Abstract
Objectives
To identify pregnancy-induced changes in biomechanical properties of the vaginal wall and compare these with Fibulin-5 knockout mice (Fbln5-/-) with and without prolapse.
Study Design
Mid-vaginal segments of nonpregnant and late-pregnant wild type (WT), Fbln5-/- with prolapse, and Fbln5-/- mice without prolapse were studied. Tissue length at failure, maximal strain, maximal stress, and tissue stiffness were determined.
Results
Compared with nonpregnant mice, vaginas of pregnant and Fbln5-/- (with prolapse) mice exhibited decreased maximal stress, increased distensibility and strain, and decreased stiffness. Tissues from Fbln5-/- mice without prolapse were similar to nonpregnant WT animals.
Conclusions
Pregnancy confers remarkable changes in the vaginal wall including increased distensibility and decreased stiffness and maximal stress. Elastinopathy alone is insufficient to cause significant changes in these properties, but prolapse confers additional alterations in distensibility and stiffness similar to those observed in pregnancy. These changes may contribute to the poor durability of many restorative surgical procedures for prolapse.
Keywords: stress-strain, vaginal muscularis, pelvic organ support, fibulin-5, elastic fibers
Introduction
The physiologic processes of pregnancy and parturition must impart dramatic adaptations of the vaginal wall and pelvic floor to allow for marked vaginal distention followed by a rapid return to a pre-pregnant-like state. Yet, numerous epidemiologic studies suggest that many women fail to completely recover from this event; indeed, vaginal distention trauma appears to play an important role in the etiology of pelvic organ prolapse with vaginal delivery conferring a 4- to 11-fold increase in the risk of developing prolapse (1, 2). In women with overt prolapse of the vaginal vault, there is pathologic distention of the vagina that fails to spontaneously recover and often progresses to a larger and more symptomatic bulge.
Recent studies indicate that there are altered histomorphological features in patients with pelvic floor disorders such as prolapsed vaginas and incontinence and that these features are accompanied by changes in the ratio of collagen subtypes and in elastic fiber homeostasis (3-8). Fewer studies have examined the changes in biomechanical properties associated with the physiologic events of pregnancy and parturition or the pathologic events of prolapse. Biomechanical properties may be useful tools for understanding the underlying structural changes that occur in both physiologic and pathologic conditions that alter function of the vaginal wall and its support.
Mice with null mutations in the gene encoding fibulin-5 (Fbln5-/-) demonstrate numerous signs of elastinopathy including lax skin, emphysematous lungs, and dilated and tortuous great vessels. Importantly, they also demonstrate pelvic organ prolapse remarkably similar to that in primates, i.e. the vagina and cervix are descended, stretched, and herniate through the pelvic floor musculature. The vaginas in prolapsed animals are patulous with a widened genital hiatus (4). Serial prolapse examinations in a large colony of Fbln5-/- mice showed that 91% of these animals reliably progress to severe pelvic organ prolapse as a function of age (9). Like in humans, increasing parity appears to accelerate this disease process. Therefore, Fbln5-/- mice are excellent models in which to examine the effects of prolapse on the biomechanical properties of the vaginal wall.
The objectives of this study were to identify pregnancy-induced changes in biomechanical properties of the vaginal wall and to compare these changes with those that occur in Fbln5-/- mice with and without pelvic organ prolapse.
Material and Methods
Mice
A total of 25 female mice were studied and sacrificed in accordance with the standards of humane animal care described by the National Institutes of Health Guide for the Care and Use of Laboratory Animals, using protocols approved by the Institutional Animal Care and Use Committee of University of Texas Southwestern Medical Center. Animals were housed under a 12-hour light cycle at 22°C. All wild type (WT) mice used in these studies were C3BL/6J. Fbln5-/- mice were of a similar mixed strain (C57BL/6 × 129SvEv). To obtain timed pregnant animals, nulliparous females were housed with males for 4 to 6 hours and checked at midday for vaginal plugs. Plug day was considered day 0.
Eight nonpregnant virginal WT mice were sacrificed and used as controls for comparisons with 6 late-pregnant nulliparous (day 18) WT, 7 virginal Fbln5-/- with severe vaginal and perineal prolapse, and 4 age-matched virginal Fbln5-/- without prolapse. After disarticulation of the pubic symphysis, uterine horns together with the bladder, cervix, and vagina were dissected down to the perineal skin. The vaginal dissection extended down to the connective tissue suspending the vaginal wall to the pubocaudalis. Using a dissection microscope, 3 mm wide transverse (i.e. cross-sectional) segments were excised from the middle third of the vagina using a scalpel blade. This section of the vaginal wall is believed to contribute to Level II support in women (10). The portion of the urethra on the anterior vaginal wall was left in place on the excised specimens.
Biomechanical Testing
The vaginal segments were kept intact as rings and mounted loosely between 2 stainless steel wires, one of which was fastened to a steel rod mounted with a calibrated mechanical drive and the other to a force transducer connected to a Grass FT.03C force transducer. Tissues were maintained in physiologic salt solution in water-jacketed baths at 37°C with 95% O2 and 5% CO2. After acclimation in the baths for 15 minutes, ring diameter was equilibrated to slack length, i.e. the length of initial resting tone. This distance was measured using the calibrated mechanical drive and confirmed using a digital caliper sensitive to 0.01 mm. All rings were then distended in 0.5 mm increments with 2 minute intervals between successive changes in length to allow the force generated to relax and come to a new steady-state. This series of step-strains was continued until failure (ring breakage) or until the force generated with further distention reached a plateau. This tissue length at failure (i.e. maximal distention) was measured with the mechanical drive and the digital caliper. The maximal force generated in grams with each increase in distention and the force at tissue failure was determined.
Calculations
Force in grams was converted to Newtons (N). The wet weight of the vaginal segments was measured after biomechanical testing, and the cross-sectional area was calculated by taking the volume of the sample (wet weight divided by specific gravity of smooth muscle tissue) and dividing this by slack length. Stress (N/m2) was then determined as force per unit area and plotted against strain (change in length of the specimen divided by its slack length). The linear segment of this sigmoid-shaped curve was considered the tissue's elastic portion, and the slope of this line computed as the modulus of elasticity, an index of tissue stiffness (N/m2). To compare the different specimens to one another, best-fit sigmoidal curves were calculated for the stress-strain relationship of each specimen using SigmaPlot software (version 10.0), and the corresponding stress was determined for every 5% increase in strain.
Statistics
Statistical analyses were made using SigmaStat software version 2.03 (Jandel Scientific, San Rafael, CA). Maximal force at failure, stress, distensiblity, strain, and stiffness values were compared for the vaginal tissues of nulliparous nonpregnant WT controls and the pregnant animals using Student t-tests (or Mann-Whitney Rank Sum Test for nonparametric data). Nonpregnant WT controls were compared to the Fbln5-/- mice with and without prolapse using one-way analysis of variance with Student-Newman-Keuls multiple comparison test for differences between groups. A P value <0.05 was considered statistically significant.
Results
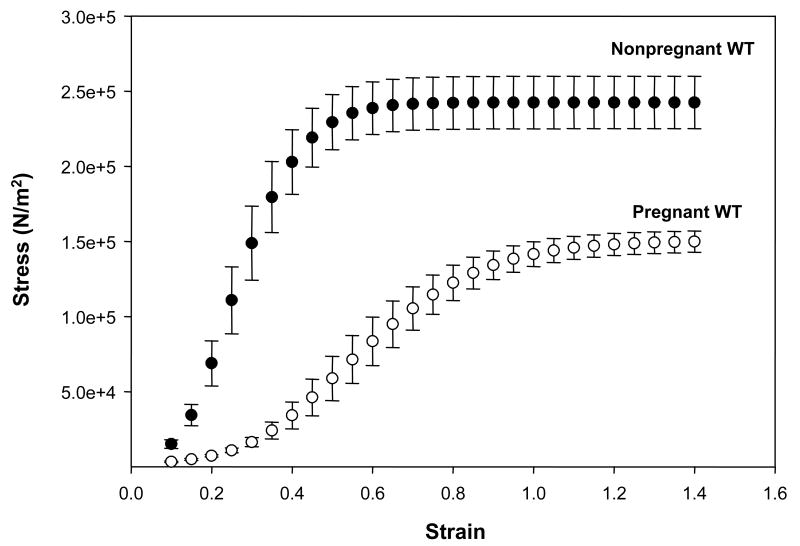
The stress-strain curves of vaginal tissues from late-pregnant animals and nonpregnant controls are shown in Figure 1. In vaginal tissues from nonpregnant WT mice, small increases in strain resulted in marked increases in stress generation indicating a stiff, nondistensible tissue. In contrast, in vaginal tissues from pregnant animals, large increases in strain resulted in relatively small increases in stress, and stress continued to increase until tissue diameters reached twice that of resting length, i.e. strain = 1.0 (Figure 1). In vaginal rings from nonpregnant mice, however, maximal increases in stress were observed with only 50% increases in resting diameter. Tissue load at failure was similar in pregnant and nonpregnant mice (Table 1). Tissue cross-sectional area, however, was increased significantly in vaginal rings from pregnant animals. Thus, maximal stress of the vaginal wall corrected for cross-sectional area was decreased markedly in pregnant animals (P <0.001, Table 1). Maximal distension of the vaginal wall was increased significantly during pregnancy (3-fold, P <0.001). Not only was resting vaginal diameter increased, but maximal strain was also increased 2-fold during pregnancy (P <0.001, Table 1). Linear stiffness was decreased 3.4-fold in the vaginal wall from pregnant mice compared with nonpregnant animals (P <0.001).
Figure 1. Stress-strain relationships of vaginal tissues from pregnant day-18 wild type mice (open circle, n = 6) compared with nonpregnant wild type controls (closed circle, n = 8).
Strain was calculated as the change in length of the specimen divided by its slack length, and stress is expressed in Newtons per cross-sectional area. Mean values are shown with standard errors.
Table I.
Biomechanical properties of vaginal tissues from nonpregnant nulliparous wild-type mice are compared with those of tissues from pregnant wild-type mice, Fbln5-/- mice without prolapse, and Fbln5-/- mice with prolapse.
| Mouse Group | n | Load at Failure (N) |
P* | Maximal Stress (×105 N/m2) |
P | Max Distention (mm) |
P | Max Strain (%) |
P | Stiffness (×105 N/m2) |
P |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WT Nonpregnant (nulliparous) | 8 | 0.54 ± 0.02 | - | 2.3 ± 0.16 | - | 2.9 ± 0.2 | - | 44 ± 4.3 | - | 9.5 ± 1.1 | - |
| WT Late Pregnant (d.18) | 6 | 0.50 ± 0.01 | NS | 1.4 ± 0.07 | <0.001 | 8.4 ± 0.6 | <0.001 | 87 ± 7.7 | <0.001 | 2.8 ± 0.50 | <0.001 |
| Fbln5-/- Without Prolapse | 4 | 0.50 ± 0.02 | NS | 2.2 ± 0.20 | 0.808 | 3.6 ± 0.7 | 0.223 | 51 ± 8.0 | 0.434 | 7.0 ± 0.76 | 0.181 |
| Fbln5-/- With Prolapse | 7 | 0.54 ± 0.02 | NS | 1.8 ± 0.13 | 0.058 | 9.6 ± 0.2 | <0.001 | 82 ± 9.7 | 0.003 | 4.9 ± 0.55 | 0.004 |
WT, wild type
Fbln5-/-, fibulin-5 knockout mice
P values compared with nulliparous WT controls
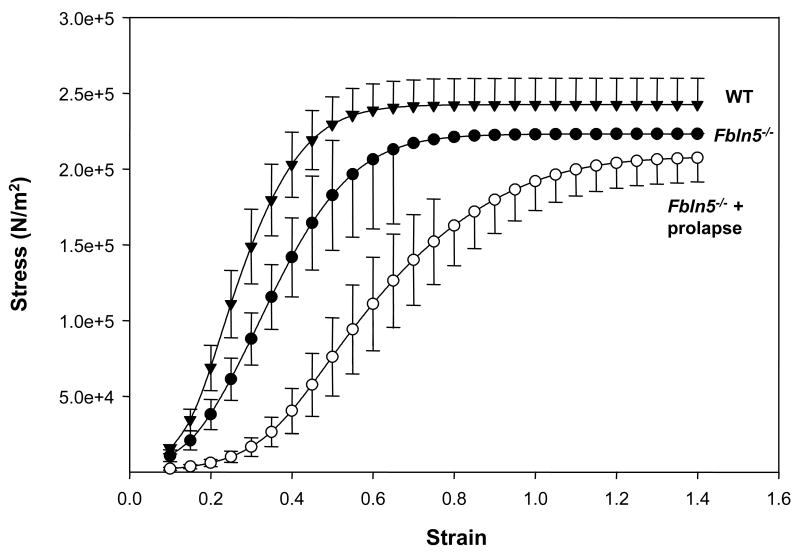
To determine if pregnancy-induced changes in biomechanical properties of the vaginal wall were similar to those observed in animal models of pelvic organ prolapse, vaginal tissues from Fbln5-/- mice were compared with nonpregnant wild type animals (Figure 2). Interestingly, vaginal tissues from Fbln5-/- mice with prolapse demonstrated a pattern of biomechanical properties very similar to those of pregnant animals. Specifically, stress at tissue failure was decreased in the prolapsed vaginal wall compared with vaginal tissues from nonpregnant WT mice (1.2-fold, P =0.058). Resting vaginal diameter was increased significantly in Fbln5-/- mice with prolapse, and maximal distention before failure was also increased 2-fold (both P <0.001). Like tissues from pregnant animals, vaginal stiffness was decreased significantly in Fbln5-/- mice (P =0.004, Table 1). These data indicate that biomechanical properties of the prolapsed vaginal wall are similar to those observed during pregnancy, i.e., decreased stiffness, decreased maximal load, increased distensibility, and increased vaginal diameter.
Figure 2. Stress-strain relationships of vaginal tissues from Fbln5-/- mice with prolapse (open circles, n = 7) and Fbln5-/- mice without prolapse (closed circles, n = 4) compared with nonpregnant wild type controls (closed triangles, n = 6).
Mean values are shown with standard errors.
Changes in the biomechanical properties of the vaginal wall in Fbln5-/- mice with prolapse may be due to the absence of normal elastic fibers in the vagina or to additional changes brought about by prolapse of the organ. Thus, we determined the biomechanical properties of vaginal tissues from Fbln5-/- mice with and without prolapse (Figure 2, Table 1). Surprisingly, biomechanical properties of vaginal tissues from Fbln5-/- mice without prolapse were similar to nonpregnant WT mice with no significant differences in load at tissue failure, maximal stress, distance distended, strain, or linear stiffness. It is possible that we failed to find small, but significant, differences in knockout animals without prolapse because of the few number of animals in this group (n = 4).
Comment
Changes with Pregnancy
In this study using full-thickness sections of isolated mouse vagina, we found that pregnancy confers remarkable physiologic changes in biomechanical properties. During pregnancy, the resting diameter of the vaginal wall is increased and may distend up to 3 times in diameter compared with vaginal tissues from nonpregnant animals (i.e., 2-fold increase in strain). Tissue stiffness is decreased (i.e. distensibility is increased). These appear to be intuitive compensations to allow for parturition. These changes, however, come at the expense of vaginal wall strength; the pregnant vagina endured less maximal stress (force per unit area). Thus, while the vagina appears to undergo substantial adaptations during pregnancy to allow maximal flexibility and distention, late-pregnancy is ripe for significant vaginal wall trauma if the vaginal wall is stretched beyond these adaptations (as in the settings of cephalo-pelvic disproportion, prolonged late-stage labor, or forceps delivery).
These findings are in agreement with a recent report by Lowder, et al in which the entire reproductive system of the rat with the vagina and its supportive connective tissue attachments were tested as a complex. The system was tensioned in a uniaxial load-to-failure test by pulling the distal vagina downward in its longitudinal axis. Using this technique, mean linear stiffness and ultimate load at failure were decreased in pregnancy and at delivery while maximal distention was increased at time of delivery (11). By testing the entire complex with the surrounding connective tissue attachments intact, their model was sensitive to the compensatory adaptations and interactions of the uterosacral ligaments, perineal membrane, vagina, and paravaginal attachments. Our study expands and complements their findings by examining the biomechanical properties of one component of this system in isolation: the vaginal wall. Our testing protocol involved interval rests between progressive increases in vaginal diameter to allow for tissue recovery between successive stretches. This protocol perhaps provides more information regarding the biomechanical properties of the vagina during parturition with cyclic and progressive distention of the vagina.
In 1974, Åke Rundgren elegantly described the biomechanical testing of the vagina, cervix, uterine horns, and some extra-reproductive tract tissues in pregnant rats at various gestational ages and up to 38 days postpartum (12). The effects of primiparity and multiparity were also compared. In that investigation, vaginal weight increased 100% over the course of gestation and vaginal length increased 60%. At term, vaginal circumference was twice that of nonpregnant rats. Similar to the current study, Rundgren found marked changes in the biomechanical properties of the pregnant rat vagina: maximal displacement increased (1.5-fold), maximal load decreased (3-4-fold), maximal endurable stress decreased 50%, and the “stress-strain relationship”, or stiffness, also decreased. Interestingly, with repeated pregnancies, there was a further decrease in the force-resisting capacity of the vaginal walls suggesting that the vagina may lose strength imparted by the extracellular matrix with each pregnancy (12).
Of note, the vagina has anisotropic mechanical properties, i.e. different responses are seen in different directions of loading. Our study's design for vaginal distention has the benefit of recapitulating the transverse stretching that occurs in parturition; the mid-vaginal cross-sectional ring was kept intact as opposed to cutting a length-wise strip of vaginal tissue or by pulling in the longitudinal axis.
Changes with Prolapse
The etiology of prolapse is complex and multifactorial, but numerous human and animal studies point to the associated trauma of childbirth as playing a significant role. DeLancey, et al. have shown that gross changes to the surrounding pelvic muscle support, i.e. the levator ani, are evident in women with prolapse. These patients are able to generate less vaginal closure force during muscle contractions, and their genital hiatus is wider than in nonprolapsed controls (13). Overall, it appears that parturition with vaginal distention can adversely affect the integrity of gross muscular support of the levator ani, the smooth and striated muscle of the urethral and vaginal walls, and the connective tissue network crucial to pelvic visceral support.
The studies of biomechanical properties that have been done in women with prolapse have yielded conflicting results. Goh, et al. studied pre- and postmenopausal women with prolapse and found similar degrees of tissue deformation (i.e. maximal strain) despite menopausal status. Tissues from women without prolapse were not tested (14). Lei, et al. described significant differences between pre- and postmenopausal women with and without prolapse; in general, prolapse conferred less elasticity and greater stiffness although vaginal tissues from women with severe prolapse exhibited low forces at failure (15). A third study showed marked variability in maximal strength and strain with no discernable trends in biomechanical properties of vaginal samples taken from 16 postmenopausal women with prolapse (16).
Part of the variability in current human studies of biomechanical properties of vaginal prolapse is likely due to the method of tissue acquisition. When vaginal wall is excised during a prolapse surgery (e.g. from an anterior colporrhaphy), the vaginal muscularis is often split or separated from the adventitia. The tissue biopsy may be highly variable depending on the ultimate amounts of epithelium, subepithelial connective tissue, and muscularis. In an animal model, full thickness vagina can be examined in all cases. In particular, there is value in using an animal model with prolapse to study the changes in biomechanical properties. The majority of Fbln5-/- mice will develop pelvic organ prolapse with increasing age, but about 10% do not. In this study, these nonprolapsed Fbln5-/- mice, which were age-matched to the prolapsed animals, were not statistically different than nonpregnant controls with respect to maximum stress, strain, or stiffness. Prolapsed knockout animals, however, demonstrated biomechanical properties similar to those of the pregnant animals when compared with nonpregnant controls: the vaginas of prolapsed mice demonstrated increased strain and decreased stiffness with decreased maximal stress. That is, defects in elastin synthesis alone are not enough to cause changes in the biomechanical properties of the vaginal wall. The presence of abnormal elastic fibers in knockout mice generally leads to an increase in matrix-metalloprotease (MMP) activity in the extracellular matrix (4); MMPs will digest collagen fibers in addition to elastin degradative products, and the abnormal or decreased collagen content may ultimately be the determinant of altered biomechanical properties (12). Once a combination of factors—be it age, parity with vaginal distention trauma, or other factors that alter collagen fiber integrity—promote the progression to overt prolapse, the biomechanical properties of the vaginal wall are fundamentally altered.
Clinically, surgical techniques for the correction of pelvic organ prolapse are imperfect and often of poor durability. Approximately 29% of women who undergo surgery for prolapse or incontinence will have repeat operations, and the time between reoperation shortens with each subsequent surgery (17). Intuitively, it seems clear that women who developed vaginal prolapse initially have inferior vaginal wall strength or weaknesses in surrounding connective tissue or muscular support and, thus, would be prone to repetitive failures of surgical repairs. Results of the current study corroborate those surgical findings; in prolapsed tissue, the vaginal wall endures less stress and is more distensible (less stiff). The results indicate that similar changes in biomechanics of pelvic floor connective tissues occur during pregnancy. Since the majority of women do not develop pelvic organ prolapse after vaginal delivery and biomechanical properties return to normal postpartum (12), reparative and restorative processes postpartum are crucial for return of vaginal support after parturition. We suggest that, in prolapse, failure of repair processes such as elastic fiber assembly and synthesis results in irreversible alterations in tissue biomechanics and may contribute to the poor reliability and longevity of restorative surgeries for prolapse.
Acknowledgments
We thank Mr. Jesús Acevedo for expert technical assistance. This work was supported by NIH grant AG028048.
Footnotes
Presented at the American Urogynecologic Society 28th Annual Scientific Meeting, Hollywood, FL, September 27-29, 2007
No reprints will be available
Condensation: Pregnancy and vaginal prolapse both cause changes in biomechanical properties of the vaginal wall: increased distensibility and strain with decreased maximal stress and stiffness.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mant J, Painter R, Vessey M. Epidemiology of genital prolapse: observations from the Oxford Family Planning Association Study. Br J Obstet Gynaecol. 1997;104(5):579–85. doi: 10.1111/j.1471-0528.1997.tb11536.x. [DOI] [PubMed] [Google Scholar]
- 2.Patel DA, Xu X, Thomason AD, Ransom SB, Ivy JS, DeLancey JO. Childbirth and pelvic floor dysfunction: an epidemiologic approach to the assessment of prevention opportunities at delivery. Am J Obstet Gynecol. 2006;195:23–8. doi: 10.1016/j.ajog.2006.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzgerald MP, Mollenhauer J, Hale DS, Benson JT, Brubaker L. Urethral collagen morphologic characteristics among women with genuine stress incontinence. Am J Obstet Gynecol. 2000;182:1565–74. doi: 10.1067/mob.2000.107327. [DOI] [PubMed] [Google Scholar]
- 4.Drewes PG, Yanagisawa H, Starcher B, Hornstra IK, Csiszar K, Marinis SI, et al. Pelvic organ prolapse in Fibulin-5 knockout mice: pregnancy changes in elastic fiber homeostasis in mouse vagina. Am J Pathol. 2007;170:578–89. doi: 10.2353/ajpath.2007.060662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boreham MK, Wai CY, Miller RT, Schaffer JI, Word RA. Morphometric analysis of smooth muscle in the anterior vaginal wall of women with pelvic organ prolapse. Am J Obstet Gynecol. 2002;187(1):56–63. doi: 10.1067/mob.2002.124843. [DOI] [PubMed] [Google Scholar]
- 6.Boreham MK, Wai CY, Miller RT, Schaffer JI, Word RA. Morphometric properties of the posterior vaginal wall in women with pelvic organ prolapse. Am J Obstet Gynecol. 2002;187(6):1501–9. doi: 10.1067/mob.2002.130005. [DOI] [PubMed] [Google Scholar]
- 7.Wong MY, Harmanli OH, Agar M, Dandolu V, Grody MH. Collagen content of nonsupport tissue in pelvic organ prolapse and stress urinary incontinence. Am J Obstet Gynecol. 2003;189:1597–9. doi: 10.1016/j.ajog.2003.09.043. [DOI] [PubMed] [Google Scholar]
- 8.Falconer C, Ekman G, Malmström A, Ulmsten U. Decreased collagen synthesis in stress-incontinent women. Obstet Gynecol. 1994;84:583–6. [PubMed] [Google Scholar]
- 9.Wieslander CK, Acevedo JF, Drewes PG, Yanagisawa HK, Word RA. Pelvic organ prolapse severity increases with age in Fibulin-5 knockock mice. Int Urogynecol J. 2006;17:S371–2. [Google Scholar]
- 10.DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am JU Obstet Gynecol. 1992;166:1717–24. doi: 10.1016/0002-9378(92)91562-o. [DOI] [PubMed] [Google Scholar]
- 11.Lowder JL, Debes KM, Moon DK, Howden N, Abramowitch SD, Moalli PA. Biomechanical adaptations of the rat vagina and supportive tissues in pregnancy to accommodate delivery. Obstet Gynecol. 2007;109:136–43. doi: 10.1097/01.AOG.0000250472.96672.6c. [DOI] [PubMed] [Google Scholar]
- 12.Rundgren A. Physical properties of connective tissue as influenced by single and repeated pregnancies in the rat. Acta Physiol Scand Suppl. 1974;417:1–138. [PubMed] [Google Scholar]
- 13.DeLancey JO, Morgan DM, Fenner DE, Kearney R, Guire K, Miller JM, et al. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol. 2007;109:295–302. doi: 10.1097/01.AOG.0000250901.57095.ba. [DOI] [PubMed] [Google Scholar]
- 14.Goh JT. Biomechanical properties of prolapsed vaginal tissue in pre- and postmenopausal women. Int Urogynecol J Pelvic Floor Dysfunct. 2002;13:76–9. doi: 10.1007/s001920200019. [DOI] [PubMed] [Google Scholar]
- 15.Lei L, Song Y, Chen R. Biomechanical properties of prolapsed vaginal tissue in pre- and postmenopausal women. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:603–7. doi: 10.1007/s00192-006-0214-7. [DOI] [PubMed] [Google Scholar]
- 16.Cosson M, Lambaudie E, Boukerrou M, Lobry P, Crépin G, Ego A. A biomechanical study of the strength of vaginal tissues. Results on 16 post-menopausal patients presenting with genital prolapse. Eur J Obstet Gynecol Reprod Biol. 2004;112(2):201–5. doi: 10.1016/s0301-2115(03)00333-6. [DOI] [PubMed] [Google Scholar]
- 17.Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–6. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]