosal cystic areas of gas and distention of the involved segments | Translucent areas of gas clustered along the distorted contours of the ascending and transverse colon | ND |
| 14 | |||||
| 1999 | |||||
| 2 | Azami | Noticeable gaseous distension of the small intestine | Noticeable gaseous distention of the small intestine with pockets of small gas bubbles in the submucosal space | No constriction in the sigmoid or lower descending colon | ND |
| 15 | |||||
| 2000 | |||||
| 3 | Maeda et al | Multiple cystic radiolucencies in the abdomen | Pneumatosis intestinalis around the bowel wall and gas within the retroperitoneum | ND | ND |
| 16 | |||||
| 2002 | |||||
| 4 | Tachibana et al | Free gas of the right peritoneal cavity and pneumatosis intestinalis throughout the ascending colon | Free gas below the right diaphragm, and pneumatosis intestinalis throughout the ascending colon | ND | Polypoid lesions in the ascending and transverse colon |
| 17 | |||||
| 2002 | |||||
| 5 | Yanaru et al | Small round radiolucent clusters in the middle abdomen | ND | Numerous submucosal protrusions of sessile of semipedunculated configurations | Numerous submucosal protrusions of sessile of semipedunculated configurations |
| 18 | |||||
| 2002 | |||||
| 6 | Matsuda et al | Noticeable gaseous distention of the colon, and curvilinear radiolucency within the bowel wall | Free gas in the peritoneal cavity, pneumatosis intestinalis throughout the bowel wall | ND | ND |
| 19 | |||||
| 2004 | |||||
| 7 | Nagahara et al | Free air below the diaphragm | Pneumatosis intestinalis throughout the ascending colon | ND | ND |
| 20 | |||||
| 2006 | |||||
| 8 | Hisamoto et al | Free air below the diaphragm, and noticeable gaseous distention of the ascending and transverse colon | Slight dialatation, mesenteric edema, and diffuse pneumatosis intestinalis throughout the ascending colon | Many cystic areas of the ascending colon | Multiple sessile polypoid lesions covered with normal-appearing mucosa in the area from the ascending colon |
| 21 | |||||
| 2006 | |||||
| 9 | Furio et al | ND | The presence of numerous intraparietal cysts, of varying size, diffuse in the varied colic segments, compatible with wall pneumatosis of the colon | ND | Multiple polypoid formations of varying sizes in the sigmoid, descending, ascending and cecum |
| 22 | |||||
| 2006 | |||||
| 10 | Miyagawa et al | Cystic radiolucencies in the colon | ND | Multiple numerous round polypoid lesions from the ascending colon to the sigmoid colon | Numerous round polypoid lesions from the ascending colon to the sigmoid colon |
| 23 | |||||
| 2006 | |||||
| 11 | Yasuoka et al | Noticeable gaseous distension of the small intestine | Free gas in the peritoneal cavity | ND | ND |
| 24 | |||||
| 2007 | |||||
| 12 | Maeda et al | Diffuse air shadows along the intestine suggesting gas accumulation in the bowel wall | Circumferential collections of air adjacent to the bowel lumen that ran parallel to the bowel wall | ND | ND |
| 25 | |||||
| 2007 | |||||
| 13 | Saito et al | Pneumoperitoneum with free air under the diaphragm and curvilinear radiolucency within the bowel wall | Intramural air in the ascending colon, and gas collection in the mesentery | ND | ND |
| 26 | |||||
| 2007 | |||||
| 14 | Our case | Small linear radiolucent gas collections along the wall of the colon | Intramural air in the sigmoid colon, and free gas in the peritoneal cavity around the sigmoid colon | ND | Multiple smooth surfaced small hemispherical protrusions in the sigmoid colon |
| 2008 |
ND: Not done.
The mean age of the 14 patients was 65.9 years, while 7 were male and 7 were female. The causative agent was voglibose in 9 cases and acarbose in 5, while none was caused by miglitol. The global market share in 2005 for voglibose and acarbose was in a ratio of roughly 3:2. Miglitol was not released in Japan until 2006, and accordingly no reports are available on PCI associated with the newest agent. As future cases are reported, we expect that there will be no significant differences in the incidence of PCI between these agents. The mean prescribed dosages were 0.64 mg/d for voglibose and 200 mg/d for acarbose. The interval between commencement of αGI therapy and the onset of PCI varied greatly, ranging from 7 d to 11 years. The most common symptoms were abdominal distention (57%) and abdominal pain (36%), while only 2 cases had hematochezia (14%) as in the case described herein (Table 1).
Different radiological and endoscopic modalities are useful in the diagnosis of PCI. To summarize the imaging findings in the 14 reported cases (Table 2), linear or round radiolucent gas collections were seen along the wall of the colon in plain abdominal radiographs in most cases, and pneumatosis was seen within or along the wall of the colon on abdominal CT scanning. Subserous pneumocystis in particular are liable to rupture, releasing free gas into the peritoneal cavity, making it important to distinguish this condition from bowel perforation[30]. Multiple rounded protrusions are a common finding in barium enema examinations of patients with PCI. The colonoscopic findings may be similar to multiple polyposis or collections of submucosal tumors, but subserous pneumatosis may go undetected.
With cessation of αGI therapy, conservative treatment could lead to resolution of PCI within 28 d. In the 2 cases where αGI therapy was continued, resolution took more than 120 d. Therefore, ceasing the αGI therapy is the key to successful treatment of PCI. One case underwent emergency surgery due to the presence of free air in the peritoneal cavity, where bowel perforation could not be ruled out[19]. In our case, there were 2 possible causes, namely, the αGI voglibose and prednisolone. Since our patient claimed to have experienced abdominal distention, increased flatus and constipation prior to the onset of PCI, we considered voglibose the causative agent. We therefore ceased αGI and continued corticosteroid therapy, and kept our patient fasting with fluid supplementation, achieving resolution of PCI after 14 d (Table 1).
The symptoms of PCI include abdominal pain, diarrhea and abdominal distention, none of which is disease specific. Diabetic patients sometimes develop autonomic neuropathy, with gastrointestinal symptoms similar to those of PCI. As αGI therapy is commonly used in Japan, it is difficult to determine whether diabetic patients complaining of gastrointestinal symptoms are suffering only from diabetes mellitus or from PCI. If the clinical picture of diabetes mellitus is consistent with that of PCI, diabetes mellitus can be detected by plain abdominal radiography. The possibility of PCI should be considered in diabetic patients complaining of gastro- intestinal symptoms, and appropriate investigations should be performed with this potential diagnosis in mind.
In this report, we presented a case of PCI associated with αGI therapy, and a review of the literature. Our patient recovered rapidly after conservative treatment, including ceasing of the voglibose, fasting, and fluid supplementation. Recently, several reports on PCI associated with αGI therapy have been published, predominantly from Japan where αGIs are commonly used[14-21,23,24]. If the use of αGIs becomes more widespread internationally, we can expect more reports of this condition globally. The possibility of PCI should be considered in diabetic patients complaining of gastrointestinal symptoms, and the gastrointestinal tract should be thoroughly investigated in these patients.
Footnotes
Peer reviewer: Gary A Abrams, Associate Professor, Department of Medicine, University of Alabama at Birmingham, 1530 3rd Ave South, Birmingham 35294, United States
S- Editor Zhong XY L- Editor Wang XL E- Editor Yin DH
References
- 1.Christl SU, Gibson GR, Murgatroyd PR, Scheppach W, Cummings JH. Impaired hydrogen metabolism in pneumatosis cystoides intestinalis. Gastroenterology. 1993;104:392–397. doi: 10.1016/0016-5085(93)90406-3. [DOI] [PubMed] [Google Scholar]
- 2.Florin TH, Hills BA. Does counterperfusion supersaturation cause gas cysts in pneumatosis cystoides coli, and can breathing heliox reduce them? Lancet. 1995;345:1220–1222. doi: 10.1016/s0140-6736(95)91996-1. [DOI] [PubMed] [Google Scholar]
- 3.Gruenberg JC, Grodsinsky C, Ponka JL. Pneumatosis intestinalis: a clinical classification. Dis Colon Rectum. 1979;22:5–9. doi: 10.1007/BF02586748. [DOI] [PubMed] [Google Scholar]
- 4.Ho LM, Mosca PJ, Thompson WM. Pneumatosis intestinalis after lung transplant. Abdom Imaging. 2005;30:598–600. doi: 10.1007/s00261-005-0311-y. [DOI] [PubMed] [Google Scholar]
- 5.Horiuchi A, Akamatsu T, Mukawa K, Ochi Y, Arakura N, Kiyosawa K. Case report: Pneumatosis cystoides intestinalis associated with post-surgical bowel anastomosis: a report of three cases and review of the Japanese literature. J Gastroenterol Hepatol. 1998;13:534–537. doi: 10.1111/j.1440-1746.1998.tb00682.x. [DOI] [PubMed] [Google Scholar]
- 6.McCollister DL, Hammerman HJ. Air, air, everywhere: pneumatosis cystoides coli after colonoscopy. Gastrointest Endosc. 1990;36:75–76. doi: 10.1016/s0016-5107(90)70936-4. [DOI] [PubMed] [Google Scholar]
- 7.Keyting WS, McCarver RR, Kovarik JL, Daywitt AL. Pneumatosis intestinalis: a new concept. Radiology. 1961;76:733–741. doi: 10.1148/76.5.733. [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi K, Shirai T, Shimakura K, Akamatsu T, Nakama H, Kono K, Sakato M, Shigeno T, Furuta S, Nakajima T. Pneumatosis cystoides intestinalis and trichloroethylene exposure. Am J Gastroenterol. 1985;80:753–757. [PubMed] [Google Scholar]
- 9.Mueller CF, Morehead R, Alter AJ, Michener W. Pneumatosis intestinalis in collagen disorders. Am J Roentgenol Radium Ther Nucl Med. 1972;115:300–305. doi: 10.2214/ajr.115.2.300. [DOI] [PubMed] [Google Scholar]
- 10.Quiroz ES, Flannery MT, Martinez EJ, Warner EA. Pneumatosis cystoides intestinalis in progressive systemic sclerosis: a case report and literature review. Am J Med Sci. 1995;310:252–255. [PubMed] [Google Scholar]
- 11.Heng Y, Schuffler MD, Haggitt RC, Rohrmann CA. Pneumatosis intestinalis: a review. Am J Gastroenterol. 1995;90:1747–1758. [PubMed] [Google Scholar]
- 12.Goodman RA, Riley TR 3rd. Lactulose-induced pneumatosis intestinalis and pneumoperitoneum. Dig Dis Sci. 2001;46:2549–2553. doi: 10.1023/a:1012308911096. [DOI] [PubMed] [Google Scholar]
- 13.Duncan B, Barton LL, Eicher ML, Chmielarczyk VT, Erdman SH, Hulett RL. Medication-induced pneumatosis intestinalis. Pediatrics. 1997;99:633–636. doi: 10.1542/peds.99.4.633. [DOI] [PubMed] [Google Scholar]
- 14.Hayakawa T, Yoneshima M, Abe T, Nomura G. Pneumatosis cystoides intestinalis after treatment with an alpha-glucosidase inhibitor. Diabetes Care. 1999;22:366–367. doi: 10.2337/diacare.22.2.366a. [DOI] [PubMed] [Google Scholar]
- 15.Azami Y. Paralytic ileus accompanied by pneumatosis cystoides intestinalis after acarbose treatment in an elderly diabetic patient with a history of heavy intake of maltitol. Intern Med. 2000;39:826–829. doi: 10.2169/internalmedicine.39.826. [DOI] [PubMed] [Google Scholar]
- 16.Maeda A, Yokoi S, Kunou T, Murata T. [A case of pneumatosis cystoides intestinalis assumed to be induced by acarbose administration for diabetes mellitus and pemphigus vulgaris] Nippon Shokakibyo Gakkai Zasshi. 2002;99:1345–1349. [PubMed] [Google Scholar]
- 17.Tachibana Y, Band H, Asai J, Notsumata K, Toya D, Tanaka N, Miura M. A case of pneumatosis cystoides intestinalis with abdominal free air. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2002;22:1103–1106. [Google Scholar]
- 18.Yanaru R, Hizawa K, Nakamura S, Yoshimura R, Watanabe K, Nakamura U, Yoshinari M, Matsumoto T. Regression of pneumatosis cystoides intestinalis after discontinuing of alpha-glucosidase inhibitor administration. J Clin Gastroenterol. 2002;35:204–205. doi: 10.1097/00004836-200208000-00020. [DOI] [PubMed] [Google Scholar]
- 19.Matsuda Y, Yoshida H, Sugimoto H, Tanaka K. A case of pneumatosis cystoides intestinalis with intraperitoneal free air in palliative care. J Jpn Surg Assoc. 2004;65:3288–3292. [Google Scholar]
- 20.Nagahara Y, Hakoda T, Imada T, Kurose H, Okuno T, Yoshida T. Pneumatosis cystoides intestinalis after treatment with an alpha-glucosidase inhibitor. Diabetes J. 2006;34:104–107. [Google Scholar]
- 21.Hisamoto A, Mizushima T, Sato K, Haruta Y, Tanimoto Y, Tanimoto M, Matsuo K. Pneumatosis cystoides intestinalis after alpha-glucosidase inhibitor treatment in a patient with interstitial pneumonitis. Intern Med. 2006;45:73–76. doi: 10.2169/internalmedicine.45.1330. [DOI] [PubMed] [Google Scholar]
- 22.Furio L, Vergura M, Russo A, Bisceglia N, Talarico S, Gatta R, Tomaiuolo M, Tomaiuolo P. Pneumatosis coli induced by acarbose administration for diabetes mellitus. Case report and literature review. Minerva Gastroenterol Dietol. 2006;52:339–346. [PubMed] [Google Scholar]
- 23.Miyagawa M, Kanemasa H, Nakagawa S, Nitan T, Matsumoto M, Tokita K, Kajita Y, Mitsufuji S, Okanoue T. A case of pneumatosis cystoides intestinalis after treatment with an α-glucosidase inhibitor. Gastroenterol Endosc. 2006;48:329–333. [Google Scholar]
- 24.Yasuoka R, Sonoyama Y, Fujiki H, Morita S, Mitsuo M, Kadotani Y. A case of intestinal emphysema with pneumoperitoneum in which α-glucosidase inhibitor participated. J Jpn Surg Assoc. 2007;68:2014–2018. [Google Scholar]
- 25.Maeda Y, Inaba N, Aoyagi M, Kanda E, Shiigai T. Fulminant pneumatosis intestinalis in a patient with diabetes mellitus and minimal change nephrotic syndrome. Intern Med. 2007;46:41–44. doi: 10.2169/internalmedicine.46.6076. [DOI] [PubMed] [Google Scholar]
- 26.Saito M, Tanikawa A, Nakasute K, Tanaka M, Nishikawa T. Additive contribution of multiple factors in the development of pneumatosis intestinalis: a case report and review of the literature. Clin Rheumatol. 2007;26:601–603. doi: 10.1007/s10067-005-0179-9. [DOI] [PubMed] [Google Scholar]
- 27.Du Vernoi GJ. Aer intestinorum tam subextima guam intima tunica inclusus. Obsergatinonae Anatomicae Acad. Scient Imp Petoropal. 1730;5:213–215. [Google Scholar]
- 28.Wysowski DK, Armstrong G, Governale L. Rapid increase in the use of oral antidiabetic drugs in the United States, 1990-2001. Diabetes Care. 2003;26:1852–1855. doi: 10.2337/diacare.26.6.1852. [DOI] [PubMed] [Google Scholar]
- 29.Smyth S, Heron A. Diabetes and obesity: the twin epidemics. Nat Med. 2006;12:75–80. doi: 10.1038/nm0106-75. [DOI] [PubMed] [Google Scholar]
- 30.Caudill JL, Rose BS. The role of computed tomography in the evaluation of pneumatosis intestinalis. J Clin Gastroenterol. 1987;9:223–226. doi: 10.1097/00004836-198704000-00024. [DOI] [PubMed] [Google Scholar]