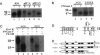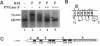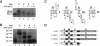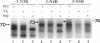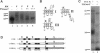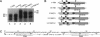Abstract
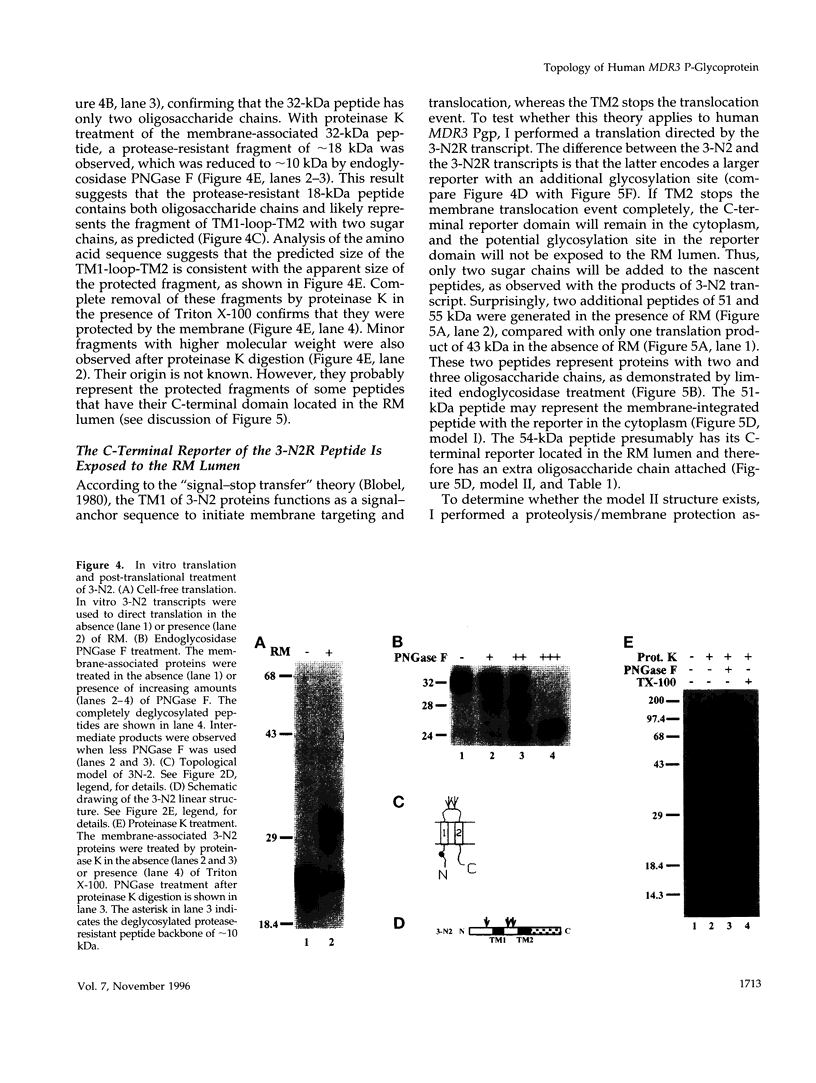
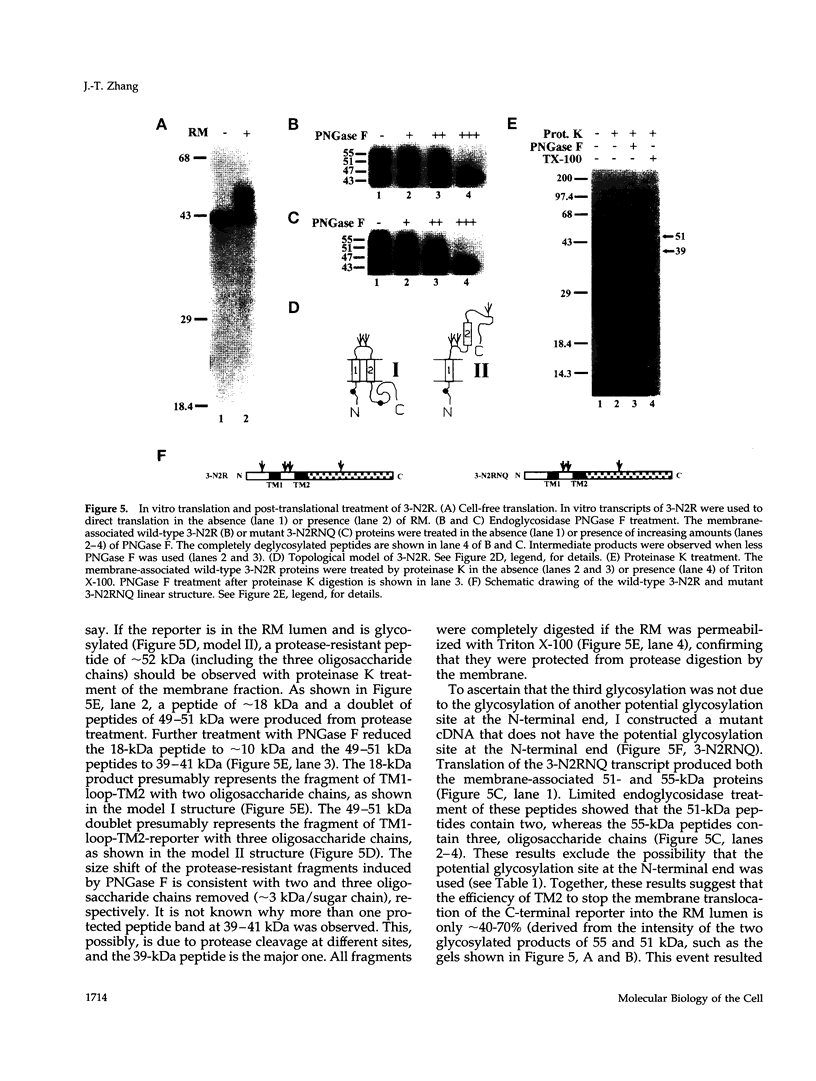
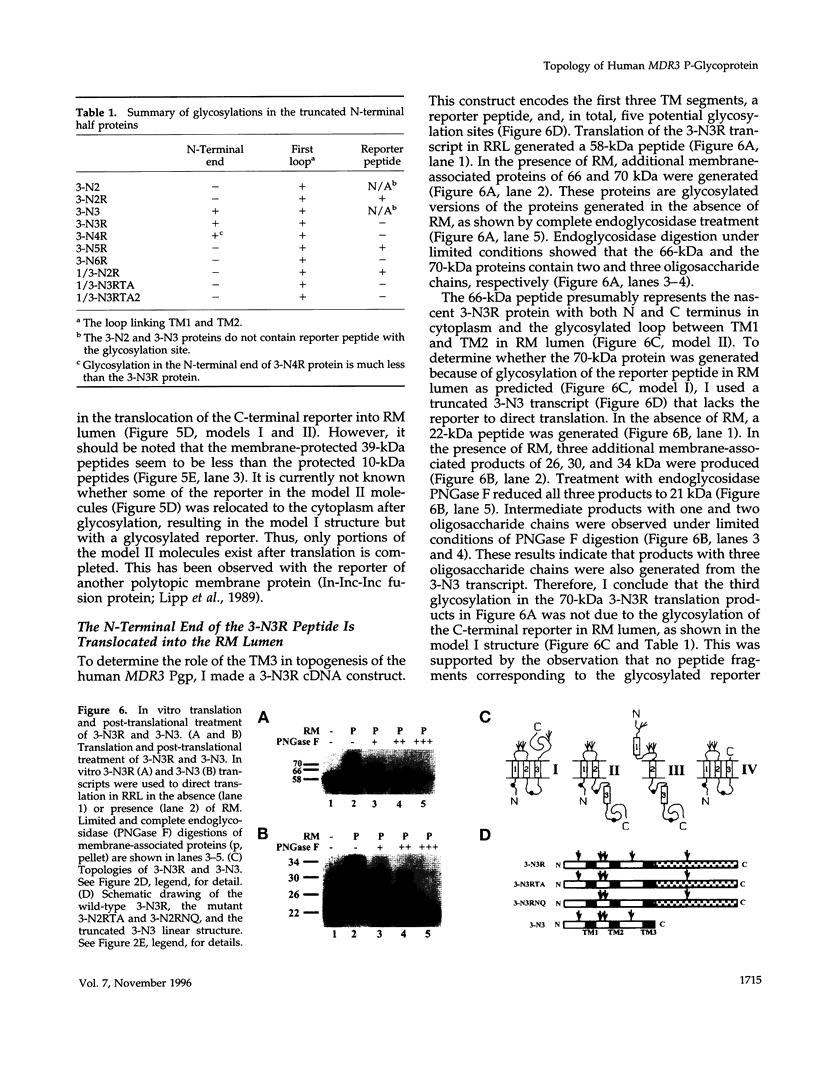
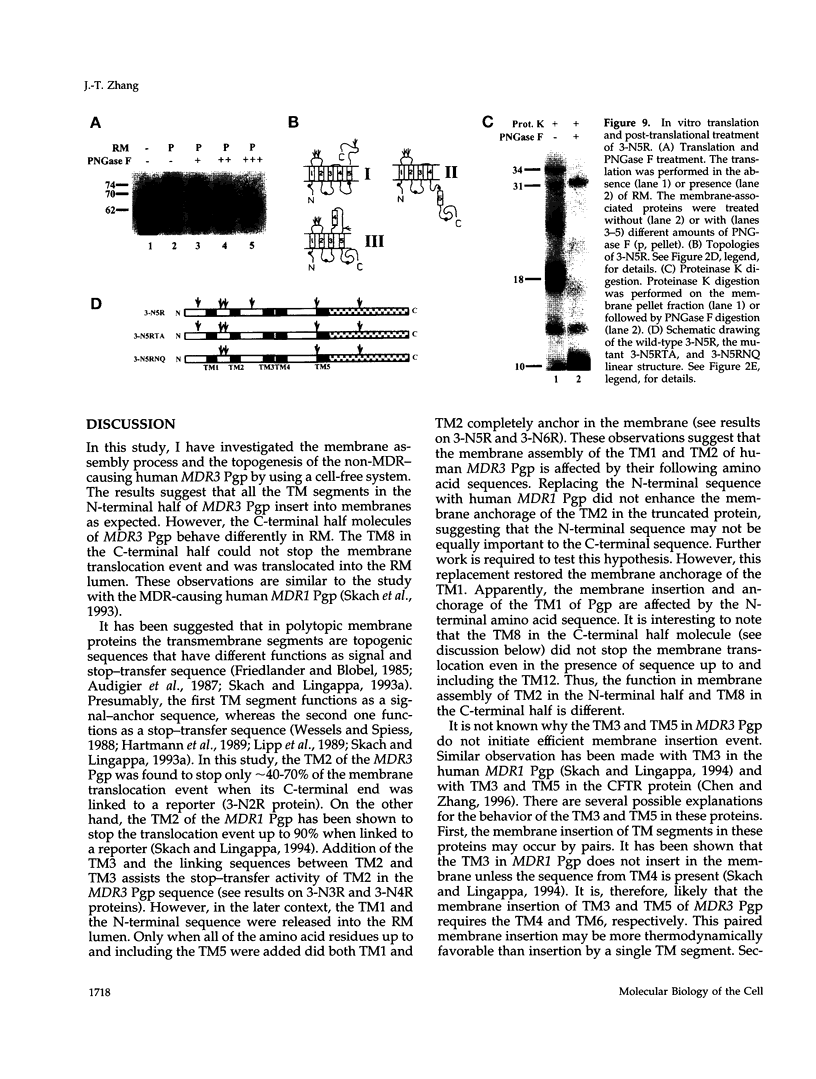
The biogenesis of membrane proteins with a single transmembrane (TM) segment is well understood. However, understanding the biogenesis and membrane assembly of membrane proteins with multiple TM segments is still incomplete because of the complexity and diversity of polytopic membrane proteins. In an attempt to investigate further the biogenesis of polytopic membrane proteins, I used the human MDR3 P-glycoprotein (Pgp) as a model polytopic membrane protein and expressed it in a coupled cell-free translation/translocation system. I showed that the topogenesis of the C-terminal half MDR3 Pgp molecule is different from that of the N-terminal half. This observation is similar to that of the human MDR1 Pgp. The membrane insertion properties of the TM1 and TM2 in the N-terminal half molecule are different. The proper membrane anchorage of both TM1 and TM2 of the MDR3 Pgp is affected by their C-terminal amino acid sequences, whereas only the membrane insertion of the TM1 is dependent on the N-terminal amino acid sequences. The efficient membrane insertion of TM3 and TM5 of MDR3 Pgp, on the other hand, requires the presence of the putative TM4 and TM6, respectively. The TM8 in the C-terminal half does not contain an efficient stop-transfer activity. These observations suggest that the membrane insertion of putative TM segments in the human MDR3 Pgp does not simply follow the prevailing sequential event of the membrane insertion by signal-anchor and stop-transfer sequences. These results, together with my previous findings, suggest that different isoforms of Pgp can be used in comparison as a model system to understand the molecular mechanism of topogenesis of polytopic membrane proteins.
Full text
PDF

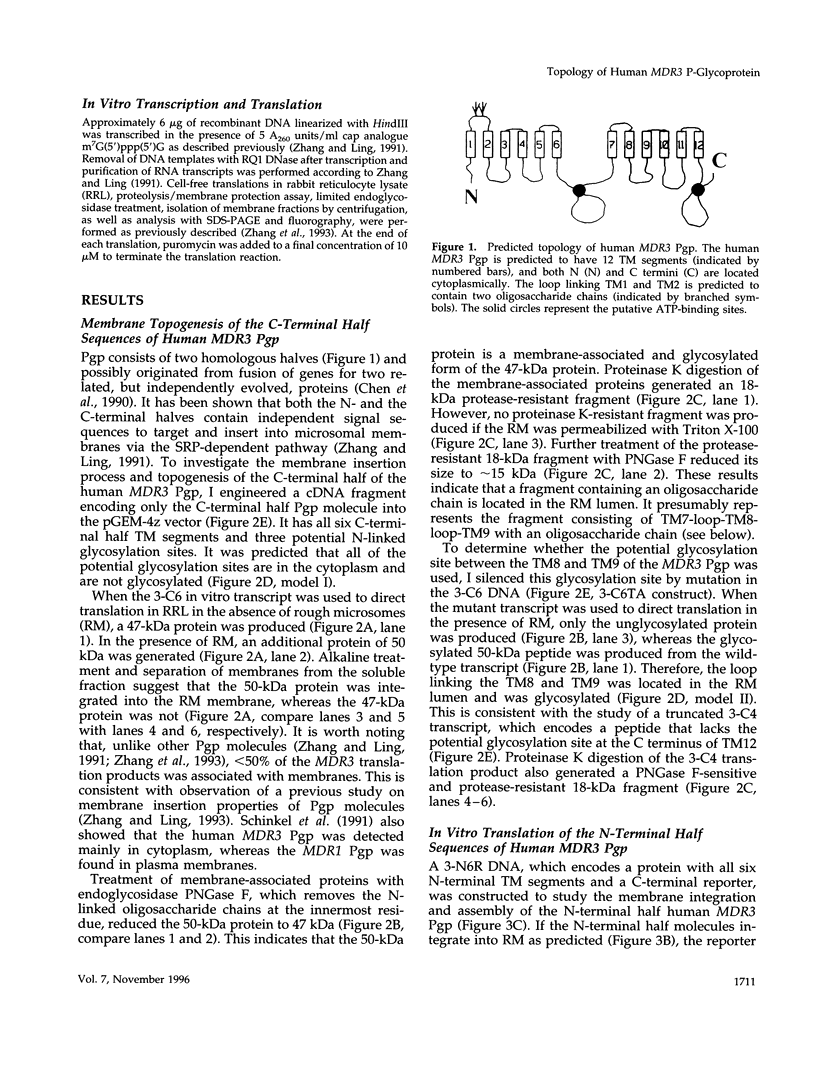
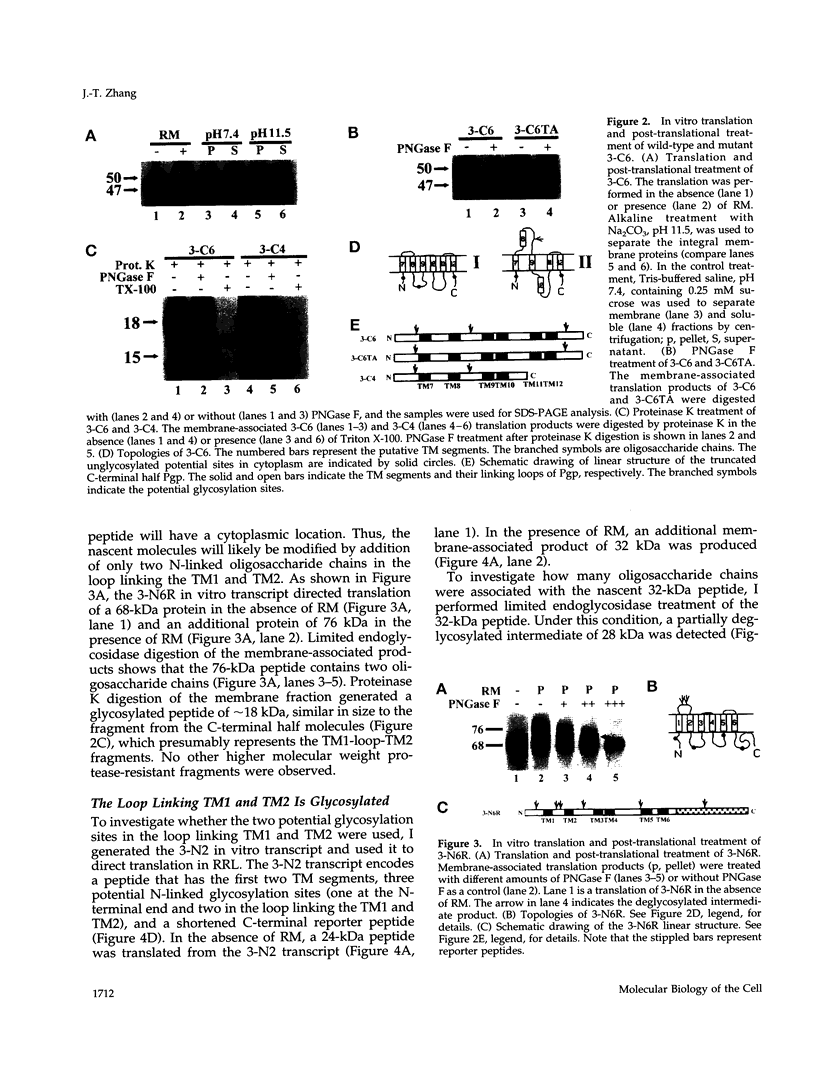




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Audigier Y., Friedlander M., Blobel G. Multiple topogenic sequences in bovine opsin. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5783–5787. doi: 10.1073/pnas.84.16.5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibi E., Béjà O. Membrane topology of multidrug resistance protein expressed in Escherichia coli. N-terminal domain. J Biol Chem. 1994 Aug 5;269(31):19910–19915. [PubMed] [Google Scholar]
- Blobel G. Intracellular protein topogenesis. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1496–1500. doi: 10.1073/pnas.77.3.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braell W. A., Lodish H. F. The erythrocyte anion transport protein is contranslationally inserted into microsomes. Cell. 1982 Jan;28(1):23–31. doi: 10.1016/0092-8674(82)90371-3. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Simoni R. D. Biogenesis of 3-hydroxy-3-methylglutaryl-coenzyme A reductase, an integral glycoprotein of the endoplasmic reticulum. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1674–1678. doi: 10.1073/pnas.81.6.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béjà O., Bibi E. Multidrug resistance protein (Mdr)-alkaline phosphatase hybrids in Escherichia coli suggest a major revision in the topology of the C-terminal half of Mdr. J Biol Chem. 1995 May 26;270(21):12351–12354. doi: 10.1074/jbc.270.21.12351. [DOI] [PubMed] [Google Scholar]
- Chavez R. A., Hall Z. W. The transmembrane topology of the amino terminus of the alpha subunit of the nicotinic acetylcholine receptor. J Biol Chem. 1991 Aug 15;266(23):15532–15538. [PubMed] [Google Scholar]
- Chen C. J., Clark D., Ueda K., Pastan I., Gottesman M. M., Roninson I. B. Genomic organization of the human multidrug resistance (MDR1) gene and origin of P-glycoproteins. J Biol Chem. 1990 Jan 5;265(1):506–514. [PubMed] [Google Scholar]
- Chen M., Zhang J. T. Membrane insertion, processing, and topology of cystic fibrosis transmembrane conductance regulator (CFTR) in microsomal membranes. Mol Membr Biol. 1996 Jan-Mar;13(1):33–40. doi: 10.3109/09687689609160572. [DOI] [PubMed] [Google Scholar]
- Friedlander M., Blobel G. Bovine opsin has more than one signal sequence. 1985 Nov 28-Dec 4Nature. 318(6044):338–343. doi: 10.1038/318338a0. [DOI] [PubMed] [Google Scholar]
- Goldman B. M., Blobel G. In vitro biosynthesis, core glycosylation, and membrane integration of opsin. J Cell Biol. 1981 Jul;90(1):236–242. doi: 10.1083/jcb.90.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann E., Rapoport T. A., Lodish H. F. Predicting the orientation of eukaryotic membrane-spanning proteins. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5786–5790. doi: 10.1073/pnas.86.15.5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A. How N-linked oligosaccharides affect glycoprotein folding in the endoplasmic reticulum. Mol Biol Cell. 1994 Mar;5(3):253–265. doi: 10.1091/mbc.5.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast C., Canfield V., Levenson R., Gros P. Membrane topology of P-glycoprotein as determined by epitope insertion: transmembrane organization of the N-terminal domain of mdr3. Biochemistry. 1995 Apr 4;34(13):4402–4411. doi: 10.1021/bi00013a032. [DOI] [PubMed] [Google Scholar]
- Kast C., Canfield V., Levenson R., Gros P. Transmembrane organization of mouse P-glycoprotein determined by epitope insertion and immunofluorescence. J Biol Chem. 1996 Apr 19;271(16):9240–9248. doi: 10.1074/jbc.271.16.9240. [DOI] [PubMed] [Google Scholar]
- Lipp J., Flint N., Haeuptle M. T., Dobberstein B. Structural requirements for membrane assembly of proteins spanning the membrane several times. J Cell Biol. 1989 Nov;109(5):2013–2022. doi: 10.1083/jcb.109.5.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo T. W., Clarke D. M. Membrane topology of a cysteine-less mutant of human P-glycoprotein. J Biol Chem. 1995 Jan 13;270(2):843–848. doi: 10.1074/jbc.270.2.843. [DOI] [PubMed] [Google Scholar]
- Schekman R. Translocation gets a push. Cell. 1994 Sep 23;78(6):911–913. doi: 10.1016/0092-8674(94)90265-8. [DOI] [PubMed] [Google Scholar]
- Schinkel A. H., Roelofs E. M., Borst P. Characterization of the human MDR3 P-glycoprotein and its recognition by P-glycoprotein-specific monoclonal antibodies. Cancer Res. 1991 May 15;51(10):2628–2635. [PubMed] [Google Scholar]
- Sengstag C., Stirling C., Schekman R., Rine J. Genetic and biochemical evaluation of eucaryotic membrane protein topology: multiple transmembrane domains of Saccharomyces cerevisiae 3-hydroxy-3-methylglutaryl coenzyme A reductase. Mol Cell Biol. 1990 Feb;10(2):672–680. doi: 10.1128/mcb.10.2.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silve S., Volland C., Garnier C., Jund R., Chevallier M. R., Haguenauer-Tsapis R. Membrane insertion of uracil permease, a polytopic yeast plasma membrane protein. Mol Cell Biol. 1991 Feb;11(2):1114–1124. doi: 10.1128/mcb.11.2.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skach W. R., Calayag M. C., Lingappa V. R. Evidence for an alternate model of human P-glycoprotein structure and biogenesis. J Biol Chem. 1993 Apr 5;268(10):6903–6908. [PubMed] [Google Scholar]
- Skach W. R., Lingappa V. R. Amino-terminal assembly of human P-glycoprotein at the endoplasmic reticulum is directed by cooperative actions of two internal sequences. J Biol Chem. 1993 Nov 5;268(31):23552–23561. [PubMed] [Google Scholar]
- Skach W. R., Lingappa V. R. Transmembrane orientation and topogenesis of the third and fourth membrane-spanning regions of human P-glycoprotein (MDR1). Cancer Res. 1994 Jun 15;54(12):3202–3209. [PubMed] [Google Scholar]
- Slatin S. L., Qiu X. Q., Jakes K. S., Finkelstein A. Identification of a translocated protein segment in a voltage-dependent channel. Nature. 1994 Sep 8;371(6493):158–161. doi: 10.1038/371158a0. [DOI] [PubMed] [Google Scholar]
- Wessels H. P., Spiess M. Insertion of a multispanning membrane protein occurs sequentially and requires only one signal sequence. Cell. 1988 Oct 7;55(1):61–70. doi: 10.1016/0092-8674(88)90009-8. [DOI] [PubMed] [Google Scholar]
- Zhang J. T., Duthie M., Ling V. Membrane topology of the N-terminal half of the hamster P-glycoprotein molecule. J Biol Chem. 1993 Jul 15;268(20):15101–15110. [PubMed] [Google Scholar]
- Zhang J. T., Lee C. H., Duthie M., Ling V. Topological determinants of internal transmembrane segments in P-glycoprotein sequences. J Biol Chem. 1995 Jan 27;270(4):1742–1746. doi: 10.1074/jbc.270.4.1742. [DOI] [PubMed] [Google Scholar]
- Zhang J. T., Ling V. Membrane orientation of transmembrane segments 11 and 12 of MDR- and non-MDR-associated P-glycoproteins. Biochim Biophys Acta. 1993 Dec 12;1153(2):191–202. doi: 10.1016/0005-2736(93)90405-o. [DOI] [PubMed] [Google Scholar]
- Zhang J. T., Ling V. Study of membrane orientation and glycosylated extracellular loops of mouse P-glycoprotein by in vitro translation. J Biol Chem. 1991 Sep 25;266(27):18224–18232. [PubMed] [Google Scholar]
- Zhang M., Wang G., Shapiro A., Zhang J. T. Topological folding and proteolysis profile of P-glycoprotein in membranes of multidrug-resistant cells: implications for the drug-transport mechanism. Biochemistry. 1996 Jul 30;35(30):9728–9736. doi: 10.1021/bi960400s. [DOI] [PubMed] [Google Scholar]