Abstract
AIM: To observe the effect of berberine on insulin secretion in rat pancreatic islets and to explore its possible molecular mechanism.
METHODS: Primary rat islets were isolated from male Sprague-Dawley rats by collagenase digestion and treated with different concentrations (1, 3, 10 and 30 μmol/L) of berberine or 1 μmol/L Glibenclamide (GB) for 24 h. Glucose-stimulated insulin secretion (GSIS) assay was conducted and insulin was determined by radioimmunoassay. 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed to evaluate cytotoxicity. The mRNA level of hepatic nuclear factor 4 alpha (HNF4α) was determined by reverse transcription polymerase chain reaction (RT-PCR). Indirect immunofluorescence staining and Western blot analysis were employed to detect protein expression of HNF4α in the islets. Glucokinase (GK) activity was measured by spectrophotometric method.
RESULTS: Berberine enhanced GSIS rather than basal insulin secretion dose-dependently in rat islets and showed no significant cytotoxicity on islet cells at the concentration of 10 μmol/L. Both mRNA and protein expressions of HNF4α were up-regulated by berberine in a dose-dependent manner, and GK activity was also increased accordingly. However, GB demonstrated no regulatory effects on HNF4α expression or GK activity.
CONCLUSION: Berberine can enhance GSIS in rat islets, and probably exerts the insulinotropic effect via a pathway involving HNF4α and GK, which is distinct from sulphonylureas (SUs).
Keywords: Berberine, Sulphonylureas, Hepatocyte nuclear factor 4 alpha, Glucokinase, Pancreatic islet
INTRODUCTION
Type 2 diabetes mellitus is a complex and heterogeneous disorder caused by the interaction of hereditary and environmental factors, pathophysiologically characterized by insulin resistance and functional defects in insulin release from pancreatic β cells.
Sulphonylureas (SUs) are the most commonly prescribed insulin secretagogues. These drugs act via augmentation of insulin secretion from pancreatic β cells. The SU receptor-1 on the ATP-sensitive potassium channels (KATP channels) is occupied by SU leading to closure of the potassium channels and subsequent opening of calcium channels, resulting in exocytosis of insulin granules[1]. Yet, the maintenance of satisfactory long-term glycaemic control in patients undergoing SU therapy is usually restricted by increased risk of hypoglycemia coupled with declined insulinotropic activity due to desensitized β cells to the agents[2,3]. Therefore, new types of insulinotropic substances with an alternative action profile are in demand.
Berberine, the major active constituent of Chinese herb Rhizoma Coptidis, is being used to treat diabetes for decades, showing obvious therapeutic actions with few reported side effects. Previous studies have demonstrated that berberine modulates cholesterol through increasing low-density lipoprotein receptor mRNA stability[4], reduces body adiposity and increases insulin sensitivity partly through activating AMP-activated protein kinase[5], and improves glucose metabolism via induction of glycolysis[6], implying a promising future for berberine in the therapy of diabetes. Moreover, a new research further revealed that berberine also possessed insulinotropic property in isolated pancreatic islets[7]. However, the underlying mechanism is not fully understood.
Recently, the hepatocyte nuclear factors (HNFs) transcriptional regulatory networks were identified in pancreatic islet tissue, providing insight into the molecular basis of abnormal β cell function. It was deemed that three members of HNFs family, HNF4α, HNF1α and HNF6, were at the center of the connected network, operating cooperatively to regulate numerous developmental and metabolic functions in human pancreatic islets. It was also revealed that HNF4α was bound to about 11% of the genes represented on the DNA microarray in pancreatic islets[8,9]. The occupancy by HNF4α of a substantial fraction of expressed genes suggests that HNF4α is a widely acting transcription factor and crucial for development and proper secretary function of pancreatic β cells. Furthermore, Bartoov-Shifman et al[10] found that HNF4α could activate insulin gene directly, through a previously unrecognized cis element. Clinical researches have indicated that mutations in gene encoding HNF4α result in maturity-onset diabetes of the young type 1, characterized by autosomal dominant inheritance, early onset and impairment of glucose-stimulated insulin secretion (GSIS)[11]. And two independent laboratory studies demonstrated that β-cell-specific HNF4α knock-out mice exhibited impaired GSIS and deficient intracellular calcium response to glucose or SU[12,13].
Increasing evidences suggest an essential role for HNF4α in the maintenance of proper secretary function of pancreatic β cell and glucose metabolism. In this study, we hypothesize that berberine facilitates insulin secretion through a pathway involving HNF4α in pancreatic islets. We introduce Glibenclamide (GB) as a control to compare the action profile of berberine with SUs, and to explore the possible molecular mechanism.
MATERIALS AND METHODS
Animals
Adult male Sprague-Dawley rats weighing 250-300 g [Grade SPF, Certificate No. SCXK (E2004-0007)] were purchased from the Experimental Animal Center, Tongji Medical College, Huazhong University of Science and Technology. The rats were housed at 22°C, and 60%-70% relative humidity, with 12 h light/dark cycle. The rats were given free access to food and tap water. All rats received humane care in compliance with the institutional animal care guidelines approved by the Experimental Animal Ethical Committee of Tongji Medical College, Huazhong University of Science and Technology.
Drug preparation
Berberine hydrochloride (Sigma, St Louis, MO, USA) and GB (Alexis CO, San Diego, CA, USA) were dissolved in dimethyl sulfoxide (DMSO, Amresco, TX, USA), with a final concentration of DMSO 0.01% (v/v) in the culture medium.
Islet isolation and culture conditions
Primary pancreatic islets were isolated as previously reported[14,15]. In brief, rats were anesthetized with intramuscular pentobarbital injection, 8-10 mL ice-cold Hanks’ balanced salt solution (HBSS) containing 0.75 mg/mL type V collagenase (Sigma, St Louis, MO, USA) was injected via pancreatic duct, extended pancreas was removed and digested in a 38°C water bath for 8-10 min. Then digestion was terminated by 30 mL ice-cold HBSS with 10% fetal bovine serum (Gibco, USA), and the suspension was filtered through a 600 μm screen to discard the undigested tissue. After twice washes with HBSS, islets were purified by Ficoll-400 (Amersham Pharmacia Biotech, Uppsala, Sweden) discontinuous gradient centrifugation at 800 × g for 20 min at 4°C, and hand-picked under dissecting microscope. About 250-400 islets were yielded from each pancreas. The purity of islets was evaluated by dithizone (DTZ, Sigma, St Louis, MO, USA) staining[16], and the viability was assessed according to the acridine orange/propidium iodide (AO/PI, Sigma, St Louis, MO, USA) fluorescent staining method[17]. Freshly isolated islets were first cultured overnight at 37°C in a 50 mL/L CO2-950 mL/L air atmosphere in serum-free RPMI 1640 (Hyclone, Gaithersburg, MD, USA) containing 2% (w/v) bovine serum albumin fraction V (BSA, Amresco, TX, USA), 11.1 mmol/L glucose, 5 mmol/L glutamine, 1 mmol/L sodium pyruvate, 100 IU/mL pennicillin, 100 μg/mL streptomycin and 15 mmol/L HEPES. Then islets were cultured for 24 h in various experimental media containing 1, 3, 10 and 30 μmol/L berberine or 1 μmol/L GB. Normal control was also set by incubating islets with medium in the absence of berberine or GB.
GSIS assay
For evaluation of insulin secretion, islets were washed twice with Krebs-Ringers Bicarbonated HEPES [KRBH, containing 120 mmol/L NaCl, 4.8 mmol/L KCl, 2.5 mmol/L CaCl2, 1.2 mmol/L MgSO4, 1.2 mmol/L KH2PO4, 25 mmol/L NaHCO3, 10 mmol/L HEPES, 2.8 mmol/L glucose, 0.5% BSA (w/v), pH 7.4] at the end of the incubation. Batches of 10 size-matched islets (six replicas per condition) were transferred into 1.5 mL Eppendorf tubes, and pre-incubated for 30 min at 37°C in KRBH with 2.8 mmol/L glucose. Subsequently, islets were incubated in KRBH supplemented with either 2.8 mmol/L or 16.7 mmol/L glucose for 1 h at 37°C. Aliquots of supernatant were collected after gentle centrifugation and stored at -20°C for insulin determination by radioimmunoassay kit (Beijing Institute of Atomic Energy, China).
3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cytotoxicity assay
Cytotoxicity of berberine and GB on islet cells was tested by a colorimetric assay that detected the conversion of MTT (Sigma, St Louis, MO, USA) into the formazan by the mitochondrial enzyme succinate dehydrogenase in viable cells[18]. After in vitro treatment, islets were dissociated into single cells by incubation in Ca2+/Mg2+-free KRBH containing 5 mmol/L EDTA and 0.25 mg/mL trypsin for 10 min at 37°C with gentle shaking, and then resuspended in RPMI 1640. Islet cells were cultured in a 96-well plate supplemented with 0.5 mg/mL MTT. After 4 h incubation, the insoluble formazan crystals within islet cells were extracted by DMSO, and absorbance was measured by ELX800 Universal Microplated Reader (BioTek Instruments Inc, USA) at wavelength of 630 nm.
Semi-quantitative reverse transcription polymerase chain reaction (RT-PCR) analysis
Semi-quantitative RT-PCR was performed to determine the mRNA level of HNF4α in the islets. Total RNA was extracted from about 300 islets by the Trizol Reagent Kit (Gibco, USA) according to the manufacturer’s instructions. Two μg of RNA from each sample was then reverse-transcribed into first-strand cDNA in 25 μL solution using Oligo (dT) Primers and MMLV reverse transcriptase (Promega, Madison, WI, USA). PCR reaction was performed in a standard 25 μL reaction solution contained 3 μL cDNA, 0.5 μL each of sense and anti-sense primers. Sequence-specific primers for cDNA amplification were as follows: HNF4α (product 464 bp, sense 5'-GCAGTGCGTGGTAGACAAAGATA-3'; anti-sense 5'-AGTGCCGAGGGACGATGTAG-3') and the housekeeping gene β-actin (product 213 bp, sense 5'-AGATCTGGCACCACACCTTCTAC-3'; anti-sense 5'-TCAGGATCTTCATGAGGTAGTCT-3'). Reaction conditions for HNF4α were as follows: predenaturing at 95°C for 5 min , denaturing at 95°C for 1 min, annealing at 60°C for 50 s, extending at 72°C for 1 min, 35 cycles, with final extending at 72°C for 10 min. Amplification of β-actin was performed by predenaturing at 94°C for 5 min, denaturing at 94°C for 1 min, annealing at 55°C for 50 s, extending at 72°C for 50 s, 30 cycles and final extending at 72°C for 10 min. PCR products were electrophoresed through 1.5% agarose gel, stained with ethidium bromide and visualized under ultraviolet illumination. Band intensity was calculated densitometrically using the SensiAnsys software (Shanghai PeiQing Science & Technology, China).
Immunofluorescence
Indirect immunofluorescence assay was performed as previously described with a few modifications[19,20]. In brief, islets were placed into 1.5 mL Eppendorf tubes, fixed in 4% paraformaldehyde/10 mmol/L PBS for 30 min, followed by a 3 h permeabilization with 0.3% Triton-X100/10 mmol/L PBS. Subsequently, islets were blocked with 5% fetal bovine serum/0.2 Triton-X100/10 mmol/L PBS overnight at 4°C and then equilibrated in antibody dilution buffer (1% BSA/0.2% Triton X-100/10 mmol/L PBS) twice for 20 min at room temperature. Primary antibodies used were as follows: goat anti-rat HNF4α IgG (1:300, Santa Cruz, San Diego, CA, USA), Guinea pig anti-insulin antibody (1:300, Sigma, St Louis, MO, USA), and the incubation was carried out for 16 h at 4°C. The secondary antibodies were Cy3 conjugated rabbit anti-goat IgG (1:400, Sigma, St Louis, MO, USA) and FITC conjugated rabbit anti-guinea pig IgG (1:250, Sigma, St Louis, MO, USA), incubation was performed for 1 h at room temperature. Finally, islets were mounted with 50% glycerol/10 mmol/L PBS, smeared onto glass slides and subjected to Confocal Laser Scanning Microscope (Olympus FV500, Japan). The excitation wavelengths for Cy3 and FITC were 552 nm and 488 nm, and the emission wavelengths were 565 nm and 525 nm, respectively. To avoid variability in fluorescent intensity caused by depth-related changes and wide range of islet diameters, a single section image was taken at the depth 1/3 the distance between the upper islet surface and its maximum diameter. Ten areas from each cover slip were randomly selected and analyzed by the HMIAS-2000 Imaging System (Champion Medical Imaging Co., Wuhan, China).
Western blot analysis
Primary pancreatic islets treated with the various experimental conditions were lysated in buffer containing 25 mmol/L Tris, 1% SDS, 5% β-mercaptoethanol, 10 mmol/L EDTA, 20 mmol/L PMSF, 10 mg/L aprotinin, 10 mg/L leupeptin, 10 mg/L antipain, 8 mol/L urea. Homogenates were centrifuged at 12 000 × g for 15 min at 4°C, supernatants were harvested. Protein concentrations were measured by the method of Bradford[21], with Coomassie brilliant blue staining kit (Jiancheng Biology Institute, Nanjing, China). And 50 μg total protein for each group was boiled for 5 min in sample buffer [50 mmol/L Tris· Cl (pH 6.8), 100 mmol/L DTT, 2% SDS, 0.1% bromchlorphenol blue, 10% glycerol] and separated by 12% SDS-PAGE. Thereafter, proteins were transferred electrophoretically onto a polyvinylidine fluoride membrane. Before immunostaining, the membranes were blocked with 5% non-fat milk in Tris-buffered saline and 0.1% Tween (TBST) overnight at 4°C, followed by incubation with appropriate dilutions of the primary specific antibody goat anti-rat HNF4α IgG (1:500, Santa Cruz Biotechnology Inc, CA, USA) in 5%BSA/TBST at 4°C for 16 h. The secondary antibody was horseradish peroxidase (HRP) conjugated rabbit anti-goat IgG (1:2000, Sigma, St Louis, MO, USA), incubation was carried out at room temperature for 2 h. GAPDH was probed with anti-GAPDH IgG as a loading control. Immunodetection was performed using an enhanced chemiluminescence detection kit (Pierce, Rockford, IL). Protein bands on films (Eastman Kodak, Rochester, NY, USA) were analyzed by densitometry (Bio-Rad, Hercules, USA) using “Quantity One” quantitation analysis software program.
Glucokinase (GK) activity assay
GK activity was measured by spectrophotometric assay as previously described[22]. Briefly, islet were washed twice with PBS, approximately 150 islets from each group were homogenized (30 strokes) in 200 μL lysis buffer containing 20 mmol/L K2HPO4, 5 mmol/L dithiothreitol, 1 mmol/L EDTA, and 110 mmol/L KCl, followed by sonication (20 KHz, 60 W) for 3 × 10 s on ice. The homogenate was then centrifuged at 12 000 × g for 10 min, and the supernatant fraction was used for GK determination. Then 10 μL of the supernatant was added to 100 μL reaction buffer, containing 50 mmol/L HEPES/HCl (pH 7.6), 100 mmol/L KCl, 7.4 mmol/L MgCl2, 15 mmol/L β-mercaptoethanol, 0.5 mmol/L NAD+, 0.05% BSA (w/v), 2 IU/mL glucose-6-phosphate dehydrogenase, and 5 mmol/L ATP. The assay was conducted for 1 h at 30°C, and reaction was stopped by adding 1 mL of 500 mmol/L NaHCO3 buffer (pH 9.4). In each assay, blanks were obtained by incubating 0.5 or 100 mmol/L glucose in the absence of ATP. Absorbance was measured at 340 nm, correction for hexokinase activity was applied by substracting the activity measured at 0.5 mmol/L glucose from the activity measured at 100 mmol/L glucose. Protein concentrations were determined by the Bradford assay.
Statistical analysis
All the data were expressed as mean ± SD, and analyzed with SPSS 13.0 software by one-way analysis of variance (ANOVA) LSD-t and SNK-q. P < 0.05 was considered statistically significant.
RESULTS
Purity and viability of freshly isolated islets
The purity of the freshly isolated islets was estimated by the percentage of DTZ-positive islets (crimson red) in the preparation. According to the method, an approximate 95% purity was assessed. For viability evaluation, islets were exposed to AO/PI, and subjected to fluorescent microscopy (Nikon ECLIPSE TE2000-U, Japan) with viable cells stained green while nonviable cells bright red. The viability exceeded 90% as assessed by the method (Figure 1).
Figure 1.
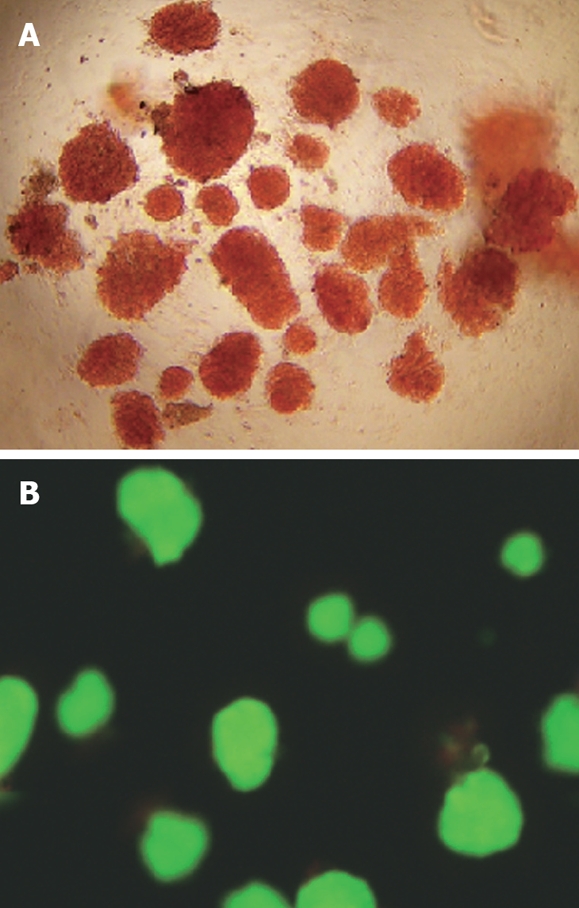
Quality assessment of freshly isolated islets. A: DTZ staining of islets under an inverted microscope (× 10); B: AO/PI double fluorescence staining of islets under a fluorescent microscopy (× 10).
Effects of berberine and GB on insulin secretion
In the experiment, we employed static incubation assay to further examine the facilitative effect of berberine on islet insulin secretion. Islets were first incubated with 2.8 mmol/L glucose, and then challenged with 16.7 mmol/L glucose. All samples were determined by immunoradioassay. As Figure 2A depicted, islets in control group exhibited a normal response to glucose stimulation, with insulin secretion of 7.21 ± 1.43 vs 30.50 ± 5.17 (mIU/L per hour per 10 islets). Treatment of islets with 1 μmol/L GB potently elevated basal insulin secretion (P < 0.01), while inhibited GSIS by about 3 folds relative to normal control (P < 0.05). In contrast, although none of the four berberine groups showed any promoting effects on basal insulin secretion, treatment with 1, 3, 10 μmol/L berberine resulted in dose-dependently increased GSIS (P < 0.05 or P < 0.01), still no enhancement was observed in islets of 30 μmol/L berberine group.
Figure 2.
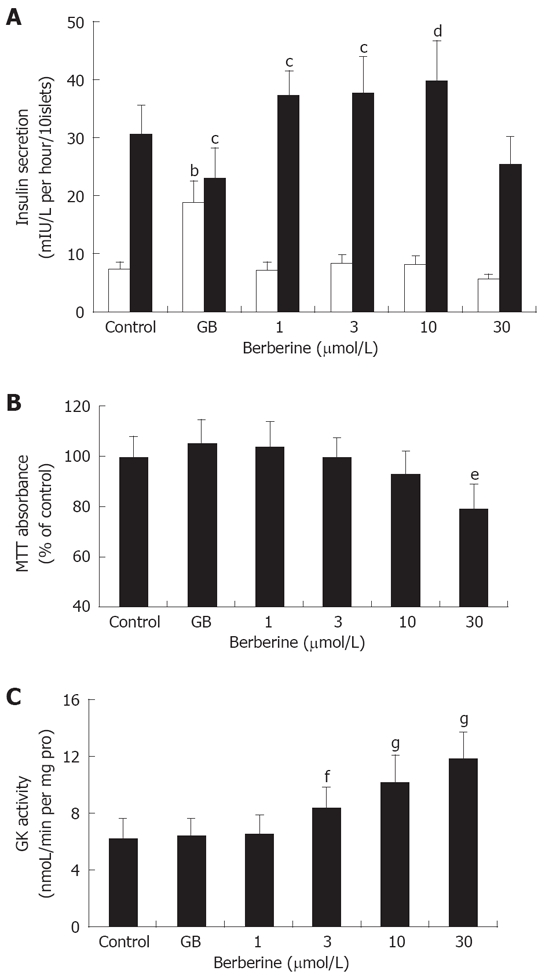
Pharmacological effects of berberine and GB on pancreatic islets. A: Effects of berberine and GB on basal (2.8 mmol/L Glucose) and GSIS (16.7 mmol/L Glucose) by pancreatic islets. Control and GB represent islets of untreated and 1 μmol/L GB treated groups, while 1, 3, 10 and 30 represent berberine groups of indicated concentrations. bP < 0.01 vs control at 2.8 mmol/L glucose; cP < 0.05, dP < 0.01 vs control at 16.7 mmol/L glucose; B: Cytotoxicity of berberine and GB on islet cells assessed by MTT assay. Results were expressed as the percentage of absorbance to the control value. Data are presented as mean ± SD (n = 6). eP< 0.01 vs control. C: Effects of berberine and GB on islet GK activity. fP < 0.05, gP < 0.01 vs control.
Cytotoxicity of berberine and GB on islet cells
We used MTT assay to analyze cytotoxicity caused by berberine and GB. Results were expressed as percentage of formazan absorbance relative to control value. It was observed that, 30 μmol/L berberine inhibited formazan absorbance by about 2 folds compared to the control, indicating significant cytotoxicity on islet cells (P < 0.01). None of the other groups demonstrated significantly diminished absorbance (Figure 2B).
Effects of berberine and GB on HNF4α gene expression
In RT-PCR experiment, it was observed that at concentrations lower than 10 μmol/L, berberine treatment induced a general dose-dependent increase relative to control in HNF4α mRNA expression (P < 0.05 or P < 0.01). However, no significant difference was observed in islets incubated with either 30 μmol/L berberine or 1 μmol/L GB compared with the control (Figure 3).
Figure 3.
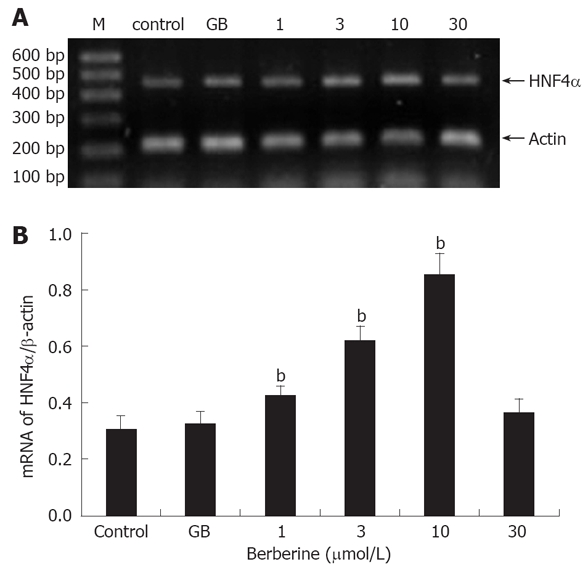
Effects of berberine and GB on HNF4α mRNA expression. A: A representative gel electrophresis profile of DNA fragment; B: Semi-quantitative mRNA determination, results were normalized to β-actin and expressed as arbitrary unit. M: DNA Marker, control and GB represent islets of untreated and 1 μmol/L GB treated groups; 1, 3, 10 and 30 represent berberine groups of indicated concentrations. Data are presented as mean ± SD (n = 6), bP < 0.01 vs control.
Immunofluorescence and confocal microscopy
Confocal images showed apparent co-localization of insulin (green) and HNF4α (red) in the islets. As Figure 4 depicts, islets exhibited a normal architecture, featuring predominant distribution of insulin throughout the entire β cell cytoplasm as well as typical nuclear localization of HNF4α in both β cell and peripheral α cell. It was noteworthy that islets of berberine treated groups demonstrated more intense and distinct fluorescence of HNF4α than control group, with the strongest red fluorescence emitted from the 10 μmol/L berberine treated group. However, no distinguishable change of HNF4α staining was observed in GB group compared with control group.
Figure 4.
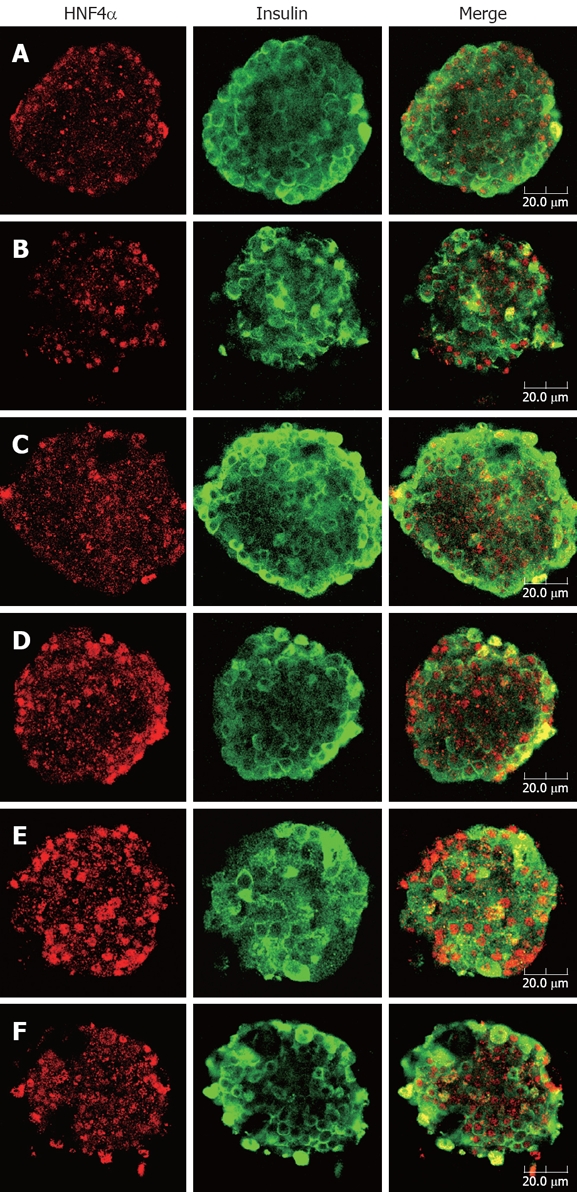
Double immunofluorescence staining for HNF4α (red) and insulin (green) of rat pancreatic islets. After treatment with indicated concentrations of berberine or GB for 24 h, islets were fixed and stained with anti-HNF4α and anti-insulin antibodies. Images of islets were taken at the corresponding depth by confocal laser microscopy (× 40). A: Control, B: 1 μmol/L GB; C to F: Represent 1, 3, 10 and 30 μmol/L berberine. Bar in the figure indicates 20 μm. Apparent nuclear localization of HNF4α could be observed in all groups of islets, while insulin fluorescence was diffusely distributed in cytoplasma of β cells. In the islets treated with various concentrations of berberine, the red fluorescence emitted was comparatively intense, suggesting up-regulated expression of HNF4α, while no obvious difference of HNF4α staining was found between control and GB treated islets.
Effects of berberine and GB on HNF4α protein expression
To further clarify the correlation of berberine’s insulinotropic effect with HNF4α expression, we quantified the protein level of HNF4α by Western blot. Similar to the result of immunofluorescence, protein expression of HNF4α also demonstrated a dose-dependent increase in the berberine treated islets (P < 0.05 or P < 0.01), with maximum expression in 10 μmol/L berberine group. Still no significant difference was found between GB group and control group (Figure 5).
Figure 5.
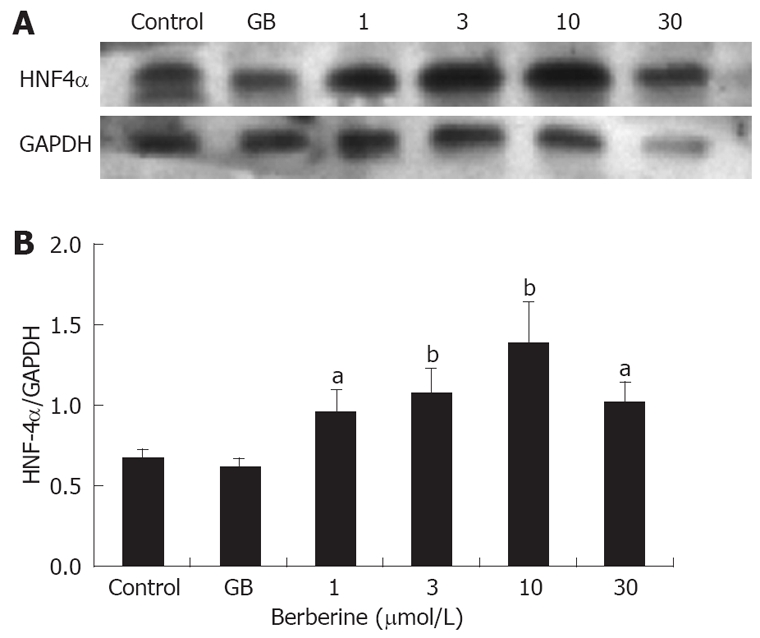
Effects of berberine and GB on protein expression of HNF4α. A: A representative Western blot of HNF4α; B: Quantification of HNF4α protein, results were adjusted to GAPDH and expressed as relative density units. Control and GB represent islets of untreated and 1 μmol/L GB treated groups, 1, 3, 10 and 30 represent berberine groups of indicated concentrations. Data are presented as mean ± SD (n = 3). aP < 0.05, bP < 0.01 vs control.
Effects of berberine and GB on islet GK activity
In the experiment, GK activity was determined in the islet homogenates. As shown in Figure 2C, compared to the control, treatment with 3, 10 and 30 μm/L berberine significantly activated islet GK activity (P < 0.05 or P < 0.01) in a dose-dependent manner, while no enhancement of GK activity was observed in the islets incubated with 1 μmol/L berberine or 1 μmol/L GB.
DISCUSSION
The SUs are a family of oral hypoglycemic agents used extensively for the treatment of type 2 diabetes. They mediate the insulinotropic effect via blocking β cell KATP channels and depolarizing the membrane. Nonetheless, because the potent insulin stimulating property is independent of glucose challenge, they enhance insulin secretion even at basal glucose levels. Therefore, patients receiving SUs therapy are at high risk of hypoglycemia. Moreover, chronic SUs therapy may lead to a selective desensitization of pancreatic β cells to SUs[1,2].
In the present study, the insulinotropic effect of berberine on rat islets was compared with GB, a classical SU derivative. It was demonstrated that 1 μmol/L GB acutely promoted basal insulin secretion by approximately 250% in isolated islets, while markedly reduced GSIS in the presence of 16.7 mmol/L glucose. This observation is in good agreement with previous reports[2,23]. The suppressed GSIS might partly result from the reduction of KATP channel activity induced by chronic exposure to GB and/or the depletion of insulin stores, reflecting the controversy of β-cell desensitization vs β-cell exhaustion[3,24]. In contrast to the action profile of GB, berberine induced no significant changes in basal insulin secretion but increased GSIS dose-dependently at the concentrations of 1-10 μmol/L. This result is consistent with a previous research by Ko et al[25], which showed that berberine exerted no stimulatory effect on basal insulin secretion (2 mmol/L glucose), but increased GSIS at the concentration of 5 and 50 μmol/L in MIN6 cells.
To define the underlying mechanism for the completely different action profile of GB and berberine, we first examined the cytotoxicity caused by the drugs. Our results indicated that only 30 μmol/L berberine demonstrated statistically significant toxicity on islet cell. Hence, it could not be the distinctions of cell metabolism that account for the different action modalities of the two agents. Accordingly, we concluded berberine probably stimulated insulin secretion via a mechanism distinct from SUs! In the following experiments, we determined the gene and protein expressions of HNF4α, a transcription factor confirmed to play an essential role in GSIS, attempting to explore if HNF4α a potential target underling the facilitating effect of berberine on GSIS. Our data conspicuously showed that both the mRNA and protein expressions increased in a dose-dependent manner, reaching their maximum at 10 μmol/L berberine then declining to the levels parallel those of control. No significant changes were observed in the islets treated with 1 μmol/L GB compared with the control. These results strongly suggest the potential involvement of HNF4α in berberine’s insulinotropic action but not GB-induced insulin secretion. The significant cytotoxicity caused by 30 μmol/L berberine might offer a plausible explanation for the reduction of HNF4α expression.
The question remains as to how HNF4α mediate the stimulatory effects of berberine on GSIS in pancreatic islets, as few evidences to date substantiate that HNF4α regulates GSIS directly in pancreatic islets. For elucidating this, we further investigated the effect of berberine on GK activity in rat islets. It is generally acknowledged that GK plays the crucial role of “glucose sensor” in pancreatic β-cell, due to its specific kinetic properties that include low affinity and positive cooperativity for glucose and a lack of inhibition by its product glucose-6-phosphate. GK regulates GSIS by modulation of the glycolytic pathway and controlling the rate of its subsequent metabolism[26,27]. It is also deemed that GK is one of the downstream targets of the HNFs transcription regulatory circuit in pancreatic islets[9]. Therefore, there is a good possibility that berberine exerts the facilitating effect on GSIS through direct action of GK! As expected, our results showed that berberine also elevated GK activity dose-dependently in islets, exhibiting the strongest effect at the concentration of 30 μmol/L. Yet, still no significant difference was observed between islets treated with 1 μmol/L GB and the control, which agrees with a previous report indicating that GB exerted no regulatory effect on GK activity in isolated islets[28]. Thus, our data further support the hypothesis that GK plays a role in the stimulatory effect of berberine on GSIS.
It appears puzzling that discrepancies also existed despite the conspicuous correlation among insulin secretion and HNF4α expression and GK activity in general. It was demonstrated that 1 μmol/L berberine significantly increased insulin secretion, HNF4α gene and protein expressions, however, no enhancement of GK activity was observed. In contrast, 30 μmol/L berberine significantly increased GK activity, while both insulin secretion and HNF4α gene expression were at a normal level. As mentioned above, HNFs form a network, function solely or cooperatively to regulate the expression of multiple target genes that are important in the maintenance of metabolism homeostasis. It is conceivable, therefore, that not only HNF4α but also other HNFs such as HNF1α or HNF6 might participate in the modulation of GK activity. However, these need to be clarified further.
Taken together, our results suggest that berberine might exert its insulinotropic effect in isolated rat islets by up-regulating the expression of HNF4α, which probably acts solely or together with other HNFs to modulate GK activity, rendering β cell more sensitive to glucose fluctuation and response more effectively to glucose challenge. Interestingly, Ko et al[25] revealed that berberine facilitated GSIS in MIN6 cells partly via an enhanced insulin/insulin-like growth factor-1 (IGF-1) signaling cascade, which seems discrepant from the pathway we proposed. However, it is speculated that insulin signaling could interact with HNF-regulated transcription in beta cells, and insulin or IGF-1 act as potential upstream inductive signals regulating the HNFs and their target genes[9]. This might at least in part provide plausible explanation for the controversy.
In conclusion, our findings indicate that berberine enhances GSIS, rather than basal insulin secretion dose-dependently in isolated rat islets. This might partly be attributable to the up-regulation of HNF4α expression and GK activity by berberine. It is also suggested that HNF4α and GK might not participate in GB-induced insulin secretion. Berberine would be a promising insulin secretagogue which works through a mechanism distinct from SUs.
COMMENTS
Background
Berberine, a main constituent isolated from Chinese herb Rhizoma coptidis, is gaining increasing attention, especially for its anti-diabetic properties, including improving insulin resistance, lowing blood glucose and modulating lipid metabolism. Recent researches further reveal that berberine also possesses insulinotropic action, yet, the molecular mechanism remains unclear.
Research frontiers
The transcriptional regulatory circuit of hepatic nuclear factors (HNFs) has recently been identified in pancreatic islets. And numerous evidences suggest an essential role for hepatic nuclear factor 4 alpha (HNF4α) in the proper secretary function of β cells. This study aims to elucidate if HNF4α underlies the mechanism of berberine’s facilitating effect on insulin secretion.
Innovations and breakthroughs
In this study, the authors found that berberine could promote glucose-stimulated insulin secretion (GSIS) rather than basal insulin secretion in primary rat islets. Furthermore, they revealed that berberine might exert the insulinotropic effect through a mechanism involving HNF4α and glucokinase (GK), which is absolutely distinct from that of the widely used sulphonylureas (SUs).
Applications
The data suggest that berberine might be a promising insulin secretagogue which works via a unique mechanism in diabetes treatment.
Terminology
HNF4α is a transcriptional factor belonging to the hepatocyte nuclear factor family. It has been demonstrated that HNF4α is expressed mainly in liver and pancreatic islet tissue, regulating the transcription of multiple target genes implicated in glucose metabolism and insulin secretion. GK is known as “glucose sensor”, modulating insulin secretion by controlling the rate of glycolysis in pancreatic β cells.
Peer review
It is a simple and elegant study. Authors observed the effect of berberine on insulin secretion by rat pancreatic islets and explored its possible molecular mechanism.
Acknowledgments
We thank the staff of the Institute of Integrated Traditional and Western Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology for their help with this study.
Footnotes
Supported by The National Natural Science Foundation of China, No. 30500685
Peer reviewer: Giovanni Tarantino, MD, Professor, Department of Clinical and Experimental Medicine, Federico II University Medical School, VIA S. PANSINI, 5, Naples 80131, Italy
S- Editor Li DL L- Editor Ma JY E- Editor Lin YP
References
- 1.Groop LC. Sulfonylureas in NIDDM. Diabetes Care. 1992;15:737–754. doi: 10.2337/diacare.15.6.737. [DOI] [PubMed] [Google Scholar]
- 2.Rabuazzo AM, Buscema M, Vinci C, Caltabiano V, Vetri M, Forte F, Vigneri R, Purrello F. Glyburide and tolbutamide induce desensitization of insulin release in rat pancreatic islets by different mechanisms. Endocrinology. 1992;131:1815–1820. doi: 10.1210/endo.131.4.1396327. [DOI] [PubMed] [Google Scholar]
- 3.Kawaki J, Nagashima K, Tanaka J, Miki T, Miyazaki M, Gonoi T, Mitsuhashi N, Nakajima N, Iwanaga T, Yano H, et al. Unresponsiveness to glibenclamide during chronic treatment induced by reduction of ATP-sensitive K+ channel activity. Diabetes. 1999;48:2001–2006. doi: 10.2337/diabetes.48.10.2001. [DOI] [PubMed] [Google Scholar]
- 4.Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C, Wang Y, Wang Z, Si S, Pan H, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344–1351. doi: 10.1038/nm1135. [DOI] [PubMed] [Google Scholar]
- 5.Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, Ye JM, Lee CH, Oh WK, Kim CT, et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55:2256–2264. doi: 10.2337/db06-0006. [DOI] [PubMed] [Google Scholar]
- 6.Yin J, Gao Z, Liu D, Liu Z, Ye J. Berberine improves glucose metabolism through induction of glycolysis. Am J Physiol Endocrinol Metab. 2008;294:E148–E156. doi: 10.1152/ajpendo.00211.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leng SH, Lu FE, Xu LJ. Therapeutic effects of berberine in impaired glucose tolerance rats and its influence on insulin secretion. Acta Pharmacol Sin. 2004;25:496–502. [PubMed] [Google Scholar]
- 8.Odom DT, Zizlsperger N, Gordon DB, Bell GW, Rinaldi NJ, Murray HL, Volkert TL, Schreiber J, Rolfe PA, Gifford DK, et al. Control of pancreas and liver gene expression by HNF transcription factors. Science. 2004;303:1378–1381. doi: 10.1126/science.1089769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kulkarni RN, Kahn CR. Molecular biology. HNFs--linking the liver and pancreatic islets in diabetes. Science. 2004;303:1311–1312. doi: 10.1126/science.1095486. [DOI] [PubMed] [Google Scholar]
- 10.Bartoov-Shifman R, Hertz R, Wang H, Wollheim CB, Bar-Tana J, Walker MD. Activation of the insulin gene promoter through a direct effect of hepatocyte nuclear factor 4 alpha. J Biol Chem. 2002;277:25914–25919. doi: 10.1074/jbc.M201582200. [DOI] [PubMed] [Google Scholar]
- 11.Yamagata K, Furuta H, Oda N, Kaisaki PJ, Menzel S, Cox NJ, Fajans SS, Signorini S, Stoffel M, Bell GI. Mutations in the hepatocyte nuclear factor-4alpha gene in maturity-onset diabetes of the young (MODY1) Nature. 1996;384:458–460. doi: 10.1038/384458a0. [DOI] [PubMed] [Google Scholar]
- 12.Gupta RK, Vatamaniuk MZ, Lee CS, Flaschen RC, Fulmer JT, Matschinsky FM, Duncan SA, Kaestner KH. The MODY1 gene HNF-4alpha regulates selected genes involved in insulin secretion. J Clin Invest. 2005;115:1006–1015. doi: 10.1172/JCI200522365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miura A, Yamagata K, Kakei M, Hatakeyama H, Takahashi N, Fukui K, Nammo T, Yoneda K, Inoue Y, Sladek FM, et al. Hepatocyte nuclear factor-4alpha is essential for glucose-stimulated insulin secretion by pancreatic beta-cells. J Biol Chem. 2006;281:5246–5257. doi: 10.1074/jbc.M507496200. [DOI] [PubMed] [Google Scholar]
- 14.Lacy PE, Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967;16:35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- 15.Gotoh M, Maki T, Satomi S, Porter J, Bonner-Weir S, O'Hara CJ, Monaco AP. Reproducible high yield of rat islets by stationary in vitro digestion following pancreatic ductal or portal venous collagenase injection. Transplantation. 1987;43:725–730. doi: 10.1097/00007890-198705000-00024. [DOI] [PubMed] [Google Scholar]
- 16.Latif ZA, Noel J, Alejandro R. A simple method of staining fresh and cultured islets. Transplantation. 1988;45:827–830. [PubMed] [Google Scholar]
- 17.Chen XB, Li YX, Jiao Y, Dong WP, Li G, Chen J, Tan JM. Influence of heme oxygenase-1 gene transfer on the viability and function of rat islets in in vitro culture. World J Gastroenterol. 2007;13:1053–1059. doi: 10.3748/wjg.v13.i7.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janjic D, Wollheim CB. Islet cell metabolism is reflected by the MTT (tetrazolium) colorimetric assay. Diabetologia. 1992;35:482–485. doi: 10.1007/BF02342448. [DOI] [PubMed] [Google Scholar]
- 19.de Vargas LM, Sobolewski J, Siegel R, Moss LG. Individual beta cells within the intact islet differentially respond to glucose. J Biol Chem. 1997;272:26573–26577. doi: 10.1074/jbc.272.42.26573. [DOI] [PubMed] [Google Scholar]
- 20.Brissova M, Fowler MJ, Nicholson WE, Chu A, Hirshberg B, Harlan DM, Powers AC. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem. 2005;53:1087–1097. doi: 10.1369/jhc.5C6684.2005. [DOI] [PubMed] [Google Scholar]
- 21.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 22.Aalinkeel R, Srinivasan M, Kalhan SC, Laychock SG, Patel MS. A dietary intervention (high carbohydrate) during the neonatal period causes islet dysfunction in rats. Am J Physiol. 1999;277:E1061–E1069. doi: 10.1152/ajpendo.1999.277.6.E1061. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Jeppesen PB, Abudula R, Dyrskog SE, Colombo M, Hermansen K. Stevioside does not cause increased basal insulin secretion or beta-cell desensitization as does the sulphonylurea, glibenclamide: studies in vitro. Life Sci. 2006;78:1748–1753. doi: 10.1016/j.lfs.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Rustenbeck I, Wienbergen A, Bleck C, Jorns A. Desensitization of insulin secretion by depolarizing insulin secretagogues. Diabetes. 2004;53 Suppl 3:S140–S150. doi: 10.2337/diabetes.53.suppl_3.s140. [DOI] [PubMed] [Google Scholar]
- 25.Ko BS, Choi SB, Park SK, Jang JS, Kim YE, Park S. Insulin sensitizing and insulinotropic action of berberine from Cortidis rhizoma. Biol Pharm Bull. 2005;28:1431–1437. doi: 10.1248/bpb.28.1431. [DOI] [PubMed] [Google Scholar]
- 26.German MS. Glucose sensing in pancreatic islet beta cells: the key role of glucokinase and the glycolytic intermediates. Proc Natl Acad Sci USA. 1993;90:1781–1785. doi: 10.1073/pnas.90.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matschinsky F, Liang Y, Kesavan P, Wang L, Froguel P, Velho G, Cohen D, Permutt MA, Tanizawa Y, Jetton TL. Glucokinase as pancreatic beta cell glucose sensor and diabetes gene. J Clin Invest. 1993;92:2092–2098. doi: 10.1172/JCI116809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patane G, Piro S, Anello M, Rabuazzo AM, Vigneri R, Purrello F. Exposure to glibenclamide increases rat beta cells sensitivity to glucose. Br J Pharmacol. 2000;129:887–892. doi: 10.1038/sj.bjp.0703131. [DOI] [PMC free article] [PubMed] [Google Scholar]


